Abstract
Thyroid hormone exerts broad effects on the adult heart, however little is known regarding the role of thyroid hormone on regulating cardiac growth early in development and in response to pathophysiological conditions. To address this issue, we determined the effects of fetal thyroidectomy on cardiac growth and growth related gene expression in control and pulmonary artery banded fetal sheep. Fetal thyroidectomy (THX) and placement of a restrictive pulmonary artery band (PAB) was performed at 126 ± 1 d gestation (term 145 d). Four groups of animals (n = 5–6 in each group): 1) control; 2) fetal THX; 3) fetal PAB; and 4) fetal PAB + THX; were monitored for 1 week prior to being euthanized. Fetal heart rate was significantly lower in the two THX groups compared with the non-THX groups while mean arterial blood pressure was similar among groups. Combined left and right ventricle free wall + septum weight, expressed per kg fetal weight, was significantly increased in PAB (6.27 ± 0.85 g/kg) compared to control animals (4.72 ± 0.12 g/kg). THX significantly attenuated the increase in cardiac mass associated with PAB (4.94 ± 0.13 g/kg) while THX alone had no detectable effect on heart mass (4.95 ± 0.27 g/kg). The percentage of binucleated cardiomyocytes was significantly decreased in THX and PAB +THX (~16%) compared to the non-THX groups (~27%). No differences in levels of activated Akt, ERK or JNK were detected among the groups. Markers of cellular proliferation but not apoptosis or expression of growth related genes were lower in the THX and THX+ PAB groups relative to thyroid intact animals. These findings suggest that in the late gestation fetal heart, thyroid hormone has important cellular growth functions in both physiologic and pathophysiologic states. Specifically, thyroid hormone is required for adaptive fetal cardiac growth in response to pressure overload.
Keywords: cardiac, development, hypothyroidism
INTRODUCTION
Marked changes in the myocardium occur during the last third of gestation as the fetus prepares for the transition to extrauterine life (Smolich et al., 1989). Accompanying increases in right (RV) and left ventricle (LV) free wall mass, there is a dramatic increase in the number of cardiomyocytes within the heart as well as a transition from mononucleated to binucleated myocytes (Jonker et al., 2007). In the sheep, where this process has been well described, the transition from mononucleated to binucleated cells begins around 100 d gestation (term ~145 d) such that at term, approximately 70% of cardiomyocytes are binucleated, or terminally differentiated (Jonker et al., 2007). As such, the number of adult cardiomyocyte numbers appears to be determined during the prenatal and perinatal period. Disruption of this process leads to a reduction in cardiomyocyte endowment in the heart and potentially increases the risk for cardiac disease during postnatal life (Li et al., 2003; Li et al., 2004; Corstius et al., 2005).
The mechanisms governing this developmentally regulated heart growth process are not well understood, though a number of mechanical and hormonal factors appear to regulate fetal cardiomyocyte proliferation and maturation, including cardiac load, angiotensin II, cortisol, insulin-like growth factor-1 and atrial natriuretic factor (Barbera et al., 2000; Sundgren et al., 2003a; Sundgren et al., 2003b; Giraud et al., 2006; O'Tierney et al., 2010). In the adult, thyroid hormone is known to have dramatic effects on the cardiovascular system, including regulating cardiac growth and inducing cardiomyocyte hypertrophy (Kahaly & Dillmann, 2005). Far less is known regarding the relationship between thyroid hormone and heart growth during fetal life. In isolated fetal sheep cardiomyocytes, exogenous triiodothyronine (T3) inhibited proliferation, as determined by BrdU uptake and expression of cyclin D1 (Ledda-Columbano et al., 2006; Chattergoon et al., 2007). However, in vivo, thyroidectomized fetal sheep heart display decreased binucleated cardiomyocyte population (a measure of maturation) and cell cycle activity (Chattergoon et al., 2011). The role of thyroid hormone in regulating pathophysiologic cardiac growth in the fetus has, to our knowledge, not been investigated.
We hypothesized that endogenous thyroid hormone modulates cardiomyocyte development and is required for adaptive cardiac growth in response to pressure overload in utero. To test this hypothesis, we evaluated the effect of fetal thyroidectomy on cardiac growth, cardiomyocyte proliferation and maturation, and expression of myocardial mRNA and proteins thought to be involved in cardiac growth and function. To specifically assess the role of thyroid hormone in regulating pathophysiologic cardiac growth, we examined the effect of thyroidectomy on these markers in fetuses that underwent placement of a pulmonary artery band that increases cardiac load and RV mass.
METHODS
Ethical approval
All procedures were performed within the regulations of the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the University of Iowa Animal Care and Use Committee. Time-bred pregnant ewes of mixed Dorset-Suffolk breed with twin were obtained from a local supplier and acclimated to the laboratory over several days.
Animals and surgical preparation
Four groups of fetal sheep were prepared surgically, including thyroidectomy alone (THX), pulmonary artery banding alone (PAB), thyroidectomy + pulmonary artery banding (THX + PAB), and control (sham thyroidectomy + sham pulmonary artery banding, CON). Pregnant ewes at 125–126 days gestation (term 145 days) with twin fetal pregnancies were used for the study (n = 12 ewes). Anesthesia was induced with 12 mg/kg of thiopental sodium (Pentothal Sodium, Abbott Laboratories) and maintained with a mixture of isoflurane (1–3%), oxygen (30%) and nitrous oxide. Under sterile conditions, the uterus was opened and the fetal head exteriorized. A ventral midline incision was made in the neck and in one group of animals the thyroid gland identified, isolated and removed (Hopkins & Thorburn, 1972). Indwelling catheters (PE-90, ID = 0.86 mm, OD = 1.27 mm, Intramedic, Franklin Lakes, NJ) were placed into the right fetal carotid artery and jugular vein and the neck incision closed. Fetal blood gases were monitored throughout the surgery following catheter placement. A catheter for measurement of amniotic pressure was secured to the fetal skin. The fetus was further exteriorized to allow access to the left chest wall and via a third interspace thoracotomy, the main pulmonary artery was exposed proximal to the ductus arteriosus and double-wrapped with an umbilical tape ligature to constrict the diameter of the artery by 50% as measured using calipers. Previous work by us has demonstrated this degree of constriction results in an acute pressure gradient of 12–14 mmHg across the constriction (Dalshaug et al., 2002). The fetal chest was closed and the hysterotomy repaired. These procedures were then repeated on the second twin, with performance of sham thyroidectomy or pulmonary artery constriction when indicated. At the completion of surgery, maternal incisions were closed in separate layers and all catheters were exteriorized through a subcutaneous tunnel and placed in a cloth pouch on the ewe's flank. Ampicillin sodium (Wyeth Laboratories, Philadelphia) was administered intra-amniotically at the completion of surgery (2 g) and to the ewe prior to surgery (2 g) and daily for three days. Pregnant ewes were returned to individual pens and allowed free access to food and water. Butorphanol (0.1 mg/kg i.v.; Torbugesic; Fort Dodge Animal Health, Fort Dodge, IA) was given for 24 h postoperatively for analgesia.
Experimental protocol
Animals were monitored daily for 1 week following surgery. Physiological measurements were begun 24 h after surgical preparation. Ewes were confined to stanchions during the course of the experiment, where they were afforded free access to food and water. Pressures were recorded with Transpac pressure transducers (Abbott, Abbott Park, IL) on a calibrated computerized system (MacLab, ADInstruments, Colorado Springs, CO; Apple, Cupertino, CA). Fetal arterial pressures were referred to amniotic fluid pressure and reported as arithmetic mean from computer tracings. Arterial pressure tracings were used to calculate fetal heart rate. Fetal arterial blood samples were taken daily for blood gases and pH (Gem Premier 3000, Instrumentation Laboratory, Bedford MA). Samples for determination of serum thyroxine (T4) were obtained one the first postoperative day (Day 1) and again prior to euthanizing the animals (Day 7). Thyroxine levels were measured in duplicate using an ovine-specific radioimmunoassay (Animal Health Diagnostic Center, Cornell University College of Veterinary Medicine, Ithaca NY, Ned Place MD, PhD, Director).
Tissue Collection
On the 7th postoperative day, ewes were again anesthetized with general anesthesia, the fetuses exteriorized and the hearts arrested in diastole with an intravenous solution of saturated potassium chloride. Fetuses were weighed and their hearts were removed. The hearts were dissected into anatomical components and each component weighed. A thin mid-ventricular wall section was fixed in ethanol-acetic acid (2% v/v) for 24 h and embedded in paraffin. The remaining ventricular tissue was immediately frozen in liquid nitrogen. Ewes were euthanized by an intravenous administration of pentobarbital sodium and phenytoin sodium (120 mg/kg barbiturate, Euthasol Solution, Virbac Corp., Fort Worth TX).
Cardiomyocyte Isolation
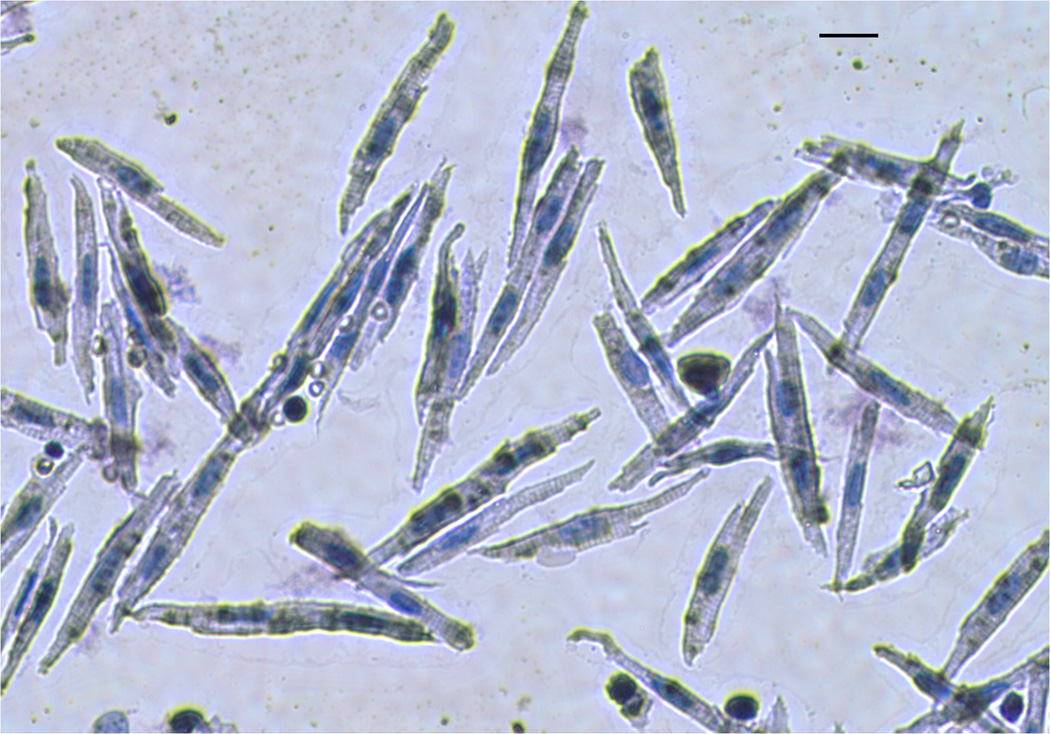
To determine the percentage of mononucleated and binucleated myocytes within each ventricular free wall, cardiomyocytes were isolated from paraffin embedded sections as described by Leeuwenburgh et al.(Leeuwenburgh et al., 2008). Briefly, 100 um thick tissue slices were deparaffinized and rehydrated using xylene and decreasing concentrations of ethanol. Tissues were incubated in a collagenase solution (450 U·mL−1, Worthington, type I) and shaken for 30 min. Supernatant was removed and the process repeated on the remaining tissue. Supernatant was again removed, combined with the first aliquot and centrifuged (1000 rpm) for 10 min. Pelleted cells were resuspended in phosphate-buffered saline, and transferred to glass slides using cytospin centrifugation. Cells were subsequently stained with hematoxylin and eosin for counting of nuclei (Figure 1). At least 300 myocytes from each ventricle of each fetus were counted.
Figure 1. Dissociated cardiomyocytes from fetal sheep.
Cells were isolated from paraffin embedded sections, stained with hematoxylin and eosin and viewed under×40 power for counting of nuclei. Bar = 20 µm.
Quantitative Immunoblot
Immunoblots were performed as described previously to quantify protein expression (Olson et al., 2006a). Cardiac samples were homogenized and then sonicated in a buffer containing soybean trypsin inhibitor, leupeptin and PMSF in 50 mM Tris, 10mM EDTA, 150 mM NaCl and 0.1% mercaptoethanol. Cellular debris was removed by centrifugation and samples were quantified spectrophotometrically. Protein was separated by SDS-PAGE and transferred to nitrocellulose membranes. These were blocked for 1 hour in 5% nonfat milk, and then incubated with primary antibodies overnight at 5°C. Bound antibody was detected by incubation with infrared-labeled secondary antibodies (IRDye 800 or IRDye 700 700DX, Li-Cor Biotechnology, Lincoln, NE). Blots were read and quantified on a Li-Cor Odyssey Imaging System (Li-Cor Biotechnology). All immunoblots were performed in duplicate with the results for each sample being averaged. Primary antibodies included antibodies from Santa Cruz Biotechnology (Santa Cruz, CA) specific to total ERK1/2 (sc-93), phosphorylated ERK1/2 (sc-7383), total JNK1/2 (sc-1648), phosphorylated JNK1/2 (sc-6254), beta-myosin heavy chain (β-MHC) (sc-71575), sarcoplasmic reticulum Ca2+-ATPase (SERCA2) (sc-8094), and phospholambam (sc-30142); from Cell Signaling Technology (Beverly, MA) specific to PCNA (2586), cyclin B1 (4135), cyclin D1 (2978), cyclin E (4129) and p21 (2978); from BD Pharmigen (San Diego, CA) specific to cleaved (activated) caspase 3 (559565) and from Imgenex Corp., (San Diego, CA) specific to cleaved caspase 8 (5703).
qRT-PCR
Total RNA was isolated from LV free wall using the ToTALLY RNA™ kit (Ambion, Inc.) followed by further purification using the RNeasy® midi kit (Qiagen Inc.). RNA was quantitated using a NanoDrop® ND-1000 spectrophotometer (Labtech International, East Sussex, U.K.). Reverse transcription reactions were performed on 1 µg total RNA with the addition of oligo dT, dNTPs, DTT, RNasin, and Superscript III™ reverse transcriptase (Life Technologies, Inc.). Gene expression levels were analyzed by quantitative real-time PCR using the TaqMan® system (Life Technologies, Inc.) which makes use of an intron-spanning primer pair and a fluorogenic probe. Primer/probe sets for growth related genes (Table 1) were either identified from the literature or were designed in our laboratory for specific ovine genes by first PCR cloning a large region of each via either published sequence or relying on highly conserved regions for degenerate primer design. Once a large section was PCR amplified, it was sequenced and intron-spanning primer/probe sets were designed for qRT-PCR using the Primer3 software following guidelines established by Applied Biosystems. To control for possible regional variations in the heart, 3 separate tissue pieces from each animal were processed and analyzed. Relative standard curves were generated for each run using pooled RNA. All individual samples were run in triplicate and each final average was calculated from 3 separate reverse transcription reactions that were generated from 3 separate RNA harvests. When compared across equally loaded (by quantitated RNA amounts) control and programmed cells, there was no significant change in GAPDH RNA quantity, supporting its use as an endogenous control.
Table 1.
Primer/probe sets for growth related genes.
| Gene | Forward | Probe | Reverse |
|---|---|---|---|
| GAPDH | GGCATCGTGGAGGGACTTAT | CATCACTGCCACCCAGAAAACTGTG | AAGCAGGGATGATGTTTTGG |
| IGF1 | TGGATGCTCTCCAGTTCGTGT | ACAGGGGCTTTTATTTCAACAAGCCCAC | CACAGCTCCGGAAGCAGC |
| IGF1R | TTGCAAGAACCATGCCTGCAGAAG | ACCTGGGAGCCAAGGCCTGAGAACTCCATCTTT | TGGGATTCTCAGGTTCTGGCCATT |
| IGF2 | CTGCCTCTACGACCGTGCTT | TCACAGCATACCCCGTGGGCAAG | TGCTTCCAGGTGTCAGATTGG |
| IGF2R | AGGACGAAGCCGTCATTCTGAGTT | ATGCCAACGGAGACACTTGTCCTCCGGAAACTGA | ACACTCCTCGTAGCTCTTCCCATT |
| PDGF | ATGGGACCGGGTCAAGTTC | AGCTCCGTCTTCATCTAAGGAGTCTCCA | CATCCGTTCCTTCGATGACCT |
| VEGF | GCTCTCTTGGGTGCATTGGA | CCTTGCCTTGCTGCTCTACCTTCACCA | TGCAGCCTGGGACCACTT |
| AT1R | GGGCTGTCTACACTGCTATGGAA | ACCGCTGGCCCTTCGGCAA | CCGGAAGCGATCTTACATAGGTA |
| AT2R | TGTTCTGGCGTTCATCATTTG | TGGCTTCCCTTCCATGTTCTGACCTTC | CCATCCAAGCTAGAGCATCCA |
GAPDH, glyceraldehyde 3-phosphate dehydrogenase; IGF1, insulin-like growth factor 1; IGF1R, insulin-like growth factor 1 receptor; IGF2, insulin-like growth factor 2; IGF2R, insulin-like growth factor 2 receptor; PDGF, platelet derived growth factor; VEGF, vascular endothelial growth factor; AT1R, angiotensin II type 1 receptor; AT2R, angiotensin II type 2 receptor.
Data analysis
All values are presented as mean ± SE. Statistical comparisons were performed by Student unpaired, two-tailed t-test or analysis of variance (ANOVA) with a post-hoc Tukey’s test if the F-statistic was found to be significant. Gene expression data were analyzed by 2-way ANOVA, factoring for thyroid status and pulmonary artery banding status. A value of p < 0.05 was considered significant.
RESULTS
A total of 23 fetuses from 12 pregnant ewes were studied, with 6 fetuses in each group except for 5 in the PAB group. One animal was not included in this group as it was acidotic and bradycardic on the morning of the last day of study. Gestational age and fetal weight were similar among all groups on Day 7 (Table 2). No significant differences in arterial pH or blood gas values were found among groups on any day (Day 1 and 7 shown in Table 2). Mean arterial blood pressure was also similar among groups, though heart rate on day 7 was significantly lower in THX and PAB + THX compared to non-THX groups. As expected fetal heart weight (RV free wall + LV free wall + septum), normalized to fetal weight, was significantly increased in PAB compared to CON. THX alone had no effect on heart weight (not significant compared to CON), though PAB + THX heart weight was significantly less than PAB alone and similar to CON and THX. Thyroxine levels in THX and PAB + THX were significantly less than levels found in CON and PAB. Weights of liver, left lung, left kidney and left adrenal, normalized for fetal weight, were not different among groups (data not shown).
Table 2.
Hemodynamic and arterial blood values.
| Control (n=6) | THX (n=6) | PAB (n=5) | PAB + THX (n=6) | |||||
|---|---|---|---|---|---|---|---|---|
| DAY 1 | DAY 7 | DAY 1 | DAY 7 | DAY 1 | DAY 7 | DAY 1 | DAY 7 | |
| Gestational age, days | 126 ± 1 | 133 ± 1 | 127 ± 1 | 134 ± 1 | 126 ± 1 | 133 ± 1 | 126 ± 1 | 133 ± 1 |
| Fetal weight, kg | 3.66 ± 0.25 | 3.36 ± 0.53 | 3.15 ± 0.35 | 3.82 ± 0.26 | ||||
| Heart weight/ Fetal weight, g/kg |
4.72 ± 0.12 | 4.95 ± 0.27 | 6.27 ± 0.85* | 4.94 ± 0.13 | ||||
| pH | 7.36 ± 0.01 | 7.31 ± 0.02 | 7.38 ± 0.02 | 7.36 ± 0.02 | 7.34 ± 0.02 | 7.36 ± 0.01 | 7.39 ± 0.11 | 7.36 ± 0.02 |
| PO2, torr | 22 ± 2 | 18 ± 2 | 19 ± 2 | 20 ± 1 | 19 ± 2 | 18 ± 2 | 19 ± 1 | 20 ± 2 |
| PCO2, torr | 51 ± 2 | 58 ± 7 | 50 ± 2 | 53 ± 2 | 52 ± 2 | 57 ± 2 | 52 ± 2 | 53 ± 3 |
| MABP, mmHg | 43 ± 2 | 43 ± 2 | 45 ± 2 | 39 ± 2 | 45 ± 2 | 46 ± 2 | 42 ± 2 | 42 ± 2 |
| HR, bpm | 163 ± 12 | 161 ± 10 | 151 ± 11 | 124 ± 8† | 178 ± 3 | 158 ± 6 | 150 ± 4 | 131 ± 5† |
| T4, ug/dL | 9.58 ± 1.02 | 8.00 ± 0.51 | 4.66 ± 0.44 | 0.12 ± 0.03† | 9.05 ± 0.93 | 7.50 ± 1.10 | 3.92 ± 0.36 | 0.21 ± 0.02† |
Values are means ± SE. n = number of animals in each group; Heart weight = RV + LV + septum weight; T4, thyroxine;
p < 0.05 compared to all other groups;
p < 0.05 compared to same group Day 1 values and Day 7 control and PAB values.
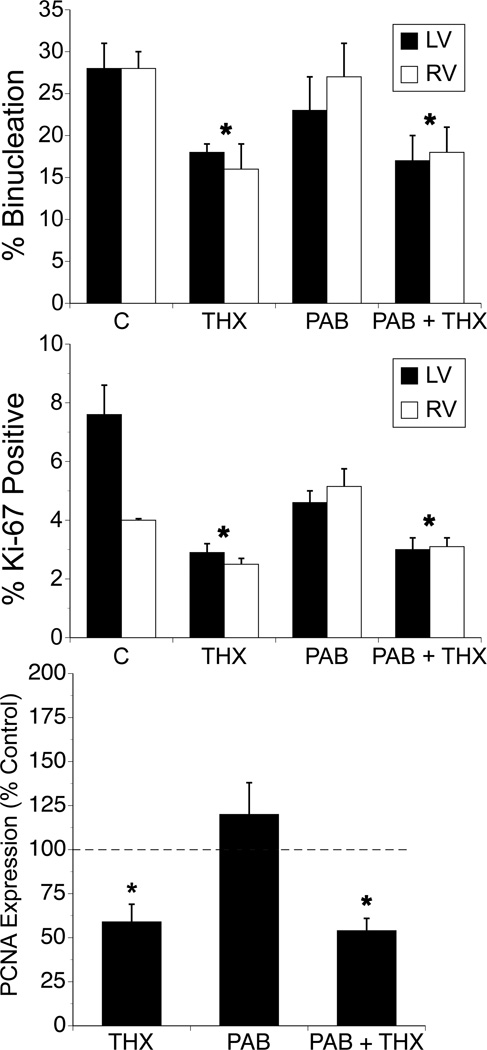
The proportion of binucleated cardiomyocytes, which remained similar between LV and RV within each group was significantly decreased in both THX and PAB + THX groups compared to non-THX groups (p< 0.05, ANOVA factoring for treatment effect; Figure 2). PAB had no effect on altering the proportion of binucleated cardiomyocytes. Cellular proliferative activity, as determined by the percentage of mononucleated cells that stained positive for the nuclear protein Ki-67 was also decreased in LV and RV cardiomyocytes from THX and PAB + THX compared to other groups (p< 0.05, ANOVA factoring for treatment effect; Figure 2). Using Western blot analysis, we found cardiac expression of the proliferation marker Proliferating Cell Nuclear Antigen (PCNA) was also significantly decreased in LV and RV from THX and PAB + THX compared to the other groups, a finding consistent with the Ki-67 data (Figure 2).
Figure 2. Effects of thyroidectomy and pulmonary artery banding on fetal cardiomyocyte maturation and proliferation.
Values expressed as mean ±SE. PCNA data represent LV only. * p<0.05 compared to other treatment groups for same ventricle. N = 6 for each respective group. No differences between LV and RV were noted within groups.
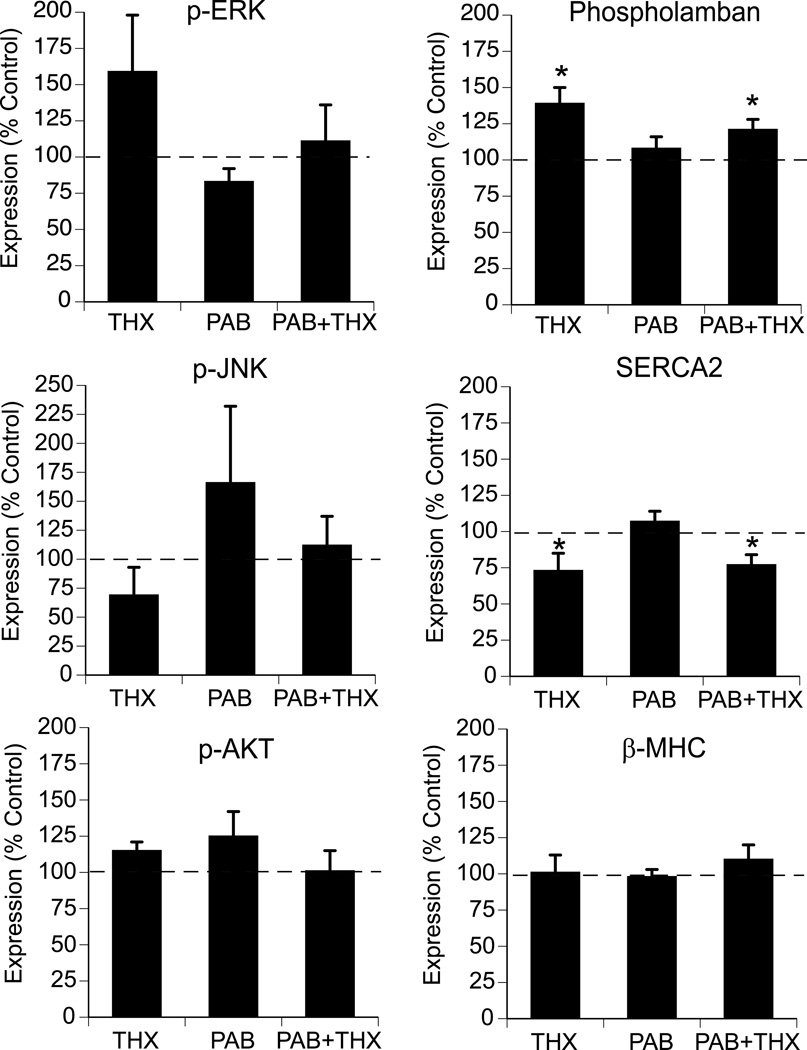
Steady state protein levels of Akt, a serine/threonine protein kinases and two terminal product mitogen-activated protein kinases present in the myocardium (ERK1/2, and JNK) were measured in the RV and LV free wall of the fetal hearts. No significant changes in RV or LV total or phosphorylated (active) MAP kinase signaling or Akt protein levels were identified among groups (Figure 3, LV data only).
Figure 3. Effects of thyroidectomy and pulmonary artery banding on expression activated (phosphorylated) ERK, JNK Akt and phopholambam, SERCA2 and β-MHC in fetal myocardium.
Values expressed as mean ±SE. Data represent LV only and are expressed as percent of control. *p<0.05 compared to control and PAB (ANOVA, treatment effect for THX). N = 6 for each respective group.
Left ventricular protein levels of phospholambam, SERCA2 and β-MHC, which have previously been shown to be regulated by thyroid hormone in the postnatal heart were also determined (Figure 3). THX and PAB + THX resulted in significantly increased expression of phospholambam but repression of SERCA2 levels. Protein levels of β-MHC were similar among groups.
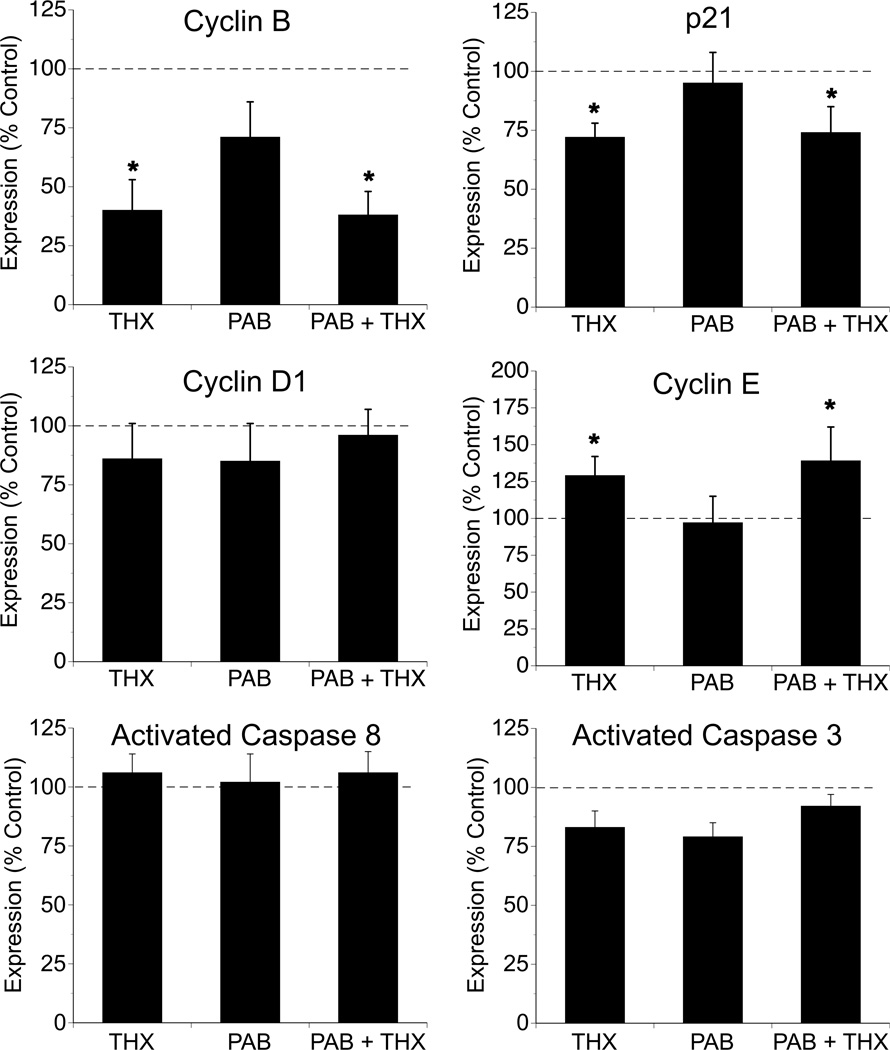
Modulation of cell cycle activity involves multiple classes of regulatory molecules, including cyclins, which regulate the activity of cyclin-dependent kinases, which in turn orchestrate the phases of mitosis. Inhibitors of the process, including the cip/kip family of proteins, bind to cyclin-cdk complexes to prevent progression of the cell cycle. To begin to examine these pathways, we explored the myocardial expression of cyclins B1, D1 and E as well as cip/kip family member p21. While protein levels of cyclin D1 were similar among groups, levels of cyclin E were significantly greater in THX and PAB + THX fetuses compared to CON and PAB (Figure 4). Conversely, myocardial protein levels of cyclin B and p21 were significantly decreased in THX and PAB + THX compared to the other groups (Figure 4).
Figure 4. Effects of thyroidectomy and pulmonary artery banding on fetal myocardial expression of cell-cycle and apoptosis related proteins.
Values expressed as mean ±SE. Data represent LV only and are expressed as percent of control. *p<0.05 compared to control and PAB (ANOVA, treatment effect for THX). N = 6 for each respective group.
To evaluate whether apoptosis contributed to the differences in myocardial mass, we examine levels of the initiator caspase 8 and the effector caspase 3. Protein levels of the activated forms of these caspases were not significantly different among groups (Figure 4).
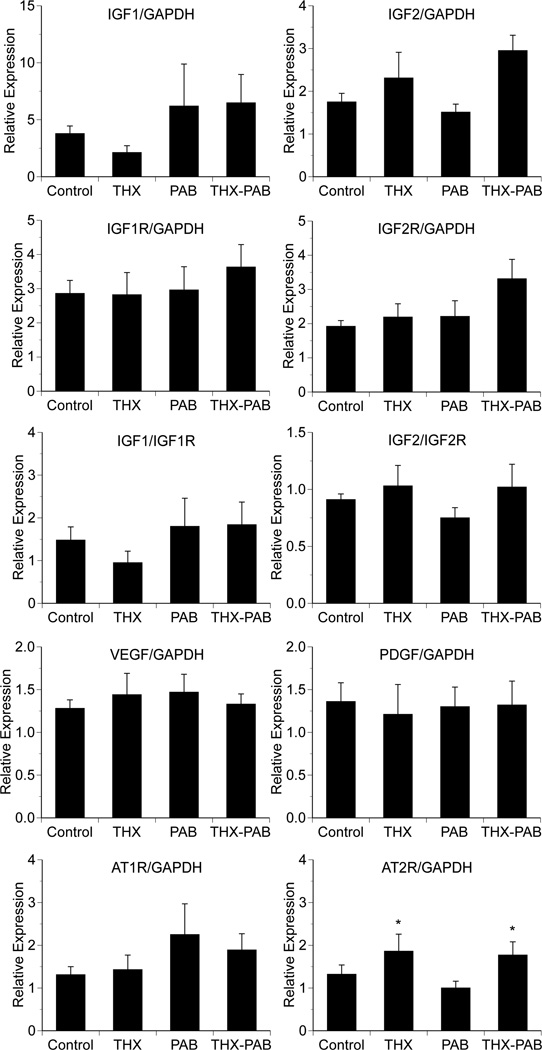
Quantitative RT-PCR was used to examine changes in expression of several genes implicated in regulating fetal cardiac growth. We were unable to identify a significant effect of either thyroidectomy or pulmonary artery banding on expression of IGF-1, IGF-1R, IGF-2, IGF-2R, AT1R, VEGF or PDGF (Figure 5). We did find a significant effect of thyroidectomy on increased myocardial expression of AT2R in THX groups compared to non-THX groups (p < 0.05).
Figure 5. Effects of thyroidectomy and pulmonary artery banding expression of cardiac growth related genes.
Values expressed as mean ±SE relative to GAPDH or IGF1R for IGF1 and IGF2R for IGF2. *p<0.05 for THX and THX-PAB compared to other groups (ANOVA, treatment effect for THX). N = 6 for each respective group.
DISCUSSION
Over the past decade, there has been a growing body of literature focused on understanding normal cardiomyocyte maturation and proliferation in the developing heart, as well as the cardiomyocyte response to mechanical stress resulting from pressure and/or volume overload. Clearly, multiple neurohumoral factors are involved in these responses. Recent studies have highlighted the important role of thyroid hormone in regulating fetal cardiomyocyte maturation (Chattergoon et al., 2011). In this study, we expand upon these findings to examine the role of endogenous thyroid hormone on the adaptive cardiac response to right ventricular pressure loading in third trimester fetal sheep. Thyroidectomy, which resulted in a significant decrease in circulating thyroxine levels, did not alter heart mass compared to control, but resulted in fewer binucleated cardiomyocytes and a slowing of cardiomyocyte proliferation. In the presence of pulmonary artery banding, which alone results in an increase in cardiac mass, thyroidectomy attenuated a compensatory increase in mass and again suppressed cardiomyocyte maturation and proliferation. These findings establish that thyroid hormone is an important and potent regulator of cardiomyocyte maturation and proliferation in the late gestation fetal heart during physiologic and pathophysiologic states and is required for adaptive cardiac growth in response to pressure overload in utero.
Normal growth of the immature heart is accompanied by cardiomyocyte proliferation and terminal differentiation, progressing from a heart composed of mononucleated cells to primarily bi- or multinucleated cells (Jonker et al., 2007; Thornburg et al., 2011). While the mechanisms and signals regulating this process are poorly understood, a number of pathophysiological conditions are known to alter this maturational process. For example, placental insufficiency results in a decreased number of binucleated cardiomyocytes, suggesting a delay of cardiomyocyte maturation (Bubb et al., 2007; Morrison et al., 2007). Chronic fetal anemia, resulting in volume overload and an increase in cardiac mass, results in an increased proportion of binucleated cardiomyocytes, cellular enlargement and accelerated proliferation (Jonker et al., 2010). Barbera et al. previously demonstrated that right ventricular pressure load in near term fetal sheep for 10 days, induced by PA banding, increased cardiac mass, increased the proportion of binucleated cardiomyocytes in the RV but not LV, and increased cardiomyocyte size (Barbera et al., 2000). In the present study, PA banding increased cardiac mass but failed to result in a significant change in the percentage of binucleated cardiomyocytes in either ventricle. Reasons for differences in the binucleation response between this study and that of Barbera et al. may relate to differences in duration of PAB, differences in afterload on the heart, or differences in the ages of the animals at the time of study. Notably, in the absence of thyroid hormone, cardiac mass failed to increase in PAB fetuses despite an increased RV pressure load, suggesting endogenous levels of thyroid hormone are necessary adaptive cardiac growth in response to pathophysiological conditions. The striking degree of attenuation of increased cardiac mass does not appear to be explained by increased rates of apoptosis, as myocardial levels of activated caspase 3 and 8 were similar among groups. Involvement of autophagy in the failure of cardiac remodeling in THX + PAB fetuses was not explored though has been shown in the stressed heart to contribute to cell death and loss of cardiac mass (Zhu et al., 2007; Nishida et al., 2009).
In the present study, we found that THX alone did not decrease cardiac mass, although THX resulted in a decrease percentage of binucleated cardiomyocytes in PAB and non-PAB hearts, suggesting a slowing of the maturational process. In somewhat younger fetal sheep (~6 days) exposed to hypothyroid conditions for a slightly longer period of time, Chattergoon found fetal thyroidectomy was associated with decreased cardiac growth (heart weight to body weight ratio) as well as decreased binucleation compared to controls (Chattergoon et al., 2011). Reasons for the discrepant results in cardiac growth likely relate to differences in fetal age and duration of hypothyroidism as well as definition of heart mass (RV + LV + septum vs. whole heart). We also did not include sham thyroidectomy alone or sham PAB alone fetuses in the design of the study due to the large number of additional animals this would have required. Inclusion of such groups, and additional comparisons of measured outcomes may have resulted in different interpretation of the findings.
Chattergoon et al. previously reported that physiologic and pharmacologic concentrations of exogenous T3 inhibited in vitro proliferation of cardiomyocytes isolated from 135 d fetal sheep as measured by BrdU uptake (Chattergoon et al., 2007). The authors speculated that the decreased proliferative capacity was consistent with T3 promoting cardiomyocyte maturation (i.e., terminal differentiation). When studied in vivo, this same group found elevated levels of T3 increased fetal cardiomyocyte binucleation and cell size while suppressing proliferation, findings consistent with induction of maturation, while fetal thyroidectomy decreased expression of markers of proliferation, namely Ki67 and phospho-histone 3 (Chattergoon et al., 2011), providing evidence that a low thyroid hormone environment suppresses maturation. We similarly found that THX, both in the absence and presence of PAB resulted in a slowing of the cardiomyocyte proliferation process, as evidenced by a decrease in the % of Ki-67 positive staining cells and PCNA expression, both markers of proliferations, compared to controls. Thus, in this in vivo model, physiological levels of thyroid hormone appear necessary for normal fetal cardiomyocyte proliferation as well as maturation.
Interactions between thyroid hormones and pressure overload (wall stress) on the heart are likely complex and multiple. Triiodothyronine is the most biologically active thyroid hormone. Conversion from T4 to T3 can occur at the tissue level via activity of the type 2 iodothyronine deiodinase. As previously noted, T3 exerts its direct effect by binding to nuclear thyroid hormone receptors TRα and TRβ, thereby influencing cardiac gene expression. In isolated adult rat cardiomyocytes, T3 has been shown to increase cyclin D1 mRNA and protein levels along with PCNA expression and BrdU uptake (Ledda-Columbano et al., 2006). These findings suggest that a population of postmitotic cardiomyocytes remain capable of proliferation. To examine regulation of cell division, we examined expression of several proteins regulating cell cycle activity. In the fetal sheep heart, composed primarily of mononucleated cardiomyocytes, we observed that hypothyroidism was associated with increase expression of cyclin E, decreased expression of cyclin B1 and the cyclin-dependent kinase p21, while cyclin D1 remained unaltered. Chattergoon et al. similarly found no effect of thyroidectomy on cyclin D1 expression, and failed to detect a change on cardiac p21 expression (Chattergoon et al., 2011). Depletion of cyclin B1 is known to inhibit cellular proliferation (Yuan et al., 2004). Thus, in the hypothyroid fetus, decreased cardiomyocyte proliferation may be regulated, in part, by decreased cyclin B1 expression and suppression of mitotic activity. Interestingly, the increased expression of cyclin E, which is important for cells making the transition from G1 to S phase, and decreased expression of p21, which functions as an inhibitor of CDK2 or CDK4, which in turn partner with a number of cyclins to in the G1 to S phase transition suggest that under these conditions, mitogenic signals are also acting to promote escape G1 arrest and cell division (Gopinathan et al., 2011).
The signal transduction pathways mediating both pressure-overload and thyroid hormone induced fetal cardiac growth are not well understood, though multiple pathways are likely involved. In earlier studies, we demonstrated exogenous angiotensin II administration produces fetal cardiac growth, though blockade of angiotensin receptors fails to attenuate pressure-overload cardiac hypertrophy (Segar et al., 1997; Segar et al., 2001). An important role for PI3K/Akt/GSK-3b/mTor activation in thyroid-hormone induced cardiac hypertrophy has been suggested in studies of isolated neonatal rat myocytes (Kenessey & Ojamaa, 2006). This pathway, as well as the extracellular signal-regulated kinase (ERK) branch of the MAPK cascade, have previously been shown to be important in mediating the pro-proliferative effects on isolated fetal sheep cardiomyocytes of other hormones, including ANG II and IGF-1 (Sundgren et al., 2003a; Sundgren et al., 2003b). Despite being associated with an attenuation of cardiomyocyte proliferation, we found thyroidectomy, regardless of PAB status, had no significant effect on cardiac levels of activated ERK, JNK or Akt, results similar to those of reported by others (Chattergoon et al., 2011). The failure to identify changes in myocardial expression of activated ERK and JNK are consistent with our previous findings in aortic and pulmonary artery banded fetal sheep and suggest that neither thyroid hormone or pressure-overload mechanisms significantly utilize MAPK signaling pathways to regulate cardiomyocyte growth (Olson et al., 2006b). We recognize that the design of the study allowed examination of myocardial protein expression at only a single time point and that failure to detect changes in the levels of protein does not necessarily signify that these pathways are not involved in regulating the response of the heart to thyroid hormone. For example, O’Tierney recently described that while atrial natriuretic peptide alone had no effect on fetal sheep cardiomyocyte proliferation, ERK or PI3K signaling, the compound inhibits angiotensin II stimulated proliferation by suppressing ERK and Akt phosphorylation (O'Tierney et al., 2010). Collectively, these studies underscore the complex balance of pro- and anti-proliferating stimuli acting upon the developing heart.
In addition to exploring the MAPK and Akt pathways, we examined the expression of several genes thought to be involved in cardiac growth. We were particularly interested in the IGF system, as both IGF1 and IGF2 have been shown to stimulate cardiomyocyte proliferation and hypertrophy in the immature heart (Liu et al., 1996; Sundgren et al., 2003a; Lumbers et al., 2009). Furthermore, expression of a number of these genes has previously been shown to be developmentally regulated and temporally associated with changes in cardiomyocyte proliferative activity (Reini et al., 2009). However, other than for AT2R, we were unable to detect a significant change in myocardial expression in any of theses genes among groups. Isolation of RNA for real-time PCR from the myocardium, and thus a number of cardiac cell types, rather than just cardiomyocytes, could contribute our findings of relatively unchanged expression in growth factors. The significance of the finding for AT2R is unclear, although increased expression of AT2R has been associated with enhanced myocardial vulnerability to ischemia reperfusion injury (Xue et al., 2011). It should be noted, however, that Chen et al. (Chen et al., 2005) found no effect of THX on expression of AT2R or AT1R protein in fetal myocardium. Furthermore, we recognize that cardiac growth is a dynamic process and that our determination of gene or protein expression at a single time point may miss important changes in select signaling pathways. Additional studies are needed to evaluate for patterns of expression over time.
In long gestation mammals, such as the human or sheep, fetal levels of thyroid hormones increase late in gestation (Fraser & Liggins, 1988; Thorpe-Beeston et al., 1991). This increased activity of the fetal thyroid contributes to growth, development and differentiation of a number of biological systems and tissues. Within the heart, thyroid hormone appears to influence calcium handling and be important to the developmental regulation of myofibrillar protein expression, particular the transition from myosin heavy chain β to myosin heavy chain α (reviewed Kahaly & Dillmann, 2005). In the postnatal heart, thyroid hormone promotes the expression of SERCA2 while decreasing levels of phospholambam, a potent inhibitor of SERCA2 (Belakavadi et al., 2010; Dillmann, 2010). Notably, in hypothyroid animals, including those in the present study, levels of SERCA2 are decreased while those of phospholambam are increased. Thus, regulation of these calcium signaling proteins by thryroid hormone appears similar in the pre-and postnatal heart. The lack of significant changes in expression of B-MHC in hypothyroid fetuses contrasts findings in the adult heart in which hypothyroidism enhances B-MHC genes expression (Haddad et al., 2010). Organ specific developmental differences as well as duration of the hypothyroid state may contribute to this difference (Haddad et al., 2008). The lack of effect of PAB on expression of these proteins is intriguing and may suggest that adaptive cardiac growth in response to acute pressure overload in the fetus is more physiologic and pathologic, as changes in the myocardial expression of SERCA2, phospholambam and B-MHC in hypertrophied heart are thought to represent a pathologic response (Bernardo et al., 2010).
It is increasing recognized that environment and well-being early in development influence health later in life. In the present study, we identified that endogenous thyroid hormone impacts heart development during physiological as well as pathophysiological growth. Specifically, thyroidectomy was associated with impaired cardiomyocyte proliferation and maturation in the late gestation fetal heart and attenuated the increase in cardiac mass in response to pressure overload. These findings may have important clinical implications, particular for women with thyroid disorders pregnant with a fetus with congenital heart disease. However, the molecular mechanisms involved with the thyroid hormone contribution to cardiac development in physiologic and pathophysiologic states remain to be identified. Factors that inhibit cardiomyocyte proliferation and potentially diminish the endowment of myocytes within the heart at birth may ultimately influence postnatal cardiac health. Better understanding of the physiologic and pathophysiologic cardiac growth process may allow for improved clinical care of infants and children with congenital heart disease and new therapeutic strategies for the prevention of cardiac disease later in life.
NEW FINDINGS.
The importance of endogenous thyroid hormone in contributing to pathophysiologic adaptive growth of the fetal heart is not known.
The study identifies that thyroid hormone is required for adaptive fetal cardiac growth in response to pressure overload. Understanding the pathophysiologic cardiac growth process may allow for improved clinical care new therapeutic strategies for fetuses and infants with congenital heart disease.
Acknowledgements
This study was supported by NIH R01 HL080657 (JLS).
REFERENCES
- Barbera A, Giraud GD, Reller MD, Maylie J, Morton MJ, Thornburg KL. Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1157–R1164. doi: 10.1152/ajpregu.2000.279.4.R1157. [DOI] [PubMed] [Google Scholar]
- Belakavadi M, Saunders J, Weisleder N, Raghava PS, Fondell JD. Repression of cardiac phospholamban gene expression is mediated by thyroid hormone receptor-{alpha}1 and involves targeted covalent histone modifications. Endocrinology. 2010;151:2946–2956. doi: 10.1210/en.2009-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Bubb KJ, Cock ML, Black MJ, Dodic M, Boon WM, Parkington HC, Harding R, Tare M. Intrauterine growth restriction delays cardiomyocyte maturation and alters coronary artery function in the fetal sheep. J Physiol. 2007;578:871–881. doi: 10.1113/jphysiol.2006.121160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattergoon NN, Giraud GD, Louey S, Stork P, Fowden AL, Thornburg KL. Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J. 2011 doi: 10.1096/fj.10-179895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattergoon NN, Giraud GD, Thornburg KL. Thyroid hormone inhibits proliferation of fetal cardiac myocytes in vitro. J Endocrinol. 2007;192:R1–R8. doi: 10.1677/JOE-06-0114. [DOI] [PubMed] [Google Scholar]
- Chen K, Carey LC, Valego NK, Liu J, Rose JC. Thyroid hormone modulates renin and ANG II receptor expression in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1006–R1014. doi: 10.1152/ajpregu.00046.2005. [DOI] [PubMed] [Google Scholar]
- Corstius HB, Zimanyi MA, Maka N, Herath T, Thomas W, van der Laarse A, Wreford NG, Black MJ. Effect of intrauterine growth restriction on the number of cardiomyocytes in rat hearts. Pediatr Res. 2005;57:796–800. doi: 10.1203/01.PDR.0000157726.65492.CD. [DOI] [PubMed] [Google Scholar]
- Dalshaug GB, Scholz TD, Smith OM, Bedell KA, Caldarone CA, Segar JL. Effects of gestational age on myocardial blood flow and coronary flow reserve in pressure-loaded ovine fetal hearts. Am J Physiol Heart Circ Physiol. 2002;282:H1359–H1369. doi: 10.1152/ajpheart.00686.2001. [DOI] [PubMed] [Google Scholar]
- Dillmann W. Cardiac hypertrophy and thyroid hormone signaling. Heart Fail Rev. 2010;15:125–132. doi: 10.1007/s10741-008-9125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser M, Liggins GC. Thyroid hormone kinetics during late pregnancy in the ovine fetus. J Dev Physiol. 1988;10:461–471. [PubMed] [Google Scholar]
- Giraud GD, Louey S, Jonker S, Schultz J, Thornburg KL. Cortisol stimulates cell cycle activity in the cardiomyocyte of the sheep fetus. Endocrinology. 2006;147:3643–3649. doi: 10.1210/en.2006-0061. [DOI] [PubMed] [Google Scholar]
- Gopinathan L, Ratnacaram CK, Kaldis P. Established and novel Cdk/cyclin complexes regulating the cell cycle and development. Results Probl Cell Differ. 2011;53:365–389. doi: 10.1007/978-3-642-19065-0_16. [DOI] [PubMed] [Google Scholar]
- Haddad F, Jiang W, Bodell PW, Qin AX, Baldwin KM. Cardiac myosin heavy chain gene regulation by thyroid hormone involves altered histone modifications. Am J Physiol Heart Circ Physiol. 2010;299:H1968–H1980. doi: 10.1152/ajpheart.00644.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad F, Qin AX, Bodell PW, Jiang W, Giger JM, Baldwin KM. Intergenic transcription and developmental regulation of cardiac myosin heavy chain genes. Am J Physiol Heart Circ Physiol. 2008;294:H29–H40. doi: 10.1152/ajpheart.01125.2007. [DOI] [PubMed] [Google Scholar]
- Hopkins PS, Thorburn GD. The effects of foetal thyroidectomy on the development of the ovine foetus. J Endocrinol. 1972;54:55–66. doi: 10.1677/joe.0.0540055. [DOI] [PubMed] [Google Scholar]
- Jonker SS, Giraud MK, Giraud GD, Chattergoon NN, Louey S, Davis LE, Faber JJ, Thornburg KL. Cardiomyocyte enlargement, proliferation and maturation during chronic fetal anaemia in sheep. Exp Physiol. 2010;95:131–139. doi: 10.1113/expphysiol.2009.049379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol. 2007;102:1130–1142. doi: 10.1152/japplphysiol.00937.2006. [DOI] [PubMed] [Google Scholar]
- Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–728. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- Kenessey A, Ojamaa K. Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J Biol Chem. 2006;281:20666–20672. doi: 10.1074/jbc.M512671200. [DOI] [PubMed] [Google Scholar]
- Ledda-Columbano GM, Molotzu F, Pibiri M, Cossu C, Perra A, Columbano A. Thyroid hormone induces cyclin D1 nuclear translocation and DNA synthesis in adult rat cardiomyocytes. FASEB J. 2006;20:87–94. doi: 10.1096/fj.05-4202com. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh BP, Helbing WA, Wenink AC, Steendijk P, de Jong R, Dreef EJ, Gittenberger-de Groot AC, Baan J, van der Laarse A. Chronic right ventricular pressure overload results in a hyperplastic rather than a hypertrophic myocardial response. J Anat. 2008;212:286–294. doi: 10.1111/j.1469-7580.2008.00853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Bae S, Zhang L. Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am J Physiol Heart Circ Physiol. 2004;286:H1712–H1719. doi: 10.1152/ajpheart.00898.2003. [DOI] [PubMed] [Google Scholar]
- Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Liu Q, Yan H, Dawes NJ, Mottino GA, Frank JS, Zhu H. Insulin-like growth factor II induces DNA synthesis in fetal ventricular myocytes in vitro. Circ Res. 1996;79:716–726. doi: 10.1161/01.res.79.4.716. [DOI] [PubMed] [Google Scholar]
- Lumbers ER, Kim MY, Burrell JH, Kumarasamy V, Boyce AC, Gibson KJ, Gatford KL, Owens JA. Effects of intrafetal IGF-I on growth of cardiac myocytes in late-gestation fetal sheep. Am J Physiol Endocrinol Metab. 2009;296:E513–E519. doi: 10.1152/ajpendo.90497.2008. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am J Physiol Regul Integr Comp Physiol. 2007;293:R306–R313. doi: 10.1152/ajpregu.00798.2006. [DOI] [PubMed] [Google Scholar]
- Nishida K, Kyoi S, Yamaguchi O, Sadoshima J, Otsu K. The role of autophagy in the heart. Cell Death Differ. 2009;16:31–38. doi: 10.1038/cdd.2008.163. [DOI] [PubMed] [Google Scholar]
- O'Tierney PF, Chattergoon NN, Louey S, Giraud GD, Thornburg KL. Atrial natriuretic peptide inhibits angiotensin II-stimulated proliferation in fetal cardiomyocytes. J Physiol. 2010;588:2879–2889. doi: 10.1113/jphysiol.2010.191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AK, Protheroe KN, Scholz TD, Segar JL. The mitogen-activated protein kinases and Akt are developmentally regulated in the chronically anemic fetal sheep heart. J Soc Gynecol Investig. 2006a;13:157–165. doi: 10.1016/j.jsgi.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Olson AK, Protheroe KN, Segar JL, Scholz TD. Mitogen-activated protein kinase activation and regulation in the pressure-loaded fetal ovine heart. Am J Physiol Heart Circ Physiol. 2006b;290:H1587–H1595. doi: 10.1152/ajpheart.00984.2005. [DOI] [PubMed] [Google Scholar]
- Reini SA, Wood CE, Keller-Wood M. The ontogeny of genes related to ovine fetal cardiac growth. Gene Expr Patterns. 2009;9:122–128. doi: 10.1016/j.gep.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segar JL, Dalshaug GB, Bedell KA, Smith OM, Scholz TD. Angiotensin II in cardiac pressure-overload hypertrophy in fetal sheep. Am J Physiol Regul Integr Comp Physiol. 2001;281:R2037–R2047. doi: 10.1152/ajpregu.2001.281.6.R2037. [DOI] [PubMed] [Google Scholar]
- Segar JL, Scholz TD, Bedell KA, Smith OM, Huss DJ, Guillery EN. Angiotensin AT1 receptor blockade fails to attenuate pressure-overload cardiac hypertrophy in fetal sheep. Am J Physiol. 1997;273:R1501–R1508. doi: 10.1152/ajpregu.1997.273.4.R1501. [DOI] [PubMed] [Google Scholar]
- Smolich JJ, Walker AM, Campbell GR, Adamson TM. Left and right ventricular myocardial morphometry in fetal, neonatal, and adult sheep. Am J Physiol. 1989;257:H1–H9. doi: 10.1152/ajpheart.1989.257.1.H1. [DOI] [PubMed] [Google Scholar]
- Sundgren NC, Giraud GD, Schultz JM, Lasarev MR, Stork PJ, Thornburg KL. Extracellular signal-regulated kinase and phosphoinositol-3 kinase mediate IGF-1 induced proliferation of fetal sheep cardiomyocytes. Am J Physiol Regul Integr Comp Physiol. 2003a;285:R1481–R1489. doi: 10.1152/ajpregu.00232.2003. [DOI] [PubMed] [Google Scholar]
- Sundgren NC, Giraud GD, Stork PJ, Maylie JG, Thornburg KL. Angiotensin II stimulates hyperplasia but not hypertrophy in immature ovine cardiomyocytes. J Physiol. 2003b;548:881–891. doi: 10.1113/jphysiol.2003.038778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg K, Jonker S, O'Tierney P, Chattergoon N, Louey S, Faber J, Giraud G. Regulation of the cardiomyocyte population in the developing heart. Prog Biophys Mol Biol. 2011;106:289–299. doi: 10.1016/j.pbiomolbio.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe-Beeston JG, Nicolaides KH, Felton CV, Butler J, McGregor AM. Maturation of the secretion of thyroid hormone and thyroid-stimulating hormone in the fetus. N Engl J Med. 1991;324:532–536. doi: 10.1056/NEJM199102213240805. [DOI] [PubMed] [Google Scholar]
- Xue Q, Dasgupta C, Chen M, Zhang L. Foetal hypoxia increases cardiac AT(2)R expression and subsequent vulnerability to adult ischaemic injury. Cardiovasc Res. 2011;89:300–308. doi: 10.1093/cvr/cvq303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Yan R, Kramer A, Eckerdt F, Roller M, Kaufmann M, Strebhardt K. Cyclin B1 depletion inhibits proliferation and induces apoptosis in human tumor cells. Oncogene. 2004;23:5843–5852. doi: 10.1038/sj.onc.1207757. [DOI] [PubMed] [Google Scholar]
- Zhu H, Tannous P, Johnstone JL, Kong Y, Shelton JM, Richardson JA, Le V, Levine B, Rothermel BA, Hill JA. Cardiac autophagy is a maladaptive response to hemodynamic stress. J Clin Invest. 2007;117:1782–1793. doi: 10.1172/JCI27523. [DOI] [PMC free article] [PubMed] [Google Scholar]