Abstract
Background & Aims
Gastric hypersensitivity (GHS) contributes to epigastric pain in patients with functional dyspepsia (FD); the etiology and cellular mechanisms of this dysfunction remain unknown. We investigated whether inflammatory insult to the colons of neonatal rats induced GHS in adult life.
Methods
We used cellular, molecular, and in vivo approaches to investigate the mechanisms of GHS in adult rats subjected to neonatal colonic insult by intraluminal administration of trinitrobenzene sulfonic acid (TNBS); controls received saline. Six to 8 weeks later, rats were evaluated for GHS and tissue was collected for molecular experiments.’
Results
Inflammatory insult to the colon on post-natal day (PND) 10 caused an aberrant increase of corticosterone on PND 15 and induced GHS in adult life. We called these FD-like rats. Inhibition of glucocorticoid receptors following neonatal insult blocked the induction of GHS in adult rats. The aberrant increase of plasma corticosterone in neonates elevated the plasma concentration of norepinephrine, nerve growth factor (NGF) in the gastric fundus muscularis externae, brain-derived neurotrophic factor (BDNF) in the thoracic dorsal root ganglia (DRG) and spinal cord, and downregulated Kv1.1 mRNA in thoracic DRG without affecting the expression of Kv1.4, Nav1.8, TrpA1, TrpV1, or P2X3 in FD-like rats. Inhibition of glucocorticoid receptors during neonatal insult or the inhibition of adrenergic receptors, NGF or BDNF in FD-like rats suppressed GHS. The intrathecal administration of small interfering RNAs against Kv1.1 increased GHS in naïve rats.
Conclusion
Inflammatory insult to the colons of rat pups leads to GHS in adult life. GHS is caused by altered expression of genes encoding neurotrophins and ion channels, and altered activity of the sympathetic nervous system.
Keywords: Functional bowel disorders, abdominal pain, visceral hypersensitivity, early-life insult
Postprandial epigastric pain is a cardinal symptom of Functional Dyspepsia (FD) that afflicts 10–25% of the population according to various estimates1, 2. These patients feel pain in the absence of any structural, morphological or known organic abnormality. However, clinical studies have identified gastric hypersensitivity (GHS) to gastric distension as a major contributor to pain of epigastric origin3, 4. The cellular mechanisms and the etiology of GHS remain unknown, primarily due to the lack of availability of visceral tissue from FD patients and normal human subjects. Animal models that mimic specific pathologies of FD, such as GHS, are essential to advance the field and identify therapeutic targets to relieve the morbidity of visceral pain5.
Early life events, such as severe psychological stress, inflammation, abuse and trauma are established risk factors for the development of Functional Bowel Disorders, including FD, in adulthood6–8. One of the most common of these events is colonic inflammation due to pathogens or food allergies. The annual episodes of diarrhea in U.S. children less than 5 years-old range from 20 to 35 million9, 10, with 22,000 of these infections severe enough to result in hospitalization. Recent findings show that early-life diarrhea is a risk factor for the development of functional bowel disorders11.
Activation of the hypothalamic-pituitary-adrenal axis (HPA-axis) and the sympatho-adrenal medullary axis (SAM-axis) is a common factor for psychological and inflammatory stressors. Acute stress prepares organisms to cope with threats and insults12. However, if the stress is severe or it persists, the stress mediators become maladaptive; they can alter the expression of their target genes to cause persistent organ dysfunction13–15. We tested the hypothesis that robust inflammatory insult to the colon in neonates modifies the SAM-axis in adulthood to elevate plasma norepinephrine. Norepinephrine, in turn 1) upregulates the expression of nerve growth factor (NGF) in the gastric fundus, as well as brain-derived neurotrophic factor (BDNF) in the thoracic dorsal root ganglia (DRG) and spinal cord and 2) suppresses ion channel Kv1.1 in the thoracic DRG to induce GHS in adulthood. We tested the hypothesis in Sprague-Dawley rats.
Experimental Design and Methods
Animals
Male Sprague Dawley rats, each weighing 250–300 g and 10-day old pups were used in these studies. The University of Texas Medical Branch IACUC approved all procedures performed on these animals.
Neonatal colonic insult
Rat pups received 0.2 ml of 130 mg/kg (32.5 to 39.5 mg for 250 to 300 g rat weight) TNBS in 10% ethanol in saline through a tube inserted 2 cm into the distal colon on post-natal day (PND) 10. Control pups received saline. Six-to-eight weeks later, the now-adult rats were tested for gastric hypersensitivity and their tissue was collected for molecular experiments. The mortality rate was 4.1% (6 out of 145).
Evaluation of gastric sensitivity to gastric distension
A 2 cm long balloon, prepared from a condom and attached to PE240 tubing, was surgically positioned in the gastric fundus and sutured in place. The tube was externalized at the nape of the neck. A pair of electrodes was sutured to the acromeotrapezious muscle. Seven-to-ten days later, gastric sensitivity to balloon distension was measured. Rats received a series of 20 sec gastric balloon distensions: 30, 40, 50, 60, 80, 100, and 120 mmHg (measured using a sphygmomanometer) with 2 min between distensions. A Biopac EMG-100C amplifier (sample rate 2000 per second, HP0.1Hz, LP500Hz) and UIM100C (both Biopac Systems, Inc., Goleta, CA) continuously recorded EMG. Traces were visualized and analyzed using Acknowledge (Biopac Systems, Inc., Goleta, CA). EMG was rectified and the area under the curve (AUC) calculated for the 20 sec distention period. Baseline activity, taken 20 sec before distention, was subtracted from the EMG induced by distension. Data were displayed as AUC in volts × seconds (V×S) as a function of distention pressure.
Intrathecal Catheter
Intrathecal catheters, gastric balloons and electrodes were installed in adult FD rats as described previously14). A previously described Kv1.1 siRNA16 AAATTTTACGAGTTGGGCGAG, and negative control siRNA ON-TARGETplus were purchased from Dharmacon (Boulder, CO). For intrathecal treatment, 2 μg of the appropriate siRNA) was mixed (1:5 v/v) with i-Fect transfection reagent (Neuromics, Edina, MN); rats received 2ug siRNA/10 ul/rat/injection.
Electrophysiology
Under general 2% isoflurane anesthesia, the lipid soluble fluorescent dye, DiI-I (1,1′dioleyl-3,3,3′,3′-tetramethylindocarbocyanine methanesulfonate, Invitrogen), 25 mg in 0.5 ml methanol was injected in 2 μl volumes at eight to ten sites in the fundus wall starting. Thoracolumbar DRG neurons were isolated from DRG T13, L1 and L2, 16 days later. The methods used for isolation of DRG and current clamp recordings were described previously17 (see supplement for more details).
Detailed statistics and methods for neonatal corticosterone measurements, RU-486 administration, NGF neutralizing antibody, Laser capture microscopic (LCM) dissection, tissue protein and RNA measurements, western blot, HPA and SAM-axis dysfunction and in vitro experiments are described in the Supplement.
Results
Gastric hypersensitivity in adult rats subjected to neonatal colonic insult
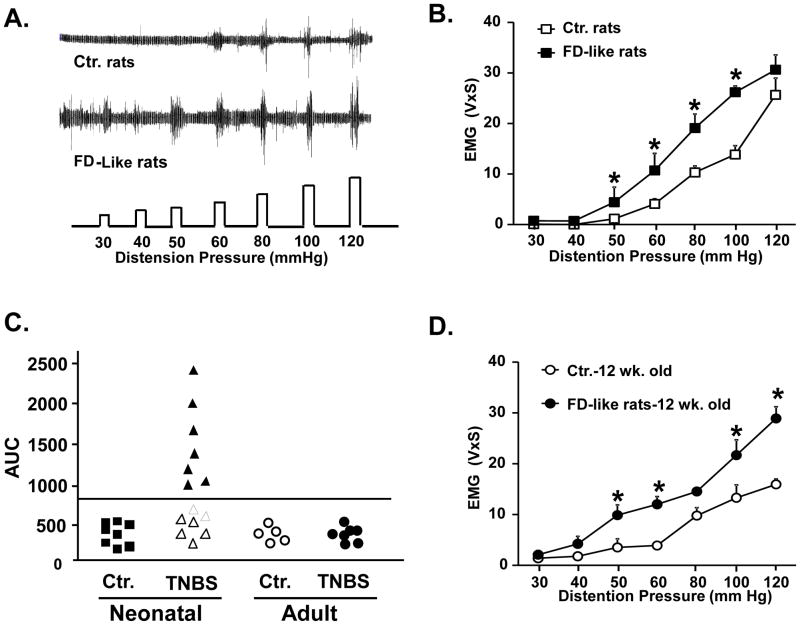
At 6–8 weeks following neonatal inflammatory insult on PND 10, rats showed significantly greater average visceromotor responses (VMR) to graded gastric distention, compared with age-matched controls subjected to neonatal saline treatment (Figures 1A and 1B). Among these FD-like rats, 50% exhibited VMR responses greater than two standard deviations above the mean of controls (Figure 1C). We termed these rats “responders”. We tested whether GHS occurs only if the inflammatory insult was applied during the neonatal stage of development. We applied similar inflammatory insult to 6–8 week old naïve adult rats. At 6–8 weeks after insult, the mean VMR responses of these rats did not differ significantly from those of age-matched naïve adult rats treated with saline (Figure 1C). Age-matched FD-like rats remained hypersensitive to gastric distention at least 12 weeks after the neonatal insult (Figure 1D). All subsequent experiments were performed 6–8 weeks after the neonatal insult and done in the entire group of responders and non-responders.
Figure 1.
Gastric hypersensitivity was detected in adult rats 6 weeks following colonic inflammatory insult on PND 10. A. Representative EMG activity recorded from the acromeotrapezious muscle in a control (saline, PND 10) and an FD-like rat (TNBS, PND 10) in response to graded phasic gastric distention. B. The VMR to gastric distention was significantly greater in FD-like rats (n=14) vs. age matched controls (n=8,*p<0.05). C. To distinguish between hypersensitive and normo-sensitive rats among the FD-like rats, we calculated the area under the distention pressure-EMG activity curve for each control and FD-like rat. Approximately 50% of the FD-like rats exhibited gastric sensitivity values greater than 2X the standard deviation of controls (outside the 95% confidence limits of controls, designated by the line). No significant differences in gastric sensitivity were observed in 12 week old rats treated with TNBS at 6 weeks of age (n=5) and 12 week old rats treated with saline at the same age (n=5). D. FD-like rats remained hypersensitive to gastric distention at 12 weeks of age compared to age-matched controls (n= 7 and n=5, respectively). AUC, area under the curve, V×S, volts × seconds.
Altered expression of BDNF and Kv1.1 in thoracic DRG and spinal cord
We used retrograde labeling with CTB-488 followed by isolation of gastric-specific thoracic neurons by laser capture microdissection (Figure 2A).
Figure 2.
Increase in BDNF expression in gastric-specific dorsal root ganglia (DRG) neurons and in thoracic spinal cord segments contributed to gastric hypersensitivity. A. Photomicrographs of sections from T9 DRG showing gastric neurons, identified by uptake of retrograde label, CTB-488 (green) and isolated by laser capture micro-dissection. B. qTR-PCR showed a significant 2.5-fold increase in BDNF mRNA and a significant 50% decrease in Kv1.1 mRNA levels in gastric neurons from FD-like rats compared to controls. The mRNA expression of other genes was not affected (n=4 rats each, *p<0.05). C. ELISA showed increased BDNF protein in thoracic spinal cord segments of FD-like rats vs. controls (*p<0.05, n=5 rats each). D. Intrathecal treatment with BDNF antagonist trkB-Fc, once daily for five consecutive days significantly reduced the VMR to gastric distention in FD-like rats compared to pre-treatment baseline and to vehicle-treated FD-like rats (*p<0.05 vs. vehicle, n=5 rats each).
BDNF
We detected a significant 2.5-fold increase in BDNF mRNA expression in the gastric thoracic DRG of FD-like rats vs. control rats (Figure 2B). We found a significant increase in BDNF protein in thoracic spinal cords of FD-like rats vs. controls (Figure 2C). The increase in BDNF expression in the gastric primary afferents may contribute to hypersensitivity through the release of protein from sensory nerve endings in the spinal cord dorsal horns to enhance synaptic transmission. Daily intrathecal administration of the trkB receptor antagonist trkB-Fc (5 ug in 10 ul sterile saline or vehicle) for 5 days, significantly suppressed the VMR to gastric distension in FD-like rats; intrathecal administration of the vehicle had no effect (Figure 2D). These data indicated that BDNF upregulation contributed to gastric hypersensitivity in FD-like rats.
Kv1.1
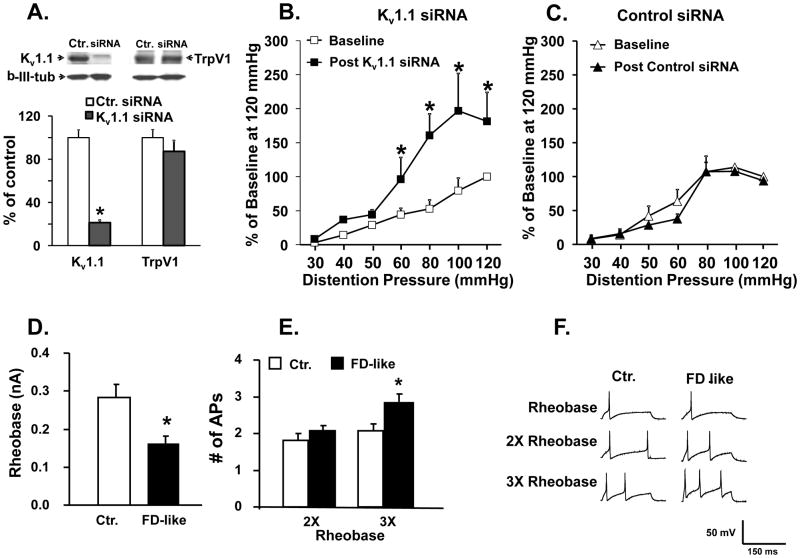
In contrast to the upregulation of BDNF, Kv1.1 expression significantly decreased in gastric DRG neurons (Figure 2B). We investigated whether downregulation of Kv1.1 contributed to GHS. Intrathecal treatment of naïve rats with Kv1.1 siRNA (2 μg/rat, twice per day for three days), but not control siRNA, significant decreased Kv1.1 protein expression in thoracic DRG without significantly altering the expression of other nociceptive genes, such as TrpV1 (Figure 3A). GHS increased significantly in naïve rats treated with Kv1.1 siRNA, but not the control siRNA, compared to pre-treatment baseline (Figure 3B), showing that decrease of Kv1.1 expression increased gastric sensitivity in naïve rats.
Figure 3.
siRNA mediated knockdown of Kv1.1 expression in thoracic DRG significantly increased gastric sensitivity in naïve adult rats. A. Western blots showed a significant decrease in Kv1.1 protein in thoracic DRG (T8-T12) following intrathecal treatment with Kv1.1 siRNA but not with control siRNA. siRNA treatment did not alter TrpV1 expression (n=5 rats each, *p <0.01 vs. control siRNA). B. Naïve rats treated with Kv1.1 siRNA showed a significant increase in VMR to gastric distention (n=5 rats each, compared to pre-treatment baseline, *p<0.05). C. Treatment with control siRNA had no significant effect on gastric hypersensitivity. D. Patch clamp recordings from freshly dissociated gastric DRG neurons from FD-like and PND 10 saline treated littermate controls showed a significant decrease in rheobase in FD-like rats, and E. a significant increase in the number of action potentials elicited by current injection at 3X the rheobase in gastric DRG neurons from FD-like rats. F. Sample voltage vs. time traces showing action potentials evoked at X1, X2, and X3 rheobase. The patch clamp data were obtained from 16 cells from 5 PND 10 saline control rats and 19 cells from 5 FD-like rats.
Kv1.1 channels regulate the electrogenesis of action potential in DRG neurons, including resting membrane potential18 and firing rates of action potential19. Therefore, we used a patch clamp to investigate whether gastric DRG neurons are sensitized in FD-like rats. We found a significant decrease in rheobase and a significant increase in the number of action potentials elicited by current injection at 3X the rheobase in gastric DRG neurons from FD-like rats vs. control rats (Figure 3D, E and F).
By contrast, no significant changes occurred in mRNA levels of TrpV1, TrpA1, P2X3, Kv1.4 or NaV1.8 in FD-like rats vs. controls (Figure 2B). In addition, on examining whole thoracic DRG, we found no significant change in expression of either BDNF or Kv1.1 (data not shown), indicating selective effects on gastric DRG neurons.
Increased NGF expression in the fundus muscularis externae contributes to GHS in FD-like rats
We investigated whether neonatal inflammatory insult increased NGF expression in the fundus muscularis externae to induce GHS in FD-like rats. Analysis using quantitative reverse transcription polymerase chain reaction (qRT-PCR) showed a significant increase in NGF mRNA, but not in glial cell-derived neurotrophic factor or artemin mRNA in the muscularis externae of the gastric fundus in FD-like rats (Figure 4A). NGF protein increased significantly in the fundus of FD-like rats but not in the corpus (Figure 4B). Treatment of FD-like rats with an NGF neutralizing antibody (16 μg//kg for 5 days) partially, but significantly, suppressed the VMR to gastric distension, while similar treatment with non-immune serum had no significant effect (Figure 4C).
Figure 4.
Increase in NGF expression in the fundus muscularis externae of FD-like rats contributed to gastric hypersensitivity and to increased expression of BDNF in the spinal cord. A. NGF mRNA increased significantly in the fundus of FD-like rats without any change in the expression of glial cell-derived neurotrophic factor and artemin (n=6,*p<0.05 vs. control). B. ELISA showed increase of NGF protein in the fundus muscularis externae of FD-like rats, but not in the corpus muscularis externae (n=7 each group, *p<0.05 vs. control rats). C. Systemic treatment of FD-like rats with an NGF neutralizing antibody (16 ug/kg/day for 5 days) partially, but significantly, reduced VMR to gastric distention compared to FD-like rats treated with non-immune serum (n=8 rats each group, *p<0.05). D. qRT- PCR showed that NGF antibody treatment significantly decreased BDNF mRNA in gastric DRG neurons in FD-like rats. This treatment produced no significant effect on Kv1.1 mRNA. E. Western blot showed significant decrease in BDNF expression in the thoracic spinal cord of FD-like rats treated with NGF neutralizing serum (*p<0.05 vs. Ctr., +p<0.05 vs. neonatal TNBS plus non-immune serum). Control rats were treated with saline on PND 10.
We investigated whether an increase in the expression of NGF in gastric muscularis externae, contributed to changes in expression of Bdnf and Kv1.1 in gastric DRG. NGF antibody treatment significantly suppressed the expression of Bdnf mRNA in gastric-specific DRG neurons (Figure. 4D) and reduced BDNF protein in the spinal cord (Figure 4E), but had no significant effect on the suppression of Kv1.1 channels in gastric-specific DRG neurons (Figure 4D).
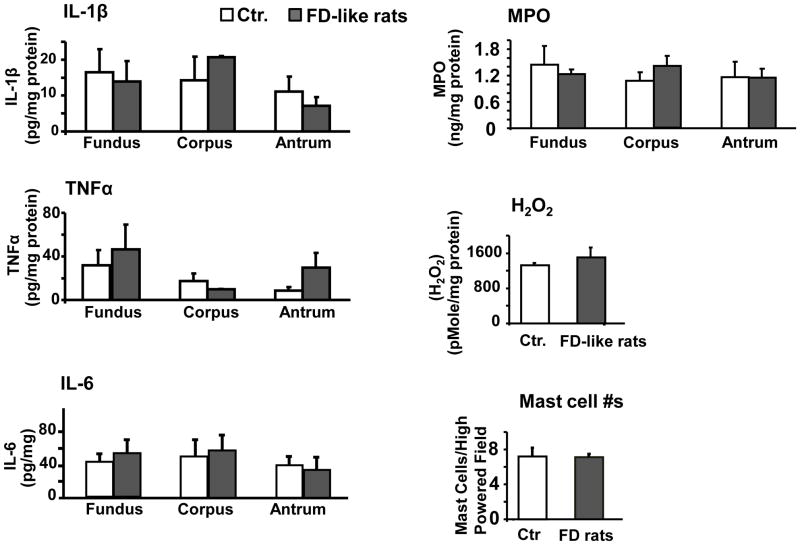
Lack of an inflammatory response in the gastric wall of FD-like rats
Since inflammatory mediators can induce NGF expression, we investigated whether neonatal inflammatory insult altered the nascent inflammatory environment in the stomach wall in FD-like rats. We found no significant increase in the pro-inflammatory cytokines IL-1β, TNFα and IL-6, or in myeloperoxidase (MPO), oxidative stress (H2O2) or mast cell numbers in the stomach wall (Figure. 5).
Figure 5.
There were no significant differences in the expression of several inflammatory cytokines (IL-1β, TNFα, or IL-6), myeloperoxidase (MPO), hydrogen peroxide (H2O2), or number of mast cells per high-powered field in the gastric muscularis externae of FD-like rats compared to PND 10 saline treated controls (N=6 rats in each group).
Neonatal factors that induce FD-like GHS in adulthood
Corticosterone plays a vital role in early-life development in preparation for normal health in adulthood20. In addition, corticosterone is a critical mediator of the stress response. We investigated whether inopportune elevation of corticosterone by neonatal inflammation was an underlying cause of GHS in FD-like rats. We found a significant 3-fold increase in plasma corticosterone on PND 15 following inflammatory insult on PND 10 vs. saline-treated control rats; by PND 17, plasma corticosterone levels were not different between the two groups (Figure 6A). Pups subjected to inflammatory insult that were treated once daily from PND 9 to PND 17 with 16 μg/kg RU-486, an antagonist of glucocorticoid receptors, did not then develop GHS in adulthood; vehicle treatment induced GHS, as usual (Figure 6B). RU-486 treatment following TNBS insult prevented the increase of NGF mRNA in the fundus muscularis, the decrease of Kv1.1 mRNA in gastric DRG neurons and the increase o BDNF mRNA in gastric DRG neurons (Figure 6C and D).
Figure 6.
Increased neonatal plasma corticosterone contributed to the development of gastric hypersensitivity in FD-like rats. A. The increase in plasma corticosterone level on PND 15 in TNBS treated pups was significantly greater than in saline treated pups (n=10 each, *p<0.05 TNBS treated vs. saline treated pups). B. Treatment of pups with TNBS + vehicle for RU-486 significantly increased VMR to gastric distension 6 weeks later vs. control rats; TNBS + the glucocorticoid receptor antagonist RU-486 treatment once per day from PND 9 to 17 blocked this increase (n=8 each). C. qRT-PCR showed that RU-486 blocked the increase of NGF in PND 10 TNBS treated pups when they grew into adults. (n=10 each). D. qRT-PCR showed that neonatal RU486 treatment prevented changes in gene expression of Kv1.1 and BDNF in gastric specific dorsal root ganglia neurons of FD- like rats. Gastric-specific DRG neurons were isolated from adult rats by laser capture microdissection. +p<0.05, TNBS+vehicle vs. controls; *p<0.05, TNBS+vehicle vs. TNBS+RU-486.
Mediators of GHS in adult FD-like rats
We investigated whether HPA-axis or SAM-axis dysfunction contributed to GHS in FD-like rats. The basal plasma levels of corticosterone and the short-term increase in plasma corticosterone in response to one-hour water avoidance stress (WAS) did not differ between the FD-like and control rats (Figure 7A). However, the basal plasma level of norepinephrine in FD-like rats was significantly greater than in control rats (Figure 7B), while the short-term elevation of norepinephrine (Figure 7B) and epinephrine (Figure 7C) in response to WAS was blunted in FD-like vs. control rats. We found that i.p. administration of a cocktail of adrenergic receptor antagonists, 2 mg/kg phentolamine (α1 and α2), 2 mg/kg propranolol (β1 and β2) and 2 mg/kg CL316243 (β3) daily for five days significantly suppressed the VMR to gastric distension in FD-like rats; vehicle administration had no significant effect (Figure 7D). This treatment also significantly suppressed the expression of NGF in the muscularis externae of the gastric fundus (Figure 7E). In vitro incubation of fundi muscularis externae tissue with norepinephrine for 24 hours concentration-dependently increased the expression of NGF (Figure 7F). Together, these findings support the hypothesis that adrenergic induction of increased NGF expression in the fundus promotes GHS in FD-like rats.
Figure 7.
Adrenergic receptor activation contributed to gastric hypersensitivity in FD-like rats. Plasma levels of stress hormones were measured before and at several time-points after one hour water avoidance stress (WAS) in adult FD-like and control rats. A. No significant differences were observed in serum corticosterone response to WAS between FD-like and postnatal day (PND) 10 saline (control) rats (n=8, +p< 0.05 vs. basal level). B. Pre-stress basal norepinephrine was significantly higher in FD-like rats [N=8, *p<0.05 vs. PND 10 saline (control) rats]. WAS evoked a significant increase in plasma norepinephrine in PND 10 saline (control) rats but not in FD-like rats. (n=8, *p<0.05) vs. FD-like rats. C. Similar results were observed for WAS-induced increase of serum epinephrine. D. Systemic treatment with an adrenergic receptor antagonist cocktail (phentolamine, 2 mg/kg, propranolol, 2 mg/kg and CL316243, 2 ug/kg) significantly reduced gastric sensitivity in FD-like rats compared to pre-treatment baseline or vehicle treatment of FD-like rats (n=6 in each group, *p<0.05 vs. vehicle, +p< 0.05 vs. baseline). E. This treatment significantly reduced nerve growth factor (NGF) protein expression in the fundus (N=6 in each group * p<0.05 vs. vehicle treated rats). F. ELISA showed increased NGF protein in fundus muscle strips treated with norepinephrine in vitro for 24 hours (n=6 in each group, *p<0.05).
Comparison of key findings between FD models of neonatal colonic inflammation and gastric irritation
We confirmed that gastric irritation in rat pups with iodoacetamide also induces GHS in adult life5 (Fig. S1A in supplement). However, key cellular mechanisms underlying GHS in the two models differed. Iodoacetamide irritation did not elevate the basal or WAS stimulated plasma level of norepinephrine (Fig. S1B) or NGF in the fundus muscularis externae in iodoacetamide treated FD-like rats (Fig. S1C); however, it elevates BDNF expression in the thoracic cord DRG.
Discussion
Our findings show that neonatal inflammatory insult to the colon induces GHS in adulthood. Earlier reports found that neonatal colonic mechanical or chemical irritation with mustard oil or acetic acid induced visceral hypersensitivity (VHS) to colorectal distension in adult life21–23. Neonatal inflammatory insult in the colon also impaired colonic smooth muscle function, resulting in diarrhea-like conditions in adult life15. Together, these findings suggest that some of the symptoms of FD and irritable bowel syndrome (IBS) may have a common etiology, which explains the clinical observation that 46% of FD patients have concurrent symptoms of IBS24. Our current findings along with those cited above support the clinical observation that early life trauma is a major risk factor for the development of Functional Bowel Disorders (motility dysfunction and abdominal pain)6–8, 25.
Visual inspection, myeloperoxidase assay, and cytokine assays indicated no organic disease or structural abnormality in FD-like rats, which agrees with the defining conditions of functional dyspepsia. Some reports have suggested low-grade inflammation in the lamina propria as the basis of VHS in functional bowel disorders, including functional dyspepsia26, 27. However, there is little evidence that H pylori infection causes the symptoms of FD; meta-analysis found no correlation between H pylori infection and the symptoms of FD. In addition, eradication of H pylori infection failed to improve the symptoms of FD28. Other reports found a low-grade inflammation in the duodenum of post-infective FD patients. However, it is not clear how low-grade inflammation in the duodenal lamina propria could sensitize the far away located sensory nerve endings in the muscularis externae of the fundus so as to cause GHS. The inflammatory mediators need to be in direct contact with their target cells in order to alter their function. Accumulating evidence shows that the low-grade inflammation and the accompanying increase of inflammatory mediators observed in the lamina propria is within the physiological range to protect the gut from the hostile luminal environment29, 30. In this case, statistical significance does not equal pathological abnormality. On the other hand, our findings show that SAM-axis dysfunction by neonatal inflammation elevated plasma norepinephrine, which upregulated the expression of NGF in the fundus muscularis externae. Increase of NGF in fundus muscularis externae upregulated BDNF expression in the thoracic dorsal root ganglia and spinal cord. Concurrently, neonatal inflammation suppressed the expression of Kv1.1 in the thoracic DRG by a yet unknown mechanism. These alterations in nociceptive genes and ion channels together caused GHS in the absence of any apparent inflammation.
Our results show that elevation of plasma corticosterone at an inopportune time during neonatal development produces gastric hypersensitivity in adults. The HPA-axis in rats is in a state of relative quiescence (stress hyporesponsive period: SHRP) from PND 3 to PND 1431; the low level of corticosterone during this period protects the rapidly growing organism. We found that plasma corticosterone is very low on PND 11, begins to increase gradually, and then increases abruptly 3-fold on PND 15. However, inflammatory insult on PND 10 more than doubles the abrupt increase of corticosterone on PND 15. This brief spike in plasma corticosterone is enough to induce GHS to gastric distension in adult life; blocking glucocorticoid receptors by RU-486 during this period prevented the induction of GHS in FD-like rats. It is noteworthy that the timing of SHRP is species-dependent; in humans it occurs in the last trimester of pregnancy32.
The epigenetic code programs the expression of genes during the fetal and neonatal stages of development in each cell type at levels appropriate for normal cellular function in adult life. However, epigenetic programming is sensitive to changes in the cellular microenvironment. If severe or persistent stress occurs during the early development, the epigenetic mechanisms reprogram the expression of genes vulnerable at that time to ensure immediate survival/adaptation of the organism33, 34. However, this programming may persist into adulthood, causing organ dysfunction. The epigenetic programming due to an increase in glucocorticoids depends on the timing of insult during fetal and neonatal developments as well as the type and intensity of the stressor. For this reason, a similar inflammatory insult in a mature adult animal did not induce GHS to gastric distension.
Our findings show that neonatal inflammatory insult on PND 10 affects the resting plasma norepinephrine and norepinephrine release by activation of the SAM-axis in response to acute stress differentially in FD-rats; neonatal inflammation elevated the resting plasma norepinephrine, but its release by acute stress was blunted. Note that the resting plasma/urine levels of norepinephrine are elevated also in IBS patients35, 36; similar data are not available for FD patients. However, clinical studies found altered autonomic function, including increased sympathetic activity, in FD patients compared to healthy controls37–39. By contrast, neonatal inflammation did not alter the resting levels of corticosterone or its short-term release by activation of the HPA-axis by acute WAS stress in FD-like rats.
We identified two nociceptive proteins, NGF and BDNF, and one ion channel, Kv1.1 that contribute to increase of GHS in FD-like rats. NGF is a key regulator of primary afferent neuronal sensitivity40; the neutralization of NGF by its antibody suppressed GHS in FD-like rats. Maternal deprivation of neonates also increased NGF in the colon wall, which, in turn, increased MPO activity and number of mast cells41. We found that the elevation of plasma norepinephrine under non-inflammatory conditions in FD-like rats enhanced the expression of NGF in the muscularis externae of the gastric fundus; blocking adrenergic receptors suppressed NGF expression. We chose to detect NGF in muscularis externae tissue because evidence from animal and human studies shows that the nociceptive nerve endings of primary afferent neurons terminate in the muscularis externae42, 43. In addition, in vitro data showed that norepinephrine concentration-dependently enhanced NGF expression in the fundus muscularis externae. In vivo experiments showed that blocking α1, β1, β2 and β3 adrenergic receptors inhibited hypersensitivity to gastric distension in FD-like rats.
Neutralizing NGF suppressed the expression of BDNF in gastric DRG, indicating that norepinephrine increased the expression of BDNF by upregulating the expression of NGF in the fundus wall, which then transports retrograde to upregulate the expression of BDNF in the gastric DRG. However, neutralizing NGF did not reverse the suppression of Kv1.1, indicating an alternate mechanism for its suppression in FD-like rats. The upregulation of NGF and BDNF and concurrent downregulation of Kv1.1 may synergistically induce GHS. The increase in neurotrophins potentiates synaptic neurotransmission14, 44, and the decrease in Kv1.1 increases the electrogenesis of action potentials18, 19.
Our findings together with previous findings5 show that GHS may result from more than one etiology, such as colonic inflammation and gastric irritation during neonatal development. Each type of insult and its location utilized different mechanisms to induce GHS. GHS due to neonatal colonic inflammation results from increase of plasma norepinephrine, followed by increase of NGF in fundus muscularis externae, followed by increase of BDNF in thoracic DRG and spinal cord and concurrent decrease of Kv1.1 channels in colon specific DRG neurons in adult life. GHS due to gastric irritation results from increase of BDNF in thoracic DRG in adult life by yet unknown mechanisms. Epidemiological studies show a significant incidence of early childhood diarrhea9, 10. Data on the incidence of gastritis in early childhood are not available. However, gastric suction at birth is a known risk factor for the development of psychosomatic and functional disorders in adulthood45.
In summary, our findings show that a robust inflammatory insult to the colon in on PND 10 caused an aberrant increase of plasma corticosterone on PND 15, around which time the HPA-axis matures. The inopportune increase of corticosterone elevated the basal plasma level of norepinephrine when the pups grew into adults. No overt inflammation was apparent in the gastric wall at this time. The increased plasma norepinephrine acting on adrenergic receptors enhanced the expression of NGF in fundus muscularis externae. The retrograde transport of NGF enhanced the expression of BDNF in gastric DRG. The neonatal inflammatory insult also suppressed the expression of Kv1.1 in thoracic DRG by yet unknown epigenetic mechanisms. The enhanced expression of NGF and BDNF and concurrent suppression of Kv1.1 ion channels synergistically enhanced gastric sensitivity to distension. The persistent alterations in the SAM-axis appear to lie at the heart of the increase in gastric sensitivity. The changes in the short-term response of the HPA-axis to acute stress did not relate to persistent GHS. Our findings mimic the underlying features of functional dyspepsia; they can be tested in patients for validity in inducing the symptom of epigastric pain and in developing potential targets for therapeutic interventions.
Acknowledgments
Supported in part by NIDDK Grant 5R01DK088796
The authors gratefully acknowledge the assistance of Dr. Sarah Toombs Smith in preparation of the manuscript and of Dr. Guang-Yin Xu in obtaining patch clamp data.
Abbreviations used in this paper
- BDNF
brain-derived nerve neurotrophic factor
- DRG
dorsal root ganglia
- FD
Functional dyspepsia
- GHS
gastric hypersensitivity
- HPA-axis
hypothalamic-pituitary-adrenal axis
- IBS
irritable bowel syndrome
- NGF
nerve growth factor
- PND
post-natal day
- SAM-axis
sympatho-adrenal medullary axis
- SHRP
stress hyporesponsive period
- TNBS
trinitrobenzene sulfonic acid
- VMR
visceromotor response
Footnotes
There are no conflicts of interest to disclose for both authors.
Study concept and design (SKS and JHW); acquisition of data (JHW); analysis and interpretation of data (SKS and JHW); drafting of the manuscript (JHW); critical revision of the manuscript for important intellectual content (SKS) statistical analysis (JHW); obtained funding (SKS)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tack J, Masaoka T, Janssen P. Functional dyspepsia. Current opinion in gastroenterology. 2011;27:549–57. doi: 10.1097/MOG.0b013e32834b7ca8. [DOI] [PubMed] [Google Scholar]
- 2.Chang L. Review article: epidemiology and quality of life in functional gastrointestinal disorders. Alimentary pharmacology & therapeutics. 2004;20 (Suppl 7):31–9. doi: 10.1111/j.1365-2036.2004.02183.x. [DOI] [PubMed] [Google Scholar]
- 3.Keohane J, Quigley EM. Functional dyspepsia: the role of visceral hypersensitivity in its pathogenesis. World journal of gastroenterology: WJG. 2006;12:2672–6. doi: 10.3748/wjg.v12.i17.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miwa H, Watari J, Fukui H, et al. Current understanding of pathogenesis of functional dyspepsia. Journal of gastroenterology and hepatology. 2011;26 (Suppl 3):53–60. doi: 10.1111/j.1440-1746.2011.06633.x. [DOI] [PubMed] [Google Scholar]
- 5.Liu LS, Winston JH, Shenoy MM, et al. A rat model of chronic gastric sensorimotor dysfunction resulting from transient neonatal gastric irritation. Gastroenterology. 2008;134:2070–9. doi: 10.1053/j.gastro.2008.02.093. [DOI] [PubMed] [Google Scholar]
- 6.Geeraerts B, Van Oudenhove L, Fischler B, et al. Influence of abuse history on gastric sensorimotor function in functional dyspepsia. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2009;21:33–41. doi: 10.1111/j.1365-2982.2008.01178.x. [DOI] [PubMed] [Google Scholar]
- 7.Chitkara DK, van Tilburg MA, Blois-Martin N, et al. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am J Gastroenterol. 2008;103:765–74. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Videlock EJ, Adeyemo M, Licudine A, et al. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137:1954–62. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pont SJ, Grijalva CG, Griffin MR, et al. National rates of diarrhea-associated ambulatory visits in children. J Pediatr. 2009;155:56–61. doi: 10.1016/j.jpeds.2009.01.075. [DOI] [PubMed] [Google Scholar]
- 10.Pont SJ, Carpenter LR, Griffin MR, et al. Trends in healthcare usage attributable to diarrhea, 1995–2004. J Pediatr. 2008;153:777–82. doi: 10.1016/j.jpeds.2008.06.037. [DOI] [PubMed] [Google Scholar]
- 11.Saps M, Lu P, Bonilla S. Cow’s-milk allergy is a risk factor for the development of FGIDs in children. Journal of pediatric gastroenterology and nutrition. 2011;52:166–9. doi: 10.1097/MPG.0b013e3181e85b55. [DOI] [PubMed] [Google Scholar]
- 12.Larauche M, Mulak A, Tache Y. Stress-related alterations of visceral sensation: animal models for irritable bowel syndrome study. Journal of neurogastroenterology and motility. 2011;17:213–34. doi: 10.5056/jnm.2011.17.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhury BK, Shi XZ, Sarna SK. Norepinephrine mediates the transcriptional effects of heterotypic chronic stress on colonic motor function. Am J Physiol Gastrointest Liver Physiol. 2009 doi: 10.1152/ajpgi.90712.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winston JH, Xu GY, Sarna SK. Adrenergic stimulation mediates visceral hypersensitivity to colorectal distension following heterotypic chronic stress. Gastroenterology. 2010;138:294–304. e3. doi: 10.1053/j.gastro.2009.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choudhury BK, Shi XZ, Sarna SK. Gene plasticity in colonic circular smooth muscle cells underlies motility dysfunction in a model of postinfective IBS. Am J Physiol Gastrointest Liver Physiol. 2009;296:G632–42. doi: 10.1152/ajpgi.90673.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi XX, Nicol GD. Manipulation of the potassium channel Kv1. 1 and its effect on neuronal excitability in rat sensory neurons. Journal of neurophysiology. 2007;98:2683–92. doi: 10.1152/jn.00437.2007. [DOI] [PubMed] [Google Scholar]
- 17.Xu GY, Winston JH, Shenoy M, et al. Transient receptor potential vanilloid 1 mediates hyperalgesia and is up-regulated in rats with chronic pancreatitis. Gastroenterology. 2007;133:1282–92. doi: 10.1053/j.gastro.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman DA, Magee JC, Colbert CM, et al. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–75. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- 19.Pongs O. Voltage-gated potassium channels: from hyperexcitability to excitement. FEBS Lett. 1999;452:31–5. doi: 10.1016/s0014-5793(99)00535-9. [DOI] [PubMed] [Google Scholar]
- 20.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–25. [PubMed] [Google Scholar]
- 21.Al-Chaer ED, Kawasaki M, Pasricha PJ. A new model of chronic visceral hypersensitivity in adult rats induced by colon irritation during postnatal development. Gastroenterology. 2000;119:1276–85. doi: 10.1053/gast.2000.19576. [DOI] [PubMed] [Google Scholar]
- 22.Winston J, Shenoy M, Medley D, et al. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–27. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Christianson JA, Bielefeldt K, Malin SA, et al. Neonatal colon insult alters growth factor expression and TRPA1 responses in adult mice. Pain. 2010;151:540–9. doi: 10.1016/j.pain.2010.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corsetti M, Caenepeel P, Fischler B, et al. Impact of coexisting irritable bowel syndrome on symptoms and pathophysiological mechanisms in functional dyspepsia. The American journal of gastroenterology. 2004;99:1152–9. doi: 10.1111/j.1572-0241.2004.30040.x. [DOI] [PubMed] [Google Scholar]
- 25.Drossman DA, Leserman J, Nachman G, et al. Sexual and physical abuse in women with functional or organic gastrointestinal disorders. Ann Intern Med. 1990;113:828–33. doi: 10.7326/0003-4819-113-11-828. [DOI] [PubMed] [Google Scholar]
- 26.Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- 27.Mearin F, Perez-Oliveras M, Perello A, et al. Dyspepsia and irritable bowel syndrome after a Salmonella gastroenteritis outbreak: one-year follow-up cohort study. Gastroenterology. 2005;129:98–104. doi: 10.1053/j.gastro.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Sarnelli G, Cuomo R, Janssens J, et al. Symptom patterns and pathophysiological mechanisms in dyspeptic patients with and without Helicobacter pylori. Dig Dis Sci. 2003;48:2229–36. doi: 10.1023/b:ddas.0000007856.71462.6c. [DOI] [PubMed] [Google Scholar]
- 29.Cremon C, Gargano L, Morselli-Labate AM, et al. Mucosal immune activation in irritable bowel syndrome: gender-dependence and association with digestive symptoms. Am J Gastroenterol. 2009;104:392–400. doi: 10.1038/ajg.2008.94. [DOI] [PubMed] [Google Scholar]
- 30.Sarna SK. Lessons Learnt from Post-Infectious IBS. Frontiers in physiology. 2011;2:49. doi: 10.3389/fphys.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walker CD, Perrin M, Vale W, et al. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–51. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- 32.Midgley PC, Holownia P, Smith J, et al. Plasma cortisol, cortisone and urinary glucocorticoid metabolites in preterm infants. Biology of the neonate. 2001;79:79–86. doi: 10.1159/000047071. [DOI] [PubMed] [Google Scholar]
- 33.Warner MJ, Ozanne SE. Mechanisms involved in the developmental programming of adulthood disease. The Biochemical journal. 2010;427:333–47. doi: 10.1042/BJ20091861. [DOI] [PubMed] [Google Scholar]
- 34.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–6. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 35.Heitkemper M, Jarrett M, Cain K, et al. Increased urine catecholamines and cortisol in women with irritable bowel syndrome. Am J Gastroenterol. 1996;91:906–13. [PubMed] [Google Scholar]
- 36.Posserud I, Agerforz P, Ekman R, et al. Altered visceral perceptual and neuroendocrine response in patients with irritable bowel syndrome during mental stress. Gut. 2004;53:1102–8. doi: 10.1136/gut.2003.017962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorena SL, Figueiredo MJ, Almeida JR, et al. Autonomic function in patients with functional dyspepsia assessed by 24-hour heart rate variability. Dig Dis Sci. 2002;47:27–31. doi: 10.1023/a:1013246900041. [DOI] [PubMed] [Google Scholar]
- 38.Greydanus MP, Vassallo M, Camilleri M, et al. Neurohormonal factors in functional dyspepsia: insights on pathophysiological mechanisms. Gastroenterology. 1991;100:1311–8. [PubMed] [Google Scholar]
- 39.Park DI, Rhee PL, Kim YH, et al. Role of autonomic dysfunction in patients with functional dyspepsia. Dig Liver Dis. 2001;33:464–71. doi: 10.1016/s1590-8658(01)80023-2. [DOI] [PubMed] [Google Scholar]
- 40.Petruska JC, Mendell LM. The many functions of nerve growth factor: multiple actions on nociceptors. Neurosci Lett. 2004;361:168–71. doi: 10.1016/j.neulet.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Barreau F, Cartier C, Ferrier L, et al. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004;127:524–34. doi: 10.1053/j.gastro.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 42.Lembo T, Munakata J, Naliboff B, et al. Sigmoid afferent mechanisms in patients with irritable bowel syndrome. Dig Dis Sci. 1997;42:1112–20. doi: 10.1023/a:1018817132213. [DOI] [PubMed] [Google Scholar]
- 43.Brierley SM, Jones RC, 3rd, Gebhart GF, et al. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology. 2004;127:166–78. doi: 10.1053/j.gastro.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Numakawa T, Suzuki S, Kumamaru E, et al. BDNF function and intracellular signaling in neurons. Histology and histopathology. 2010;25:237–58. doi: 10.14670/HH-25.237. [DOI] [PubMed] [Google Scholar]
- 45.Anand KJ, Runeson B, Jacobson B. Gastric suction at birth associated with long-term risk for functional intestinal disorders in later life. J Pediatr. 2004;144:449–54. doi: 10.1016/j.jpeds.2003.12.035. [DOI] [PubMed] [Google Scholar]