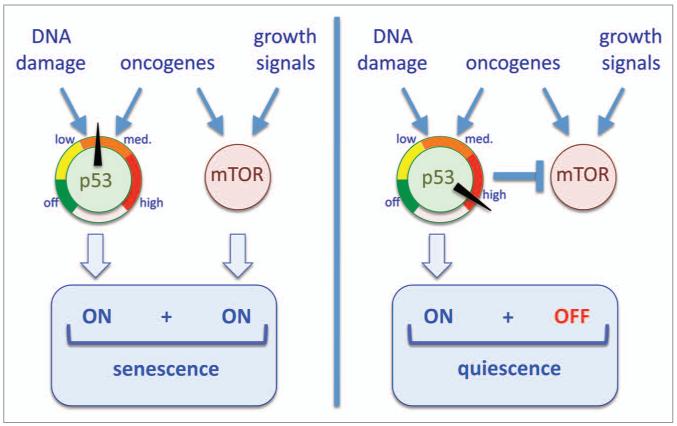
During the last years, cellular senescence has taken center stage as a main defense against cancer. A variety of recent data, both in cellular systems and in mouse models, have converged in highlighting the importance of mTOR-driven hypertrophy for senescence. A report from the laboratories of Mikhail Blagosklonny and collaborators in this issue of Cell Cycle goes one step ahead by demonstrating that p53, when maximally activated, inhibits mTOR and, consequently, prevents hypertrophy and senescence.1 The emerging picture indicates that modest p53 activation preserves mTOR activity and therefore results in senescence, while strong p53 activation inhibits mTOR and results in quiescence (Fig. 1).
Figure 1.
The p53 dial shifts senescence to quiescence. (Left) Stress stimuli that activate a medium intensity p53 response produce cell cycle arrest without inhibiting mTOR, and the final outcome is senescence. (Right) High activation of p53 not only inhibits the cell cycle but also mTOR, and the final outcome is quiescence.
Senescence and Quiescence
Senescence is a cellular state characterized by a permanent and largely irreversible cell cycle arrest. Senescence is triggered by a multitude of stresses, notably including DNA damage and oncogenic signaling, both highly prevalent in tumor cells2 (Fig. 1). During the early stages of tumorigenesis, tumor cells still conserve the capacity to undergo senescence, which together with the increasingly higher levels of cellular stress, results in the actual induction of tumor cell senescence. Thus, senescent cells are particularly abundant and readily detectable in a wide variety of pre-malignant murine and human tumors.3 Tumor cell senescence efficiently halts tumor progression, and only those cells that disable or bypass senescence evolve to a malignant state, i.e., cancer. Accordingly, while senescent cells are abundant in pre-malignant tumors, they are rare in malignant ones.3 Large efforts have been devoted to understand the mechanistic pathways that establish senescence and these investigations have solidified a strong association between senescence mediators and tumor suppression, both in simple cell culture assays as well as in mouse cancer models.2
Senescence is irreversible in the sense that discontinuance of the inducing stimulus does not revert cells to their original proliferative state. This is a critical functional distinction with quiescence, which is a stable but reversible arrest. For many years, an open question in the field has been to understand why some stress stimuli trigger senescence and others quiescence. Understanding the mechanistic difference between these two types of arrest could have implications for designing cancer therapies. A treatment that induces senescence is expected to be more efficient than one that induces quiescence. This is so not only because of the stability and, for the most part, irreversibility of the senescence arrest, but also because senescent cells can be eliminated by phagocytosis, resulting in tumor regression.4
The Key to Unlock Senescence is mTOR
Previous work by Demidenko and Blagosklonny had demonstrated that the hypertrophic cytoplasm characteristic of senescent cells in culture was a consequence of mTOR activity in a context of cell cycle blockade, or, in other words, to the uncoupling between cell growth and cell division.5 Thus, for example, in the presence of serum (i.e., active mTOR), transient overexpression of the cell cycle inhibitor p21 resulted in permanent, irreversible senescence, whereas transient overexpression of p21 in the absence of serum or in the presence of rapamycin (i.e., inactive mTOR) produced a reversible arrest or quiescence.5
This paradigm has received important support from the analyses of mouse models where in vivo senescence is abrogated or attenuated by rapamycin. This is the case in mice with persistent expression of Wnt1 in the epidermal stem cells of hair follicles.6 Sustained Wnt1/β-catenin activity resulted in rapid hair growth followed by stem cell exhaustion, hair follicle senescence and, finally, hair loss. Interestingly, this in vivo senescence response to aberrant oncogenic signaling was accompanied by strong mTOR activity and, importantly, senescence and hair loss were prevented by treatment with rapamycin.6 Conceptually similar results have been also reported in pre-malignant prostate tumors (prostate intraepithelial neoplasias or PIN) induced by Pten loss.7 These PINs are characterized by a strong mTOR activity and a prevalent senescent response. Remarkably, treatment of these mice with rapamycin abrogated senescence but did not reactivate proliferation, which is consistent with a shift from a senescent arrest to a quiescent arrest.
Based on the above in vitro and in vivo evidence, it can be concluded that mTOR activity is a necessary condition for the establishment of senescence (Fig. 1). Oncogenic signaling is one of the most efficient and widely reported means for inducing senescence.2,8 In this regard, it is worth noting that oncogenic signaling generally impinges both on p53 (to activate an anti-proliferative defense) and, importantly, also on mTOR (as part of the oncogenic program). This dual activation of p53 and mTOR may explain the high efficiency of oncogenic signaling in inducing senescence.3
The p53 Dial
It has been widely assumed that a modest, or partial p53 activation results in quiescence, while a strong, full p53 response produces senescence. This assumption, however, has been turned upside down by a recent report from Blagosklonny’s laboratory.9 In particular, they observed that in a given cancer cell line, overexpression of the p53 transcriptional target p21 (i.e., analogous to a partial p53 response) resulted in senescence, while simultaneous overexpression of p53 or treatment with the p53-activating drug nutlin (i.e., full p53 response) resulted in quiescence. Based on their previous work on the pro-senescence activity of mTOR,5 they went on to uncover the fact that full p53 activation results in mTOR inhibition.9 In fact, previous investigators had reported that p53 is able to inhibit mTOR through several p53 transcriptional targets, including PTEN,10,11 AMPK subunits,11 TSC211 and sestrins,12,13 all of which are upstream negative regulators of mTOR. Therefore, similar to serum deprivation or rapamycin (see above), a potent activation of p53 produces mTOR inhibition, thus preventing the hypertrophic phenotype and favoring quiescence over senescence (Fig. 1).
In the current report,1 Blagosklonny and co-workers go one step ahead and demonstrate that the same p53-activating treatment can induce senescence or quiescence depending on the strength of the p53 response. Thus, moderate doses of the DNA damaging agent doxorubicin or the p53-stabilizing compound nutlin induce a moderate p53 response that does not inhibit mTOR and, therefore, results in senescence (Fig. 1). In contrast, high doses of the above-mentioned compounds produce a strong p53 response, which inhibits mTOR and results in quiescence (Fig. 1). The fact that p53 may produce senescence or quiescence depending on the strength of its response may reconcile some apparently conflicting observations in the literature.
Implications
Importantly, the present publication1 may have implications for chemotherapy. Specifically, the standard use of maximally tolerated doses of chemotherapy may need to be reconsidered because the strongest possible activation of p53 may not necessarily be the most therapeutically effective strategy. In the particular case of apoptosis-resistant cancers, the goal should be to induce senescence. For this, the impact of chemotherapy on the intratumoral mTOR activity (e.g. by immunohistochemistry) should be monitored. If maximally tolerated doses inhibit mTOR, then, even if counterintuitive, administering sub-maximal doses that do not inhibit mTOR is a scientifically sound approach worth testing, given its simplicity and its potential benefits for patients.
References
- 1.Leontieva OV, et al. Cell Cycle. 2010;9:4323–7. doi: 10.4161/cc.9.21.13584. [DOI] [PubMed] [Google Scholar]
- 2.Collado M, et al. Cell. 2007;130:223–33. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Collado M, et al. Nat Rev Cancer. 2010;10:51–7. doi: 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xue W, et al. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demidenko ZN, et al. Cell Cycle. 2008;7:3355–61. doi: 10.4161/cc.7.21.6919. [DOI] [PubMed] [Google Scholar]
- 6.Castilho RM, et al. Cell Stem Cell. 2009;5:279–89. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alimonti A, et al. J Clin Invest. 2010;120:681–93. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serrano M, et al. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 9.Demidenko ZN, et al. Proc Natl Acad Sci USA. 2010;107:9660–4. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stambolic V, et al. Mol Cell. 2001;8:317–25. doi: 10.1016/s1097-2765(01)00323-9. [DOI] [PubMed] [Google Scholar]
- 11.Feng Z, et al. Cancer Res. 2007;67:3043–53. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 12.Budanov AV, et al. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- 13.Budanov AV, et al. Cell. 2008;134:451–60. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]