Abstract
Objective
The objective was to evaluate the effects of bazedoxifene acetate (BZA), a new selective estrogen receptor modulator, on coronary and peripheral artery atherosclerosis and to determine if it would antagonize the atheroprotective effects of conjugated equine estrogens (CEE) in a monkey model.
Methods
Ninety-eight surgically postmenopausal monkeys (Macaca fascicularis) were fed a moderately atherogenic diet and then randomized to receive no treatment, or women’s equivalent doses of BZA (20 mg/day), CEE (0.45 mg/day) or BZA+CEE. The experiment period was for 20 months (approximately equivalent to 5 years of patient experience) during which interim measures were made of cardiovascular risk factors. At the end of the experimental period, the extent and severity of coronary and iliac artery atherosclerosis was quantified.
Results
Body weight, adiposity, fasting glucose concentrations and plasma lipid profiles were not different among treatment conditions. BZA had no adverse effects on coronary artery nor common iliac artery atherosclerosis extent or severity when compared to no-treatment. CEE, administered soon after inducing menopause, had a robust atheroprotective effect on both iliac and coronary artery extent and severity. The addition of BZA to the CEE treatment antagonized the atheroprotective effect of the CEE.
Conclusions
In this nonhuman primate trial, treatment with BZA alone, CEE alone and BZA and CEE in combination did not have significant effects on plasma lipid profiles. CEE markedly inhibited the progression and complication of both coronary and iliac artery atherosclerosis. BZA had no adverse effects on atherosclerosis but attenuated the atheroprotective effects of CEE.
Keywords: Coronary atherosclerosis, menopause, estrogens, SERMS, bazedoxifene
INTRODUCTION
Postmenopausal hormone therapy (PHT) is the only FDA and Health Canada-approved treatment for menopausal symptoms and osteoporosis prevention. Traditional PHT includes estrogen-only therapy (ET) and, for women with a uterus, estrogen + progestogen (EPT). Over the past decade, the results of a number of studies of both women and nonhuman primates have raised concerns about the potential adverse effects of the progestogen component of EPT. Those concerns are focused primarily on issues about breast cancer risk and the possibility of increased cardiovascular events. The most commonly prescribed estrogen + progestin co-therapy in the United States has been conjugated equine estrogens (CEE) given with medroxyprogesterone acetate (MPA)1 . In 1996, Cline et al.2 reported the results of a study of surgically postmenopausal cynomolgus monkeys in which CEE+MPA increased breast epithelial proliferation to a greater extent than CEE alone, leading them to conclude that women treated with the combination of CEE+MPA may be at greater risk of developing breast neoplasms than women treated with CEE alone. That conclusion was confirmed some years later with data from the Women’s Health Initiative (WHI) randomized clinical trial3 .
CEE therapy, initiated soon after inducing surgical menopause in cynomolgus monkeys, has a robust beneficial effect of inhibiting the initiation and progression of coronary artery atherosclerosis4. In one major study using surgically postmenopausal cynomolgus monkeys, MPA was found to antagonize the inhibitory effects of CEE on coronary artery atherosclerosis5. More recently, there have been reports on subgroups from the WHI trial all suggesting cardiovascular benefits with CEE but not with CEE+MPA6-8.
Taken together, these concerns have resulted in a major research effort to identify a means of protecting the breast and uterus during postmenopausal estrogen therapy. The ideal means would be an agent that would act in a tissue selective manner when combined with estrogen therapy without increasing the risk for breast and endometrial cancer or adversely affecting the progression of atherosclerosis. It has been suggested that a reasonable approach would be a selective estrogen receptor modulator (SERM) that binds to estrogen receptors alpha and beta with high affinity and acts either as an estrogen agonist or antagonist depending on the target tissue9. Phase III clinical trials evaluating the new third-generation SERM, bazedoxifene acetate (BZA), have shown promising results in the treatment of osteoporosis, and in combination with CEE for the reduction of menopausal symptoms and prevention of osteoporosis10-13.
We have completed recently a randomized, parallel-arm trial with surgically postmenopausal cynomolgus monkeys (Macaca fascicularis) designed to determine the effects of BZA given alone, CEE given alone and BZA and CEE in combination on the breast, uterus and cardiovascular system. We have reported that BZA given to monkeys at a clinically relevant dose is an estrogen antagonist capable of abrogating the effects of CEE on the monkey’s breast14, inhibits estrogen effects on the endometrium, and lacks uterotropic effects when given alone and with CEE15. Since CEE given alone to monkeys in the early stage of surgical menopause and to women in the early stage of either surgical or natural menopause effectively reduces the progression of atherosclerosis, we report in this communication on the effect of adding BZA to CEE on the extent and severity of coronary and peripheral artery atherosclerosis. Further, because BZA is used for the prevention and treatment of osteoporosis, we report on its lack of adverse effects on the progression of atherosclerosis in the monkey model.
METHODS
Animal Subjects
One hundred and five (105) premenopausal cynomolgus monkeys (Macaca fasicularis) were obtained through our collaborative association with the Institut Pertanian Bogor, Bogor, Indonesia. Mean estimated age was determined by dentition to be 12 years. During a pre-experimental phase, the animals were evaluated for their health status and were ovariectomized to make them surgically menopausal. Following ovariectomy, plasma concentrations of estradiol were monitored to document completeness of the ovariectomy. Five monkeys were excluded due to the presence of ectopic ovarian tissue, one because it developed diabetes, and one was euthanized for chronic weight loss due to intestinal disease. All procedures were approved by the Institutional Animal Care and Use Committee of Wake Forest University and conducted in accordance with federal, state and institutional guidelines. Wake Forest University is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Study Design
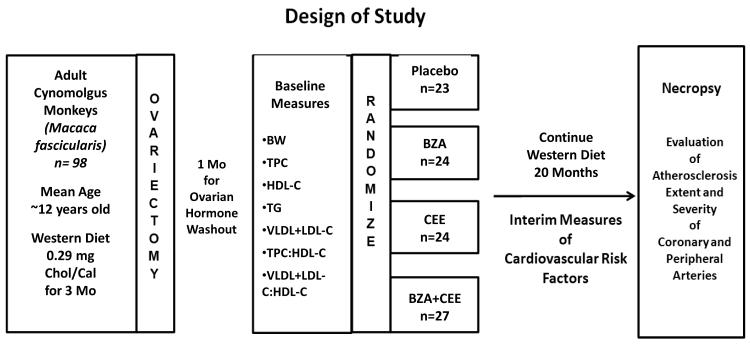
The design of the study was a four-group, parallel-arm design with treatments lasting for 20 months (Figure 1). Social groups of monkeys (n = 2 to 5 per group) were randomly assigned to treatment using a permuted block randomization scheme. This was done to minimize differences in time when treatments were initiated, as groups were started into treatment on a staggered schedule of two social groups every week. . Social groups were then assigned to one of four experimental groups to receive no treatment (control, n=23) or treatment with BZA, 20 mg/day (n=24), CEE, 0.45 mg/day (n=24), or the combination of BZA, 20 mg/day + CEE, 0.45 mg/day (n=27). All doses are described as the woman’s equivalent dose. In order to account for the differences in metabolic rate between monkeys and women, the woman’s dose of CEE (0.45 mg/day) was presented to the monkeys scaled to 1,800 Calories of diet (the usual intake of U.S. women). The BZA dose was based on a dose finding study to determine the amount needed to approximate the blood levels of women administered 20 mg of BZA/day as we have described previously14. All monkeys were fed a semi-purified diet formulated to be similar to diets consumed by women in North America but containing an exaggerated amount of cholesterol necessary to induce sufficient atherosclerotic plaques for evaluation within the timeframe of this trial. The diet was prepared in the Wake Forest Primate Center Diet Laboratory and contained 0.29 mg cholesterol per Calorie and derived 19% of its energy from protein (casein and lactalbumin), ~35% from fat and ~47% from carbohydrates. The monkeys were offered 120 Cal of diet/kg body weight daily.
Figure 1.
Randomized Parallel-Arm Study Design.
Abbreviations: BW= body weight (Kg), TPC= total plasma cholesterol (mg/dl), HDL-C= high density lipoprotein cholesterol (mg/dl), TG= triglycerides (mg/dl), VLDL+LDL-C= very low density lipoprotein cholesterol + low density lipoprotein cholesterol (mg/dl), TPC:HDL-C= ratio of TPC to HDL-C, VLDL+LDL-C: HDL-C= ratio of VLDL+LDL-C to HDL-C. BZA= Bazedoxifene acetate, CEE= conjugated equine estrogens.
Women equivalent doses: CEE (0.45 mg/day), BZA (20 mg/day), CEE + BZA; Control
Assessment of Cardiovascular Risk Variables
Body Weight and Adiposity
Body weights were determined at baseline and monthly during the experimental period. The degree of adiposity was assessed at 12 and 20 months post treatment. Our measure of adiposity is generally comparable to the body mass index of human subjects. Trunk length was measured as the distance from the supra-sternal notch to the pubic symphysis. The adiposity index was calculated as body weight (Kg)/trunk length (m2). Duplicate measures of adiposity were made by two technicians and the mean of the two measurements was recorded.
Plasma Lipids/Lipoproteins and Fasting Glucose
Following 18-24 hour fasting periods, blood samples were collected into EDTA at baseline (4 weeks after ovariectomy), 3, 6, 12, and 20 months post treatment to determine fasting lipid/lipoprotein and glucose concentrations. Total plasma cholesterol (TPC) concentrations, high-density lipoprotein cholesterol (HDL-C) concentrations, and plasma triglycerides (TGs) were determined in the Comparative Medicine Clinical Chemistry and Endocrinology Laboratory using reagents (ACE cholesterol, ACE HDL-C, and ACE triglycerides) and instrumentation (ACE ALERA autoanalyzer) from Alfa Wasserman Diagnostic Technologies (West Caldwell, NJ). TPC and HDL-C were standardized to calibrated controls from the Centers for Disease Control and Prevention-National Heart, Lung, and Blood Lipid Standardization Program. Intra- and inter-assay coefficients of variation were less than 5% for all analyses. Non-HDL-C, which approximates the sum of low-density lipoprotein (LDL) cholesterol and very-low-density lipoprotein (VLDL) cholesterol, was calculated by subtracting HDL-C from TPC. Plasma glucose concentrations were determined by the glucose oxidase method using a Glucose Analyzer 2 (Beckman Instruments, Brea CA, USA).
Necropsy Procedures and Atherosclerosis Evaluations
Necropsy Procedure
After 20 months of postmenopausal treatment, the monkeys were euthanized using sodium pentobarbital (100 mg/kg, intravenously), a method consistent with the recommendations of the panel on euthanasia of the American Veterinary Medical Association. The circulatory system was flushed with lactated Ringers, the heart was removed, and the coronary arteries were perfused for 1 hour at 100 mm/Hg pressure using 10% neutral buffered formalin.
Coronary Arteries
Fifteen blocks (each 3 mm in length) of coronary artery were cut perpendicular to the long axis of the arteries. Five of these were serial blocks from the left circumflex (LCX), five were from the left anterior descending (LAD), and five were from the right coronary artery (RCA). Each of the 15 blocks was embedded in paraffin, and 5-μm sections were made and stained with Verhoeff-van Gieson’s stain. Each section was captured digitally using a Nikon DS Fi1 camera mounted on an Oympus BH-2 microscope equipped with a mechanical stage. Morphometric measurements were made using Image-Pro Plus version 7.0 imaging software (Media Cybernetics, Inc., Bethesda, MD). Measurements were made blind to treatment by a single technician with more than 20 years experience and were randomly re-evaluated by one of us (T. Clarkson).
Plaque extent
Plaque extent was expressed as the cross-sectional area (mm2) of the coronary artery plaque. Plaque extent is expressed as the median plaque areas for each of the three main epicardial coronary arteries (left anterior descending [LAD], left circumflex [LCX] and right coronary artery [RCA]). The details of this approach have been reported previously16,17.
Plaque severity
Because plaque complications occur independently from plaque extent, we used a well-established system for assigning a severity grade on a 0 to V scale18. Coronary arteries with a normal intima were given a score of 0. Intimal lesions that consisted of smooth muscle adaptive thickening were given a grade of I. If the smooth muscle adaptive thickening contained macrophage foam cells, a grade of II was given. For those cases in which there had been necrosis of macrophages and the accumulation of small pools of extracellular lipid, a grade of III was recorded. The grade of IV was recorded for those lesions in which the small pools of extracellular lipid had coalesced into a core of extracellular lipid. The most complicated plaques observed in this study were given the grade of V, based on the core of extracellular lipid being separated from the lumen by a definitive fibromuscular cap. Plaque severity scores were expressed as the mean maximum severity grade for each of the three main epicardial coronary arteries (LAD, LCX and RCA). We chose to use the maximum grade since the clinical significance for any one of the coronary arteries relates to its most complicated lesion and not its average lesion. Grades were determined blind to treatment by three independent observers.
Iliac Arteries
The common iliac artery was opened longitudinally, laid flat on cardboard, and immersion fixed in 10% neutral buffered formalin. The common iliac artery was divided into 3 congruent blocks cut perpendicular to the long axis of the artery. Blocks were embedded in paraffin, sectioned, stained and measured as described for the coronary arteries.
Plaque extent
Plaque extent (mm2) was determined morphometrically as described for the coronary arteries and the mean of the three congruent blocks was recorded.
Plaque severity
Plaque severity was determined as described for the coronary arteries. The mean maximum grade was recorded.
Statistical Analysis
Analyses of coronary artery atherosclerosis extent and severity were done for each of the three coronary arteries separately. The extent of coronary artery atherosclerosis in the LAD, LCX and RCA was calculated as the average plaque extent (mm2) across the five blocks. Given the highly skewed distribution of the plaque size data, a non-parametric Kruskal-Wallis test was used to compare treatment group differences, and extent of atherosclerosis was reported as the median plaque size for each treatment group. Subsequent multiple comparisons relative to the control group were performed using Wilcoson Rank Sum tests, and Bonferroni corrections were made accordingly. Atherosclerosis severity was assessed by analyzing the maximum severity score across the five blocks using analysis of variance (ANOVA) to assess the treatment effect. Multiple comparisons with the control group were performed using Dunnett’s method.
Iliac artery atherosclerosis extent was computed as the mean plaque area (mm2) across the three blocks for each monkey. Square root transformations were used to achieve better normality and subsequently analyzed using ANOVA. Dunnett’s method was used to adjust for multiple comparisons with the control group.
For risk factors such as lipids and glucose, the baseline differences among treatment groups were analyzed using ANOVA. Both baseline and follow-up measures were then analyzed together using linear mixed effects models to assess the pattern of change over time for different treatment groups. Body weight and adiposity index were analyzed with similar ANOVA and mixed effect models.
RESULTS
Baseline Assessment of Risk Variables
For all of the variables except plasma triglyceride concentrations (TG), there were no significant difference among the groups (Table 1). There were small but statistically significant group differences among the groups in TG concentrations. The small differences were not considered physiologically significant.
Table 1.
Baseline Characteristics of the Groups
| Characteristic | Control | BZA | CEE | CEE+BZA | p-value |
|---|---|---|---|---|---|
| BW | 3.13 ± 0.12 | 3.09 ± 0.12 | 2.92 ± 0.12 | 2.96 ± 0.11 | 0.796 |
| TPC | 330.9 ± 29.9 | 359.4 ± 29.3 | 301.4 ± 29.3 | 322.9 ± 27.9 | 0.832 |
| HDL-C | 53.0 ± 3.9 | 48.2 ± 3.8 | 54.2 ± 3.8 | 57.6 ± 3.6 | 0.653 |
| TG | 47.2 ± 3.9 | 33.0 ± 3.8 | 39.4 ± 3.8 | 34.2 ± 3.6 | 0.016 |
| VLDL+LDL-C | 277.9 ± 31.5 | 311.3 ± 30.8 | 247.1 ± 30.8 | 265.2 ± 29.4 | 0.896 |
| TPC:HDL-C | 7.2 ± 1.1 | 8.2 ± 1.1 | 6.6 ± 1.1 | 7.5 ± 1.0 | 0.425 |
| VLDL+LDL-C:HDL-C | 6.2 ± 1.1 | 7.2 ± 1.1 | 5.6 ± 1.1 | 6.5 ± 1.0 | 0.798 |
Data presented are means ± standard error of the means.
Abbreviations: BW=body weight (Kg), TPC=total plasma cholesterol (mg/dl), HDL-C=high density lipoprotein cholesterol (mg/dl), TG=triglycerides (mg/dl), VLDL+LDL-C= very low density lipoprotein cholesterol + low density lipoprotein cholesterol (mg/dl), TPC:HDL-C=ratio of TPC to HDL-C, VLDL+LDL-C: HDL-C=ratio of VLDL+LDL-C to HDL-C. BZA= Bazedoxifene acetate, CEE= conjugated equine estrogens.
Experimental Assessment of Risk Variables
Presented in Table 2 are the means and standard errors over the 20-month study for the risk factors evaluated. There were no significant group differences for body weight or for adiposity (a measure comparable to body mass index for human patients). There were no significant differences in plasma lipid profiles among the groups. The plasma triglyceride concentrations tended to be somewhat higher in the BZA and CEE groups but the differences did not reach statistical significance (p=0.068). Fasting plasma glucose concentrations were the same for all of the groups.
Table 2.
Effect of Treatment on Cardiovascular Risk Factors
| Risk Factor | Control | BZA | CEE | CEE+BZA | p-value |
|---|---|---|---|---|---|
| BW | 3.13 ± 0.11 | 3.06± 0.11 | 2.97 ± 0.11 | 3.02 ± 0.10 | 0.778 |
| Adiposity | 43.9 ± 1.3 | 41.7 ± 1.3 | 41.2 ± 1.2 | 39.7 ± 1.2 | 0.129 |
| TPC | 341.4 ± 24.1 | 391.6 ± 23.7 | 339.3 ± 23.6 | 358.9 ± 22.3 | 0.379 |
| HDL-C | 50.9 ± 3.3 | 47.5 ± 3.3 | 52.6 ± 3.3 | 48.8 ± 3.1 | 0.699 |
| TG | 52.6 ± 5.1 | 64.2 ± 8.4 | 63.0 ± 3.4 | 49.8 ± 3.4 | 0.068 |
| VLDL+LDL-C | 290.5 ± 25.4 | 344.1 ± 24.9 | 286.7 ± 24.8 | 310.1 ± 23.4 | 0.350 |
| TPC:HDL-C | 7.7 ± 0.8 | 9.4 ± 0.8 | 8.0 ± 0.8 | 8.9 ± 0.8 | 0.426 |
| VLDL+LDL-C:HDL-C | 6.7 ± 0.8 | 8.4 ± 0.8 | 7.0 ± 0.8 | 7.9 ± 0.8 | 0.426 |
| Fasting Glucose | 74.7 ± 1.8 | 74.1 ± 1.8 | 72.6 ± 1.8 | 76.4 ± 1.7 | 0.481 |
Data presented are means ± standard error of the means.
Abbreviations: BW= body weight (Kg),Adiposity= kilograms of BW/ distance from the suprasternal notch to the symphysis pubis in meters squared, TPC= total plasma cholesterol (mg/dl), HDL-C= high density lipoprotein cholesterol (mg/dl), TG= triglycerides (mg/dl), VLDL+LDL-C= very low density lipoprotein cholesterol + low density lipoprotein cholesterol (mg/dl), TPC:HDL-C= ratio of TPC to HDL-C, VLDL+LDL-C: HDL-C= ratio of VLDL+LDL-C to HDL-C. BZA= Bazedoxifene acetate, CEE= conjugated equine estrogens.
Coronary Artery Atherosclerosis Extent
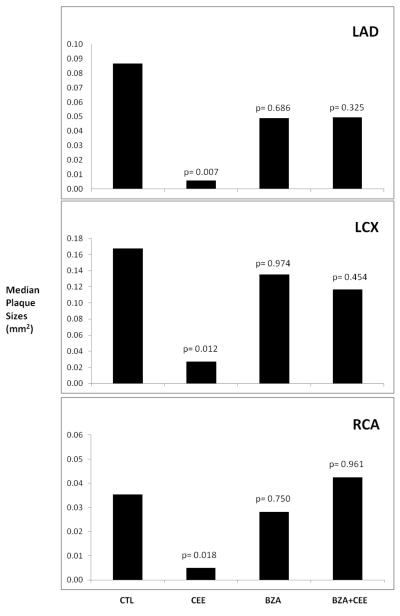
The extent of coronary artery atherosclerosis for each monkey was calculated as the mean cross sectional area of plaques in five congruent sections of the three main epicardial coronary arteries (LAD, LCX, and RCA). Because the data were not distributed normally, the data presented in Figure 2 are the median plaque sizes for the control and treatment groups for each of the three main coronary arteries. Neither BZA alone or BZA + CEE had any effect on plaque sizes (extent) compared to the control group. There was a highly significant reduction in the size of the coronary artery plaques of all three main coronary arteries in the CEE treated monkeys.
Figure 2.
Because of the skewed distribution of the plaque size measures the median plaque size for each of the epicardial coronary arteries is presented. The probability estimates are non-parametric p-values comparing each treatment group to the control group.
Abbreviations: LAD= left anterior descending coronary artery, LCX= left circumflex coronary artery, RCA= right coronary artery. CTL= control, CEE= conjugated equine estrogens, BZA= Bazedoxifene acetate.
Coronary Artery Atherosclerosis Severity
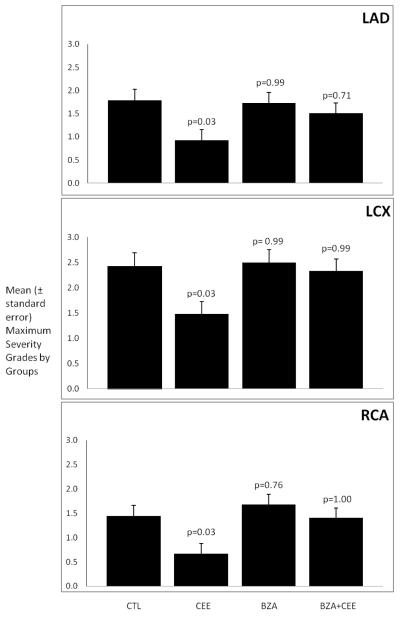
The severity of the coronary artery atherosclerosis for each monkey was determined using the well established grading system of the American Heart Association. For each monkey, a grade was given for each of the five congruent sections of the three main epicardial coronary arteries (LAD, LCX and RCA). Because the clinical significance of plaque severity for an individual coronary artery relates to the maximum grade for that artery, the data presented in Figure 3 are the mean maximum grades for each of the three coronary arteries. Neither BZA administered alone nor BZA + CEE had an effect on the severity of the atherosclerotic plaques. Treatment with CEE alone significantly reduced plaque severity.
Figure 3.
The data presented are the mean maximum severity grades by groups. The variability is expressed as the standard error of the mean. The probability estimates are p-values comparing each treatment group to the control group using Dunnett’s method.
Abbreviations: LAD= left anterior descending coronary artery, LCX= left circumflex coronary artery, RCA= right coronary artery. CTL= control, CEE= conjugated equine estrogens, BZA= Bazedoxifene acetate.
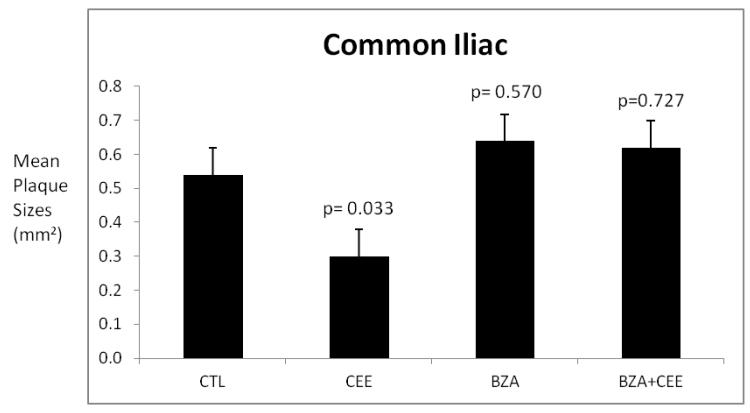
Common Iliac Artery Atherosclerosis Extent
The extent of common iliac artery atherosclerosis for each monkey was calculated as the mean cross sectional area of plaques in three congruent sections of the artery. The data presented in Figure 4 are the mean plaque sizes for the control and treatment groups. Much like the coronary arteries, neither BZA alone nor BZA combined with CEE had any effect on plaque sizes (extent) compared to the control group. Treatment with CEE significantly reduced plaque size compared with the control group.
Figure 4.
The data presented are the mean common iliac artery plaque sizes (cross sectional areas of intima) by groups. The variability is expressed as the standard error of the mean. The probability estimates are p-values comparing each treatment group to the control group using Dunnett’s method.
Abbreviations: CTL= control, CEE= conjugated equine estrogens, BZA= Bazedoxifene acetate.
Common Iliac Artery Atherosclerosis Severity
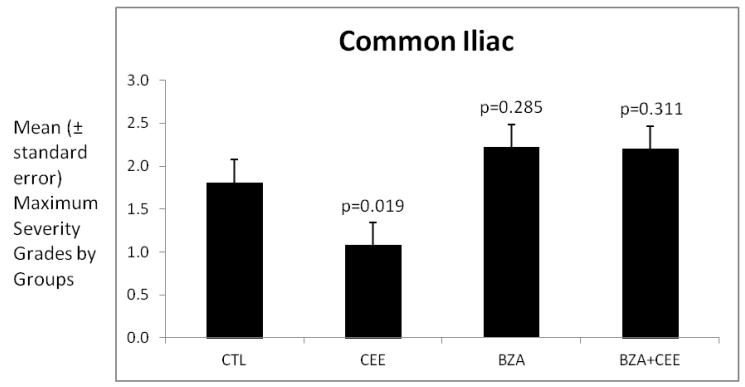
For each monkey, a grade was given for each of the three congruent sections of the common iliac artery. Because the clinical significance of plaque severity for an individual coronary artery relates to the maximum grade for that artery, the data presented in Figure 5 are the mean maximum grades for the common iliac artery. There was no effect on plaque severity of treatment with either BZA or BZA+CEE. Treatment with CEE significantly reduced plaque severity compared with the control group.
Figure 5.
The data presented are the mean maximum severity grades of the common iliac artery plaques by groups. The variability is expressed as the standard error of the mean. The probability estimates are p-values comparing each treatment group to the control group using Dunnett’s method.
Abbreviations: CTL= control, CEE= conjugated equine estrogens, BZA= Bazedoxifene acetate.
DISCUSSION
While postmenopausal hormone therapy (PHT) is generally accepted as the most effective means of treating menopausal symptoms and for the prevention of osteoporosis, few issues have been more controversial than the cardiovascular implications of estrogens given alone (ET) or combined with a progestin (EPT). The primary purpose of the study reported here was to investigate the cardiovascular effects of a tissue selective SERM (BZA) given alone or in combination with CEE to surgically postmenopausal cynomolgus monkeys.
There was no effect of BZA administered alone or in combination with CEE on body weight or adiposity of the monkeys. The lack of an effect on body weight could be considered an important advantage of the combination compared with CEE+MPA since CEE+MPA results in significant increases in body weight of the monkey model compared to controls or to those treated with CEE alone19. Long-term studies of the effects of BZA or BZA+CEE on body weight or body mass index of women have not been reported.
The lack of an effect of BZA and of BZA+CEE on the plasma lipid profiles of the monkeys is in contrast to the reported effects of these two treatments on the plasma lipid profiles of women. Christiansen and colleagues20 found that BZA given at a dose of 20 mg/day to postmenopausal women resulted in a median percent reduction in TPC concentrations of 3.8, median percent reductions of LDL-C of 5.4 and an increase in HDL-C of 5.1. That we did not observe a significant effect of CEE treatment on the plasma lipid profiles of the monkeys is consistent with most of our prior studies using the cynomolgus monkey model16,21,22. In most of our prior studies with CEE we modeled a woman’s dose of 0.625 mg/day. In the study reported here, we modeled a woman’s dose of CEE of 0.45 mg/day. However, we have reported previously that lower doses of CEE administered to the monkey model are as atheroprotective as the woman’s equivalent of 0.625 mg/day23. While we found no effect on lipid profiles of the monkeys given BZA+CEE, Lobo and co-workers13 reported rather robust beneficial effects of BZA+CEE treatment of postmenopausal women at the dose of the combination that we modeled in the monkeys (BZA, 20 mg/day + CEE, 0.45 mg/day). They reported decreases of about 10% in plasma concentrations of LDL-C and increases of about 11% in HDL-C. The differences in the effects of the treatments on the plasma lipid profiles of our monkey model in this trial probably relates to the exaggerated amount of dietary cholesterol used to induce sufficient atherosclerosis for evaluation within the timeframe of the trial.
We found no evidence that BZA treatment had any adverse effects on either the extent or severity of coronary and common iliac artery atherosclerosis. That finding is consistent with an earlier study evaluating raloxifene, a second-generation SERM, in cynomolgus macaques24. Despite the lack of a beneficial effect of CEE treatment on the plasma lipid profiles of the monkeys, there was a robust inhibition of the atherosclerosis progression (extent) and plaque severity/complications in all three main epicardial coronary arteries and the common iliac artery. That finding is comparable to a number of studies we have reported previously finding that estrogen treatment initiated immediately after making the monkeys surgically menopausal results is an average inhibition of about 70% in the progression of coronary artery atherosclerosis21,25-27. Interestingly, a similar estimate was made for the beneficial effects of estrogen treatment on the progression of carotid artery intimal media thickness of postmenopausal women28.
The robust atherosclerosis inhibitory effect of CEE in the monkey model is lost with increasing time since menopause, an observation that lead to the so-called “timing hypothesis”29,30. Our observation was that the delay of estrogen treatment for two years (comparable to six years for women) after surgical menopause, a period that allowed the plaques to progress to a complicated stage, resulted in no beneficial effect of the estrogen treatment4. We have interpreted that finding as likely being related to the loss of estrogen receptors with advancing atherosclerosis as described by Losordo et al.31 for human subjects with advancing atherosclerosis.
Analysis of a large number of monkey studies in which ET was initiated soon after surgical menopause led us to conclude that only about 25% of the atheroprotective effects of estrogens relate to their plasma lipid effects and that about 75% of the beneficial effects are plasma lipid-independent, presumably direct effects on the arteries via estrogen receptors32. Our finding that BZA attenuated the beneficial atheroprotective effects of the CEE was unexpected and likely relates to the fact that the CEE beneficial effects in the monkey are primarily receptor-mediated. Much in the same way that BZA protects the breast and uterus during CEE treatment in the monkey model, it appears to interfere with the estrogen agonist effect on coronary and iliac arteries.
Some aspects of the translational significance of the monkey study reported here seem clear and some are unclear. It is clear that there were no adverse effects of BZA on the progression or severity of atherosclerosis of coronary and peripheral arteries. It is unclear whether some beneficial effects of BZA might have been seen had BZA had the same effect on the monkeys’ plasma lipid profiles as has been observed for women. It is clear that the robust atheroprotective effects of CEE administered soon after induction of the menopause has again been replicated. In this monkey trial, the addition of BZA to the CEE treatment completely attenuated the atheroprotective effects of the CEE. It is unclear if that outcome would have been different if the reductions in LDL-C and increases in HDL-C reported to occur in women had been the case with the monkeys. However, it seems doubtful to us that the outcome would have been different since the protective effects of CEE are primarily by its direct effects on the artery wall not by changes in plasma lipids and lipoproteins.
CONCLUSIONS
In this preclinical, nonhuman primate trial, treatment with BZA alone, CEE alone and BZA and CEE in combination did not have significant effects on plasma lipid profiles. CEE treatment markedly inhibited the progression and complication of both coronary and iliac artery atherosclerosis. BZA treatment had no adverse effects on atherosclerosis but attenuated the atheroprotective effects of CEE on both the coronary and iliac arteries. Similar to breast and uterine effects, BZA also seems to block the effects of CEE on the artery wall. The translational implications for postmenopausal women seem clear. When BZA alone is used to prevent or treat osteoporosis there is no indication that atherosclerosis progression will be exacerbated. When CEE is administered in combination with BZA for the treatment of osteoporosis or for menopausal symptoms, the potential cardiovascular benefits of CEE may be diminished.
Summary.
Conjugated equine estrogens markedly inhibited the progression of both coronary and iliac artery atherosclerosis of postmenopausal monkeys fed a moderately atherogenic diet. BZA had no adverse effects on atherosclerosis but attenuated the atheroprotective effects of CEE.
ACKNOWLEDGMENTS
The authors would like to thank the following: Dewayne Cairnes, Margaret (Chrissy) May, Margaret Mehaffey and Maryanne Post for their technical skills and Martha Henderson for preparation of the manuscript.
Source of Funding: This work was supported by grants from Pfizer, Inc. (an investigator-originated grant to TBC) and the National Institutes of Health, Office of the Director (8T32OD010957 to KE).
Footnotes
Conflicts of Interest/Financial Disclosure: TBC received an investigator-originated grant from Pfizer and has another investigator-originated cardiovascular grant from Merck.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Thomas B. Clarkson, Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
Kelly F. Ethun, Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
Haiying Chen, Department of Biostatistical Sciences, Wake Forest School of Medicine
Debbie Golden, Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
Edison Floyd, Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
Susan E. Appt, Department of Pathology/Comparative Medicine, Wake Forest School of Medicine
REFERENCES
- 1.Hersh AL, Stefanick ML, Stafford RS. National use of posmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Cline JM, Söderqvist G, von Schoultz E, Skoog L, von Schoultz B. Effects of hormone replacement therapy on the mammary gland of surgically postmenopausal cynomolgus macaques. Am J Obstet Gynecol. 1996;174:93–100. doi: 10.1016/s0002-9378(96)70379-4. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Hendrix SL, Langer RD, Stefanick ML, Gass M, Lane D, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 4.Clarkson TB, Appt SE. Controversies about HRT – lessons from monkey models. Maturitas. 2005;51:64–74. doi: 10.1016/j.maturitas.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 5.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol. 1997;17:217–221. doi: 10.1161/01.atv.17.1.217. [DOI] [PubMed] [Google Scholar]
- 6.Women’s Health Initiative Steering Committee Effects of conjugated equine estrogens in postmenopausal women with hysterectomy. The Women’s Health Initiative Randomized Controlled Trial. JAMA. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 7.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary artery calcification. N Engl J Med. 2007;356:2591–2602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 8.Hodis HN, Mack WJ. Postmenopausal hormone therapy in clinical perspective. Menopause. 2007;14:944–957. doi: 10.1097/gme.0b013e31802e8508. [DOI] [PubMed] [Google Scholar]
- 9.Palacios S. The future of the new selective estrogen receptor modulators. Menopause Int. 2007;13:27–34. doi: 10.1258/175404507780456791. [DOI] [PubMed] [Google Scholar]
- 10.Pinkerton JV, Utian WH, Constantine GD, Olivier S, Pickar JH. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause. 2009;16:11116–11124. doi: 10.1097/gme.0b013e3181a7df0d. [DOI] [PubMed] [Google Scholar]
- 11.Bachmann G, Bobula J, Mirkin S. Effects of bazedoxifene/conjugated estrogens on quality of life in postmenopausal women with symptoms of vulvar/vaginal atrophy. Climacteric. 2010;13:132–140. doi: 10.3109/13697130903305627. [DOI] [PubMed] [Google Scholar]
- 12.Utian W, Yu H, Bobula J, Mirkin S, Olivier S, Pickar JH. Bazedoxifene/conjugated estrogens and quality of life in postmenopausal women. Maturitas. 2009;63:329–335. doi: 10.1016/j.maturitas.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Lobo RA, Pinkerton JV, Gass ML, Dorin MH, Ronkin S, Pickar JH, et al. Evaluation of bazedoxifene/conjugated estrogens for the treatment of menopausal symptoms and effects on metabolic parameters and overall safety profile. Fertil Steril. 2009;92:1025–1038. doi: 10.1016/j.fertnstert.2009.03.113. [DOI] [PubMed] [Google Scholar]
- 14.Ethun KF, Wood CE, Register TC, Cline JM, Appt SE, Clarkson TB. Effects of bazedoxifene acetate with and without conjugated equine estrogens on the breast of postmenopausal monkeys. Menopause. 2012 Jul 9; doi: 10.1097/gme.0b013e318252e46d. [Epub ahead of print] 2012;19:doi:10.1097/GME.0b013e318252e46d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ethun KF, Wood CE, Appt SE, Cline JM, Chen H, Clarkson TB. Effects of combination therapy with bazedoxifene and conjugated equine estrogens on the endometrial and vaginal epithelium of surgically postmenopausal monkeys. Endocr Rev. 2010;31(3 Suppl 1):S1303. [Google Scholar]
- 16.Clarkson TB, Anthony MS, Wagner JD. A comparison of tibolone and conjugated equine estrogens effects on coronary artery atherosclerosis and bone density of postmenopausal monkeys. J Clin Endocrinol Metab. 2001;86:5396–5404. doi: 10.1210/jcem.86.11.8021. [DOI] [PubMed] [Google Scholar]
- 17.Clarkson TB, Bond MG, Marzetta CA, Bullock BC. Approaches to the study of atherosclerosis regression in rhesus monkeys: Interpretation of morphometric measurements of coronary arteries. In: Gotto AM Jr, Smith LC, Allen B, editors. Atherosclerosis V: The fifth international symposium on atherosclerosis; New York: Springer-Verlag; 1980. pp. 739–748. [Google Scholar]
- 18.Stary HC, Chandler AB, Dinsmore RE, et al. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. Arterioscler Thromb Vasc Biol. 1995;15:1512–1531. doi: 10.1161/01.atv.15.9.1512. [DOI] [PubMed] [Google Scholar]
- 19.Shadoan MK, Anthony MS, Rankin SE, Clarkson TB, Wagner JD. Effects of tibolone and conjugated equine estrogens with or without medroxyprogeterone acetate on body composition and fasting carbohydrate measures in surgically postmenopausal monkeys. Metabolism. 2003;52(9):1085–1091. doi: 10.1016/s0026-0495(03)00181-1. [DOI] [PubMed] [Google Scholar]
- 20.Christiansen C, Chesnut CH, III, Adachi JD, et al. Safety of bazedoxifene in a randomized, double-blind, placebo- and active-controlled Phase 3 study of postmenopausal women with osteoporosis. BMC Musculoskeletal Disord. 2010;11:130. doi: 10.1186/1471-2474-11-130. http://www.biomedcentral.com/1471-2474/11/130 doi: 10.1186/1471-2474-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression - A comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86:41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 22.Shelton KA, Clarkson TB, Kaplan JR. Nonhuman primate models of atherosclerosis. In: Abee CR, Mansfield K, Tardif SD, Morris T, editors. Nonhuman Primates in Biomedical Research. 2nd. Elsevier; in press. [Google Scholar]
- 23.Appt SE, Clarkson TB, Lees CL, Anthony MS. Low dose estrogens inhibit coronary artery atherosclerosis in postmenopausal monkeys. Maturitas. 2006;55:187–194. doi: 10.1016/j.maturitas.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Clarkson TB, Anthony MS, Jerome CP. Lack of effect of raloxifene on coronary artery atherosclerosis of postmenopausal monkeys. J Clin Endocrinol Metab. 1998;83:721–726. doi: 10.1210/jcem.83.3.4617. [DOI] [PubMed] [Google Scholar]
- 25.Adams MR, Kaplan JR, Manuck SB, et al. Inhibition of coronary artery atherosclerosis by 17-beta estradiol in ovariectomized monkeys: Lack of an effect of added progesterone. Arteriosclerosis. 1990;10:1051–1057. doi: 10.1161/01.atv.10.6.1051. [DOI] [PubMed] [Google Scholar]
- 26.Clarkson TB, Kaplan JR, Shively CA, Klein KP. Benefits of exogenous estrogen in inhibiting stress-related coronary artery atherosclerosis. Br J Obstet Gynaecol. 1996;103(Suppl 13):73–79. [PubMed] [Google Scholar]
- 27.Clarkson TB. Estrogens, progestins and coronary heart disease in cynomolgus monkeys. Fertil Steril. 1994;62:147S–151S. [PubMed] [Google Scholar]
- 28.Karim R, Mack WJ, Lobo RA, et al. Determinant of the effect of estrogen on the progression of subclinical atherosclerosis: Estrogen in the Prevention of Atherosclerosis Trial. Menopause. 2005;12:366–373. doi: 10.1097/01.GME.0000153934.76086.A4. [DOI] [PubMed] [Google Scholar]
- 29.Clarkson TB. The new conundrum: do estrogens have any cardiovascular benefits. Int J Fertil. 2002;47(2):61–68. [PubMed] [Google Scholar]
- 30.Williams JK, Anthony MS, Honoré EK, et al. Regression of atherosclerosis in female monkeys. Arterioscler Thromb Vasc Biol. 1995;15:827–836. doi: 10.1161/01.atv.15.7.827. [DOI] [PubMed] [Google Scholar]
- 31.Losordo DW, Kearney M, Kim KA, Jekanowski J, Isner JM. Variable expression of the estrogen receptor in normal and atherosclerotic coronary arteries of premenopausal women. Circulation. 1994;89:1501–1510. doi: 10.1161/01.cir.89.4.1501. [DOI] [PubMed] [Google Scholar]
- 32.Clarkson TB, Anthony MS. Effects on the cardiovascular system -- basic aspects. In: Dempster D, Lindsay R, Jordan VC, editors. Estrogens and anti-estrogens. Lippincott-Raven Publishers; Philadelphia: 1997. pp. 89–118. [Google Scholar]