Abstract
The lateral division of the bed nucleus of the stria terminalis (BNST), which forms part of the circuitry regulating fear and anxiety, contains a large number of neurons expressing corticotropin releasing factor (CRF), a neuropeptide that plays a prominent role in the etiology of fear- and anxiety-related psychopathologies. Stress increases CRF expression within BNST neurons, implicating these cells in stress- and anxiety-related behaviors. These experiments examined the effect of chronically enhanced CRF expression within BNST neurons on conditioned and unconditioned anxiety-related behavior by using a lentiviral vector containing a promoter that targets CRF gene over-expression (OE) to CRFergic cells. We found that BNST CRF over-expression did not affect unconditioned anxiety-like responses in the elevated plus maze or basal acoustic startle amplitude. CRF OE induced prior to training weakened sustained fear (conditioned anxiety); when induced after conditioning, CRF OE increased expression of the conditioned emotional memory. Increased BNST CRF expression did not affect plasma corticosterone concentration but did decrease CRFR1 receptor density within the BNST and CRFR2 receptor density within the dorsal portion of the caudal dorsal raphe nucleus. These data raise the possibility that the observed behavioral effects may be mediated by enhanced CRF receptor signaling or compensatory changes in CRF receptor density within these structures. Together, these studies demonstrate that CRF neurons within the lateral BNST modulate conditioned anxiety-like behaviors and also suggest that enhanced CRF expression within these neurons may contribute to inappropriate regulation of emotional memories.
Keywords: bed nucleus of the stria terminalis, corticotropin releasing factor, fear, anxiety, startle, HPA axis
Introduction
The bed nucleus of the stria terminalis (BNST) is a component of the circuitry involved in stress, anxiety, and fear, and is particularly important for maintaining long-duration fear responses that resemble anxiety (1, 2). In rodents, lesioning, inactivation, or pharmacological manipulation of the BNST alters anxiety-like behavior in several paradigms (2–5), while in human and non-human primates, imaging studies have suggested that the BNST is activated by anxiogenic stimuli (6–9).
The lateral BNST contains a large number of neurons that express the neuropeptide corticotropin releasing factor (CRF; 10–12). CRF has been strongly implicated as a regulator of neural circuits that govern autonomic, neuroendocrine, and behavioral stress responses (13–17). CRF receptor signaling influences several aspects of fear learning and expression (18–22). Specifically, extra-hypothalamic CRF over-expression (OE) within the central nervous system increases fear- and anxiety-related behavior (14, 23, 24), and chronic CRF hyperactivity has been linked to stress- and fear-related psychopathologies such as post-traumatic stress disorder (PTSD; 17, 25–29).
The effects of stress on associative emotional learning tend to parallel those produced by CRF receptor signaling (30). In fact, stressful experience elevates CRF expression within fear-and anxiety-associated structures, including both the dorsolateral and ventrolateral subdivisions of the BNST (31–34). This raises the possibility, then, that enhanced CRF expression within these BNST subdivisions modulates conditioned or unconditioned anxiety and possibly contributes to stress-related psychopathology. Therefore, the primary goal of the present studies was to investigate the role of BNST CRF over-expression (OE) in acquisition and expression of conditioned anxiety (modeled by sustained fear) and in unconditioned anxiety behaviors. Because BNST CRF neurons within the lateral subdivision project to a number of target structures involved in fear modulation (35–37) including the hypothalamic paraventricular nucleus where the HPA response is initiated (38), a secondary goal was to determine if BNST CRF OE would alter plasma corticosterone levels or CRF receptor density in fear- and anxiety-related target structures. To accomplish these goals, we used a lentiviral vector (LV-CRF) to chronically over-express CRF within CRF-expressing BNST neurons. We hypothesized that BNST CRF OE effects on anxiety and conditioned fear would in some ways resemble those produced by chronic stress.
Materials and Methods
Plasmid Construction
Viral vectors were derived from the HIV-based lentiviral backbones optimized by the laboratory of Dr Didier Trono (39). The Lenti-CMV-GFP viral plasmid was the ‘pCM02’ vector, which was a generous gift from the lab of Dr Joshy Jacob. PCM02 was created by inserting the 1.4 kb BamHI/XhoI fragment containing GFP-WPRE from the pHR′-CMV-GFP-WPRE plasmid (40) into BamHI/XhoI sites of the pHR-GFP-SIN backbone in place of the green fluorescent protein (GFP) fragment (40). The resulting pCM02 lentivirus-packaging vector contained a cytomegalovirus (CMV) promoter driving GFP expression followed by a woodchuck posttranscriptional regulatory element (WPRE).
The LVCRFp3.0CRF construct was created as previously described (41). Briefly, a 3.0kB region upstream of the CRF gene transcription start site containing several key regulatory elements (42) was amplified from mouse BAC clone # 129A14 and then ligated to the CRF coding sequence (from a plasmid generously provided by Wylie Vale, Salk Institute). This construct was then inserted into a lentiviral vector packaging construct, pCMV-GFP-dNhe (the kind gift of Inder Verma, Salk Institute, La Jolla, CA; (43), in place of the CMVGFP sequence. Correctly oriented clones were verified with restriction digests. The LVCRFp3.0CRF lentivirus-packaging vector thus contains a CRF neuron-specific promoter driving CRF gene expression (see Figure 1).
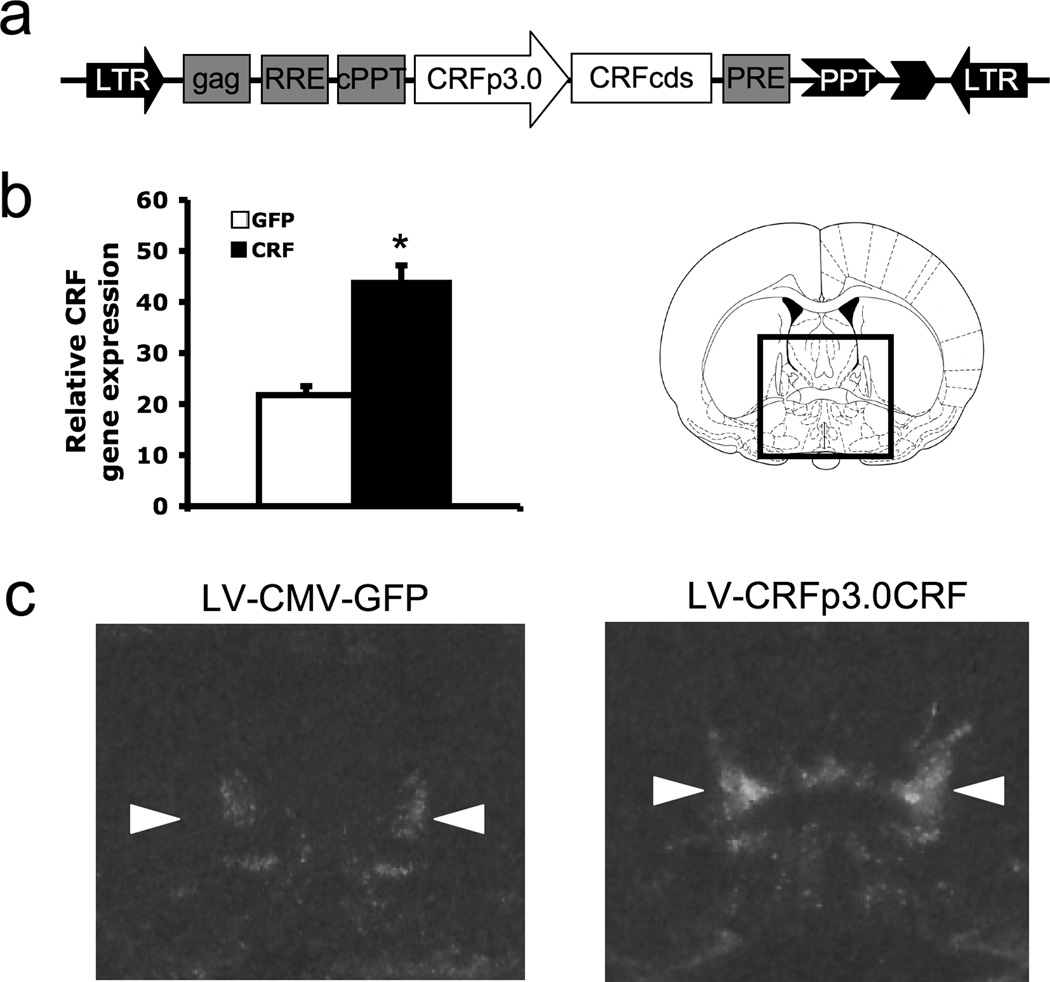
Figure 1.
The lentivirus, LVCRFp3.0CRF, produces site-specific enhancement of CRF gene expression within the BNST a) Schematic diagram of the DNA plasmid encoding the LVCRFp3.0CRF lentiviral construct for constitutive CRF expression targeted to the BNST; CRFp3.0: a 3.0 kb sequence upstream of CRF gene transcription start site used as a CRF neuron-specific promoter; CRFcds: CRF cDNA; LTR, long terminal repeat; b) Relative CRF gene expression, taken as the average difference in optical density (± SEM) between the BNST and background region of uniformly low expression (mPFC) * p < 0.001 c) Representative autoradiographs of brain sections containing lateral BNST (measurements taken from dorsolateral BNST, indicated by arrows) from LV-GFP and LV-CRF-infected animals respectively.
Preparation of Lentiviral Stocks
Virus was generated by transient co-transfection of the expression plasmid (25 µg), VSV-G pseudotyping construct (12.5 µg) and the packaging construct pCMVΔR8.92 (18.8 µg) into a 150 mm plate of 90% confluent 293 T cells as previously described (39, 44, 45). Medium was collected 48 and 72h post-transfection, cleared of debris by low-speed centrifugation and filtered through 0.45-µm filters. High-titer stocks were prepared by an initial ultracentrifugation for 1h at 23 000 r.p.m. (SW-28 rotor), and a secondary tabletop centrifugation at 13 000 r.p.m. for 30 min. Viral pellet was resuspended in 1% bovine serum albumin (BSA)/phosphate-buffered saline (PBS), and stored at −80°C. Viral titers were determined by infection of 293 T cells. Titers of stocks used in these studies were all about 2 × 109 infectious particles per mL.
Animals and Housing
Male Sprague-Dawley rats (Charles River, Raleigh, NC), weighing between 275 and 350 g at the time of surgery and housed in groups of 4 in 45 × 20 × 24-cm (depth × width × height) polycarbonate cages, were maintained on a 12-h light/dark cycle (lights on at 0800 h) with food and water available ad libitum. A total of 50 animals were used in these experiments. All procedures were conducted in accordance with U.S. Department of Agriculture, Emory University, and National Institutes of Health standards for the care and use of laboratory animals.
Surgical Procedures
Prior to surgery, rats were anesthetized with 75 mg/kg (i.p.) ketamine (Bionichepharma) and 0.5 mg/kg (i.p.) Dexdomitor (Orion Pharma) and also given an analgesic dose of 1.0 mg/kg (s.c.) meloxicam (Boehringer Ingelheim) to reduce post-operative discomfort. Once unresponsive to tailpinch, rats were placed in a Kopf Instruments stereotaxic frame with the nose bar set to −3.8 mm (flat-skull position). A 5 µL Hamilton microsyringe with a 26-gauge needle was sterilized with 100% ethanol, coated with 1% BSA, loaded with virus, and then lowered into the BNST (20° coronal angle – to avoid the lateral ventricle, 0.3 mm caudal, 6.3 mm ventral, and 3.8 mm lateral to bregma). Each animal was infused bilaterally with 2 µL of virus at a rate of 0.2 µL/min (Ultramicropump3/Micro4 Controller; World Precision Instruments, Sarasota, FL). The needle was left in place for 10 min after the injection and then slowly removed over a 2-min period. The skin was closed using a 5-0 Nylon suture (Ethilon, Johnson & Johnson, Piscataway, NJ). After surgery was complete, 0.5 mg/kg Antisedan (Orion Pharma) was given to reverse the anesthetic effects. Animals were given 14 days for recovery and to allow sufficient time for viral infection and induction of CRF or GFP expression. These lentiviruses typically cause CRF or GFP expression for the life of the animal.
Equipment
Startle Apparatus
Rats were trained and tested in four identical 8 × 15 × 15-cm Plexiglas and wire mesh cages that were each suspended between compression springs within a steel frame located within a custom-designed sound-attenuating chamber. The floor of each cage consisted of four 6.0-mm diameter stainless steel bars spaced 18 mm apart. Startle responses were evoked by 50-msec 95-dB white-noise bursts (5-msec rise-decay time, 0–22 kHz) generated by a Macintosh G3 computer sound file, amplified by a Radio Shack amplifier (Model MPA-200; Tandy), and delivered through Radio Shack Supertweeter speakers located 4 cm in front of each cage. Background noise (60-dB wideband) was produced by an ACO Pacific white-noise generator (Model 3024), and the conditioned stimulus (CS) was a 70-dB, 60-Hz clicker stimulus delivered through the same speakers as those used to provide startle and background noise. Sound level measurements were made with a Brüel & Kjaer model 2235 sound-level meter (A scale; random input) with the microphone (Type 4176) located 10 cm from the center of the speaker, which approximates the distance of the rat’s ear from the speaker during testing. The unconditioned stimulus was a 0.5-sec 0.4-mA scrambled shock delivered through the floor bars. Shock intensity was measured with a 1 kΩ resistor across a differential channel of an oscilloscope in series with a 100 kΩ resistor connected between adjacent floor bars within each cage. Current was defined as the root–mean–square voltage across the 1 kΩ resistor where mA = 0.707 × 0.5 × peak–to–peak voltage. Shocks were produced by LeHigh Valley shock generators (SGS 004).
Startle response and shock reactivity amplitudes were quantified using an accelerometer (model U321AO2; PCB Piezotronics) affixed to the bottom of each cage. Cage movement (e.g., produced by the rats’ startle response or reaction to shock) resulted in displacement of the accelerometer, which in turn produced a voltage output proportional to the velocity of cage movement. This output was amplified (PCB Piezotronics, Model 483B21) and digitized on a scale of 0–9.98 units by an InstruNET device (Model 100B; GW Instruments) interfaced to a Macintosh G3 computer. Startle amplitude was defined as the maximal peak-to-peak voltage that occurred during the first 200 msec after onset of the startle-eliciting white-noise burst. Similarly, shock reactivity amplitude was defined as the maximal peak-to-peak voltage that occurred during the 500 msec foot shock presentation. The presentation and sequencing of all stimuli were under the control of the Macintosh G3 computer using custom-designed software (The Experimenter; Glassbeads Inc.).
Elevated Plus Maze
The elevated plus maze (EPM) test was conducted on a plus-shaped apparatus consisting of two 50 × 11 cm open arms and two 50 × 11 × 40 cm enclosed arms elevated 40 cm from the floor.
Defensive Withdrawal
The defensive withdrawal (DW) apparatus consisted of a 60 × 90 cm gray plastic arena with 37 cm high walls. A white PVC tube (10 cm in diameter × 33 cm in length, closed at one end) was placed in one corner parallel to the wall with the open end facing outward.
Behavioral Procedures
Fear Conditioning and Startle Testing
On each of three consecutive days, rats received a baseline acoustic startle test in which they were exposed to 30 startle-eliciting white-noise bursts (95-dB, 50-msec duration) at an inter-stimulus interval of 30 seconds. Based on mean startle amplitude to these stimuli, rats were assigned to different treatment groups (LV-GFP or LV-CRF) such that the average pre-infusion startle amplitude of each group was comparable. The next day, rats received a pre-conditioning test consisting of a 5-min acclimation period, followed by 24 presentations of the startle-eliciting noise bursts (inter-stimlus interval of 30 seconds). The last 8 were given during a 4-min presentation of a 70-dB, 60-Hz clicker stimulus. After this test, rats received 3 fear conditioning sessions on consecutive days. During each conditioning session, the rats received 8 presentations of a variable-duration clicker stimulus (3-sec, 10-sec, 20-sec, 1-min, 2-min, 4-min, 6-min, 8-min), each co-terminating with a 0.4-mA 500-ms footshock unconditioned stimulus (US) (inter-trial interval = 3 minutes). Shock reactivity (jump amplitude in response to the first foot shock of each conditioning session) was recorded for each shock presentation throughout the three conditioning sessions. Clicker presentations occurred in order of ascending duration on the first training day, and randomly thereafter. Startle-eliciting noise bursts were also presented during training (one every 30 s). 2 (Experiment 1) or 15–16 (Experiment 2) days later rats received a test identical to that described above (i.e., startle measured for 8 min without the clicker and for an additional 4 min in the presence of the clicker). To minimize startle potentiation due to contextual fear conditioning, cages were illuminating by a fluorescent light (150 lux as measured from the middle of the cage) and scented with 30% acetic acid during conditioning, whereas test sessions were conducted in dim red light without any explicit olfactory cue, the floor bars were covered with sandpaper, and 5-cm chains hung from the test cage ceiling.
Rats in Experiment 1 (8 LV-GFP, 8 LV-CRF) were infused with virus prior to conditioning (see experimental timeline, Figure 2a). In Experiment 2, rats were infused with virus 24–48 hrs after conditioning (see experimental timeline, Figure 3a). Experiment 2 was conducted in two replications. The results from each were very similar and have been combined for the statistical analyses that follow (combined Ns = 24 LV-GFP, 23 LV-CRF).
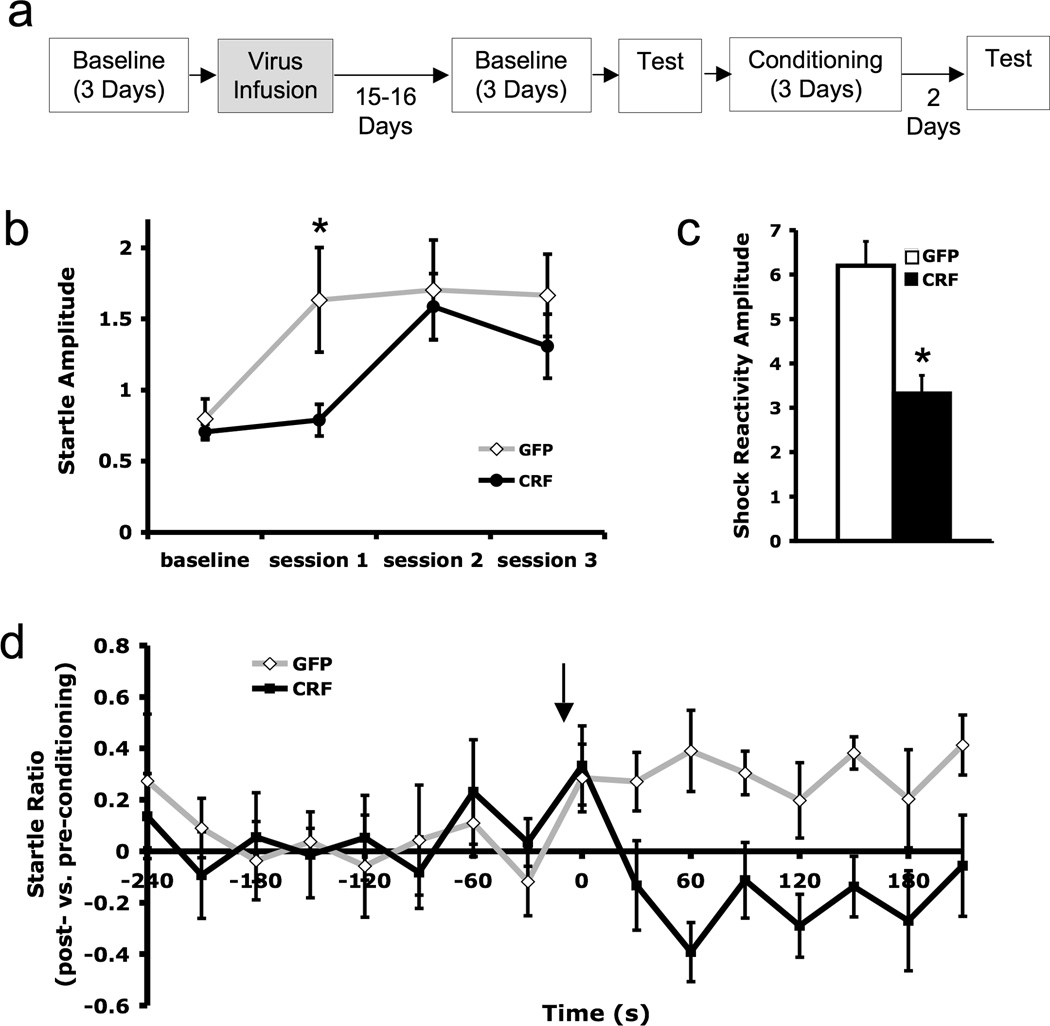
Figure 2.
CRF over-expression within the BNST induced prior to conditioning impaired acquisition of a sustained fear response but did not affect baseline acoustic startle. a) Experimental timeline. Time between events is one day unless otherwise specified. b) Average startle amplitude (± SEM) measured prior to conditioning (baseline) and for each of the three conditioning sessions c) Shock reactivity amplitude (± SEM) averaged over all three conditioning sessions d) Startle compared to pre-conditioning levels [log(post-conditioning startle/pre-conditioning startle) ± SEM] measured in a test session conducted two days after conditioning. Arrow indicates beginning of 4-min CS presentation. * p < 0.05
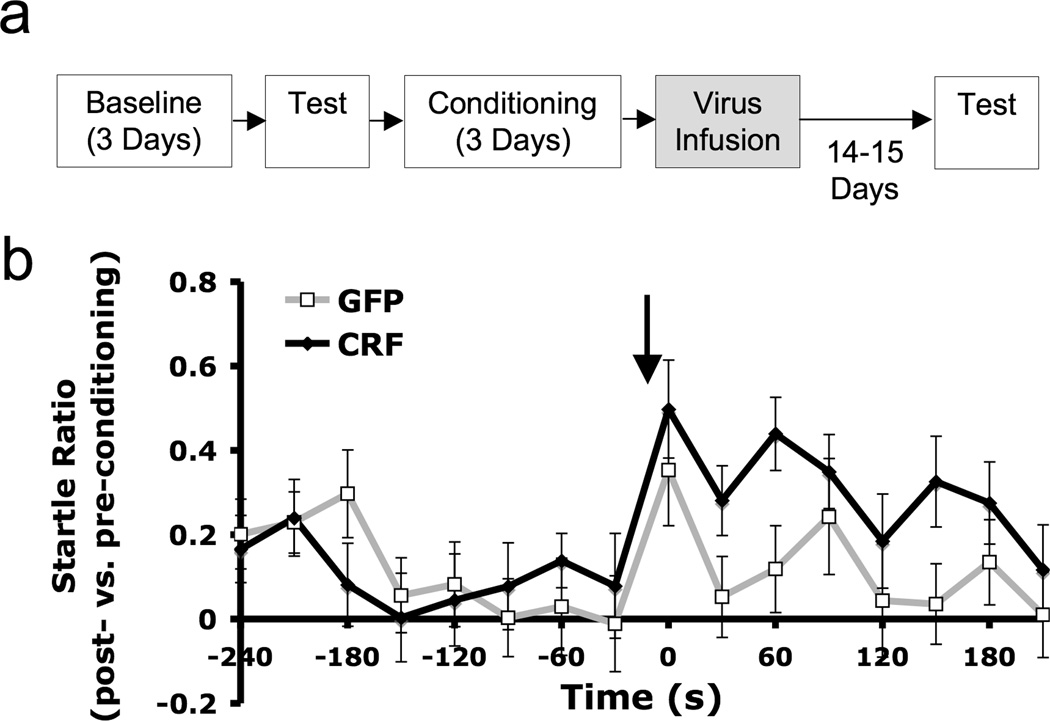
Figure 3.
While there were no differences prior to CS presentation, BNST CRF over-expression induced after conditioning enhanced sustained expression of a conditioned fear response in a test conducted 15–16 days after training. a) Experimental timeline. Time between events is one day unless otherwise specified. b) Startle compared to pre-conditioning test amplitudes. Data are expressed as [log(post-conditioning startle/pre-conditioning startle) ± SEM]. Arrow indicates beginning of 4-min CS presentation.
For Experiment 1, baseline acoustic startle amplitude (averaged over 3 test sessions) was expressed as the log of the post-infection amplitude to pre-infection amplitude ratio and analyzed using independent samples t-test. The shock reactivity averaged over all three conditioning sessions was also analyzed using an independent samples t-test. (see 46, 47 for a discussion of the merit of log transforms for these sorts of ratio scores). Startle amplitude was also analyzed for each conditioning session, using independent samples t-tests.
For conditioned fear tests in Experiments 1 and 2, startle amplitude was expressed as the log of the post-conditioning amplitude to pre-conditioning amplitude ratio and then analyzed using separate 2 × 2 factorial ANOVAs for each experiment, with treatment (i.e., CRF OE vs control) as a between-subjects factor and test phase (before vs during the clicker CS) as a within-subjects factor. A similar 2 × 2 factorial ANOVA with repeated measures on test phase was used to compare the average startle amplitude prior to CS presentation with the startle response evoked within 3 s of CS onset. As suggested elsewhere for analysis of ratio measures and in keeping with our previously published analyses of similar data (46, 47), log transforms were used for this analysis as well.
Elevated Plus Maze/Defensive Withdrawal
25 rats (15 LV-GFP, 10 LV-CRF) were tested for anxiety-like behavior in two separate assays: the elevated plus maze and the defensive withdrawal box, both of which have been shown to be sensitive to pharmacological CRF manipulations (48, 49). All animals were handled for 2 min on each of four days prior to testing. On the 5th and 6th days, they were taken to the test room 30 min prior to test sessions to acclimate to the room. Each rat was tested once for two consecutive days on either DW or EPM, in counterbalanced fashion. Data were first analyzed to determine if order of testing impacted results. No order effects were found, so data from both test days were analyzed together for each assay.
Elevated plus maze testing was conducted in a room illuminated by a single red light bulb over the center of the maze. Each rat was placed on the maze for 5 min. The apparatus was novel to the subjects at the time of testing, and each subject was tested only once. Between subjects, the maze was wiped with Quatricide (Pharmacal). All testing was conducted within two hrs after the beginning of the dark cycle (i.e. between 7 and 9 p.m.). Sessions were video recorded, and scored for (1) time spent in the open arms, (2) time spent in the closed arms, (3) number of entries into the open arms, and (4) number of entries into the closed arms, by an observer blind to the animal’s treatment. A rat was considered to have entered or spent time in an arm only when all four paws were in the respective arm. According to the ethopharmacological factor analysis of Cruz et al (50) percent of total time spent in open arms, percent of total entries into open arms, and number of entries into closed arms were analyzed using independent samples t-tests.
Defensive withdrawal testing was conducted under ambient room lighting between 2 and 6 pm. To begin the trial, the white PVC tube was placed in front of the rat while the experimenter gently guided the animal into the tube. The tube was then placed into a corner of the field, with the open end of the tube facing away from the corner along one of the walls. Each video-recorded trial lasted 10 min. During scoring, a piece of acetate film marked with perpendicular lines divided the arena into 54 10-cm squares for the purposes of estimating locomotor activity. An experimenter blind to the treatment condition scored the videos for (1) the total number of exits from the PVC tube into the arena, (2) the total time spent outside of the tube, and (3) the total number of line crossings during the trial. A rat was considered to have crossed a line if both of its front feet crossed over the line, and was considered to be outside of the tube if two-thirds of its body was outside the tube.
Plasma Corticosterone Analysis
12 rats (6 LV-GFP, 6 LV-CRF) from Experiment 2 were subjected to 30 min of intermittent foot shock stress (0.4 mA, 1 s duration, average period: 1 min) and then sacrificed 10 min after the end of the session. An additional 12 animals (6 LV-GFP and 6 LV-CRF) were sacrificed 2 weeks after virus infusion without experiencing any stress procedures or behavioral testing. Blood was collected via cardiac puncture into EDTA-coated tubes, and centrifuge-separated plasma was frozen at −80C until analysis. Because circulating corticosterone levels vary diurnally, all blood collections were performed between 12–2:30pm. Corticosterone was measured using a Coat-a-Count Radioimmunoassay kit (Siemens Healthcare Diagnostics, Deerfield, IL). All samples were run in triplicate together in a single assay on a Wizard2 Gamma Counter (Perkin Elmer, Waltham, MA). Two levels of a commercial quality control serum were run along with each assay. Factorial ANOVA with 2 stress levels (baseline or footshock-stressed) and 2 treatment conditions (BNST CRF-OE or control) was used to analyze plasma corticosterone levels.
In Situ Hybridization
Rats were euthanized by administration of isoflurane and brains rapidly extracted, frozen on dry ice, and stored at −80°C. In some cases, animals were perfused prior to tissue extraction. 20-µm thick sections from throughout the entire rostro-caudal extent of the BNST were cut at −20°C and mounted onto gelatin-coated slides. In situ hybridization was performed as previously described (45, 51). Briefly, 35S-UTP (1250Ci/mmol, 12.5mCi/ml, NEN, Boston, MA) labeled riboprobes were prepared from linearized clones using T3 polymerase at high specific activity by only using radioactive UTP in the polymerase reaction, with approximately 20–40% incorporation. Following preparation of full-length antisense RNA strands, the RNA was base hydrolyzed to average lengths of 50–100 bp and then purified using a sephadex spin column (Illustra ProbeQuant G-50 microcolumn, GE Healthcare, UK). 1×105 cpm/ml of radioactive probe was applied to each slide, which was immediately covered with parafilm to incubate at 52° C overnight. Slides were stringently washed, placed against Kodak (Rochester, NY) MR autoradiographic film for 1–5 days, and then developed in a Minolta SRX-101A film processor. Autoradiography films were scanned using a high-resolution Epson 3700 flat-bed scanner at 6000 dpi. The signal intensities of brain regions were calculated on the basis of gray values (GVs) between 0 (brightest) and 255 (darkest) obtained from the luminosity histogram feature of Adobe Photoshop. For each animal, hybridization signal was determined bilaterally by measuring the most intense 540-pixel circular region within each dorsolateral BNST region, as well as a background area with little or no hybridization signal (cortex). The anatomical level of analysis was verified using the rat brain atlas of Paxinos & Watson (52). An independent measures t-test was performed on bilaterally averaged BNST CRF mRNA levels for all animals included in these experiments to determine if the LV-CRF virus significantly increased BNST CRF mRNA expression. Individual LV-CRF treated rats were excluded from behavioral analysis if their average CRF mRNA level for both hemispheres was not greater than the control group mean.
CRFR1 and CRFR2 receptor binding autoradiography
CRFR1 receptor binding autoradiography was performed as previously described (53, 54) on fresh frozen brain tissue obtained from 12 (6 LV-GFP, 6 LV-CRF) behaviorally naïve animals sacrificed two weeks after virus infusion (same animals from which blood samples were obtained for corticosterone analysis). Sections containing basolateral amygdala, BNST, dorsal raphe nucleus, locus coeruleus, parabrachial nucleus, hypothalamic paraventricular nucleus, and ventrolateral periaqueductal gray were cut at −20°C and mounted onto four serial slide sets. These regions were chosen for their high CRF receptor density, their reception of BNST CRF projections, and/or their role in fear/anxiety behavior (36–38, 55–57). For each region, two slide sets were thawed at room temperature until dry, fixed in 0.1% paraformaldehyde-PBS (pH7.4) for 2 min, then washed in a pre-incubation medium (19°C 2 × 10 min) consisting of 50 mM Tris-HCl, pH 7.4. The slide-mounted sections were then incubated at 19°C for 2 h in a tracer buffer (50 mM Tris base, mM MgCl, 0.1% bovine serum albumin) containing 0.2 nM 125I-Sauvagine (Perkin Elmer, Waltham, MA). Because Sauvagine is a non-selective CRF agonist, cold selective CRF receptor antagonists were added to the tracer buffer to confer receptor binding specificity. To achieve selective CRFR1 binding, 500 nM of the CRFR2 antagonist Astressin-2B (Sigma-Aldrich) was added to the tracer buffer during incubation, and selective CRFR2 binding was achieved by adding 500 nM of the CRFR1 antagonist CP-154,526 (a generous gift from Michael J. Owens, Emory University). Following incubation, slides were washed in 50 mM Tris base with 10 mM MgCl (pH 7.4) 4 × 5 min plus 30 min with slow stirring. Finally, slides were briefly dipped in deionized water, dried, and apposed to Kodak MR autoradiographic film along with 125I-microscale standards (GE Healthcare) for 85 hours. Autoradiograms were quantified using previously published procedures (53, 54, 58). Briefly, films and microscale standards were digitized (MTI CCD72 camera) and quantified using AIS 6.0 (MCID, Canada). For each region of interest, unilateral optical density measurements were taken and averaged across 3 adjacent sections. A standard curve created using 8 125I standard concentrations was used to convert optical density to decays per minute per mg of tissue (dpm/mg). To control for non-specific background binding, optical density from an area of low binding adjacent to the region of interest was subtracted prior to statistical analysis. For each region, the corrected dpm/mg was analyzed using Mann-Whitney U-tests.
Results
Verification of CRF constitutive over-expression within BNST
We achieved CRF over-expression within the BNST using a lentiviral vector containing a 3.0kb promoter region upstream of the CRF gene transcription start site driving CRF gene expression (Figure 1a). This promoter has previously been shown to confer restricted CRF cell-specific expression in transgenic animals (59), and this same LVCRFp3.0CRF lentivirus construct has produced robust CRF OE within the central nucleus of the amygdala and the hypothalamic paraventricular nucleus (41). LVCRFp3.0CRF-induced CRF OE within the BNST was assessed using in situ hybridization. CRF mRNA expression levels were significantly higher in CRF OE rats compared to controls (optical brightness compared to background, GFP: 21.8 vs. CRF: 44.8; t = 5.2, p < 0.001; Figure 1b, and see Figure 1c for representative sections from LV-CRF and LV-GFP animals). Previous work from our group with a similar viral vector has demonstrated virus-induced CRF peptide expression in vivo (14).
CRF over-expression induced prior to conditioning impairs fear acquisition
There were no significant differences between BNST CRF OE and control animals in baseline acoustic startle response (t = 0.06, p = 0.95), This suggests that CRF OE in and of itself was not anxiogenic. During the first conditioning session, however, CRF-OE animals exhibited diminished startle sensitization as measured by the average acoustic startle amplitude after the first foot shock of the session (t = 2.34 p = 0.047; Figure 2b). However, by the second and third conditioning sessions, both groups were exhibiting similar startle levels (session 2: t = 2.34, p = 0.79; session 3: t = 1.038, p = 0.32). BNST CRF OE rats also exhibited a markedly blunted shock reactivity in response to the initial foot shock of the conditioning sessions (Figure 2c; t = 2.65, p = 0.02).
In harmony with these acquisition data, which might suggest weaker conditioning in the CRF-OE group, fear-potentiated startle during the sustained CS was rapidly attenuated in BNST CRF-OE animals. While both treatment groups exhibited similarly enhanced fear during the initial 15 s of CS presentation (potentiation of startle vs. pre-conditioning test: LV-GFP, 0.36 ± 0.13 vs. LV-CRF, 0.24 ± 0.15; CS effect: F1,14 = 7.86, p = 0.032; treatment effect: F1,14 = 0.091, p = 0.74; treatment × CS interaction: F1,14 = 0.72, p = 0.41), over the duration of the entire 4-min CS the average startle potentiation in CRF-OE rats rapidly declined to near pre-CS levels while startle in controls remained elevated (average startle potentiation vs. pre-conditioning test: LV-GFP: 0.31 ± 0.07 vs. LV-CRF: −0.12 ± 0.08; treatment effect: F1,14 = 5.39, p = 0.036; treatment × CS interaction: F1,14 = 10.41, p = 0.006; figure 2d). Pre-CS startle levels after conditioning were comparable to pre-conditioning levels in both groups (LV-GFP: 0.03 ± 0.09 vs. LV-CRF: 0.08 ± 0.1). Thus, there was no evidence in either group of context conditioning. Together, data from the conditioning and test sessions suggest that over-expression of CRF within the BNST prior to training impairs acquisition of the associative fear memory.
Over-expression of CRF after conditioning enhances conditioned fear expression
To determine whether over-expression of CRF would also affect retention/expression of BNST-dependent conditioned sustained fear, we performed another experiment in which animals were fear-conditioned, infused with virus, then tested 15–16 days later. During the test session (Figure 3b), both treatment groups exhibited enhanced fear compared to pre-CS levels during the initial 3 s of the clicker CS (LV-GFP, 0.35 ± 0.13 vs. LV-CRF, 0.50 ± 0.12; CS effect: F1,45 = 13.78, p = 0.001; treatment effect: F1,45 = 0.49, p = 0.49; treatment × CS interaction: F1,45 = 0.82, p = 0.37). However, over the entire 4-min CS duration, GFP-infected animals’ startle response returned to baseline, while fear-potentiated startle in CRF-OE rats remained elevated. Factorial ANOVA revealed a significant CS effect (F1,45 = 4.85, p = 0.03) and a significant interaction between treatment and CS (F1,45 = 5.14, p = 0.03). As in Experiment 1, BNST CRF OE and control animals had similarly low levels of startle prior to CS onset, demonstrating minimal context conditioning (LV-GFP: 0.11 ± 0.05 vs. LV-CRF: 0.10 ± 0.06). These data suggest that over-expression of CRF within the BNST following training enhances fear memory retention or later expression of conditioned fear to a sustained CS.
BNST CRF over-expression does not affect unconditioned anxiety
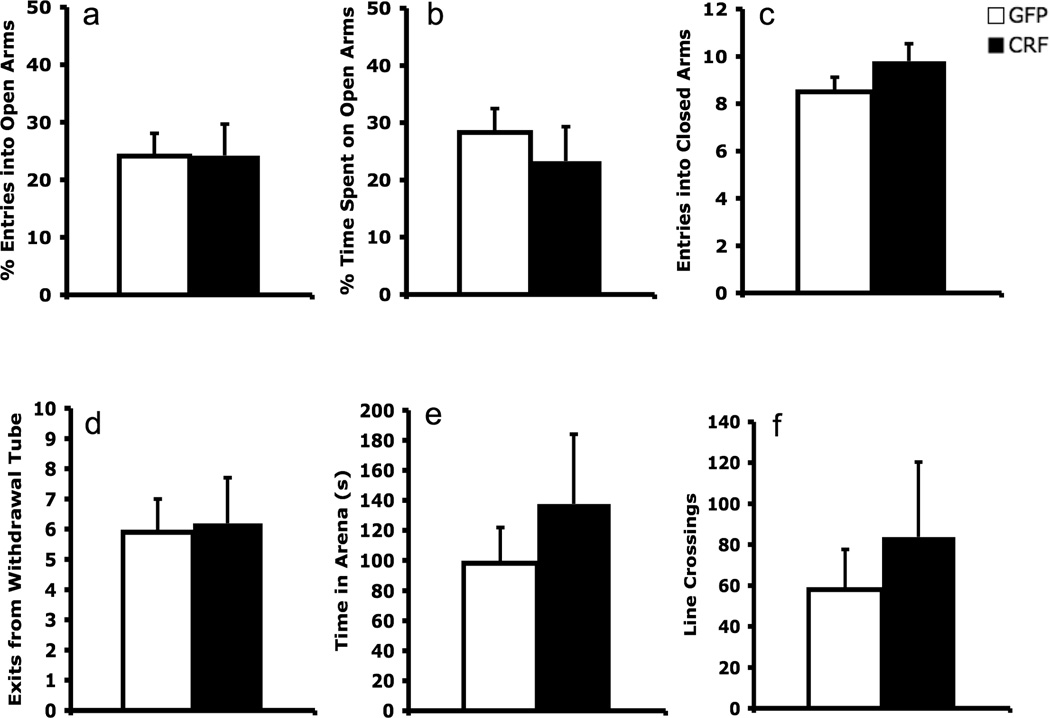
Consistent with the lack of a CRF-OE effect on baseline startle amplitude prior to conditioning or in the absence of the CS during testing (see Figures 2b, 2d, 3b), BNST CRF-OE did not affect unconditioned anxiety measures in the elevated plus maze or defensive withdrawal tests (Figure 4). On the elevated plus maze, BNST CRF OE animals exhibited a similar percentage of entries into open arms (t = 0.02, p = 0.99), percentage of time spent on open arms (t = .72, p = 0.48) and number of closed arm entries (t = 1.38, p = 0.18) compared to controls. Similarly, in the defensive withdrawal test there were no differences between groups in the number of withdrawal tube exits (t = 0.15, p = 0.88) the duration of time spent outside of the tube (t = 0.83, p = .42), or the total number of line crossings in the arena (t = 0.67, p = 0.51). These results imply that BNST CRF OE did not affect unconditioned anxiety.
Figure 4.
CRF over-expression within the BNST did not affect anxiety-like behaviors as assessed in the elevated plus maze (a–c) and defensive withdrawal (d–f) assays.
BNST CRF over-expression does not affect HPA axis activity
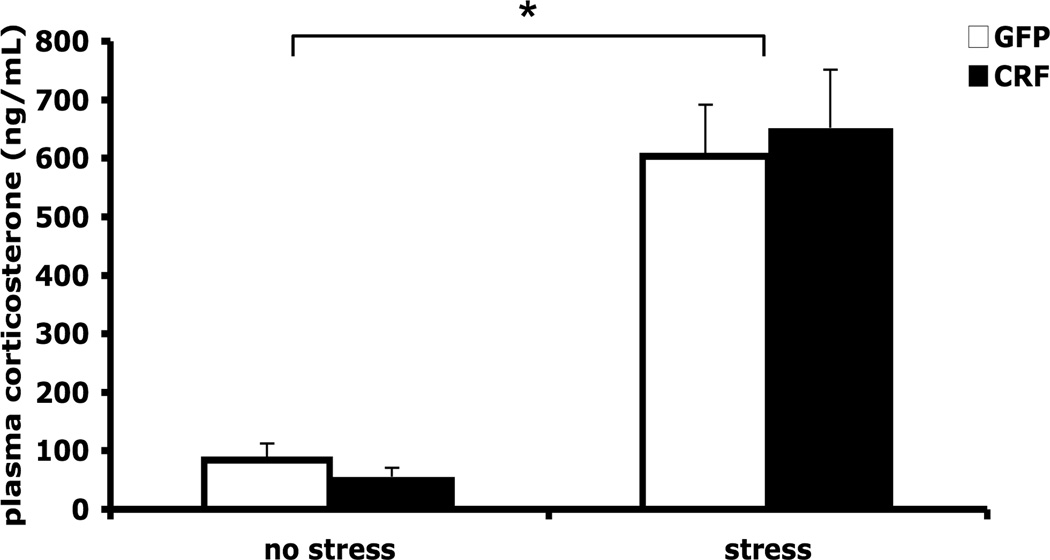
Because BNST CRF neurons project to the hypothalamic paraventricular nucleus where HPA axis responses are initiated, we examined whether continuous BNST CRF OE affects plasma corticosterone levels under basal (low stress) or stressful conditions. As expected, animals that were subjected to 30-min foot-shock stress prior to blood collection had significantly higher plasma corticosterone levels compared to unstressed rats (stress effect: F1,20 = 79.9, p < 0.001); however, there were no significant differences between LV-CRF and LV-GFP-treated animals under either basal or stress conditions (LV treatment effect: F1,20 = 0.028, p = 0.868; LV treatment × stress interaction: F1,20 = 0.389, p = 0.540; Figure 5).
Figure 5.
HPA activation was not affected by BNST CRF over-expression. Plasma corticosterone concentration (ng/mL) did not differ between groups under either baseline (low stress) conditions or after 30 min of foot shock stress. * p < 0.001 compared to baseline conditions.
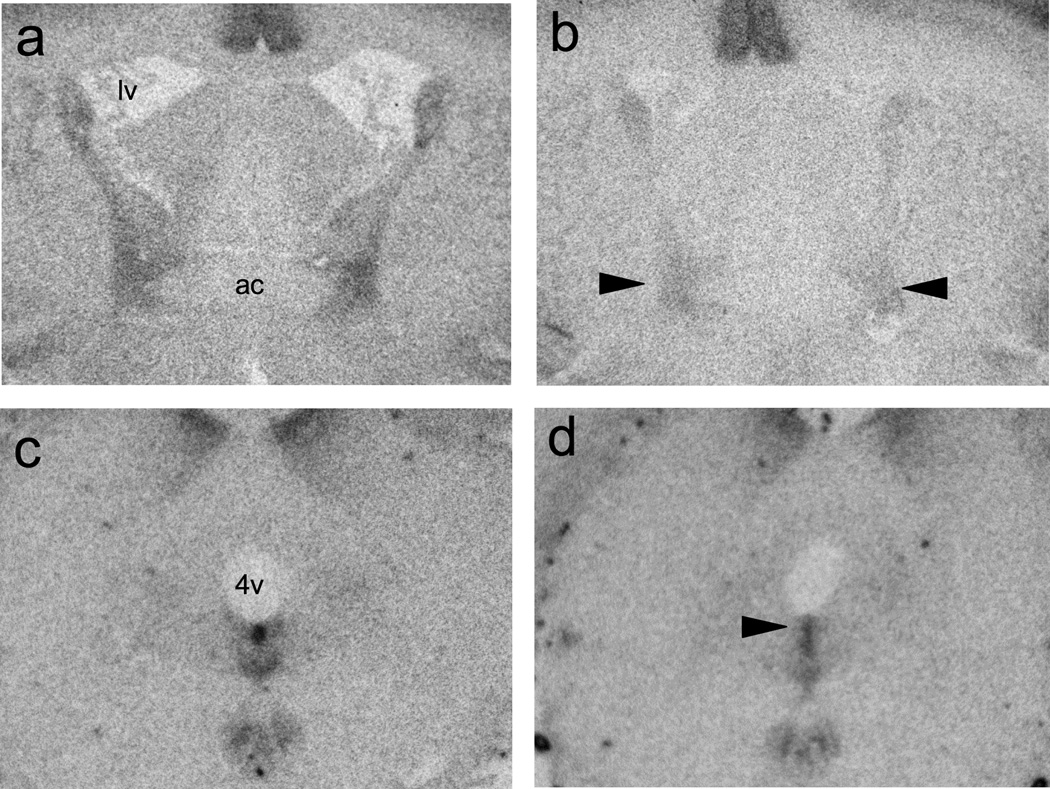
BNST CRF over-expression alters CRF receptor densities within BNST and DRN
CRFR1 and CRFR2 receptor densities for selected regions of interest are summarized in Table I. Mann-Whitney U-tests revealed that BNST CRF OE reduced CRFR1 binding density within the BNST (p = 0.004) and CRFR2 binding density in the caudal DRN (p = 0.018). Representative autoradiographs from these regions are shown in Figure 6.
Table I.
CRF receptor autoradiographic binding densities
| receptor density (dpm/mg ± SEM) |
||
|---|---|---|
| LV-GFP | LV-CRF | |
| CRFR1 | ||
| basolateral amygdala | 1770 ± 166 | 1870 ± 290 |
| bed nucleus stria terminalis | 472 ± 55 | 186 ± 21* |
| dorsal raphe nucleus | 177 ± 20 | 176 ± 21 |
| parabrachial nucleus | 770 ± 102 | 494 ± 61 |
| paraventricular hypothalamic nucleus | 266 ± 26 | 212 ± 33 |
| ventrolateral periaqueductal gray | 66 ± 4 | 59 ± 17 |
| CRFR2 | ||
| dorsal raphe nucleus | 1012 ± 127 | 414 ± 126* |
| paraventricular hypothalamic nucleus | 209 ± 35 | 131 ± 19 |
p < 0.01
Figure 6.
Representative autoradiographs showing reduced CRF receptor density in animals with enhanced BNST CRF expression (b, d) compared to controls (a, c). BNST CRF OE reduced CRFR1 receptor binding density lateral BNST (indicated by arrows in b), and CRFR2 receptor binding was lower in the dorsal portion of caudal DRN (indicated by arrow in d) of BNST CRF OE animals. lv: lateral ventricle; ac: anterior commissure; 4v: fourth ventricle.
Discussion
The lateral BNST, which is an important neural node in anxiety circuitry, contains a dense population of CRF-expressing neurons. CRF signaling in the central nervous system has been implicated in the regulation of behavioral and neuroendocrine stress responses, and CRF expression is increased by stress. This study was designed to mimic those stress effects on CRF expression within the BNST in an effort to ascertain how these neurons influence anxiety-related behaviors. We used a lentivirus containing a CRF-specific promoter in order to target CRF over-expression to the CRFergic neurons of the BNST. Previous work using this promoter has demonstrated 85–90% gene targeting specificity for CRF-producing cells (59), suggesting that the findings from the present experiments are largely the result of CRF over-production within CRFergic cells of the BNST. We found that BNST CRF OE did not affect baseline acoustic startle response or unconditioned anxiety behavior in the elevated plus maze or defensive withdrawal tests. However, BNST CRF OE impaired sustained startle potentiation (modeling conditioned anxiety) when induced prior to training but enhanced sustained startle potentiation when induced after training. These surprising paradoxical effects suggest that BNST CRF may potentially influence conditioned anxiety through separate, dissociable mechanisms.
When rats were infected with LV-CRF two weeks prior to conditioning, they exhibited blunted startle sensitization during the first day of conditioning, decreased reactivity to the initial foot shock of the conditioning sessions, and rapidly attenuated fear-potentiated startle in response to the sustained CS during testing two days later. These results, which are similar to those observed in animals over-expressing CRF in the forebrain (60) or central nucleus of the amygdala (61) prior to training, suggest that BNST CRF-OE induced associative fear learning deficits, possibly due to reduced sensitivity to the foot shock US or unspecified learning impairments.
In contrast to the impairment of fear conditioning produced by BNST CRF OE prior to training, over-expression of CRF within BNST following conditioning significantly increased fear-potentiated startle throughout the 4-min CS in a test session conducted 15–16 days after conditioning (Figure 3). This finding suggests that BNST CRF OE enhanced expression of the fear memory, in line with studies showing that intra-cranially administered CRF enhances associative fear (62–64). Stress can also strengthen emotional memories in animals as well as in people (65–67), and in the extreme can lead to fear-related psychopathogy such as PTSD. Considering that BNST CRF neurons are stress-sensitive (31–34), over-activation of these CRF neurons may represent a mechanism by which stress strengthens expression of associative fear memories.
BNST CRF OE appeared to specifically influence associative anxiety-like responses, as baseline startle amplitude and unconditioned anxiety-related behavior on the elevated plus maze were not affected. These results align with a recently published study reporting that BNST CRF OE in mice produced no significant effects on anxiety-like behavior in elevated plus maze, open field, or dark-light transfer tests (23). However, some studies have shown that CRF OE transgenic mice exhibit an anxious phenotype in tests of unconditioned anxiety (24, 68). This difference between site-specific BNST CRF OE and whole brain CRF OE may indicate that CRF neurons external to the BNST (i.e., amygdala, hippocampus, or paraventricular hypothalamus) regulate unconditioned anxiety. Indeed, we have found previously that CRF OE targeted to the central nucleus of the amygdala (CeA) did enhance basal acoustic startle (14) and anxiety-like behavior in the elevated plus maze and defensive withdrawal test (59), suggesting that CeA CRF neurons may at least partially mediate the anxious phenotype of some CRF OE transgenic animals.
There is also a considerable body of literature showing that exogenously administered CRF enhances anxiety in a number of behavioral paradigms including unconditioned acoustic startle (22, 69–71), and approach-avoidance conflict tests (72, 73). Although behavioral results from site-specific CRF OE and exogenously administered CRF (locally or ICV) do share some similarities, it must be noted that these procedures are fundamentally different in that CRF released by infected BNST neurons acts not only locally but may activate receptors in more distal structures targeted by these neurons. Furthermore, chronic CRF over-expression may induce compensatory changes in CRF receptor sensitivity or even in non-CRFergic signaling systems. Thus, any attempt to directly compare results obtained by pharmacological CRF administration with studies of targeted CRF gene OE should consider these differences. In contrast to pharmacological studies, we believe that genetically driven enhancement of CRF within CRFergic neurons most closely mimics a state of physiologically enhanced CRF activity, which cannot be modeled as well by pharmacological approaches.
Although the lateral BNST exerts a significant influence on HPA axis activity (74, 75), over-expression of CRF within this region did not produce differences in basal or stress-induced plasma corticosterone levels (Figure 5). These findings are consistent with previous data which showed that BNST CRF-OE in mice had no effect on plasma corticosterone under either basal conditions or following acute restraint stress (23). The similarity of corticosterone data from these two studies argues that CRF from BNST neurons does not play a significant role in HPA regulation, at least under the conditions tested. Also, the failure of BNST-OE to influence corticosterone levels in this experiment might imply that the CRF-OE-induced changes in sustained fear-potentiated startle observed in Experiments 1 and 2 are not the result of CRF-OE-induced alterations in HPA activity. Such a conclusion is not without precedent, in that prior studies have also shown a dissociation of fear behavior and neuroendocrine response under some conditions (76–78).
We also examined whether enhanced BNST CRF expression induces compensatory changes in CRF receptor density within target structures associated with fear and anxiety. It was found that CRF OE within the BNST produced lower CRFR1 density within the BNST, and decreased CRFR2 density in the dorsal portion of the caudal DRN. Because CRFR1 and CRFR2 receptors are known to internalize in response to CRF binding (79–82), this could suggest that CRF receptors within the BNST and DRN were down-regulated as a compensatory response to elevated localized concentrations of CRF peptide. The lower density of CRFR1 receptors within BNST matches findings from a previous study showing that BNST CRF OE lowered BNST CRFR1 mRNA expression in mice (23), and aligns with previous studies implicating CRFR1 receptors as actuators of stress-induced anxiety (83) and CRF-enhanced startle (22, 84). These data suggest that chronically enhanced BNST CRF expression may modulate fear- and anxiety in part by altering CRF signaling processes locally within the BNST. CRFR2 downregulation within the caudal DRN suggests BNST CRF OE causes higher CRF peptide concentrations in this region as well. CRF within the DRN plays an important role in stress-induced regulation of serotonin (85–88) a critical modulator of fear- and anxiety-related behaviors (89, 90); and DRN administration of CRF increases fear-like freezing behavior (91). Given that CRFR2 receptor activation also influences defensive behavior including startle (20, 84), regulation of DRN serotonergic neurotransmission by BNST CRF neuronal efferents is an intriguing possibility that deserves attention, as this may be a potential mechanism modulating fear- and anxiety-related behaviors. Further studies will be necessary to determine whether these hypothetical mechanisms actually contribute to anxiety-related behavioral alterations such as those produced by BNST CRF-OE in the present experiments.
In conclusion, these studies demonstrate that CRF neurons of the BNST are involved in the modulation of sustained fear-potentiated startle (modeling conditioned anxiety), but not unconditioned anxiety behaviors. As sustained startle potentiation has previously been found in both rats and humans to be especially sensitive to benzodiazepine and chronic SSRI administration, and to be selectively exaggerated in post-traumatic stress and panic disorder patients (92), CRF over-expression in the BNST may mimic at least some aspects of these disorders (which in the case of PTSD includes elevated startle as a diagnostic criterion), and may be a useful tool for exploring their neural substrates and for screening novel therapeutic agents.
CRF receptor binding autoradiography showing lower receptor density within the BNST and the DRN suggests that these behavioral effects may involve CRF receptor signaling within these structures. However, HPA-axis activation does not appear to play a significant role in these processes. Future work should focus on how these BNST CRF neurons influence signaling in target structures. In light of the associations between CRF signal perturbation and stress-associated fear and anxiety disorders (25–29), these findings implicate BNST CRF neurons as important members of the neural network that governs not only adaptive associative fear but also the development of fear-related psychopathologies such as PTSD.
Acknowledgments
This research was supported by NIH awards MH47840 and MH069056 to MD, the Science and Technology Center (The Center for Behavioral Neuroscience of the National Science Foundation under Agreement No. IBN-9876754), and the Yerkes Regional Primate Facility Base Grant, 2P51RR000165-51. This research project was also supported in part by the Viral Vector Core of the Emory Neuroscience NINDS Core Facilities grant, P30NS055077.
References
- 1.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010 Jan;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker DL, Davis M. Double dissociation between the involvement of the bed nucleus of the stria terminalis and the central nucleus of the amygdala in startle increases produced by conditioned versus unconditioned fear. J Neurosci. 1997 Dec 1;17(23):9375–9383. doi: 10.1523/JNEUROSCI.17-23-09375.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sullivan GM, Apergis J, Bush DE, Johnson LR, Hou M, Ledoux JE. Lesions in the bed nucleus of the stria terminalis disrupt corticosterone and freezing responses elicited by a contextual but not by a specific cue-conditioned fear stimulus. Neuroscience. 2004;128(1):7–14. doi: 10.1016/j.neuroscience.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 4.Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006 Apr;120(2):324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- 5.Sink KS, Walker DL, YY MD. Calcitonin gene-related peptide in the bed nucleus of the stria terminalis produces an anxiety-like pattern of behavior and increases neural activation in anxiety-related structures. J Neurosci. 2011 doi: 10.1523/JNEUROSCI.5274-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J Neurosci. 2010 May 19;30(20):7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol Psychiatry. 2005 Nov 15;58(10):796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Somerville LH, Whalen PJ, Kelley WM. Human Bed Nucleus of the Stria Terminalis Indexes Hypervigilant Threat Monitoring. Biol Psychiatry. 2010 May 22; doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Straube T, Mentzel HJ, Miltner WH. Waiting for spiders: brain activation during anticipatory anxiety in spider phobics. Neuroimage. 2007 Oct 1;37(4):1427–1436. doi: 10.1016/j.neuroimage.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Gray TS, Magnuson DJ. Neuropeptide neuronal efferents from the bed nucleus of the stria terminalis and central amygdaloid nucleus to the dorsal vagal complex in the rat. J Comp Neurol. 1987 Aug 15;262(3):365–374. doi: 10.1002/cne.902620304. [DOI] [PubMed] [Google Scholar]
- 11.Merchenthaler I, Vigh S, Petrusz P, Schally AV. Immunocytochemical localization of corticotropin-releasing factor (CRF) in the rat brain. Am J Anat. 1982 Dec;165(4):385–396. doi: 10.1002/aja.1001650404. [DOI] [PubMed] [Google Scholar]
- 12.Phelix CF, Paull WK. Demonstration of distinct corticotropin releasing factor--containing neuron populations in the bed nucleus of the stria terminalis. A light and electron microscopic immunocytochemical study in the rat. Histochemistry. 1990;94(4):345–364. doi: 10.1007/BF00266441. [DOI] [PubMed] [Google Scholar]
- 13.Dunn AJ, Berridge CW. Is corticotropin-releasing factor a mediator of stress responses? Ann N Y Acad Sci. 1990;579:183–191. doi: 10.1111/j.1749-6632.1990.tb48360.x. [DOI] [PubMed] [Google Scholar]
- 14.Keen-Rhinehart E, Michopoulos V, Toufexis DJ, Martin EI, Nair H, Ressler KJ, et al. Continuous expression of corticotropin-releasing factor in the central nucleus of the amygdala emulates the dysregulation of the stress and reproductive axes. Mol Psychiatry. 2009 Jan;14(1):37–50. doi: 10.1038/mp.2008.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991 Dec;43(4):425–473. [PubMed] [Google Scholar]
- 16.Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotropin-releasing factor and noradrenergic mediation of stress responses. Ann N Y Acad Sci. 1993 Oct 29;697:173–188. doi: 10.1111/j.1749-6632.1993.tb49931.x. [DOI] [PubMed] [Google Scholar]
- 17.Nemeroff CB, Vale WW. The neurobiology of depression: inroads to treatment and new drug discovery. J Clin Psychiatry. 2005;66(Suppl 7):5–13. [PubMed] [Google Scholar]
- 18.Hubbard DT, Nakashima BR, Lee I, Takahashi LK. Activation of basolateral amygdala corticotropin-releasing factor 1 receptors modulates the consolidation of contextual fear. Neuroscience. 2007 Dec 19;150(4):818–828. doi: 10.1016/j.neuroscience.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitts MW, Takahashi LK. The central amygdala nucleus via corticotropin-releasing factor is necessary for time-limited consolidation processing but not storage of contextual fear memory. Neurobiol Learn Mem. 2010 Nov 17; doi: 10.1016/j.nlm.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Risbrough VB, Geyer MA, Hauger RL, Coste S, Stenzel-Poore M, Wurst W, et al. CRF1 and CRF2 receptors are required for potentiated startle to contextual but not discrete cues. Neuropsychopharmacology. 2009 May;34(6):1494–1503. doi: 10.1038/npp.2008.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skorzewska A, Bidzinski A, Hamed A, Lehner M, Turzynska D, Sobolewska A, et al. The effect of CRF and alpha-helical CRF((9–41)) on rat fear responses and amino acids release in the central nucleus of the amygdala. Neuropharmacology. 2009 Aug;57(2):148–156. doi: 10.1016/j.neuropharm.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 22.Walker DL, Miles LA, Davis M. Selective participation of the bed nucleus of the stria terminalis and CRF in sustained anxiety-like versus phasic fear-like responses. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Nov 13;33(8):1291–1308. doi: 10.1016/j.pnpbp.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Regev L, Neufeld-Cohen A, Tsoory M, Kuperman Y, Getselter D, Gil S, et al. Prolonged and site-specific over-expression of corticotropin-releasing factor reveals differential roles for extended amygdala nuclei in emotional regulation. Mol Psychiatry. 2010 Jun 15; doi: 10.1038/mp.2010.64. [DOI] [PubMed] [Google Scholar]
- 24.Vicentini E, Arban R, Angelici O, Maraia G, Perico M, Mugnaini M, et al. Transient forebrain over-expression of CRF induces plasma corticosterone and mild behavioural changes in adult conditional CRF transgenic mice. Pharmacol Biochem Behav. 2009 Jul;93(1):17–24. doi: 10.1016/j.pbb.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 25.Bale TL. Sensitivity to stress: dysregulation of CRF pathways and disease development. Horm Behav. 2005 Jun;48(1):1–10. doi: 10.1016/j.yhbeh.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Heinrichs SC, Koob GF. Corticotropin-releasing factor in brain: a role in activation, arousal, and affect regulation. J Pharmacol Exp Ther. 2004 Nov;311(2):427–440. doi: 10.1124/jpet.103.052092. [DOI] [PubMed] [Google Scholar]
- 27.Holmes A, Heilig M, Rupniak NM, Steckler T, Griebel G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol Sci. 2003 Nov;24(11):580–588. doi: 10.1016/j.tips.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Risbrough VB, Stein MB. Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav. 2006 Nov;50(4):550–561. doi: 10.1016/j.yhbeh.2006.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004 Jul;13(7):799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues SM, LeDoux JE, Sapolsky RM. The influence of stress hormones on fear circuitry. Annu Rev Neurosci. 2009;32:289–313. doi: 10.1146/annurev.neuro.051508.135620. [DOI] [PubMed] [Google Scholar]
- 31.Choi DC, Nguyen MM, Tamashiro KL, Ma LY, Sakai RR, Herman JP. Chronic social stress in the visible burrow system modulates stress-related gene expression in the bed nucleus of the stria terminalis. Physiol Behav. 2006 Oct 30;89(3):301–310. doi: 10.1016/j.physbeh.2006.05.046. [DOI] [PubMed] [Google Scholar]
- 32.Funk D, Li Z, Le AD. Effects of environmental and pharmacological stressors on c-fos and corticotropin-releasing factor mRNA in rat brain: Relationship to the reinstatement of alcohol seeking. Neuroscience. 2006;138(1):235–243. doi: 10.1016/j.neuroscience.2005.10.062. [DOI] [PubMed] [Google Scholar]
- 33.Kim SJ, Park SH, Choi SH, Moon BH, Lee KJ, Kang SW, et al. Effects of repeated tianeptine treatment on CRF mRNA expression in non-stressed and chronic mild stress-exposed rats. Neuropharmacology. 2006 Jun;50(7):824–833. doi: 10.1016/j.neuropharm.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 34.Shepard JD, Schulkin J, Myers DA. Chronically elevated corticosterone in the amygdala increases corticotropin releasing factor mRNA in the dorsolateral bed nucleus of stria terminalis following duress. Behav Brain Res. 2006 Nov 1;174(1):193–196. doi: 10.1016/j.bbr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Gray TS, Magnuson DJ. Peptide immunoreactive neurons in the amygdala and the bed nucleus of the stria terminalis project to the midbrain central gray in the rat. Peptides. 1992 May-Jun;13(3):451–460. doi: 10.1016/0196-9781(92)90074-d. [DOI] [PubMed] [Google Scholar]
- 36.Moga MM, Saper CB, Gray TS. Bed nucleus of the stria terminalis: cytoarchitecture, immunohistochemistry, and projection to the parabrachial nucleus in the rat. J Comp Neurol. 1989 May 15;283(3):315–332. doi: 10.1002/cne.902830302. [DOI] [PubMed] [Google Scholar]
- 37.Panguluri S, Saggu S, Lundy R. Comparison of somatostatin and corticotrophin-releasing hormone immunoreactivity in forebrain neurons projecting to taste-responsive and non-responsive regions of the parabrachial nucleus in rat. Brain Res. 2009 Nov 17;1298:57–69. doi: 10.1016/j.brainres.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Champagne D, Beaulieu J, Drolet G. CRFergic innervation of the paraventricular nucleus of the rat hypothalamus: a tract-tracing study. J Neuroendocrinol. 1998 Feb;10(2):119–131. doi: 10.1046/j.1365-2826.1998.00179.x. [DOI] [PubMed] [Google Scholar]
- 39.Naldini L, Blomer U, Gage FH, Trono D, Verma IM. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc Natl Acad Sci U S A. 1996 Oct 15;93(21):11382–11388. doi: 10.1073/pnas.93.21.11382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zufferey R, Dull T, Mandel RJ, Bukovsky A, Quiroz D, Naldini L, et al. Self-inactivating lentivirus vector for safe and efficient in vivo gene delivery. J Virol. 1998 Dec;72(12):9873–9880. doi: 10.1128/jvi.72.12.9873-9880.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB. Chronic overexpression of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology. May 25; doi: 10.1016/j.psyneuen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seth KA, Majzoub JA. Repressor element silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) can act as an enhancer as well as a repressor of corticotropin-releasing hormone gene transcription. J Biol Chem. 2001 Apr 27;276(17):13917–13923. doi: 10.1074/jbc.M007745200. [DOI] [PubMed] [Google Scholar]
- 43.Tiscornia G, Singer O, Ikawa M, Verma IM. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc Natl Acad Sci U S A. 2003 Feb 18;100(4):1844–1848. doi: 10.1073/pnas.0437912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol Psychiatry. 2007 Jul;12(7):656–670. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rattiner LM, Davis M, French CT, Ressler KJ. Brain-derived neurotrophic factor and tyrosine kinase receptor B involvement in amygdala-dependent fear conditioning. J Neurosci. 2004 May 19;24(20):4796–4806. doi: 10.1523/JNEUROSCI.5654-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keene O. The log transformation is special. Stat Med. 1995;14:811–819. doi: 10.1002/sim.4780140810. [DOI] [PubMed] [Google Scholar]
- 47.Miles L, Davis M, Walker D. Phasic and sustained fear are pharmacologically dissociable in rats. Neuropsychopharmacology. Jul;36(8):1563–1574. doi: 10.1038/npp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gutman DA, Owens MJ, Thrivikraman KV, Nemeroff CB. Persistent anxiolytic affects after chronic administration of the CRF receptor antagonist R121919 in rats. Neuropharmacology. Jun;60(7–8):1135–1141. doi: 10.1016/j.neuropharm.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992 May 29;581(2):190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- 50.Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994 Sep;49(1):171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 51.Ressler KJ, Paschall G, Zhou XL, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J Neurosci. 2002 Sep 15;22(18):7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd edn. San Diego: Academic Press; 1997. [Google Scholar]
- 53.Ahern TH, Young LJ. The impact of early life family structure on adult social attachment, alloparental behavior, and the neuropeptide systems regulating affiliative behaviors in the monogamous prairie vole (microtus ochrogaster) Front Behav Neurosci. 2009;3:17. doi: 10.3389/neuro.08.017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lim MM, Nair HP, Young LJ. Species and sex differences in brain distribution of corticotropin-releasing factor receptor subtypes 1 and 2 in monogamous and promiscuous vole species. J Comp Neurol. 2005 Jun 20;487(1):75–92. doi: 10.1002/cne.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chalmers DT, Lovenberg TW, De Souza EB. Localization of novel corticotropin-releasing factor receptor (CRF2) mRNA expression to specific subcortical nuclei in rat brain: comparison with CRF1 receptor mRNA expression. J Neurosci. 1995 Oct;15(10):6340–6350. doi: 10.1523/JNEUROSCI.15-10-06340.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Souza MS, de Souza DW, Carvalho Odos S, Neves J, Massara CL. [Viability of Ascaris lumbricoides ova after human treatment with specific drugs. I--Levamisole and pyrantel pamoate] Rev Inst Med Trop Sao Paulo. 1985 Jul-Aug;27(4):197–200. doi: 10.1590/s0036-46651985000400007. [DOI] [PubMed] [Google Scholar]
- 57.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, et al. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000 Dec 11;428(2):191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 58.Phelps SM, Young LJ. Extraordinary diversity in vasopressin (V1a) receptor distributions among wild prairie voles (Microtus ochrogaster): patterns of variation and covariation. J Comp Neurol. 2003 Nov 24;466(4):564–576. doi: 10.1002/cne.10902. [DOI] [PubMed] [Google Scholar]
- 59.Martin EI, Ressler KJ, Jasnow AM, Dabrowska J, Hazra R, Rainnie DG, et al. A novel transgenic mouse for gene-targeting within cells that express corticotropin-releasing factor. Biol Psychiatry. 2010 Jun 15;67(12):1212–1216. doi: 10.1016/j.biopsych.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gresack JE, Scott CN, Niemeyer CM, Steen HA, Vicentini E, Mangiarini L, et al. Transient forebrain CRF overexpression in adulthood selectively impairs contextual fear memeory and context habituation. Society for Neuroscience Annual Meeting. 2010 [Google Scholar]
- 61.Laryea G, Muglia L. Tetracycline-regulated corticotropin-releasing hormone expression in transgenic mice as a model for isolating brain-region specific function in the stress response. Society for Neuroscience Annual Meeting. 2010 [Google Scholar]
- 62.Cole BJ, Koob GF. Propranolol antagonizes the enhanced conditioned fear produced by corticotropin releasing factor. J Pharmacol Exp Ther. 1988 Dec;247(3):902–910. [PubMed] [Google Scholar]
- 63.Radulovic J, Ruhmann A, Liepold T, Spiess J. Modulation of learning and anxiety by corticotropin-releasing factor (CRF) and stress: differential roles of CRF receptors 1 and 2. J Neurosci. 1999 Jun 15;19(12):5016–5025. doi: 10.1523/JNEUROSCI.19-12-05016.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sherman JE, Kalin NH. ICV-CRH alters stress-induced freezing behavior without affecting pain sensitivity. Pharmacol Biochem Behav. 1988 Aug;30(4):801–807. doi: 10.1016/0091-3057(88)90103-7. [DOI] [PubMed] [Google Scholar]
- 65.Cahill L, Gorski L, Le K. Enhanced human memory consolidation with post-learning stress: interaction with the degree of arousal at encoding. Learn Mem. 2003 Jul-Aug;10(4):270–274. doi: 10.1101/lm.62403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hui IR, Hui GK, Roozendaal B, McGaugh JL, Weinberger NM. Posttraining handling facilitates memory for auditory-cue fear conditioning in rats. Neurobiol Learn Mem. 2006 Sep;86(2):160–163. doi: 10.1016/j.nlm.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Rau V, DeCola JP, Fanselow MS. Stress-induced enhancement of fear learning: an animal model of posttraumatic stress disorder. Neurosci Biobehav Rev. 2005;29(8):1207–1223. doi: 10.1016/j.neubiorev.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 68.Stenzel-Poore MP, Heinrichs SC, Rivest S, Koob GF, Vale WW. Overproduction of corticotropin-releasing factor in transgenic mice: a genetic model of anxiogenic behavior. J Neurosci. 1994 May;14(5 Pt 1):2579–2584. doi: 10.1523/JNEUROSCI.14-05-02579.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee Y, Davis M. Role of the hippocampus, the bed nucleus of the stria terminalis, and the amygdala in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997 Aug 15;17(16):6434–6446. doi: 10.1523/JNEUROSCI.17-16-06434.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee Y, Davis M. Role of the septum in the excitatory effect of corticotropin-releasing hormone on the acoustic startle reflex. J Neurosci. 1997 Aug 15;17(16):6424–6433. doi: 10.1523/JNEUROSCI.17-16-06424.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang KC, Melia KR, Miserendino MJ, Falls WA, Campeau S, Davis M. Corticotropin-releasing factor: long-lasting facilitation of the acoustic startle reflex. J Neurosci. 1992 Jun;12(6):2303–2312. doi: 10.1523/JNEUROSCI.12-06-02303.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baldwin HA, Rassnick S, Rivier J, Koob GF, Britton KT. CRF antagonist reverses the "anxiogenic" response to ethanol withdrawal in the rat. Psychopharmacology (Berl) 1991;103(2):227–232. doi: 10.1007/BF02244208. [DOI] [PubMed] [Google Scholar]
- 73.Koob GF, Bloom FE. Corticotropin-releasing factor and behavior. Fed Proc. 1985 Jan;44(1 Pt 2):259–263. [PubMed] [Google Scholar]
- 74.Choi DC, Furay AR, Evanson NK, Ostrander MM, Ulrich-Lai YM, Herman JP. Bed nucleus of the stria terminalis subregions differentially regulate hypothalamic-pituitary-adrenal axis activity: implications for the integration of limbic inputs. J Neurosci. 2007 Feb 21;27(8):2025–2034. doi: 10.1523/JNEUROSCI.4301-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gray TS, Piechowski RA, Yracheta JM, Rittenhouse PA, Bethea CL, Van de Kar LD. Ibotenic acid lesions in the bed nucleus of the stria terminalis attenuate conditioned stress-induced increases in prolactin, ACTH and corticosterone. Neuroendocrinology. 1993 Mar;57(3):517–524. doi: 10.1159/000126400. [DOI] [PubMed] [Google Scholar]
- 76.Gagliano H, Fuentes S, Nadal R, Armario A. Previous exposure to immobilisation and repeated exposure to a novel environment demonstrate a marked dissociation between behavioral and pituitary-adrenal responses. Behav Brain Res. 2008 Mar 5;187(2):239–245. doi: 10.1016/j.bbr.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 77.Kalin NH, Takahashi LK. Fear-motivated behavior induced by prior shock experience is mediated by corticotropin-releasing hormone systems. Brain Res. 1990 Feb 12;509(1):80–84. doi: 10.1016/0006-8993(90)90311-x. [DOI] [PubMed] [Google Scholar]
- 78.Lee Y, Schulkin J, Davis M. Effect of corticosterone on the enhancement of the acoustic startle reflex by corticotropin releasing factor (CRF) Brain Res. 1994 Dec 12;666(1):93–98. doi: 10.1016/0006-8993(94)90286-0. [DOI] [PubMed] [Google Scholar]
- 79.Markovic D, Punn A, Lehnert H, Grammatopoulos DK. Intracellular mechanisms regulating corticotropin-releasing hormone receptor-2beta endocytosis and interaction with extracellularly regulated kinase 1/2 and p38 mitogen-activated protein kinase signaling cascades. Mol Endocrinol. 2008 Mar;22(3):689–706. doi: 10.1210/me.2007-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Milan-Lobo L, Gsandtner I, Gaubitzer E, Runzler D, Buchmayer F, Kohler G, et al. Subtype-specific differences in corticotropin-releasing factor receptor complexes detected by fluorescence spectroscopy. Mol Pharmacol. 2009 Dec;76(6):1196–1210. doi: 10.1124/mol.109.059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oakley RH, Olivares-Reyes JA, Hudson CC, Flores-Vega F, Dautzenberg FM, Hauger RL. Carboxyl-terminal and intracellular loop sites for CRF1 receptor phosphorylation and beta-arrestin-2 recruitment: a mechanism regulating stress and anxiety responses. Am J Physiol Regul Integr Comp Physiol. 2007 Jul;293(1):R209–R222. doi: 10.1152/ajpregu.00099.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perry SJ, Junger S, Kohout TA, Hoare SR, Struthers RS, Grigoriadis DE, et al. Distinct conformations of the corticotropin releasing factor type 1 receptor adopted following agonist and antagonist binding are differentially regulated. J Biol Chem. 2005 Mar 25;280(12):11560–11568. doi: 10.1074/jbc.M412914200. [DOI] [PubMed] [Google Scholar]
- 83.Adamec R, Fougere D, Risbrough V. CRF receptor blockade prevents initiation and consolidation of stress effects on affect in the predator stress model of PTSD. Int J Neuropsychopharmacol. Jul;13(6):747–757. doi: 10.1017/S1461145709990496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Risbrough VB, Hauger RL, Roberts AL, Vale WW, Geyer MA. Corticotropin-releasing factor receptors CRF1 and CRF2 exert both additive and opposing influences on defensive startle behavior. J Neurosci. 2004 Jul 21;24(29):6545–6552. doi: 10.1523/JNEUROSCI.5760-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hammack SE, Pepin JL, DesMarteau JS, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected into the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behav Brain Res. 2003 Dec 17;147(1–2):55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 86.Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF. The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci. 2002 Feb 1;22(3):1020–1026. doi: 10.1523/JNEUROSCI.22-03-01020.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mo B, Feng N, Renner K, Forster G. Restraint stress increases serotonin release in the central nucleus of the amygdala via activation of corticotropin-releasing factor receptors. Brain Res Bull. 2008 Jul 30;76(5):493–498. doi: 10.1016/j.brainresbull.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Price ML, Kirby LG, Valentino RJ, Lucki I. Evidence for corticotropin-releasing factor regulation of serotonin in the lateral septum during acute swim stress: adaptation produced by repeated swimming. Psychopharmacology (Berl) 2002 Aug;162(4):406–414. doi: 10.1007/s00213-002-1114-2. [DOI] [PubMed] [Google Scholar]
- 89.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003 Feb 28;463(1–3):235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 90.Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003 Jun;70(2):83–244. doi: 10.1016/s0301-0082(03)00087-x. [DOI] [PubMed] [Google Scholar]
- 91.Forster GL, Feng N, Watt MJ, Korzan WJ, Mouw NJ, Summers CH, et al. Corticotropin-releasing factor in the dorsal raphe elicits temporally distinct serotonergic responses in the limbic system in relation to fear behavior. Neuroscience. 2006 Aug 25;141(2):1047–1055. doi: 10.1016/j.neuroscience.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 92.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. Jan;35(1):105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]