Summary
Objective
Cryoablation is commonly used at present in the surgical treatment of atrial fibrillation. However, there have been few studies examining the efficacy of the commonly used ablation devices. This report compares the efficacy of two cryoprobes in creating transmural endocardial lesions on the beating heart in a chronic porcine model.
Methods
In 6 Hanford-miniature-swine, the right atrial appendage and the inferior vena cava were isolated using a bipolar radiofrequency-clamp to create areas of known conduction block. A connecting ablation line was performed endocardially via a purse-string with the novel malleable 10cm Cryo1-probe for 2 minutes at −40°C. Additional ablation lines were created with the Cryo1 and the 3.5cm 3011 Maze-Linear probe on the right and left atrial wall. Epicardial activation mapping was performed prior to and immediately after ablation as well as 14 days postoperatively. Histology was performed 14 days postoperatively.
Results
Transmural lesions were confirmed in 83/84 cross-sections (99%) for the Cryo1-probe and in 40/41 cross-sections (98%) for the 3011 Maze-Linear probe. There was no difference between the devices in lesion width (Cryo1: 10.7±3.5mm; 3011: 10.0±3.9mm; p=0.31), lesion depth (Cryo1: 4.5±1.7mm; 3011: 4.6±1.5mm; p=0.74) or atrial wall thickness (Cryo1: 4.5±1.8mm; 3011: 4.7±1.7mm; p=0.74). There was a conduction delay across the right atrial ablation line (20±2ms vs. 51±8ms, p<0.001) that remained unchanged at 14 days (51±8ms vs. 52±10ms, p=0.88).
Conclusions
The Cryo1-probe created transmural lesions on the beating heart resulting in sustained conduction delay. Both probes had similar performance in terms of lesion geometry in this chronic animal model.
Keywords: Ablative therapy, Arrhythmia therapy, Atrial fibrillation
Introduction
Atrial fibrillation (AF) is the most common sustained arrhythmia worldwide. The first successful nonpharmacological treatment for this arrhythmia was introduced at our institution in 1987 by Dr. James Cox. The final version of his cut and sew technique, termed the Cox-maze procedure III, has been proven to be highly efficacious.1-3 However, this procedure was technically demanding and associated with a considerable morbidity, and was not widely adopted by cardiac surgeons. The development of alternative energy sources has simplified and shortened the procedure by replacing most of the incisions of the Cox-maze procedure III with linear lines of ablation.4-7 These alternative energy sources included radiofrequency, focused ultrasound, microwave, laser and cryothermy.
Cryoablation destroys cardiac tissue by the formation of intra- and extracellular ice crystals, which disrupt cell membranes and organelles. With the development of specially designed cryoprobes, discrete lines of ablation could be achieved with homogenous scar formation8, 9. This made this energy source potentially useful for the treatment of cardiac arrhythmia. As opposed to unipolar radiofrequency energy that resulted in complications such as pulmonary vein stenosis and esophageal perforation10, cryoenergy has an excellent safety profile.11 It is the only available energy source that does not disrupt tissue collagen, thus preserving normal tissue architecture and allowing a safe ablation close to valvular tissue or the fibrous skeleton of the heart with a low arrhythmogenic potential.12-14 To be effective for the treatment of AF, an energy source must reliably create transmural lesions leading to conduction block. Studies investigating the efficacy of endocardial cryoablation in producing reliable transmural lesions on the beating heart and their impact on atrial activation have been limited and many of the commonly employed devices have not been investigated.
The purpose of this chronic animal study was to examine the ablation performance of two cryo probes on the beating heart: a newly introduced malleable cryoprobe and a rigid linear probe, both of which are clinically available for the surgical ablation of atrial fibrillation.
Methods
Experimental Protocol
Six Hanford miniature swine weighing 50–70kg were used in this study. The protocol was in compliance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington, DC, 1996) and approved by the Washington University School of Medicine Animal Studies Committee.
Two different cryoprobes were used in this study. Both devices were cooled using nitrous oxide delivered through a controlling console (AtriCure, Inc., Cincinnati, OH USA). The newly developed Cryo1 probe is a disposable malleable aluminum device with a 10cm probe length. The nitrous oxide gas is evenly dispersed to avoid temperature variations along the shaft and the use of aluminum provides a high thermal conductance. The rigid 3011 Maze Linear probe is reusable with a probe length of 3.5cm consisting of gold plated copper that is already widely in use for the surgical ablation of arrhythmias.
Each animal underwent biatrial ablation on the beating heart. Following overnight fasting, the animals were premedicated intramuscularly with Ketamine (15mg/kg) and anesthetized with endotracheally applied isoflurane (2-4%) after intubation. Preoperative intramuscular antibiotic treatment with Ceftiofur (2mg/kg) was given to all animals. Continuous monitoring throughout the procedure consisted of ECG, invasive arterial blood pressure from the left internal thoracic artery and routine blood gas analyses. Animals were heparinized intravenously (initial bolus 250U/kg, additional boluses as needed) to reach and maintain an activated clotting time of more than 350 seconds.
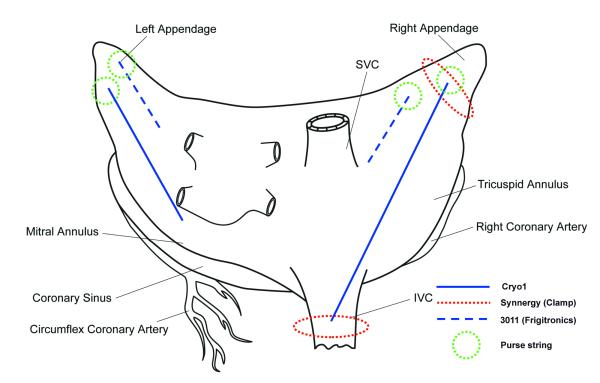
After median sternotomy, the lesion set diagrammed in Figure 1 was applied to all animals. After measuring pacing thresholds with a pen, a bipolar radiofrequency clamp (Isolator SynergyTM, AtriCure, Inc., Cincinnati, OH USA) was used to circumferentially isolate the right atrial appendage and the inferior vena cava. Electrical isolation was documented by pacing with stimulus strength of twice the previous measured threshold to confirm exit block. Epicardial unipolar electrograms from the right atrium were recorded in sinus rhythm and during pacing at 120bpm. These two lesions, representing areas of known conduction block, were connected by creating an endocardial line of ablation using the malleable cryoprobe Cryo1. Epicardial mapping was then performed to determine if conduction block was present along the length of the lesion. The probe was inserted into the right atrium via a purse-string suture placed at the right atrial appendage. The ablation was performed for 2 minutes at −60°C, which was confirmed by thermocouples placed along the surface of the device. Epicardial activation mapping of the right atrium was repeated during sinus rhythm and pacing at 120bpm. Through a separate purse string suture, a parallel endocardial line of ablation on the free right atrial wall was performed for 2 minutes at −40°C using the 3011 Maze Linear probe. An additional lesion was performed with each cryoprobe on the left atrial free wall through two separate purse string sutures for 2 minutes at −40°C. All lesions were created by a single application of the assigned device. The ending of each ablation line was marked with Prolene sutures.
Figure 1.
Lesion set on the atria.
After recovery from the procedure all animals were allowed to survive 14 days. Two doses of Ceftiofur (2mg/kg) were given intramuscularly within 48 hours postoperatively. Starting on the day of operation, each animal received a daily oral dose of 325mg of aspirin. No antiarrhythmic drugs were administered during postoperative care.
On postoperative day 14, all animals were anaesthetized, intubated and underwent redo sternotomy. Simultaneous recording of epicardial voltage at the right atrium was repeated during sinus rhythm and while pacing with bipolar pacing at 120bpm.
The animals were then euthanized using an infusion of 3M potassium chloride. The aorta was cross-clamped, the coronary arteries were perfused with a solution of 2,3,5-triphenyl-tetrazolium chloride (1%) and the heart was then removed en bloc for inspection and histologic evaluation.
Epicardial Conduction Mapping
Custom-made silicon patches with 128 embedded unipolar electrodes were used to simultaneously record epicardial electrograms. Electrodes were separated by 5mm. The patch was secured to the epicardium of the right atrium using Prolene suture. Recordings were made for at least 10 seconds during normal sinus rhythm and during atrial pacing at 120bpm from the medial side of the lesion. Data were recorded at a frequency response or 0.5-500Hz and digitized at 1000Hz.
The epicardial electrograms were acquired and analyzed by two programs developed in our laboratory (DASH and Glas, respectively; Washington University, St. Louis, MO USA). Time of epicardial activation was selected by identifying the peak negative derivative of voltage over time. Automatically selected activation times were manually reviewed and edited for accuracy. Activation maps and activation movies were then generated and displayed on a three-dimensional anatomical porcine model constructed by software developed in our laboratory (Getpic3 and Map3; Washington University, St. Louis, MO USA) based on porcine CT scans.
Microscopic and Histologic Analysis
Following resection, the heart was thoroughly inspected for intraatrial thrombus formation and then incubated in 2,3,5-triphenyl-tetrazolium chloride (1%) at room temperature for 45 minutes. Each marked lesion was sectioned at 5mm intervals perpendicular to the longitudinal axis of each ablation line. The cross sections were examined under the microscope and each slide was scanned with a high-resolution scanner and evaluated for transmurality, lesion depth and lesion width using Adobe Photoshop (Version 7, Adobe Systems, Inc., San Jose, CA USA).
All cross sections were fixed in formalin, molded in paraffin, and H&E and Trichrome stained. A histological examination of the specimen was performed by a veterinary pathologist.
Data analysis
All continuous data were expressed as mean ± standard deviation, unless otherwise specified. Categorical data were expressed as absolute numbers and proportions. Comparisons were made with the unpaired t-test. All data analyses were performed with the SYSTAT system for statistics (SYSTAT version 13, SYSTAT, Inc., Chicago, IL USA).
Results
Operative Results
All animals survived the surgical ablation procedure and the 14-day postoperative period. There was no intraoperative or postoperative cardiac arrhythmia. All animals remained in sinus rhythm until they were sacrificed. There were no signs of neurological dysfunction in the postoperative setting in any animal.
No thrombus formation was found at any location in the right or left atria when macroscopically inspected. The ablation lines of both devices appeared equally discrete, linear and pale and could be visualized endocardially and epicardially.
Epicardial Conduction Mapping
Electrical isolation was documented for the circumferential lesions around the right atrial appendage and around the inferior vena cava and confirmed by exit block with stimulus strength of twice the previous measured pacing threshold in all animals. Thus, two areas of known conduction block were established by the bipolar radiofrequency clamp.
Activation maps of the right atrium generated from surface electrogram recordings identified a significant conduction delay from 20±2ms before creation of the ablation line to 51±8ms afterwards (p<0.001) between two defined points on either side of the ablation line created by the Cryo1 probe. All six animals were found to have complete electrical block across the Cryo1 lesion. Fourteen days postoperatively, this conduction block remained unchanged in all animals and the right atrial conduction delay was similar (52±10ms) to the acute post-ablation activation map (p=0.88, Figure 2).
Figure 2.
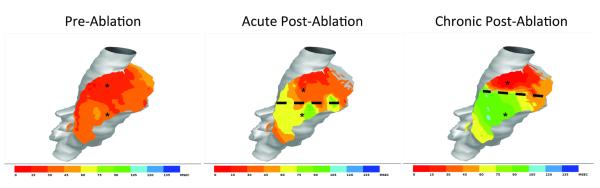
Epicardial conduction maps of the right atrium during pre-ablation, acute post-ablation and chronic post-ablation period (14 days postoperative). * denotes points of measurement. Dashed line approximates the ablation line (Cryo1).
Microscopic and Histological Examination
After the biatrial lesions created by the 3011 Maze Linear probe were sectioned at 5mm intervals, 41 cross-sections were inspected microscopically. A well-marginated transmural lesion was confirmed in all but one cross section (97.6%; Figure 3). The only section that failed transmurality was located at the very end of the left atrial ablation line (Figure 5).
Figure 3.
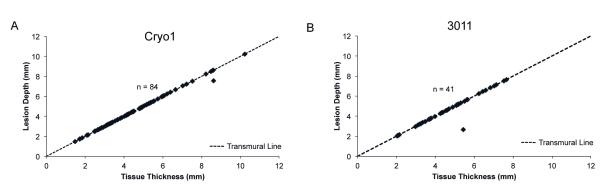
Plots determining transmurality of lesions with the cryoablation devices. A: Cryo1. B: 3011 Maze Linear.
Figure 5.
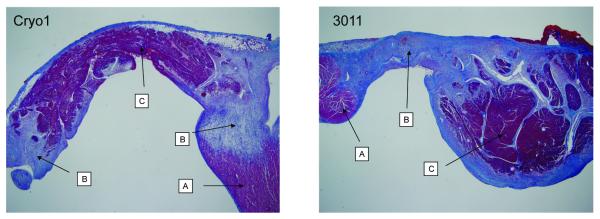
Trichrome stain of the right atrium (Cryo1) and left atrium (3011 Maze Linear). A: Normal tissue. B: Ablation line with fibrous tissue. C: Necrotic tissue in the ablation site. 2x original magnification.
The biatrial lesions created by the Cryo1 probe were sectioned at 5mm intervals as well. Microscopic examination of 84 cross sections also revealed transmurality in all but one specimen (98.8%; Figure 3). Again, the only cross section that could not confirm transmurality was located at the end of the left atrial ablation line.
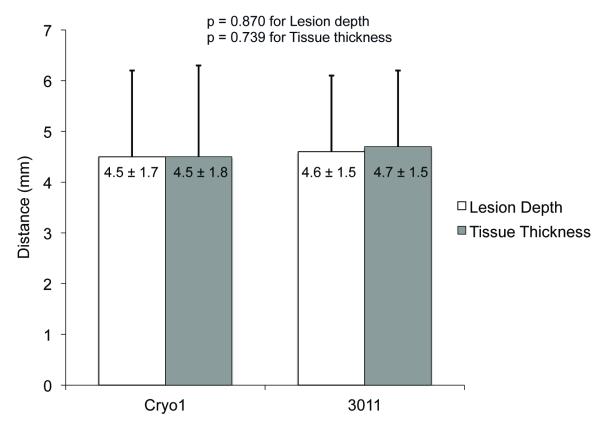
The atrial wall thickness encountered was not significantly different for the two devices (Cryo1: 4.5±1.8mm; 3011 Maze Linear: 4.7±1.5mm; p=0.74). There was also no significant difference in lesion depth (Cryo1: 4.5±1.7mm; 3011 Maze Linear: 4.6±1.5mm; p=0.74) or lesion width (Cryo1: 10.7±3.5mm; 3011 Maze Linear: 10.0±3.9mm; p=0.31) between the Cryo1-probe and the 3011 Maze Linear probe (Figure 4).
Figure 4.
Comparison of lesion depth and tissue thickness of two cryo probes.
The histological examination revealed no significant differences between the Cryo1 and 3011 Maze Linear probes. Both probes caused a distinct, well-marginated transmural ablation. The endothelial layer was intact in almost all cases for both probes. A significant observation for both probes was the development of necrotic cardiomyocytes in the center of the ablation site. These sites were characterized by occasional pyknotic cells and loss of the vessel endothelium and presumably loss of function of the vasculature. Mild lymphocyte infiltration was often present at the margins of these areas (Figure 5).
Discussion
The advent of ablation technology has resulted in the development of less invasive surgical approaches for AF by replacing surgical incisions with transmural lines of ablation.5-7, 15 Tissue injury and cell death are either caused by heat (i.e. laser, microwave or focused ultrasound) or by freezing when cryoenergy is applied. Cryogenic techniques have been reliably used in surgery for decades to treat cardiac arrhythmias. The mechanisms of cell injury during cryoablation are complex. Extracellular fluid freezes at −20°C, creating a hyperosmotic environment that causes cell shrinkage and ultimately cell death. Rapid freezing to −40°C causes expansion of intracellular ice formation that disrupts organelles and cell membranes before osmotic imbalance occurs16, and is the mode of ablation in both devices used in this study. Because of its unique method of cell destruction, cryoenergy has an excellent safety profile and is the only available energy source that does not disrupt tissue collagen. Thus, it can be applied safely in the vicinity of the heart valves. Although Doll and colleagues reported mild esophageal lesions when applying epicardial cryoenergy in a sheep model17, the risk of collateral damage of surrounding tissue is minimal as opposed to reports for unipolar radiofrequency.10
To be a treatment option, any alternative energy source must reliably create transmural lesions. The efficacy of cryoenergy depends mainly on the cooling capability of the device, on the thermal conductivity of the probe material, and on established surface contact. Of three earlier published animal studies investigating the effectiveness of cryoenergy on the beating heart, only one reported all samples to be transmural.18 While this study investigated endocardial isolation of the pulmonary veins, the remaining two studies used cryoenergy epicardially. While electrical isolation and transmurality could be documented for thin-walled structures such as the pulmonary veins and the vena cava, results for thicker tissue at the atrial wall were rather disappointing with only 25–84% of the lesions being transmural.17, 19 One reason for the failure rate of epicardial linear cryoablation on the beating heart might be the heat sink effect caused by the blood flow in the cardiac cavum. To overcome this effect, we choose an endocardial approach to mimic the endocardial application of cryoenergy that is routinely performed in concomitant cardiac surgery as well as stand-alone procedures. Due to the complexity and morbidity of surviving animals after cardiopulmonary bypass, this study was performed on the beating heart. This provided an additional thermal load to the cryoprobes, as the flowing blood pool impacts device performance. The goal of this study was to evaluate the ablation performance of a new malleable aluminum probe (Cryo1) using a nitrous oxide cooling system when used endocardially within the beating heart and to compare it to a cryo probe that has been in successful use for surgical cryoablation for many years (3011 Maze Linear).
In the present study, we were able to reliably create transmural lesions with both devices. The only two cross sections that could not confirm transmurality were located at the very end of an ablation line, emphasizing the need for overlapping connecting lesions to ensure a complete line of conduction block. At our institution, we mark the endpoints of an ablation line with methylene blue to ensure precise connection and overlapping when creating a lesion set. The ablation with the Cryo1 probe resulted in a significant increase in conduction delay as would be expected for a transmural lesion. Earlier investigations in our laboratory showed that an acute conduction delay was not an accurate indicator for the evaluation of chronic lesion integrity.20 In this study, both the acute conductance block and delay remained unchanged at 14 days, evidence of chronic line of block.
The thermal conduction coefficient is the predictor of the rate of heat loss through a unit thickness of material and is reciprocally proportional to the thermal resistance. Although the copper used as the medium in the 3011 Maze Linear probe has a 2-fold higher thermal conduction coefficient as the aluminum used in the Cryo1 probe, we could not find any significant differences in the performance or efficacy between the two probes. However, the length of the probe and its malleability makes the design of the Cryo1 probe potentially more useful for some surgical approaches.
There are limitations to this study and these results must be cautiously applied to the clinical application. There are significant differences between the human and porcine atrial anatomy. This study was performed on healthy pigs with normal atria that cannot be thoroughly compared to the diseased and thickened atria of patients with AF. However, the successful use of cryoablation in creating transmural lesions has been reported for clinical applications as well.21 A significant shortcoming for the clinical use is the endocardial application via purse string sutures. Although this would allow for a minimally invasive approach without the need for cardiopulmonary bypass and could be performed with little difficulties on the right atrium it would be dangerous and ill advised on the left atrium, as there would be a chance for both, bleeding and air embolism. The epicardial application of cryoenergy on the beating heart has already failed to demonstrate transmurality on thicker wall structures like the lateral and posterior atrial wall because of the heat sink effect.17 Thus, the creation of a full maze lesion set with epicardial cryoablation is not promising. The design of this study was rather to mimic an endocardial approach with cardiopulmonary bypass as it is used for concomitant as well as for standalone procedures.
Clinical Perspective.
“Evaluation of a novel cryoablation system: in-vivo testing in a chronic porcine model” (Schuessler)
This timely article from Drs. Schuessler’s and Damiano’s lab details the in vivo performance of a new, flexible, dispoable cryoprobe with a previously available, rigid, reusable probe. Using nitrous oxide as the refrigerant, the probes were used to form biatrial ablation lines in the beating heart porcine model. The results of the study provide histological and electrophysiologic data demonstrating both probes produce reliably uniform, transmural scars that block electrical conduction. The only failures of transmurality were the tissues ablated at the distal end of the probe, emphasizing the importance of overlapping the lesions. Of note, in order to avoid the complexity of cardiopulmonary bypass, the lesions were formed by applying the probes endocardially via pursestrings. Though this technique is well described in clinical applications, the authors do not recommend it, particularly for left atrial lesions, due to concerns of bleeding and air embolism. The authors astutely emphasize that epicardial applications of cryoprobes on beating hearts are unreliable due to the heat sink effect of the blood stream. Limitations of the study include the fact that the average lesion depth was only 4.5 mm, substantially less than the wall thickness often encountered in diseased human atria, particularly in the mitral isthmus and atrioventricular fat pad. This emphasizes our concern that 2 minute endocardial applications of the cryoprobes in this study may be inadequate for producing uniform transmural lesions in some clinical situations. With the knowledge that cryothermia is the only power source that does not destroy tissue collagen, this is an important contribution to our understanding of the effects of cryothermy in producing electrically silent lesions in atrial tissue.
Acknowledgment
We thank Diane Toeniskotter and Naomi Still for their technical assistance.
Supported in part by the National Heart, Lung and Blood Institute at the National Institutes of Health [grant numbers RO1 HL032257, RO1 HL085113, and T32 HL07776].
Footnotes
Disclosures: Richard B. Schuessler, MD, receives research support from AtriCure, Inc., Cincinnati, OH USA. Ralph J. Damiano, MD, is a consultant for AtriCure, Inc., Cincinnati, OH USA, and Medtronic, Inc., Minneapolis, MN USA, has received research grants and equipment from AtriCure, Inc. Timo Weimar MD, Anson M. Lee MD and Shuddhadeb Ray MD declare no conflict of interest.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cox JL, Schuessler RB, D’Agostino HJ, Jr., et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg. 1991;101:569–583. [PubMed] [Google Scholar]
- 2.Cox JL, Schuessler RB, Lappas DG, et al. An 8 1/2-year clinical experience with surgery for atrial fibrillation. Ann Surg. 1996;224:267–275. doi: 10.1097/00000658-199609000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad SM, Maniar HS, Camillo CJ, et al. The Cox maze III procedure for atrial fibrillation: long-term efficacy in patients undergoing lone versus concomitant procedures. J Thorac Cardiovasc Surg. 2003;126:1822–1828. doi: 10.1016/s0022-5223(03)01287-x. [DOI] [PubMed] [Google Scholar]
- 4.Hindricks G, Mohr FW, Autschbach R, et al. Antiarrhythmic surgery for treatment of atrial fibrillation--new concepts. Thorac Cardiovasc Surg. 1999;47(Suppl 3):365–369. doi: 10.1055/s-2007-1013201. [DOI] [PubMed] [Google Scholar]
- 5.Khargi K, Hutten BA, Lemke B, et al. Surgical treatment of atrial fibrillation; a systematic review. Eur J Cardiothorac Surg. 2005;27:258–265. doi: 10.1016/j.ejcts.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Williams MR, Garrido M, Oz MC, et al. Alternative energy sources for surgical atrial ablation. J Card Surg. 2004;19:201–206. doi: 10.1111/j.0886-0440.2004.04037.x. [DOI] [PubMed] [Google Scholar]
- 7.Cummings JE, Pacifico A, Drago JL, et al. Alternative energy sources for the ablation of arrhythmias. Pacing Clin Electrophysiol. 2005;28:434–443. doi: 10.1111/j.1540-8159.2005.09481.x. [DOI] [PubMed] [Google Scholar]
- 8.Holman WL, Ikeshita M, Douglas JM, Jr., et al. Cardiac cryosurgery: effects of myocardial temperature on cryolesion size. Surgery. 1983;93:268–272. [PubMed] [Google Scholar]
- 9.Lall SC, Damiano RJ., Jr. Surgical ablation devices for atrial fibrillation. J Interv Card Electrophysiol. 2007;20:73–82. doi: 10.1007/s10840-007-9186-x. [DOI] [PubMed] [Google Scholar]
- 10.Doll N, Borger MA, Fabricius A, et al. Esophageal perforation during left atrial radiofrequency ablation: Is the risk too high? J Thorac Cardiovasc Surg. 2003;125:836–842. doi: 10.1067/mtc.2003.165. [DOI] [PubMed] [Google Scholar]
- 11.Mack CA, Milla F, Ko W, et al. Surgical treatment of atrial fibrillation using argon-based cryoablation during concomitant cardiac procedures. Circulation. 2005;112:I1–6. doi: 10.1161/CIRCULATIONAHA.104.524363. [DOI] [PubMed] [Google Scholar]
- 12.Holman WL, Ikeshita M, Douglas JM, Jr., et al. Ventricular cryosurgery: short-term effects on intramural electrophysiology. Ann Thorac Surg. 1983;35:386–393. doi: 10.1016/s0003-4975(10)61589-5. [DOI] [PubMed] [Google Scholar]
- 13.Wetstein L, Mark R, Kaplan A, et al. Nonarrhythmogenicity of therapeutic cryothermic lesions of the myocardium. J Surg Res. 1985;39:543–554. doi: 10.1016/0022-4804(85)90123-4. [DOI] [PubMed] [Google Scholar]
- 14.Lustgarten DL, Keane D, Ruskin J. Cryothermal ablation: mechanism of tissue injury and current experience in the treatment of tachyarrhythmias. Prog Cardiovasc Dis. 1999;41:481–498. doi: 10.1016/s0033-0620(99)70024-1. [DOI] [PubMed] [Google Scholar]
- 15.Arcidi JM, Jr., Millar RC. Evolution of the maze III procedure: are modifications necessary? Thorac Cardiovasc Surg. 1999;47(Suppl 3):362–364. doi: 10.1055/s-2007-1013200. [DOI] [PubMed] [Google Scholar]
- 16.Gage AA, Baust J. Mechanisms of tissue injury in cryosurgery. Cryobiology. 1998;37:171–186. doi: 10.1006/cryo.1998.2115. [DOI] [PubMed] [Google Scholar]
- 17.Doll N, Kornherr P, Aupperle H, et al. Epicardial treatment of atrial fibrillation using cryoablation in an acute off-pump sheep model. Thorac Cardiovasc Surg. 2003;51:267–273. doi: 10.1055/s-2003-43086. [DOI] [PubMed] [Google Scholar]
- 18.Guiraudon GM, Jones DL, Skanes AC, et al. En bloc exclusion of the pulmonary vein region in the pig using off pump, beating, intra-cardiac surgery: a pilot study of minimally invasive surgery for atrial fibrillation. Ann Thorac Surg. 2005;80:1417–1423. doi: 10.1016/j.athoracsur.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 19.Milla F, Skubas N, Briggs WM, et al. Epicardial beating heart cryoablation using a novel argon-based cryoclamp and linear probe. J Thorac Cardiovasc Surg. 2006;131:403–411. doi: 10.1016/j.jtcvs.2005.10.048. [DOI] [PubMed] [Google Scholar]
- 20.Lee AM, Aziz A, Clark KL, Schuessler RB, Damiano RJ., Jr. Chronic performance of a novel radiofrequency ablation device on the beating heart: Limitations of conduction delay to assess transmurality. J Thorac Cardiovasc Surg. 2012;144:859–865. doi: 10.1016/j.jtcvs.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sueda T, Imai K, Ishii O, et al. Efficacy of pulmonary vein isolation for the elimination of chronic atrial fibrillation in cardiac valvular surgery. Ann Thorac Surg. 2001;71:1189–1193. doi: 10.1016/s0003-4975(00)02606-0. [DOI] [PubMed] [Google Scholar]