Abstract
Aminoacyl-tRNA synthetases classically regulate protein synthesis but some also engage in alternative signaling functions related to immune responses and angiogenesis. Threonyl-tRNA synthetase (TARS) is an autoantigen in the autoimmune disorder myositis, and borrelidin, a potent inhibitor of TARS, inhibits angiogenesis. We explored a mechanistic link between these findings by testing whether TARS directly affects angiogenesis through inflammatory mediators. When human vascular endothelial cells were exposed to tumor necrosis factor-α (TNF-α) or vascular endothelial growth factor (VEGF), TARS was secreted into the cell media. Furthermore, exogenous TARS stimulated endothelial cell migration and angiogenesis in both in vitro and in vivo assays. The borrelidin derivative BC194 reduced the angiogenic effect of both VEGF and TARS, but not a borrelidin-resistant TARS mutant. Our findings reveal a previously undiscovered function for TARS as an angiogenic, pro-migratory extracellular signaling molecule. TARS thus provides a potential target for detecting or interdicting disease-related inflammatory or angiogenic responses.
The canonical function of aminoacyl-tRNA synthetases (aaRSs), in organisms of all three kingdoms of life, is to attach amino acids to their cognate tRNAs to support protein synthesis. In addition to this universal function, many aaRSs in eukaryotes have distinct roles in inflammation, transcriptional regulation, and gene-specific translational silencing1,2,3,4. These secondary functions may account for the increasing number of reported links between aaRSs and diverse human diseases. For example, mutations in aaRSs are linked to the nervous system-related disorders Charcot-Marie-Tooth disease, leukoencephalopathy, infantile encephalopathy, and a Type III Usher-like syndrome5,6,7. The molecular mechanisms that connect aaRS activity with the pathogenesis of these diseases and other non-canonical activities are not straightforward, providing evidence that aaRSs are dynamic signaling molecules with functions that reach beyond the conventional dogma.
Several class I aaRSs have extracellular signaling functions that are stimulated in response to inflammatory cytokines, and they exhibit disparate roles in angiogenesis. Tyrosyl-tRNA synthetase (YARS) and tryptophanyl-tRNA synthetase (WARS) are secreted in response to the cytokines TNF-α and interferon, respectively. A secreted cleavage fragment of YARS is angiogenic, whereas a secreted splice variant of WARS is angiostatic2,8,9. Angiogenesis is known to be a complex phenomenon that involves release of growth factors such as vascular endothelial growth factor (VEGF) from hypoxic cells and local secretion of cytokines from inflammatory cells that direct endothelial cell migration and neovascularization10. Thus, it is possible that inflammatory signaling could regulate angiogenesis through aaRSs using separate pathways.
Threonyl-tRNA synthetase (TARS) is a class II aaRS that has not been previously associated with extracellular signaling, although there are clues that it is involved in angiogenesis and tumor cell growth. The macrolide antibiotic borrelidin, a potent non-competitive inhibitor of TARS, inhibits endothelial cell tube formation and reduces metastasis in a mouse model of melanoma11,12,13. The borrelidin derivative BC194 retains the anti-angiogenic properties of borrelidin but is less cytotoxic, providing a useful tool to explore the molecular basis of TARS angiogenic signaling14,15. In particular, the prospect that TARS has a direct role in promoting inflammatory responses and angiogenesis has not been investigated.
TARS has also been associated with autoimmune disorders including polymyositis and dermatomyositis through its identification as the target for the myositis autoantibody PL-716. The etiology of these diseases includes attack of auto-reactive T cells in muscle tissue through tumor necrosis factor-α (TNF-α) and nuclear factor κB (NFκB) signaling, and there is an epidemiological linkage between these diseases and several different cancers17,18. Despite decades of study, the mechanism by which a ubiquitious cellular protein like TARS becomes over-represented as an autoantigen in this disease and its relationship with tumorigenesis remains a puzzling research question.
These diverse observations raised the possibility that the ability of a TARS inhibitor to block angiogenesis and the recurrence of TARS as an autoantigen in myositis disorders are phenomena linked to an unrecognized secondary function of TARS. Here we report that TARS is secreted in response to inflammatory or angiogenic signaling, and that the anti-angiogenic effect of TARS inhibition is due to an extracellular TARS signaling mechanism that includes induction of endothelial cell migration.
Results
TARS is secreted from vascular endothelial cells in response to TNF-α or VEGF. One explanation for the autoantigenicity of TARS and the angiostatic potency of the TARS inhibitor BC194 is that some fraction of the total TARS intracellular pool is secreted into the extracellular space to carry out secondary functions. In previous screens for aaRSs that are secreted under basal conditions, TARS was not detected3,19. To explore the possibility that TARS is actively secreted by vascular endothelial cells in response to inflammatory or angiogenic signals, human umbilical vein endothelial cells (HUVECs) were incubated with TNF-α or VEGF followed by measurement of TARS in the media using ELISA and Western blot. As shown in Fig. 1a, both TNF-α and VEGF stimulated a significant increase in TARS in the media by ELISA after 6 h. As confirmed by a cytotoxicity assay, TARS present in the media was not a result of cell lysis (Fig. 1b). TARS could also be detected in concentrated conditioned media by Western blot after 16 h exposure to VEGF and TNF-α, and although TNF-α induced mild toxicity at this time point, there was no evidence of β-tubulin in the media (Fig. 1c). Neither VEGF nor TNF-α induced an increase in TARS mRNA, suggesting that the presence of TARS in the media was not due to an increase in TARS expression (Fig. 1d). Furthermore, adding purified recombinant TARS to the cell media did not induce secretion of VEGF as measured by ELISA, indicating that TARS is not upstream of VEGF in the angiogenic pathway (Fig. 1e). Together, these results support a pathway leading to TARS secretion that is stimulated by endothelial cell signaling through VEGF and/or TNF-α receptors.
Figure 1. TARS is secreted by endothelial cells in response to VEGF and TNF-α.
(a) HUVECs were treated with VEGF or TNF-α (50 ng/ml) where indicated. After 6 h the level of TARS in the supernatant was determined by ELISA. Graph represents mean ± s.e.m.; n = 3, *P < 0.05 (Student's t-test). (b) Cell membrane integrity for the experiments in (a and c) was confirmed using the lactate dehydrogenase assay CytoTox-ONETM. Numbers represent mean percent cytotoxicity ± s.e.m. relative to a lysis control, n = 3, *P < 0.05 (Student's t-test). (c) HUVECs grown on a 10 cm dish were exposed to 50 ng/ml of VEGF or TNF-α in 0% serum EGM-2 media for 16 h. Shown is a representative TARS Western blot of cell lysates and media samples, n = 4. Media was concentrated 20-fold to accommodate 25% onto the gel and compared to 5% of the cell lysate. Purified TARS (50 fmol) was used to gauge TARS concentration within samples (See Supplementary Fig. S1). β-tubulin was measured as a loading and lysis control. (d) VEGF and TNF-α do not induce TARS transcription. HUVECs were exposed to 50 ng/ml of VEGF or TNF-α followed by RNA extraction and RT-qPCR to measure TARS mRNA levels. Shown are mean Rq values ± s.e.m. relative to a β-2 microglobulin control; n = 3, n.s. (Student's t-test). (e) TARS does not induce VEGF secretion. HUVECs were exposed to the indicated concentrations of purified recombinant human TARS for 24 h and the level of VEGF in the supernatant determined by ELISA; mean ± s.e.m., n = 3, n.s. (Student's t-test).
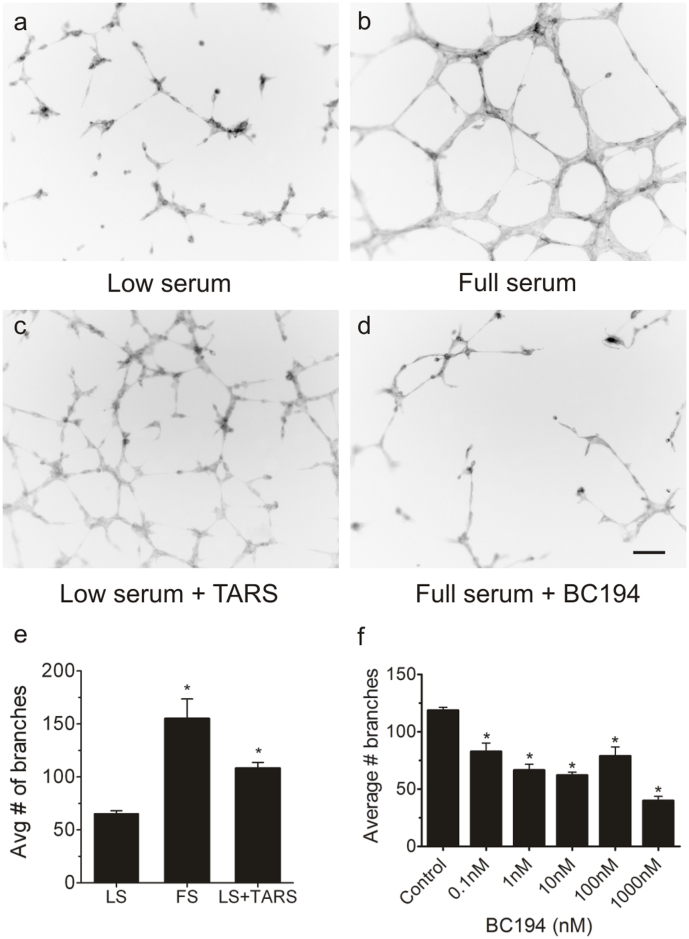
Exogenously added TARS stimulates angiogenesis. The finding that TARS is actively secreted led to the possibility that TARS has an extracellular angiogenic function that is BC194-sensitive. To test this hypothesis, human His-tagged wildtype TARS was expressed in E. coli and purified by a sequence of nickel-NTA affinity and hydroxyapatite chromatography (see Supplementary Fig. S1). Addition of purified TARS to low serum media in the endothelial cell tube assay led to a significant increase in the number of branches, a level comparable to that induced by serum (Fig. 2a–c,e). These data signified that TARS itself is angiogenic, and implicate an extracellular mechanism for BC194's angiostatic effect on endothelial cells. As shown in Fig. 2d,f, the number of serum-induced branches was significantly reduced by subnanomolar concentrations of BC194, confirming its potent angiostatic activity.
Figure 2. Exogenous TARS promotes angiogenesis in an in vitro endothelial tube formation assay, and tube formation is sensitive to subnanomolar concentrations of BC194.
HUVECs were plated onto MatrigelTM in Low serum (0.2% FBS) or EGM-2 Full serum media (2% FBS). Where indicated, 100 nM purified recombinant human TARS or 10 nM BC194 was added to the media. After 6 h, cells were fixed and stained with Oregon Green 488 Phalloidin. (a–d) are representative images; Scale bar = 100 μm. (e,f) show quantification of branches determined using the Simple Neurite Tracer plug-in on ImageJ software. Numbers represent average data from 3 separate experiments performed in duplicate. (e), Histogram of quantified branches for TARS effect; mean ± s.e.m., n = 3, *P < 0.01 compared to Low serum (LS) (Student's t-test). (f), Histogram of quantified branches for a range of BC194 concentrations added to Full serum media. Numbers represent mean ± s.e.m., n = 3, *P < 0.05 (one-way ANOVA, Tukey test).
To rule out the possibility that the angiostatic effect of BC194 was through cell toxicity induced by the unfolded protein response or apoptosis pathways, several different approaches were used to test the sensitivity of endothelial cells to BC194. Importantly, cell viability, proliferation, and nascent protein synthesis were not affected by BC194 at concentrations below 100 nM (Fig. 3a,b). Furthermore, the concentration of BC194 required to prevent branch formation was 100-fold lower than that required to detect markers for the unfolded protein response (phospho-eIF2α) and apoptosis (cleaved caspase-3) (Fig. 3c–f). These data suggest that BC194's effects on angiogenesis are not dependent on generalized toxicity and stress responses, and highlight a potential direct role for TARS in angiogenesis.
Figure 3. High concentrations of BC194 are required to stimulate the unfolded protein response and apoptosis in endothelial cells.
(a) Effects of BC194 on proliferation. HUVECs were exposed to the indicated concentrations of BC194 and relative proliferation was quantified over time using an alamarBlue® assay (a measure of mitochondrial oxidase activity); mean ± s.e.m., n = 3, *P < 0.05 (one-way ANOVA, Tukey test). (b) Effects of BC194 on nascent protein synthesis. HUVECs were exposed to the indicated concentrations of BC194 and new protein synthesis was detected using a Click-iT® metabolic labeling kit. Proteins were separated by SDS-PAGE and visualized using streptavidin-HRP. Cycloheximide (50 μM) was used as a control for complete inhibition of protein synthesis. (c–f) HUVECs grown in full serum media were exposed to the indicated concentrations of BC194, followed by Western Blot of cell extracts using antibodies recognizing phospho-eIF2α (c), cleaved caspase-3 (d) and β-actin or β-tubulin as a loading control. (e,f) Quantification of phospho-eIF2α and c-caspase-3 relative to the loading controls; mean ± s.e.m., n = 3, *P < 0.05 (one-way ANOVA, Tukey test).
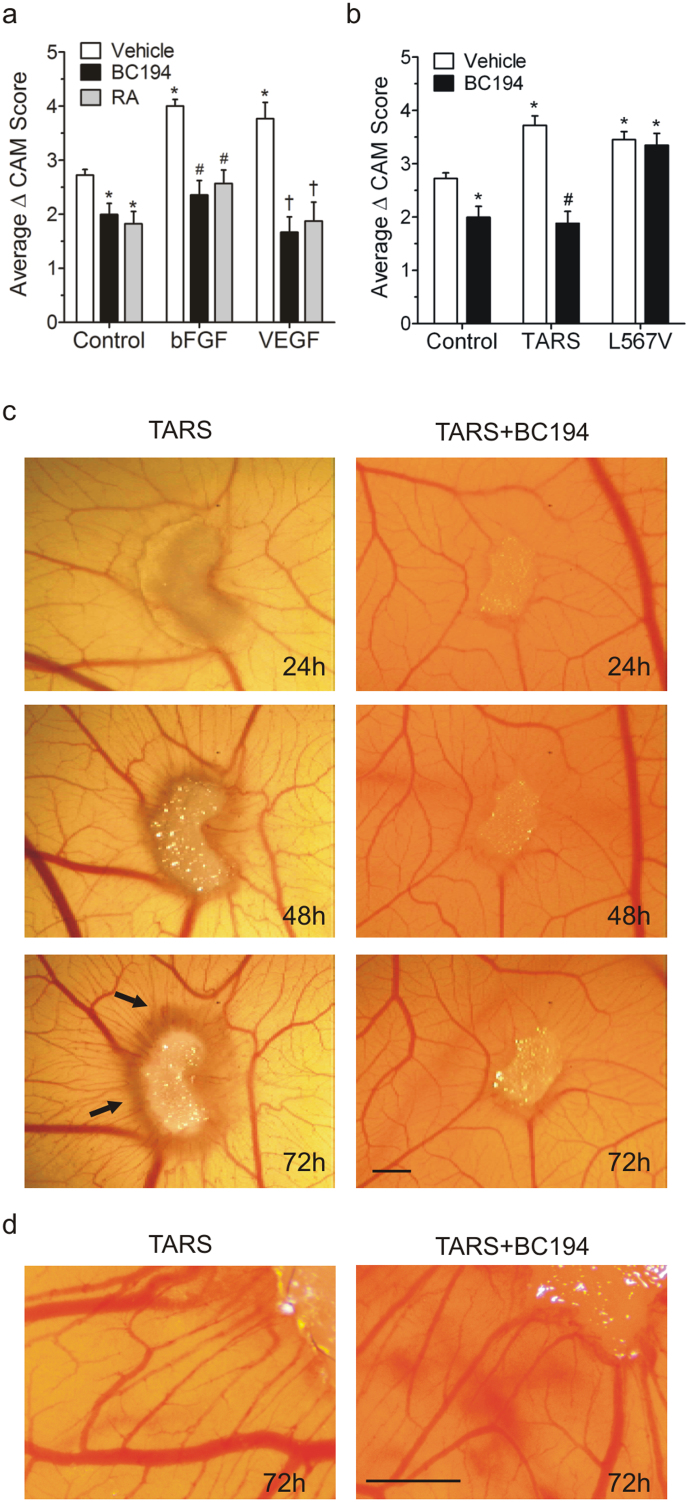
To confirm and expand these results, a chorioallantoic membrane (CAM) assay was used to examine a role for TARS in an in vivo angiogenesis environment. The daily application of BC194 to a gel sponge on the CAM over 72 h inhibited vessel formation at both the basal level and after stimulation by known angiogenic growth factors, basic fibroblast growth factor (bFGF) or VEGF (Fig. 4a, Fig. S2). Application of TARS to the CAM independently stimulated vessel formation, and the angiogenic effect exhibited sensitivity to co-administration with BC194 (Fig. 4b–d). Higher resolution images show both increased diameter of existing vessels and amplification of microvessels leading to the TARS source (Fig. 4d). These results indicate that the inhibition of angiogenesis by BC194 is not due to off-target effects, a conclusion further supported by the finding that the stimulation of vessel formation by a L567V TARS, a BC194-resistant mutant of TARS, was not inhibited by application of BC194 (Fig. 4b). Application of human leucyl-tRNA synthetase (LARS) to the CAM had no observable impact on vascularization, suggesting that the angiogenic effect observed is not a general property of all human aaRSs or an artifact of the protein preparation (Fig. S3). Collectively, these data support a specific role for extracellular TARS in the activation of the in vivo endothelial angiogenic response.
Figure 4. TARS affects in vivo angiogenesis in a CAM assay.
Fertilized chicken embryos were cultured ex-ova. At developmental day 10, agents were applied daily to gelfoam sponges on the CAM. Images were recorded daily and graphs represent the change in CAM vascularity score over 72 h. (a) BC194 (0.1 pmol) was applied to the CAM along with PBS (Control), bFGF (400 ng), and VEGF (20 ng). The angiostatic control retinoic acid (RA) was used at 1 μg; n ≥ 8, *,#,† P < 0.001 compared to Control, bFGF and VEGF respectively (one-way ANOVA, Tukey test). Representative images for (a) are shown in Fig. S2. For (b,c) 10 pmol of purified recombinant TARS, BC194-resistant mutant TARS (L567V) and/or BC194 were applied. (b) Histogram of change in CAM vascularity score over 72 h; mean ± s.e.m., n ≥ 15, *P < 0.001 compared to PBS Control; #p < 0.001 compared to TARS (one-way ANOVA, Tukey test). (c) Representative CAM images over time; arrows denote spoke-wheel response. Scale bar = 1.0 mm. (d) 72 h CAM images taken at higher magnification and resolution to show individual microvessels. Scale bar = 1.0 mm.
TARS induces endothelial cell migration. Potential mechanisms by which extracellular TARS might increase angiogenesis include an increase in cell proliferation or an increase in cell migration. In contrast to VEGF, TARS did not exert a significant effect on cell proliferation, and BC194 did not reduce the VEGF proliferative response (Fig. 5a). However, TARS significantly increased migration of HUVECs in a transwell assay to an extent that was similar to VEGF (Fig. 5b,c). LARS did not affect migration, indicating that the TARS-mediated effect was not a non-selective result of synthetase activity. Of note, BC194 reduced the migration effects of both VEGF and TARS, although the VEGF effect was less pronounced, suggesting that TARS may play a significant role in VEGF-mediated endothelial cell migration. These results provide evidence to support a mechanism for TARS angiogenic activity that includes stimulation of endothelial cell migration.
Figure 5. TARS selectively induces migration, but not proliferation of endothelial cells.
(a) HUVECs were cultured in low serum (0.2% FBS) media containing 50 ng/ml VEGF and 10 nM BC194 where indicated; relative cell proliferation was measured over time using an alamarBlue® assay; mean ± s.e.m., n = 3, *P < 0.05 compared to Control (Student's t-test). (b,c) TARS induces endothelial cell migration. HUVEC migration was measured using a transwell assay. The migration compartment contained 50 ng/ml VEGF, 100 nM LARS or 100 nM TARS and 10 nM BC194 where indicated (b) Shown are representative images of DAPI stained nuclei from migrated cells after 4 h. (c) Histogram representing number of migrated cells after 4 h for the conditions indicated; mean ± s.e.m., n ≥ 3, *P < 0.05 compared to Control, #P < 0.05 compared to VEGF (one-way ANOVA, Tukey test).
Discussion
In this study, we used several experimental approaches to demonstrate that TARS is actively secreted and exerts angiogenic activity including stimulation of endothelial cell migration. The data provide evidence that TARS secretion is within the sequence of molecular events associated with VEGF and TNF-α signaling and a testable model is shown in Fig. 6. The form of TARS that is secreted, conditions that stimulate secretion, putative receptors and responsiveness of other cell types to TARS remain to be identified. The results have several important implications including the finding of a novel extracellular function for TARS, confirming TARS as the anti-angiogenic target of the borrelidin derivative BC194, and potentially linking TARS secretion with angiogenic and inflammatory signaling in cancer and autoimmune diseases.
Figure 6. Proposed model for TARS angiogenic activity.
VEGF or TNF-α secretion by hypoxic or immune cells (a) leads to receptor-activated stimulation of TARS secretion (b). Secreted TARS has autocrine and possibly paracrine functions (c) that promote angiogenic signaling (i.e. migration) (d). BC194 binds and inactivates TARS, preventing its angiogenic function.
There are similarities and differences between this work and other studies examining a role for aaRSs in angiogenesis. YARS has been shown to be secreted in response to TNF-α, but is subsequently cleaved into two fragments with distinct cytokine activities. The amino-terminal fragment, referred to as mini-YARS, displays angiogenic activity purportedly through cooperative binding to VEGFR22,4,8. In contrast, WARS is secreted as an alternative splice variant called mini-WARS in response to interferon-γ (IFN-γ) and inhibits tumor angiogenesis20. The molecular mechanism for the angiostatic effect of mini-WARS is linked to its formation of a complex with VE-cadherin which causes interference with VEGFR2 signaling20. A low level of mini-WARS is also correlated with poor prognoses in patients with colorectal cancer, suggesting that it may serve an anti-cancer role21. By yet another mechanism, glutamyl-prolyl-tRNA synthetase is phosphorylated in response to IFN-γ causing its release from the multisynthetase complex. The resulting gamma-interferon-activated inhibitor of translation (GAIT) complex suppresses the translation of inflammatory genes, including VEGF, leading to reduced angiogenic signaling1,22. TARS displays properties most comparable to YARS, and although the experiments performed here used full-length purified TARS, there remains a possibility that proteolytic cleavage and/or post-translational modification (e.g., phosphorylation) of TARS might be a component of its secretion and/or angiogenic activity.
An important distinction between this study and previous studies of non-canonical functions of aaRSs is that the effects of TARS were explored both independently and in the context of the specific TARS inhibitor BC194. Our results using purified TARS and a BC194-resistant mutant provide strong evidence that BC194's anti-angiogenic effects are TARS-dependent. Other anti-tumorigenic effects of borrelidin, including inhibition of osteosarcoma cell migration, induction of apoptosis in acute lymphoblastic leukemia and reduction of metastasis in a mouse model of melanoma may thus also be related to the extracellular functions of TARS12,13,23. Prior to this report, it was suggested that borrelidin's angiostatic effects may be the consequence of inhibiting or activating secondary targets or through promoting anti-angiogenic isoforms of VEGF24,25. In those studies, effects on the secondary targets required μM concentrations of borrelidin, known to induce cell toxicity, thus the effects may be related to effects on intracellular TARS function or through an apoptotic response. Overall, these observations justify further exploration of the use of borrelidin derivatives such as BC194 as anti-angiogenic or anti-neoplastic agents and warrant studies of clinical associations between TARS functions and cancer.
The data presented also provide clues to explain the mystifying connection between TARS and autoimmune diseases such as polymyositis. The observation that TARS is secreted following TNF-α stimulation provides a means by which TARS could be recognized for immune cell responses. This concept is supported by reports that high TNF-α level is a diagnostic indicator of anti-synthetase syndrome in myositis and that anti-TNF-α therapy is most effective in myositis patients who express anti-TARS PL-7 antibodies26,27. Furthermore, other aaRSs have been directly associated with immune cell responses. For example, lysyl-, histidyl- and asparaginyl-tRNA synthetases promote immune cell migration and/or cytokine release19,28. Our study focused on endothelial-mediated angiogenic responses; however, the correlations between TARS and immune signaling indicate a strong likelihood that TARS also has important signaling functions related to autoimmune responses.
Methods
See Supplemental Information for Expanded Materials and Methods
Cell Culture and Reagents- Human umbilical vein endothelial cells (HUVEC) (supplied by C. Holmes, University of Vermont) were grown in Clonetics® EGM®-2 complete media (Lonza) and used between passages 4 and 6. BC194 was generated by Biotica (Cambridge, UK) according to published procedures14.
ELISA and Lactate Dehydrogenase Assays- Confluent HUVEC cultures were incubated at 37°C in Clonetics® EGM®-2 with low serum (0.2% FBS). Levels of secreted TARS protein were measured in culture media supernatants using the threonyl-tRNA synthetase ELISA Kit (USCN Life Science) according to manufacturer's instructions. Cell membrane integrity was confirmed using the lactate dehydrogenase assay CytoTox-ONETM (Promega) and reported as percent cytotoxicity relative to a lysis control. Levels of secreted VEGF were measured after 24 h using the Human VEGF ELISA kit (Thermo Scientific).
Aminoacyl-tRNA Synthetase Expression and Protein Purification- N-terminal His6-tagged human threonyl-tRNA synthetase (TARS, also known as ThrRS) and mutant L567V TARS were expressed from the plasmid pET28a (obtained from Dieter Soll, Yale Univ.). Proteins were expressed and purified from transformed E. coli RossettaTM 2(DE3)pLysS competent cells (EMD) using a modification of the method described in Bovee et al., 200329. The L567V mutant was derived from the wildtype TARS plasmid using Quikchange II Site-Directed Mutagenesis (Stratagene).
In vitro Tube Formation Assay- Tube formation assays were performed as described30,31. Briefly, HUVECs were seeded in 48-well plates coated with MatrigelTM and incubated in EGM®-2 with reduced serum (0.2% FBS) to test angiogenesis activators and Clonetics® EGM®-2 full serum media (2% FBS) to test angiogenesis inhibitors. Cells were incubated at 37°C for 6 h, fixed in formalin, stained with Oregon Green 488 Phalloidin (Molecular Probes), and imaged using fluorescence microscopy. Number of tube branches (in pixels) was quantified using the Simple Neurite Tracer plug-in on ImageJ software (NIH)32.
Endothelial Cell Proliferation and Toxicity Assays- The MTT-based alamarBlue® (Invitrogen) reagent was used to assess HUVEC cell proliferation33. Nascent protein synthesis was measured using Invitrogen Click-iT® metabolic labeling reagents according to the manufacturer's instructions34. For Western blots, cell lysates or media were separated by 10% SDS-PAGE and transferred to nitrocellulose membrane and proteins detected by antibodies as described35. Primary antibodies were: rabbit monoclonal anti-P-eIF2α (1:1000; Cell Signaling), rabbit monoclonal anti-Cleaved Caspase-3 (1:1000; Cell Signaling), rabbit polyclonal anti-ThrRS (1:1000; Santa Cruz). Loading control antibodies were rabbit monoclonal anti-β-actin and anti-β-tubulin (1:1000; Cell Signaling). Secondary antibody was HRP-goat-anti-rabbit IgG (1:5,000; Jackson Laboratories).
Chick Chorioallantoic Membrane Assay- SurgifoamTM sponges were placed within the outer one-third of the membrane between large vessels of chicken embryos at developmental day 10. Compounds were applied in 10 μl to the CAM every 24 h for 72 h. Images were taken using a Leica MZ6 stereomicroscope. Compounds were scored according to a modified version of Intensity Scoring as previously described36. Total score is averaged individual experimental condition scores from at least 8 replicates.
Transwell Migration Assay- HUVEC migration was measured using gelatin-coated 24-well 8 μm TranswellTM inserts (Corning)37. Cultures were incubated for 4 h, fixed in 10% formalin, and stained with DAPI solution (Roche) after removal of cells from the top layer of the chamber. DAPI-stained nuclei were imaged and counted using ImageJ software.
Quantitative RT-PCR-Total RNA was extracted from cells using the RNeasy column protocol and cDNA was generated using an Omniscript reverse transcriptase assay according to the manufacturer's instructions (Qiagen). Primers and probes for TARS and β2-microglobulin were Assays-on-Demand (Applied Biosystems). RT-qPCR was performed using an ABI prism 7700 Sequence Detection System (Applied Biosystems). The relative quantity of mRNA level was determined using the comparative CT (ΔΔCT) method using β2-microglobulin to normalize mRNA level31.
Statistical Analysis- All experiments were repeated at least 3 times with specific n-values reported within the Figure Legends. Data are presented as mean ± s.e.m., and P < 0.05 was considered significant. Results with P > 0.05 are indicated as not significant (n.s.). Except where noted, one-way ANOVA for multiple comparisons was performed on all data using GraphPad Prism 5 Software. Multiple comparisons were performed using the Tukey test. A Kruskal-Wallis adjustment was used where necessary. Pairwise comparisons were assessed using the Student's t-test.
Author Contributions
T.F.W., C.S.F. and K.M.L. designed the research; B.W. provided reagents, assisted in design and reviewed the paper; T.F.W. and A.C.M. performed the research; T.F.W., K.M.L., A.C.M. and C.S.F. analyzed the data; T.F.W., K.M.L., A.C.M. and C.S.F. wrote the paper.
Supplementary Material
Supplementary Information
Acknowledgments
The authors wish to thank Theresa Wellman and Brianna Blakely for their technical assistance. We also thank Dr. Jeffrey Spees and Dr. Chris Holmes, from the University of Vermont, for supplying the bFGF and HUVEC cells. Plasmids for expression of human TARS and LARS were provided by Dieter Soll, Yale Univ. and Susan Martinis, Univ. of Illinois, Urbana-Champaign respectively. RT-qPCR and Flow cytometry were performed in the Vermont Cancer Center DNA and Flow cytometry facilities. These studies were primarily supported by a New Research Initiative grant from the University of Vermont with additional funding from the Totman Medical Research Trust Fund, the Vermont Cancer Center (LCCRO) and NIH R01HL67352. TFW and ACM were supported by NIH Environmental Pathology Training Grant T32 ES007122-23. CF contributions were supported by NIGMS 2R01GM54899.
References
- Sampath P. et al. Noncanonical function of glutamyl-prolyl-tRNA synthetase: gene-specific silencing of translation. Cell 119, 195–208 (2004). [DOI] [PubMed] [Google Scholar]
- Wakasugi K. & Schimmel P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science 284, 147–51 (1999). [DOI] [PubMed] [Google Scholar]
- Park S. G., Ewalt K. L. & Kim S. Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers. Trends Biochem Sci 30, 569–74 (2005). [DOI] [PubMed] [Google Scholar]
- Greenberg Y. et al. The novel fragment of tyrosyl tRNA synthetase, mini-TyrRS, is secreted to induce an angiogenic response in endothelial cells. FASEB J 22, 1597–605 (2008). [DOI] [PubMed] [Google Scholar]
- Jordanova A. et al. Disrupted function and axonal distribution of mutant tyrosyl-tRNA synthetase in dominant intermediate Charcot-Marie-Tooth neuropathy. Nat Genet 38, 197–202 (2006). [DOI] [PubMed] [Google Scholar]
- Antonellis A. & Green E. D. The role of aminoacyl-tRNA synthetases in genetic diseases. Annu Rev Genomics Hum Genet 9, 87–107 (2008). [DOI] [PubMed] [Google Scholar]
- Puffenberger E. G. et al. Genetic mapping and exome sequencing identify variants associated with five novel diseases. PLoS One 7, e28936 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakasugi K. et al. A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc Natl Acad Sci U S A 99, 173–7 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., You S. & Hwang D. Aminoacyl-tRNA synthetases and tumorigenesis: more than housekeeping. Nat Rev Cancer 11, 708–18 (2011). [DOI] [PubMed] [Google Scholar]
- Cassavaugh J. & Lounsbury K. M. Hypoxia-mediated biological control. J Cell Biochem 112, 735–44 (2011). [DOI] [PubMed] [Google Scholar]
- Funahashi Y. et al. Establishment of a quantitative mouse dorsal air sac model and its application to evaluate a new angiogenesis inhibitor. Oncol Res 11, 319–29 (1999). [PubMed] [Google Scholar]
- Habibi D. et al. Borrelidin, a small molecule nitrile-containing macrolide inhibitor of threonyl-tRNA synthetase, is a potent inducer of apoptosis in acute lymphoblastic leukemia. Invest New Drugs 30, 1361–70 (2011). [DOI] [PubMed] [Google Scholar]
- Harisi R. et al. Differential inhibition of single and cluster type tumor cell migration. Anticancer Res 29, 2981–5 (2009). [PubMed] [Google Scholar]
- Moss S. J. et al. Biosynthesis of the angiogenesis inhibitor borrelidin: directed biosynthesis of novel analogues. Chem Commun (Camb), 2341–3 (2006). [DOI] [PubMed] [Google Scholar]
- Wilkinson B. et al. Separation of anti-angiogenic and cytotoxic activities of borrelidin by modification at the C17 side chain. Bioorg Med Chem Lett 16, 5814–7 (2006). [DOI] [PubMed] [Google Scholar]
- Labirua A. & Lundberg I. E. Interstitial lung disease and idiopathic inflammatory myopathies: progress and pitfalls. Curr Opin Rheumatol 22, 633–8 (2010). [DOI] [PubMed] [Google Scholar]
- Sigurgeirsson B., Lindelof B., Edhag O. & Allander E. Risk of cancer in patients with dermatomyositis or polymyositis. A population-based study. N Engl J Med 326, 363–7 (1992). [DOI] [PubMed] [Google Scholar]
- Suber T. L., Casciola-Rosen L. & Rosen A. Mechanisms of disease: autoantigens as clues to the pathogenesis of myositis. Nat Clin Pract Rheumatol 4, 201–9 (2008). [DOI] [PubMed] [Google Scholar]
- Park S. G. et al. Human lysyl-tRNA synthetase is secreted to trigger proinflammatory response. Proc Natl Acad Sci U S A 102, 6356–61 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E. & Schimmel P. Inhibition of tumor angiogenesis by a natural fragment of a tRNA synthetase. Trends Biochem Sci 31, 7–10 (2006). [DOI] [PubMed] [Google Scholar]
- Ghanipour A. et al. The prognostic significance of tryptophanyl-tRNA synthetase in colorectal cancer. Cancer Epidemiol Biomarkers Prev 18, 2949–56 (2009). [DOI] [PubMed] [Google Scholar]
- Arif A. et al. Two-site phosphorylation of EPRS coordinates multimodal regulation of noncanonical translational control activity. Mol Cell 35, 164–80 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi T. et al. Borrelidin is an angiogenesis inhibitor; disruption of angiogenic capillary vessels in a rat aorta matrix culture model. J Antibiot (Tokyo) 50, 671–6 (1997). [DOI] [PubMed] [Google Scholar]
- Kawamura T. et al. Anti-angiogenesis effects of borrelidin are mediated through distinct pathways: threonyl-tRNA synthetase and caspases are independently involved in suppression of proliferation and induction of apoptosis in endothelial cells. J Antibiot (Tokyo) 56, 709–15 (2003). [DOI] [PubMed] [Google Scholar]
- Woolard J. et al. Borrelidin modulates the alternative splicing of VEGF in favour of anti-angiogenic isoforms. Chem Sci 2011, 273–278 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Reichlin M., Hughes G. R. & Bernstein R. M. Anti-threonyl-tRNA synthetase, a second myositis-related autoantibody. J Exp Med 160, 420–34 (1984). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paepe B., Creus K. K. & De Bleecker J. L. The tumor necrosis factor superfamily of cytokines in the inflammatory myopathies: potential targets for therapy. Clin Dev Immunol 2012, 369432 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard O. M. Z. et al. Histidyl-tRNA Synthetase and Asparaginyl-tRNA Synthetase, Autoantigens in Myositis, Activate Chemokine Receptors on T Lymphocytes and Immature Dendritic Cells. The Journal of Experimental Medicine 196, 781–791 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovee M. L., Pierce M. A. & Francklyn C. S. Induced fit and kinetic mechanism of adenylation catalyzed by Escherichia coli threonyl-tRNA synthetase. Biochemistry 42, 15102–13 (2003). [DOI] [PubMed] [Google Scholar]
- Arnaoutova I. & Kleinman H. K. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 5, 628–35 (2010). [DOI] [PubMed] [Google Scholar]
- Cassavaugh J. M. et al. Negative regulation of HIF-1alpha by an FBW7-mediated degradation pathway during hypoxia. J Cell Biochem 112, 3882–90 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longair M. H., Baker D. A. & Armstrong J. D. Simple Neurite Tracer: open source software for reconstruction, visualization and analysis of neuronal processes. Bioinformatics 27, 2453–4 (2011). [DOI] [PubMed] [Google Scholar]
- Ahmed S. A., Gogal R. M. Jr & Walsh J. E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H]thymidine incorporation assay. J Immunol Methods 170, 211–24 (1994). [DOI] [PubMed] [Google Scholar]
- Dieterich D. C. et al. Labeling, detection and identification of newly synthesized proteomes with bioorthogonal non-canonical amino-acid tagging. Nat Protoc 2, 532–40 (2007). [DOI] [PubMed] [Google Scholar]
- Lounsbury K. M., Beddow A. L. & Macara I. G. A family of proteins that stabilize the Ran/TC4 GTPase in its GTP-bound conformation. J Biol Chem 269, 11285–90 (1994). [PubMed] [Google Scholar]
- Ribatti D., Nico B., Vacca A. & Presta M. The gelatin sponge-chorioallantoic membrane assay. Nat Protoc 1, 85–91 (2006). [DOI] [PubMed] [Google Scholar]
- Svensson K. J. et al. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc Natl Acad Sci U S A 108, 13147–52 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information