Abstract
Inhibitory optogenetics was used to examine the roles of the prelimbic cortex (PL), the nucleus accumbens core (NAcore) and the PL projections to the NAcore in the reinstatement of cocaine seeking. Rats were microinjected into the PL or NAcore with an adeno-associated virus containing halorhodopsin or archaerhodopsin. After 12 days of cocaine self-administration, followed by extinction training, animals underwent reinstatement testing along with the presence/absence of optically induced inhibition via laser light. Bilateral optical inhibition of the PL, NAcore or the PL fibers in the NAcore inhibited the reinstatement of cocaine seeking.
Keywords: ArchT, eNpHR3.0, halorhodopsin, prelimbic cortex, nucleus accumbens core, reinstatement
INTRODUCTION
The present study applied inhibitory optogenetics to the circuitry underlying relapse to cocaine seeking and tested hypotheses regarding obligatory involvement of the projection from the prelimbic cortex (PL) to the core subcompartment of the nucleus accumbens core (NAcore) (Kalivas, Volkow & Seamans 2005). Pharmacological inactivation of either the PL or the NAcore prevents reinstated cocaine seeking (Kalivas et al. 2005). While this work implies a relationship between the PL and NAcore, pharmacological inactivation cannot isolate a role for axonal projections between nuclei.
Optogenetics allows functional analysis of connections between structures by optically controlling axon terminal fields rather than pharmacologically or electrophysiologically regulating nuclear cell groups to infer connections (Stuber et al. 2011). We employed in vivo optogenetic inhibition with either the chloride pump halorhodopsin (eNpHR3.0) or the outward proton pump archaerhodopsin (ArchT) to examine the PL-to-NAcore circuit in reinstated cocaine seeking. Both opsins have been previously shown to inhibit neural activity (Chow et al. 2010; Gradinaru et al. 2010), and, therefore, we explored the use of each in the inhibition of cocaine seeking. It was hypothesized that optogenetic inhibition of the PL, NAcore and the projection between the two nuclei inhibits reinstated cocaine seeking.
MATERIALS AND METHODS (SEE APPENDIX S1 FOR DETAILS)
All methods used were in compliance with National Institutes of Health guidelines for care of laboratory animals and were approved by the Medical University of South Carolina or the University of Iowa Internal Animal Care and Use Committee. Male Sprague-Dawley rats were single, housed with a 12-hour reverse light/dark cycle. Animals were anesthetized and virus (rAAV2-hSyn-eNpHR3.0-EYFP, rAAV5-hSyn-EYFP, rAAV2-CAG-ArchT-GFP or rAAV5-CMV-GFP in |1012 viral molecules in 0.7 μl, University of North Carolina Vector Core) was stereotaxically delivered into the PL or NAcore. Twelve daily self-administration sessions lasted 2 hours or until the rats had taken a maximum of 200 infusions. Cocaine was self-administered using a Fixed Ratio 1 schedule with a 20-second timeout. Active lever presses produced a 0.05-ml infusion of 200 μg of cocaine and a 5-second tone/light cue. Rats underwent 10 d of extinction and then underwent either a cocaine- (10 mg/kg, ip) or a cocaine + cue-induced reinstatement where lever presses had no consequences. Rats underwent two reinstatement sessions for one or both types of reinstatement, counterbalanced with respect to whether illumination was provided. Illumination (10 mW of 561 nm light) was provided for the entire duration of the 2-hour reinstatement session. After reinstatement testing, animals underwent open-field testing followed by a cocaine injection (15 mg/kg, ip). Fiber optics were inserted, and either sham or laser illumination provided during the measurement of locomotor behavior (Deisseroth 2012).
Whole cell patch recordings were made in the PL or NAcore in rats previously microinjected with virus into each area. Pyramidal neurons or medium spiny neurons, respectively, were recorded (Moran et al. 2005). Extracellular field potentials were recorded in urethane anesthetized rats (Moussawi et al. 2009). Rats’ PL was microinjected with rAAV2-hSyn-eNpHR3.0-EYFP, concentric bipolar stimulating electrodes were placed in the PL, and glass recording electrodes were aimed at the NAcore.
Rats were transcardially perfused with 4% paraformaldehyde. Coronal sections were evaluated for yellow fluorescent protein (YFP) either by immunocytochemistry or using a helium/neon 543 nm laser line with a YFP excitation filter. Because a Kolmogorov–Smirnov test revealed that some of the reinstatement data were not normally distributed, a Friedman repeated measures nonparametric test was employed followed by a Dunn’s multiple comparison. The locomotor data were evaluated using a two-way analysis of variance with repeated measures over time, followed by a Bonferroni multiple comparisons test.
RESULTS
Validation of opsin location and function
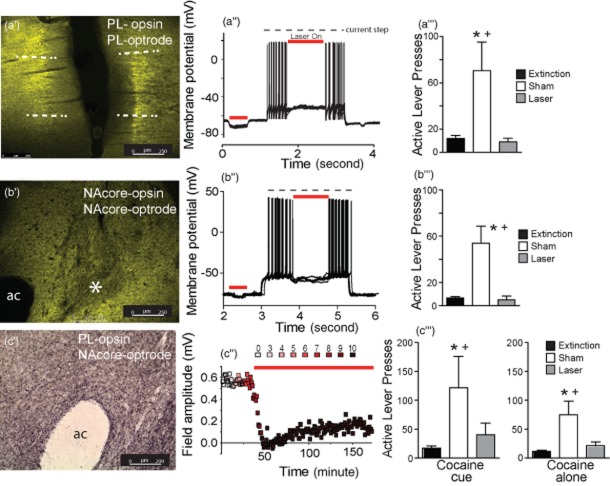
Figure 1 shows representative anatomical evidence for eNpHR3.0 expression in the PL (panel A’), eNpHR3.0 expression in NAcore neurons (panel B’) and ArchT expression in PL fibers terminating in the NAcore (panel C’). Whole cell patch clamp of a pyramidal neuron in the PL shows that optically inhibiting the infected region hyperpolarizes neurons and decreases action potential generation (Fig. 1a’’). Similarly, optically inhibiting NAcore medium spiny neurons reduced membrane potential and inhibited neuron firing (Fig. 1b’’). Figure 1c shows the results of optically inhibiting field potential amplitude in the NAcore that was elicited by electrically stimulating the PL. eNpHR3.0 was administered in the PL, and an optical fiber and recording electrode were inserted into the NAcore. Increasing laser intensity produced a progressive reduction in field amplitude. The capacity of activating eNpHR3.0 to reduce field potentials endured for at least 2 hours.
Figure 1.
Histological and electrophysiological verification of the opsin expression and inhibition of reinstated cocaine seeking. (a’) eNpHR3.0-YFP expression (green) after virus injection into the prelimbic cortex (PL). Dashed lines indicate approximate boundaries of the PL. (a’’) Laser-mediated hyperpolarization and inhibition of firing in a single eNpHR3.0-infected pyramidal cell. (a’’’) Laser light in the PL suppressed cocaine + cue-induced reinstated behavior. (b’) eNpHR3.0-YFP expression in the nucleus accumbens core (NAcore) after virus injection into the NAcore. ac, anterior commissure. *Area of relatively low fluorescence corresponding to the location of the fiber optic. (b’’) Laser-mediated hyperpolarization and inhibition of firing of a single eNpHR3.0-infected medium spiny cell. (b’’’) Laser light into the NAcore suppressed reinstated behavior. (c’) Immunostaining of archaerhodopsin expression in axonal fibers the NAcore after virus injection into the PL. (c’’) Optical inhibition of field potentials recorded in NAcore after electrical stimulation in the eNpHR3.0-infected PL of a rat. Units of laser intensity are arbitrary and correspond to step increases until the field potential disappeared and remained at this intensity for the duration of the experiment. (c’’’) Laser illumination in the NAcore suppressed both cocaine- and cocaine + cue-induced reinstatement.
Optical inhibition of PL and NAcore reduces reinstated cocaine seeking
Figure 1a’’’ shows that optical inhibition of eNpHR3.0-expressing PL reduced responding during cocaine-primed reinstatement (Friedman statistic = 11.19, P < 0.001, n = 7). Similarly, inhibition of the NAcore after intra-NAcore administration of either ArchT (n = 6) or eNpHR3.0 (n = 6) diminished responding during cocaine + cue-primed reinstatement sessions (Fig. 1b’’’). A Kruskal–Wallis test revealed no significant difference in responding between the ArchT or eNpHR3.0 animals, and thus, the data from these groups were pooled for analysis (Friedman statistic = 18.17, P < 0.001, n = 12). Figure 1c’’’ shows that optical inhibition of ArchT-expressing PL fibers in the NAcore reduced cocaine seeking induced by either a cocaine-priming injection (10 mg/kg; Friedman statistic = 9.80, P = 0.006, n = 10) or a combination cocaine-priming + cues (Friedman statistic = 9.90, P = 0.003, n = 10). In no experiment was inactive lever pressing affected by laser stimulation of fibers in the NAcore (data not shown).
Control virus experiments
Figure S1 shows that laser stimulation of cells in the NAcore infected with control virus or fibers in the NAcore following PL infection had no effect on cocaine alone or cocaine + cue-reinstated cocaine seeking, indicating that heating from the continuous laser light did not affect cocaine-seeking behavior. Figure S2 shows that optical inhibition of the NAcore, as in Fig. 1B, did not affect open-field behavior or cocaine-induced locomotor activity, suggesting the lack of these manipulations on nonspecific motor behavior. Inactive lever presses were not affected by laser stimulation in either the PL or NAcore groups (data not shown).
Histology
Figure S3 shows the location of optical fiber placement in each experiment.
Discussion
The present study is the first to use optogenetic inhibition to examine the neural circuitry underlying reinstated drug seeking and demonstrates that optogenetically inhibiting neurons in the PL or NAcore, or PL axons terminating in the NAcore prevents the reinstatement of cocaine seeking. This finding validates previous claims using pharmacological inhibition of the NAcore and PL that this projection is critical to reinstated cocaine seeking (see Introduction) and demonstrates the utility of optogenetic inhibition for characterizing the neural circuitry underlying cocaine seeking in greater detail than was previously possible using pharmacological inhibition or electrical stimulation.
Acknowledgments
This work was supported by grants DA027055 (RTL), DA015369 (PWK), DA003906 (PWK) and T32 DA7288 from the National Institutes of Health.
Conflict of Interest
None.
Authors Contribution
MTS, PWK and RTL were responsible for the study concept and design. KM and YMK conducted the electrophysiology studies. MTS, KCS, RLM, MLH and RTL conducted the behavioral studies and histology. KD provided the construct for the virus. MTS, PWK and RTL wrote the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Control virus-infected rats show no effect in response to laser stimulation
Figure S2 Locomotor activity in a novel open-field or following a cocaine injection (15 mg/kg, ip) was not altered by optical activation of archaerhodopsin (ArchT) in the nucleus accumbens core (NAcore) of animals previously microinjected with adeno-associated virus-ArchT in the NAcore.
Figure S3 Location of optical fibers in the prelimbic cortex (PL) or nucleus accumbens core (NAcore).
Appendix S1 Supplemental methods.
References
- Chow BY, Han X, Dobry AS, Qian X, Chuong AS, Li M, Henninger MA, Belfort GM, Lin Y, Monahan PE, Boyden ES. High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature. 2010;463:98–102. doi: 10.1038/nature08652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisseroth K. 2012. Predicted irradiance values: model based on direct measurements in mammalian brain tissue. Available at: http://www.stanford.edu/group/dlab/cgi-bin/graph/chart.php. Accessed 2 January 2012.
- Gradinaru V, Zhang F, Ramakrishnan C, Mattis J, Prakash R, Diester I, Goshen I, Thompson KR, Deisseroth K. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141:154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi K, Pacchioni A, Moran M, Olive MF, Gass JT, Lavin A, Kalivas PW. N-Acetylcysteine reverses cocaine-induced metaplasticity. Nat Neurosci. 2009;12:182–189. doi: 10.1038/nn.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A. Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature. 2011;475:377–380. doi: 10.1038/nature10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Control virus-infected rats show no effect in response to laser stimulation
Figure S2 Locomotor activity in a novel open-field or following a cocaine injection (15 mg/kg, ip) was not altered by optical activation of archaerhodopsin (ArchT) in the nucleus accumbens core (NAcore) of animals previously microinjected with adeno-associated virus-ArchT in the NAcore.
Figure S3 Location of optical fibers in the prelimbic cortex (PL) or nucleus accumbens core (NAcore).
Appendix S1 Supplemental methods.