Abstract
Because the circadian clock in the mammalian brain derives from a network of interacting cellular oscillators, characterizing the nature and bases of circadian coupling is fundamental to understanding how the pacemaker operates. Various phenomena involving plasticity in circadian waveform have been theorized to reflect changes in oscillator coupling; however, it remains unclear whether these different behavioral paradigms reference a unitary underlying process. To test if disparate coupling assays index a common mechanism, we examined whether there is co-variation among behavioral responses to various lighting conditions that produce changes in circadian waveform. Siberian hamsters, Phodopus sungorus, were transferred from long to short photoperiods to distinguish short photoperiod responders (SP-R) from non-responders (SP-NR). Short photoperiod chronotyped hamsters were subsequently transferred, along with unselected controls, to 24 h light:dark:light:dark cycles (LDLD) with dim nighttime illumination, a procedure that induces bifurcated entrainment. Under LDLD, SP-R hamsters were more likely to bifurcate their rhythms than SP-NR hamsters or unselected controls. After transfer from LDLD to constant dim light, SP-R hamsters were also more likely to become arrhythmic compared to SP-NR hamsters and unselected controls. In contrast, short photoperiod chronotype did not influence more transient changes in circadian waveform. The present data reveal a clear relationship in the plasticity of circadian waveform across three distinct lighting conditions, suggesting a common mechanism wherein individual differences reflect variation in circadian coupling.
Keywords: Short photoperiod chronotype, non-responsiveness, LDLD, bifurcated entrainment, dim nighttime illumination, circadian coupling
Introduction
A circadian pacemaker in the suprachiasmatic nucleus (SCN) of mammals orchestrates daily rhythms in physiology and behavior (Klein et al. 1991). The SCN is entrained with a predictable phase to the solar day through the phase-resetting actions of light, and changes in circadian function can affect entrainment patterns. At the molecular level, transcriptional, translational, and post-translational interactions among a number of clock genes and their protein products generate an approximately 24 h cycle in cellular activity (Ko and Takahashi 2006). Mutations in clock genes can yield large changes in circadian period that translate into altered phases of entrainment (Spoelstra et al. 2004). Among out-bred animal species and humans, there may be considerable inter-individual variation in entrainment chronotype (e.g., larks versus owls) and in free-running period, some of which is likely heritable (Brown et al. 2008; Xu et al. 2005).
Pacemaker function is also affected by changes at the network level. In isolation, SCN neurons express a broad range of period lengths (Herzog et al. 2004; Welsh et al. 1995). To form a functional central pacemaker with a coherent circadian period, these oscillators must interact (i.e., couple) to synchronize with one another (Bouskila and Dudek 1995; Enright 1980). Moreover, the coupled network encodes a circadian waveform as an emergent property of the phase relations among SCN neurons, which is thought to control system-level responses to photoperiod (Inagaki et al. 2007; Rohling et al. 2006). Although circadian waveform is widely recognized to reflect a central organizational dimension of pacemaker function, an understanding of its mechanistic basis has lagged behind that of circadian period and phase.
Daylength-encoding variation in circadian waveform is a principal mechanism by which non-equatorial mammals adjust their physiology and behavior to be appropriate to the season. Short-lived rodents, for example, commonly suppress reproduction and molt to a thicker pelage under autumn and winter photoperiods (Goldman 2001). In many rodent species, however, a subset of animals fails to inhibit reproductive function in short photoperiods (Nelson 1987). The population frequency of photoperiodic non-responsiveness is responsive to artificial selection, indicating that this trait is under genetic control (Kliman and Lynch 1992; Puchalski and Lynch 1986). In Siberian hamsters, Phodopus sungorus, short photoperiod nonresponsive (SP-NR) individuals fail to express the long activity duration (α) and long interval of melatonin secretion necessary to induce the suite of winter-typical traits displayed by short photoperiod responsive (SP-R) hamsters. Individual differences in short photoperiod responsiveness is posited to reflect variation in the strength of SCN coupling (Margraf et al. 1991; Puchalski and Lynch 1994).
Other circadian phenomena reflecting plasticity in circadian waveform are likewise thought to involve coupling among SCN oscillators. Prolonged constant light can induce either arrhythmia or “splitting” of locomotor activity rhythms into two distinct components (Pittendrigh and Daan 1976), which reflects temporal dissociation of SCN oscillators (de la Iglesia et al. 2000; Ohta et al. 2005; Yan et al. 2005). Phase-advancing light pulses commonly produce transient shortening of subjective night, putatively reflecting changes in the phase relations of coupled and differentially shifted evening and morning oscillators (Pittendrigh and Daan 1976; Elliott and Tamarkin 1994; Sumova and Illnerova 1998). Also, hamsters and mice can be readily and reliably induced to bifurcate their activity rhythms under 24 h light:dark:light:dark (LDLD) cycles (Gorman and Elliott 2003), and the antiphase activity bouts that emerge under LDLD correspond to temporally dissociated SCN oscillators (Watanabe et al. 2007; Yan et al. 2010). After release from LDLD into constant conditions, the component oscillators quickly rejoin under the influence of mutual coupling (Evans et al. 2010).
Despite the common usage of “coupling” as an explanatory concept in multiple circadian paradigms, it is unknown whether the underlying mechanisms are empirically related. In one previous study, SP-NR Siberian hamsters were less likely than SP-R hamsters to exhibit split rhythms in constant bright light (Puchalski and Lynch 1988), which is compatible with the view that individual differences under both lighting conditions are influenced by a common coupling mechanism. In the present study, we systematically examine co-variation of circadian waveform responses across five distinct lighting conditions to assess whether these different “coupling” paradigms are influenced by a common process. We posit that high co-variation across multiple conditions would argue for a common mechanism of coupling, whereas no co-variation would suggest that there are independent mechanisms through which circadian waveform is altered. We find that plasticity in circadian waveform is highly related under a subset of these lighting conditions, suggesting that these responses index a common coupling mechanism that differs among individuals.
Materials and Methods
Procedures
Animals and Initial Conditions
Male and female Siberian hamsters (Phodopus sungorus) were bred from stock maintained at the University of California, San Diego since 1994. At weaning, hamsters were group-housed in clear polycarbonate cages (27 cm × 20 cm × 15 cm, 2–4 hamsters/cage). Temperature was maintained at 22±2°C, with ad libitum access to water and food (Purina Chow no. 5015). The breeding colony was maintained on a 14 h light and 10 h dark light:dark cycle (LD14:10; lights on: 0600 PST, lights off: 2000 PST) with completely dark nights. Under both colony and experimental conditions, illumination within cages during the photophase was 50–100 lux. Under experimental conditions, the daily scotophase was either completely dark or dimly lit (see below) with narrowband, green light-emitting diodes (LEDs, 0.03 W, peak λ = 560 ± 23 nm half bandwidth) of an intensity comparable to that of dim moonlight (< 0.05 lux, < 9.0 × 10−9 W/cm2, < 2.5 × 1010 photons/cm2sec).
Overview of Procedures
Siberian hamsters were exposed sequentially to a number of lighting conditions that produce changes in the waveform of locomotor activity rhythms (Figure 1, S1). As described in detail below, the measured behavioral responses were 1) increases in activity duration (α) after transfer from long to short photoperiods, 2) incidence of bifurcated entrainment under 24 h LDLD cycles, 3) acute changes in α after release from LDLD into constant dim illumination, 4) transient α compression during bright-light resetting, and 5) incidence of arrhythmia under long-term constant dim illumination.
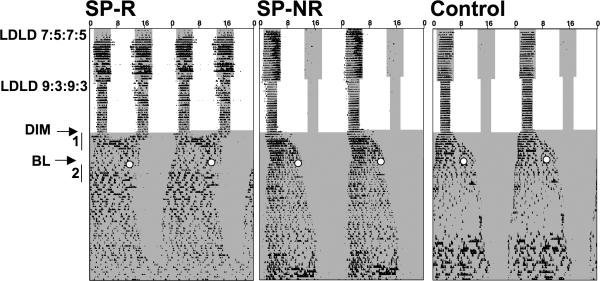
Figure 1.
Representative double-plotted actograms illustrating the lighting conditions to which Siberian hamsters were exposed. Changes in internal shading represent changes in lighting conditions, with dim nighttime illumination present throughout. Wheel-running rhythms (scale 0 to 75 wheel revolutions/6 min) were recorded after hamsters were transferred to LDLD and released into constant DIM illumination (indicated by DIM and arrow on left). After two weeks under constant DIM, hamsters were provided 15-min bright light pulses (indicated by BL, arrow at left, and white circle within actogram). Numbered sections of record indicate intervals used to measure changes in activity duration after release into DIM (1) and after bright light pulses (2). Note that the SP-R hamster, like all other SP-R hamsters, displays a bifurcated rhythm with an activity component in each scotophase starting on Day3 of LDLD; whereas the SP-NR and control hamster, like the majority of hamsters in these two groups, fail to display two activity components for the entire duration of LDLD.
Short Photoperiod Chronotypy
Since short photoperiod non-responsiveness in this species requires initial exposure to day lengths ≥16 h (Goldman and Goldman 2003; Gorman and Zucker 1997), group-housed hamsters (N = 156, 6–10 wks of age) were held for six weeks under a LD19:5 (lights on: 0600 PST, lights off 0100 PST) and then subsequently exposed to LD10:14 (lights on: 0800 PST, lights off: 1800) to distinguish short photoperiod responders from nonresponders (see criteria for chronotypy below). For logistical purposes, hamsters remained group-housed for the first 10 weeks under LD10:14 before transfer to individual cages so that locomotor activity could be recorded for chronotyping (Figure S1). Up until this point, scotophases were completely dark since dim nighttime illumination prevents the induction of short photoperiod non-responsiveness in this species (Gorman and Elliott 2004). Two weeks later, hamsters were exposed to dim nighttime illumination for six weeks and re-chronotyped in an attempt to equalize entrainment patterns across hamsters (Figure S1). Finally, chronotyped hamsters were entrained to LD19:5 for 12 days to homogenize entrainment state prior to LDLD.
Bifurcated Entrainment in LDLD
To test whether short photoperiod chronotype predicted the incidence of rhythm bifurcation, all chronotyped hamsters were next transferred to cylindrical cages (21 cm diam), equipped with a running wheel (17 cm diam). Transfer coincided with the “daytime” scotophase of LDLD7:5:7:5 (lights off: 1000 PST, lights on: 1500 PST, lights off: 2200 PST, lights on: 0300 PST) with dim nighttime illumination (same as above). A separate sample of hamsters (n = 14) was transferred from LD14:10 to identical LDLD conditions to serve as unselected, age-matched controls. For all hamsters, the photocycle was changed three weeks later to LDLD9:3:9:3 (lights off: 1100 PST, lights on: 1400 PST, lights off: 2300 PST, lights on: 0200 PST). After three more weeks, hamsters were transferred from LDLD into constant dim illumination (DIM) at the beginning of the nighttime scotophase.
Bright Light-Induced Resetting Transients
After two weeks of constant DIM, free-running hamsters were transferred within their home cage to a separate chamber and given a 15-min, 350-lux light pulse. To account for differences in α, light pulses were initiated ¾ through each animal's active phase, which is a phase at which light is expected to elicit advances in all hamsters (Puchalski and Lynch 1991a). After the bright light pulse, hamsters were returned to the housing chamber and left to free-run for seven weeks under constant DIM.
Constant DIM-Induced Arrhythmia
With prolonged exposure to constant DIM, many hamsters in the present study became arrhythmic. To assess whether arrhythmia was produced by dim illumination or another factor (e.g., age, hormonal status), approximately half the hamsters within each chronotype group were exposed to constant complete darkness (DARK) by extinguishing the LEDs. After seven weeks under constant DARK, constant DIM was reinstated for an additional seven weeks by re-powering the LEDs.
Data Collection and Analyses
During the last 8 weeks under LD10:14 and the subsequent 12 days under LD19:5, general locomotor rhythms were monitored with passive infrared motion detectors (Coral Plus; Visonic, Bloomfield, Conn., USA) mounted on filter tops. Upon transfer to LDLD, activity rhythms were monitored through the use of home cage running-wheels. Both passive infrared and wheel-running counts were recorded and compiled into 6-min bins by VitalView (Mini Mitter, Bend, OR). Activity rhythms were plotted and analyzed using ClockLab (Actimetrics, Evanston, IL).
Short Photoperiod Chronotypy
SP-R and SP-NR hamsters were identified by monitoring behavioral and physiological indices associated with a winter phenotype. All hamsters were weighed at 2–4 week intervals, starting with transfer to LD10:14 and ending immediately before transfer to LDLD. Additionally, male hamsters were lightly anesthetized with isoflurane vapors (Aerane, Fort Dodge, Iowa, USA) so that the length and width of the left testis could be measured externally with calipers. The product of testis length multiplied by squared testis width was used to produce estimated testis volume (ETV), which yields a reliable index of testis size (Gorman and Zucker 1997). To assess circadian patterns of general locomotion for each hamster, a 24-h activity profile was produced by averaging activity counts in each 6-min bin over seven days of the following experimental intervals: Over the final week of LD10:14 with dark nights (Figure S1), over the final week of LD10:14 with dimly lit nights (Figure S1), and over the final week of LD19:5, which preceded transfer to LDLD. For each activity profile, activity onset was identified as the first bin after 1600 PST above overall daily mean levels, followed within 30 min by at least three bins likewise above this threshold and preceded by at least 6 h of activity below this threshold. Activity offset was the last time point preceded by a bin exceeding this threshold and followed by at least 6 h of activity below threshold. The time difference between activity offset and onset was used to calculate α. Similar to conventions used in previous studies (Gorman and Zucker 1997; Prendergast and Freeman 1999), hamsters were categorized as SP-NR if they displayed α < 9 h whereas SP-R hamsters were classified as those that exhibited α > 11 h (Table S1). Due to limited recording space, hamsters that displayed arrhythmia or intermediate α values (9 h < α < 11 h) under LD10:14 with dark nights were removed from the study. The addition of dim nighttime illumination after the 12th week under LD10:14 increased α in only a minority of SP-NR hamsters. Those hamsters that increased α by at least 3.5 h under dimly lit nights were classified as SP-Converters (n=6; Figure S1, Table S1).
Bifurcated Entrainment in LDLD
Consistent with previous reports (Gorman and Elliott 2003, 2004), there was no ambiguity in classifying rhythms as bifurcated or unbifurcated. Activity rhythms were categorized as bifurcated if, for a minimum of five consecutive days, hamsters expressed two separate wheel-running bouts, one associated with each scotophase and each lasting longer than 30 min.
Acute α Changes in Constant DIM after LDLD
As discussed in (Evans et al. 2010), it is difficult to visually identify the exact cycle on which bifurcated rhythms become fused, but latency to the fused state may be operationalized as the number of cycles until circadian waveform stabilizes. To quantify changes in circadian waveform under free-running conditions, day-to-day measures of α were calculated on each of 23 consecutive days, starting with the day before LDLD release into constant DIM. Activity onset was identified by the first 6-min bin exceeding 5 counts/min and preceded by at least 6 h of activity below threshold, and activity offset was identified by a similar but opposite rule. Day-to-day changes in α were analyzed as % α displayed on the day before the experimental manipulation (i.e., either release from LDLD or exposure to the 15-min bright light pulse).
Bright Light-Induced Phase Shifts
While α is positively correlated with phase shift magnitude (Pittendrigh et al. 1984; Puchalski and Lynch 1991a), it is not known whether either of these variables influences the degree to which α changes during phase-advancing transients. To determine whether analyses of phase-advancing transients would need to account for differences in α, we assessed whether phase shift magnitude was related to α displayed before the light pulse. A phase shift was measured for each animal by the displacement between regression lines fit to 5–7 consecutive activity onsets before and after the 15-min bright light pulse, excluding the first two post-pulse days to allow for initial transients. The slope of the pre-pulse regression line was also used to measure free-running period length under DIM conditions
Arrhythmia in Constant DIM
Hamsters were categorized as arrhythmic if they displayed no subjectively discernible inactive phase (i.e., subjective day) over at least 3 consecutive days of constant DIM. Determinations were conducted by two different observers unaware of short photoperiod chronotype. Free-running period length under DARK conditions was measured by the slope of a linear regression line fit to 7 consecutive activity onsets during the 5th week under DARK, which was a time at which all hamsters were rhythmic.
Statistical analyses
Statistical analyses were conducted with JMP 5.0 software (SAS Institute, Cary, NC). Male and female hamsters did not differ in the response to most lighting manipulations, although a greater number of females displayed arrhythmia under constant DIM conditions (χ2(1) = 5.19, p < 0.05). Short photoperiod chronotype measures were assessed using analysis of variance (ANOVA). Group differences in the incidence of bifurcated entrainment and arrhythmia was assessed with contingency statistics (Pearson's χ2), as was latency to rhythm bifurcation due to heterogeneity of variance between groups. Day-to-day measures of % change in α were initially submitted to repeated measures ANOVA to test for an effect of entrainment history under LDLD (Factors: LDLD, Time, LDLD*Time). Separate repeated measures ANOVA were then conducted for hamsters with and without bifurcated rhythms under LDLD (Factors: Chronotype, Time, Chronotype*Time). Since prior entrainment state was not randomly assigned, some groups had a sample size of one, and these groups were excluded from statistical analyses of day-to-day measures (i.e., one SP-R with an unbifurcated rhythm at the time of release from LDLD and one SP-Converter that never displayed bifurcated entrainment). Correlational analyses were conducted with Pearson's correlation coefficient. Figures and text indicate mean ± SEM.
Results
Short Photoperiod Chronotypy
To identify short photoperiod responsive (SP-R) and non-responsive (SP-NR) individuals, hamsters were exposed to LD10:14 with dark nights for 12 weeks. In the 12th week of LD10:14 with completely dark nights, 27% and 26% of hamsters were categorized as SP-R (α > 11 h) and SP-NR (α < 9 h), respectively (Table S1, Figure S1). SP-R and SP-NR hamsters were then provided LD10:14 with dim nighttime illumination for six weeks (Figure S1) in an attempt to equalize entrainment patterns between groups (Gorman and Elliott 2004). However, the addition of dim nighttime illumination increased α in only six SP-NR hamsters, which were classifed as SP-Converters (Figure S1). In addition to α, physiological measures also distinguished SP-R and SP-NR hamsters (Table S1). All SP-R, SP-NR, and SP-Converters were re-entrained to LD19:5 to equalize entrainment state before the next lighting manipulation. Under LD19:5, α decreased and did not differ between chronotype groups (SP-NR: 6.14 h ± 0.27 h, SP-R: 6.42 h ± 0.57 h, SP-Converter: 7.65 h ± 0.82 h; F(2,31) = 1.6, p > 0.2).
Bifurcated Entrainment in LDLD
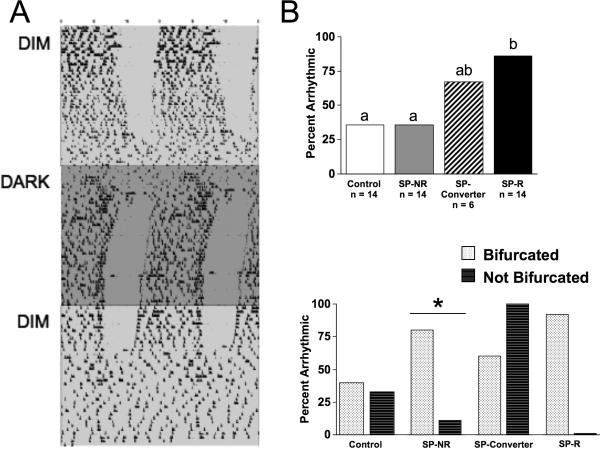
To determine if short photoperiod responsiveness would predict differences in circadian waveform under other lighting conditions, hamsters were transferred next to LDLD. Rhythm bifurcation was induced in 54% of hamsters within three weeks of LDLD7:5:7:5 and in an additional 6% (3 more hamsters) in LDLD9:3:9:3 (Figure 1, 2). Notably, 100% of SP-R hamsters and 83% of SP-Converters displayed bifurcated entrainment under LDLD, compared to only 35% of SP-NR hamsters and unselected controls (Figure 2; χ2(3) = 17.6, p < 0.001). Moreover, chronotype influenced the latency to rhythm bifurcation (Figure 2; χ2(3) = 12.5, p < 0.01), and latency to bifurcate was negatively correlated with α under the last week under LD10:14 (r2= 0.28, p = 0.008). Over the course of continued exposure to LDLD, one SP-R hamster subsequently consolidated activity into a single scotophase, but all other hamsters continued to display bifurcated rhythms.
Figure 2.
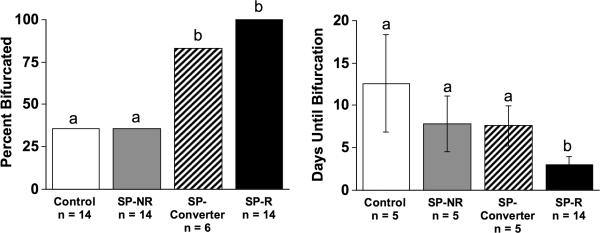
Incidence (percent) and timing (mean ± SEM) of bifurcated entrainment under LDLD. Sample sizes are indicated below each bar. Distinct letters above each bar distinguish groups that differed in post hoc pairwise χ2 tests (p < 0.05).
Release from LDLD and Bright Light-Induced Resetting Transients
To determine if short photoperiod responsiveness would predict differences in changes in circadian waveform under free-running conditions, hamsters were next released from LDLD and provided with bright light pulses. As expected, values of α after release were influenced by LDLD entrainment state (Figure S2; LDLD Entrainment State: F(1,43) = 93.1; p < 0.0001; Time: F(7,37) = 4.5, p = 0.001; LDLD Entrainment State × Time: F(7,37) = 11.6, p < 0.0001). However, the pattern of change in % α was not influenced by short photoperiod chronotype for either the previously bifurcated group (Figure S2; SP-Chronotype: F(3,24) = 1.9; p = 0.16; Time: F(7,18) = 1.7; p = 0.18; SPChronotype × Time: (F(7,20) = 1.4, p = 0.95) or the unbifurcated group (Figure S2; SP-Chronotype: F(1,15) = 0, p = 0.99; SP-Chronotype × Time: (F(6,10) = 0.37, p = 0.71).
At the time of the bright-light resetting manipulation, prior entrainment effects of LDLD were still evident (Table S2), and hamsters with previously bifurcated rhythms displayed longer α (F(1,44) = 18.6, p < 0.0001) and larger bright light-induced phase shifts (F(1,44) = 6.6, p < 0.05). Among hamsters with previously bifurcated rhythms, short photoperiod chronotype did not influence α (F(3,24) = 1.4, p = 0.27) or phase shift magnitude (F(3,24) = 0.2, p = 0.88). Among hamsters without bifurcated rhythms, SP-NR hamsters had a shorter α than unselected controls (F(1,15) = 5.6, p < 0.05) but did not differ in phase shift magnitude (F(1,16) = 0.02, p = 0.89). When α was used as a covariate, phase shift magnitude was no longer significantly affected by LDLD entrainment state (F(1,33) = 1.0, p = 0.32). No group differences were evident in free-running period under constant DIM (Table S3, F(4,17) = 0.37, p = 0.69).
Following phase-advancing bright light pulses, α decreased for several cycles and returned to steady state values within one week (Figure S2). Changes in α during phase-advancing transients were influenced by LDLD entrainment state (LDLD Entrainment State: F(1,43) = 27.3, p < 0.0001; Time: F(7,37) = 6.0, p = 0.0001; LDLD Entrainment State × Time: F(7,37) = 1.1, p = 0.39). However, the pattern of change in % α was not influenced by short photoperiod chronotype for either the previously bifurcated group (Figure S2, SP-Chronotype: F(3,24) = 2.0, p = 0.15; Time: F(7,18) = 2.6, p < 0.05; SP-Chronotype – Time: F(7,20) = 1.0, p = 0.47) or the unbifurcated group (Figure S2, SP-Chronotype: F(1,15) = 2.16, p = 0.16; Time: F(6,10) = 9.73, p < 0.0001; SP-Chronotype – Time: F(6,10) = 2.85, p = 0.07).
Long Term Exposure to DIM
After several weeks under constant DIM, 54% of hamsters developed arrhythmic activity patterns characterized by the lack of a discernible inactive phase (Figure 3). Hamsters that displayed arrhythmia did not differ in free-running period under DIM (t(20) = −0.88, p = 0.39), nor was DIM period related to the day on which arrhythmia emerged (r2= 0.02, p = 0.65). In contrast, latency to arrhythmia was negatively correlated with α displayed during the last week under LD10:14 (r2= 0.24, p = 0.01). Consistent with this, short photoperiod chronotype influenced the incidence of arrhythmia (χ2(3) = 9.83, p < 0.05). SP-R hamsters were more likely to become arrhythmic than SP-NR hamsters and unselected controls, but not more likely than SP-Converters (Figure 3). SP-NR hamsters that had adopted bifurcated rhythms in LDLD were more likely to develop arrhythmia than SP-NR hamsters without bifurcated rhythms (χ2(1) = 5.9, p < 0.05) but arrhythmia in unselected controls did not depend on entrainment state under LDLD (χ2(1) = 0.6, p = 0.8).
Figure 3.
A. Representative wheel-running record of an animal that developed an arrhythmic activity pattern under constant DIM. When constant DIM was extinguished (constant DARK), a rhythmic activity pattern rapidly developed. B. Incidence of constant DIM-induced arrhythmia differed by short photoperiod chronotype (top, conventions as in Figure 2) and entrainment state (bottom). * p < 0.01.
After seven weeks under constant DIM, half of the hamsters were transferred to constant DARK to determine if arrhythmia was produced by dim illumination. Within two weeks under constant DARK, clear circadian rhythmicity emerged in 12/16 arrhythmic hamsters (Figure 3), which was a larger proportion compared to hamsters that remained in constant DIM (χ2(1) = 9.5, p < 0.005). No group differences were evident in free-running period under constant DARK (Table S3, F(4,14) = 0.45, p = 0.77), with no significant effect of chronotype group (F(2,16) = 0.93, p = 0.42), LDLD entrainment state (t(17) = −1.06, p = 0.31), or DIM-induced arrhythmia (t(17) = −1.56, p = 0.14). When constant DIM was reinstated several weeks later, arrhythmia re-emerged in 7/12 hamsters that had become rhythmic under constant DARK (Figure 3; Latency to arrhythmia re-emergence: 16 days ± 2.4 days).
Discussion
Circadian arrhythmia, LDLD-induced rhythm bifurcation and photoperiodic regulation of α each entail changes in circadian waveform that are associated with a temporal reorganization of oscillators within the central pacemaker (Ohta et al. 2005; Watanabe et al. 2007; Yan et al. 2010; Inagaki et al. 2007). The present data demonstrate a robust empirical relationship between inter-individual variation in the plasticity of circadian waveform as measured across these three different lighting conditions. Compared to SP-NR hamsters, SP-R hamsters were more likely to display bifurcated rhythms under LDLD and reversible arrhythmia under constant dim light. On the other hand, the response to short photoperiods did not predict differential changes in circadian waveform during light-induced phase advances or after transfer from LDLD to constant conditions. This is one of only a few studies that demonstrate that inter-individual differences in mammals influence a diverse collection of entrained and free-running responses (e.g., Puchalski and Lynch 1988, 1991b; Ruby et al. 2004). As described in detail below, the pattern of results suggest that the responses that co-vary index a common coupling mechanism that differs among individuals.
Why should the circadian response under one of these paradigms predict the circadian response in another? One possibility is that there is a shared and stable trait that varies between individuals to differentially influence circadian waveform under each of the three related paradigms. Alternatively, because we employed a longitudinal design, the prior entrainment state of an animal could condition it to respond in a particular fashion to the subsequent lighting manipulation. Our intention was to exclude the latter possibility by testing hamsters in one paradigm, return them to a homogeneous circadian state, and then expose them to the next entrainment challenge. Specifically, because photoperiod non-responsiveness was virtually eliminated in an earlier study with dimly lit scotophases (Gorman and Elliott 2004), we hoped to reverse the non-responder phenotype with dim nighttime illumination, and thereby deliver all hamsters to an equivalent entrainment state. Contrary to this expectation, dim nighttime illumination reversed short photoperiod non-responsiveness in only a handful of hamsters, suggesting that dim illumination only prevents non-responsiveness when provided from the beginning of the screen. Because of this unexpected outcome, we exploited the rapid photoperiodic re-entrainment that occurs following transfer to longer day lengths (Sumova et al. 1995). Consistent with expectation, exposure to LD19:5 eliminated the difference in α between SP-R and SP-NR hamsters but yet these two groups still displayed a large difference in the incidence of rhythm bifurcation under LDLD. Accepting the caveat that photoperiodic history effects may not have been eliminated fully, this finding suggests that entrainment history alone does not produce the correlated response to LDLD. Likewise, differential rates of DIM-induced arrhythmia emerged months after hamsters were initially chronotyped and after various intervening lighting manipulations. This suggests that co-variance in the plasticity of circadian waveform is influenced by a circadian trait rather than determined exclusively by entrainment history.
Any of several intrinsic factors are potential mediators of the correlated responses, including differences in free-running period and phase resetting responses. SP-NR hamsters reportedly have longer free-running periods than SP-R hamsters, which could contribute to the aberrant entrainment under short photoperiods (Freeman and Goldman 1997; Kliman and Lynch 1991). However, the free-running period of SP-R and SP-NR hamsters used in this study did not differ under either DIM or DARK constant conditions. Phase resetting responses to bright light have also been reported to differ between SP-R and SP-NR hamsters, with the latter having a smaller amplitude phase response curve (Puchalski and Lynch 1991a) and a higher light intensity threshold for LL-induced splitting (Puchalski and Lynch 1988, 1991b). However, after adjusting for the effect of α on phase shift magnitude (Pittendrigh et al. 1984), we find no evidence that chronotype groups differ in the response to bright light pulses. This indicates that individual differences in the plasticity of circadian waveform relate to a circadian parameter other than period length or photic phase resetting.
The three circadian responses shown here to co-vary are all modulated by a common extrinsic factor – namely, dim illumination. In previous studies using this species, provision of dim nighttime illumination prevented the induction of short photoperiod non-responsiveness (Gorman and Elliott 2004), accelerated the expansion of α under a short photoperiod (Gorman and Elliott 2004), and facilitated the induction of bifurcated entrainment under LDLD (Gorman and Elliott 2004). The present study extends the effects of dim illumination to include the loss of rhythmicity under constant conditions. Arrhythmicity of locomotor activity is known to arise in the Siberian hamster under a variety of conditions: Following a phase shift of the LD cycle (Barakat et al. 2005), after exposure to very long day lengths (present study; Gorman and Zucker 1997; Prendergast and Freeman 1999), and following prolonged exposure to constant bright light (Puchalski and Lynch 1988, 1991b). In contrast to the conditions described above, in which arrhythmicity follows reductions in α, arrhythmicity under constant dim light emerged gradually as α became progressively longer. Arrhythmicity in the present study was related to dim illumination rather than age or some uncontrolled factor, because overt circadian rhythmicity returned with the removal of dim illumination and disappeared once more in the majority of hamsters when dim light was provided a second time. Since modulation of circadian waveform is markedly altered by dim illumination in each of these paradigms, inter-individual variation in sensitivity to dim light could mechanistically underlie the correlated responses. However, the initial designation of hamsters as SP-R or SP-NR was conducted in the absence of dim nighttime illumination and thus must be independent of individual variation in sensitivity to it.
In aggregate, the results lend empirical credence to the idea that a fundamental circadian parameter related to coupling within the central pacemaker varies between individuals, and individual differences in this parameter influence plasticity under a variety of lighting conditions. Many of the seemingly disparate uses of the term “coupling” in the literature may reference a common mechanism, and further research should investigate its underlying physiological and molecular basis. The circadian responses that did not co-vary with short photoperiod responsiveness– changes in circadian waveform during phase advances and after release into constant conditions– also have been argued to represent coupling dynamics between oscillators (Elliott and Tamarkin 1994; Evans et al. 2010; Meijer and De Vries 1995). The failure to co-vary here with the other measured responses suggests either that these responses are independent of circadian coupling or that they index a mechanistically distinct aspect of oscillator coupling. The latter possibility would indicate that circadian waveform is modulated by multiple coupling mechanisms. Although neural mechanisms are beyond the scope of the present study, the results predict that manipulation of any specific coupling factor (e.g., VIP or GABA (Aton and Herzog 2005)) should produce similar patterns of effects across the behavioral assays shown to co-vary here.
Although several circadian responses were treated in the present study as categorical and dichotomous (SP-R versus SP-NR; bifurcated versus unbifurcated; arrhythmic versus rhythmic), there may be continuous variation in the underlying circadian mechanisms. Selection experiments have demonstrated that short photoperiod non-responsiveness displays additive genetic variation consistent with a threshold circadian response (Freeman and Goldman 1997; Goldman et al. 2000; Kliman and Lynch 1992). We selected extremes in short photoperiod responsiveness in order to maximize statistical power for a given sample, but even so, we uncovered additional levels of individual variation in the short photoperiod non-responsive group (SP-NR) after the incorporation of dim nighttime illumination (i.e., the identification of SP-Converters). The observation that unselected, control hamsters – a group representing the full range of individual variation in response to short photoperiods – were generally more similar in their response to SP-NR hamsters suggests that bifurcated entrainment and arrhythmia emerge predominantly in a subset of highly responsive individuals.
In summary, these findings establish that a dimension of circadian organization varies between individuals to modulate the plasticity of circadian waveform under multiple lighting conditions. Conceptually distinct and less studied than other circadian parameters (i.e., period, phase, amplitude), rhythm waveform may be equally significant in the regulation of behavior and physiology. The inter-individual variation in circadian organization that exists in out-bred species (Labyak and Lee 1997; Smale et al. 2001) mirrors the genetic variation within human populations that likely contributes to individual differences in human entrainment (Roenneberg et al. 2003). A deeper understanding of circadian waveform, the underlying coupling mechanisms, and individual differences in this parameter may yield novel strategies for manipulating circadian clocks for human benefit.
Supplementary Material
Acknowledgements
This work was supported by NSF grant IBN- 0346391 and NIH grant NICHD-36460. We thank Antonio Mora and Tony Mora for providing excellent animal care and David Piecarski and Tristan Shuman for technical assistance.
Abbreviations
- SCN
Suprachiasmatic nuclei
- SP-NR
Short-photoperiod nonresponder
- SP-R
Short-photoperiod responder
- LDLD
24 h light:dark:light:dark cycles
- α
Duration of active phase
- ETV
Estimated testis volume
References
- Aton SJ, Herzog ED. Come together, right…now: Synchronization of rhythms in a mammalian circadian clock. Neuron. 2005;48:531–534. doi: 10.1016/j.neuron.2005.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat MT, O'Hara BF, Cao VH, Heller HC, Ruby NF. Light induces c-fos and per1 expression in the suprachiasmatic nucleus of arrhythmic hamsters. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1381–1386. doi: 10.1152/ajpregu.00695.2004. [DOI] [PubMed] [Google Scholar]
- Bouskila Y, Dudek FE. Can a population of suprachiasmatic nucleus neurons with different period lengths produce a stable circadian rhythm? Brain Res. 1995;670:333–336. doi: 10.1016/0006-8993(94)01356-m. [DOI] [PubMed] [Google Scholar]
- Brown SA, Kunz D, Dumas A, Westermark PO, Vanselow K, Tilmann-Wahnschaffe A, Herzel H, Kramer A. Molecular insights into human daily behavior. Proc Natl Acad Sci U S A. 2008;105:1602–1607. doi: 10.1073/pnas.0707772105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Iglesia HO, Meyer J, Carpino A, Jr., Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000;290:799–801. doi: 10.1126/science.290.5492.799. [DOI] [PubMed] [Google Scholar]
- Elliott J, Tamarkin L. Complex circadian regulation of pineal melatonin and wheel-running in Syrian hamsters. J Comp Physiol A. 1994;174:469–484. doi: 10.1007/BF00191713. [DOI] [PubMed] [Google Scholar]
- Enright JT. Temporal precision in circadian systems: A reliable neuronal clock from unreliable components? Science. 1980;209:1542–1545. doi: 10.1126/science.7433976. [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Dynamic interactions between coupled oscillators within the hamster circadian pacemaker. Behav Neurosci. 2010;124:87–96. doi: 10.1037/a0018088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DA, Goldman BD. Evidence that the circadian system mediates photoperiodic nonresponsiveness in Siberian hamsters: The effect of running wheel access on photoperiodic responsiveness. J Biol Rhythms. 1997;12:100–109. doi: 10.1177/074873049701200202. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: Formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Goldman SL, Dhandapani K, Goldman BD. Genetic and environmental influences on short-day responsiveness in Siberian hamsters (Phodopus sungorus) J Biol Rhythms. 2000;15:417–428. doi: 10.1177/074873000129001503. [DOI] [PubMed] [Google Scholar]
- Goldman SL, Goldman BD. Early photoperiod history and short-day responsiveness in Siberian hamsters. J Exp Zoolog A Comp Exp Biol. 2003;296:38–45. doi: 10.1002/jez.a.10202. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Elliott JA. Entrainment of 2 subjective nights by daily light:dark:light:dark cycles in 3 rodent species. J Biol Rhythms. 2003;18:502–512. doi: 10.1177/0748730403260219. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Elliott JA. Dim nocturnal illumination alters coupling of circadian pacemakers in Siberian hamsters, Phodopus sungorus. J Comp Physiol [A] 2004;190:631–639. doi: 10.1007/s00359-004-0522-7. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Environmental induction of photononresponsiveness in the Siberian hamster, Phodopus sungorus. Am J Physiol. 1997;272:R887–895. doi: 10.1152/ajpregu.1997.272.3.R887. [DOI] [PubMed] [Google Scholar]
- Herzog ED, Aton SJ, Numano R, Sakaki Y, Tei H. Temporal precision in the mammalian circadian system: A reliable clock from less reliable neurons. J Biol Rhythms. 2004;19:35–46. doi: 10.1177/0748730403260776. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Honma S, Ono D, Tanahashi Y, Honma K. Separate oscillating cell groups in mouse suprachiasmatic nucleus couple photoperiodically to the onset and end of daily activity. Proc Natl Acad Sci U S A. 2007;104:7664–7669. doi: 10.1073/pnas.0607713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind's Clock. University Oxford Press; New York: 1991. [Google Scholar]
- Kliman RM, Lynch GR. Evidence for independence of circadian characters and extent of photoresponsiveness in the Djungarian hamster, Phodopus sungorus. J Biol Rhythms. 1991;6:159–166. doi: 10.1177/074873049100600206. [DOI] [PubMed] [Google Scholar]
- Kliman RM, Lynch GR. Evidence for genetic variation in the occurrence of the photoresponse of the Djungarian hamster, Phodopus sungorus. J Biol Rhythms. 1992;7:161–173. doi: 10.1177/074873049200700207. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Spec No 2):R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Labyak SE, Lee TM. Individual variation in reentrainment after phase shifts of light-dark cycle in a diurnal rodent Octodon degus. Am J Physiol. 1997;273:R739–746. doi: 10.1152/ajpregu.1997.273.2.R739. [DOI] [PubMed] [Google Scholar]
- Margraf RR, Zlomanczuk P, Liskin LA, Lynch GR. Circadian differences in neuronal activity of the suprachiasmatic nucleus in brain slices prepared from photo-responsive and photo-non-responsive Djungarian hamsters. Brain Res. 1991;544:42–48. doi: 10.1016/0006-8993(91)90883-w. [DOI] [PubMed] [Google Scholar]
- Meijer JH, De Vries MJ. Light-induced phase shifts in onset and offset of running-wheel activity in the Syrian hamster. J Biol Rhythms. 1995;10:4–16. doi: 10.1177/074873049501000101. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. Photoperiod-nonresponsive morphs: A possible variable in microtine population-density fluctutations. The American Naturalist. 1987;130:350–369. [Google Scholar]
- Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents: V. Pacemaker Structure: A Clock for All Seasons. J Comp Physiol [A] 1976;106:333–355. [Google Scholar]
- Pittendrigh CS, Elliott JA, Takamura T. The circadian component in photoperiodic induction. CIBA Foundation Symposium. 1984;104:26–47. [Google Scholar]
- Prendergast BJ, Freeman DA. Pineal-independent regulation of photo-nonresponsiveness in the Siberian hamster (Phodopus sungorus) J Biol Rhythms. 1999;14:62–71. doi: 10.1177/074873099129000452. [DOI] [PubMed] [Google Scholar]
- Puchalski W, Lynch GR. Evidence for differences in the circadian organization of hamsters exposed to short day photoperiod. J Comp Physiol [A] 1986;159:7–11. doi: 10.1007/BF00612490. [DOI] [PubMed] [Google Scholar]
- Puchalski W, Lynch GR. Characterization of circadian function in Djungarian hamsters insensitive to short day photoperiod. J Comp Physiol [A] 1988;162:309–316. doi: 10.1007/BF00606119. [DOI] [PubMed] [Google Scholar]
- Puchalski W, Lynch GR. Circadian characteristics of Djungarian hamsters: Effects of photoperiodic pretreatment and artificial selection. Am J Physiol. 1991a;261:R670–676. doi: 10.1152/ajpregu.1991.261.3.R670. [DOI] [PubMed] [Google Scholar]
- Puchalski W, Lynch GR. Expression of circadian rhythmicity in Djungarian hamsters under constant light: Effects of light intensity and the circadian system's state. J Comp Physiol [A] 1991b;169:185–189. doi: 10.1007/BF00215865. [DOI] [PubMed] [Google Scholar]
- Puchalski W, Lynch GR. Photoperiodic time measurement in Djungarian hamsters evaluated from T-cycle studies. Am J Physiol. 1994;267:R191–201. doi: 10.1152/ajpregu.1994.267.1.R191. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: Daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18:80–90. doi: 10.1177/0748730402239679. [DOI] [PubMed] [Google Scholar]
- Rohling J, Wolters L, Meijer JH. Simulation of day-length encoding in the SCN: From single-cell to tissue-level organization. J Biol Rhythms. 2006;21:301–313. doi: 10.1177/0748730406290317. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Barakat MT, Heller HC. Phenotypic differences in reentrainment behavior and sensitivity to nighttime light pulses in siberian hamsters. J Biol Rhythms. 2004;19:530–541. doi: 10.1177/0748730404268055. [DOI] [PubMed] [Google Scholar]
- Smale L, McElhinny T, Nixon J, Gubik B, Rose S. Patterns of wheel running are related to Fos expression in neuropeptide-Y-containing neurons in the intergeniculate leaflet of Arvicanthis niloticus. J Biol Rhythms. 2001;16:163–172. doi: 10.1177/074873001129001863. [DOI] [PubMed] [Google Scholar]
- Spoelstra K, Albrecht U, van der Horst GT, Brauer V, Daan S. Phase responses to light pulses in mice lacking functional per or cry genes. J Biol Rhythms. 2004;19:518–529. doi: 10.1177/0748730404268122. [DOI] [PubMed] [Google Scholar]
- Sumova A, Illnerova H. Photic resetting of intrinsic rhythmicity of the rat suprachiasmatic nucleus under various photoperiods. Am J Physiol. 1998;274:R857–863. doi: 10.1152/ajpregu.1998.274.3.R857. [DOI] [PubMed] [Google Scholar]
- Sumova A, Travnickova Z, Illnerova H. Memory on long but not on short days is stored in the rat suprachiasmatic nucleus. Neurosci Lett. 1995;200:191–194. doi: 10.1016/0304-3940(95)12109-h. [DOI] [PubMed] [Google Scholar]
- Watanabe T, Naito E, Nakao N, Tei H, Yoshimura T, Ebihara S. Bimodal clock gene expression in mouse suprachiasmatic nucleus and peripheral tissues under a 7-hour light and 5-hour dark schedule. J Biol Rhythms. 2007;22:58–68. doi: 10.1177/0748730406295435. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Xu Y, Padiath QS, Shapiro RE, Jones CR, Wu SC, Saigoh N, Saigoh K, Ptacek LJ, Fu YH. Functional consequences of a CKIdelta mutation causing familial advanced sleep phase syndrome. Nature. 2005;434:640–644. doi: 10.1038/nature03453. [DOI] [PubMed] [Google Scholar]
- Yan L, Foley NC, Bobula JM, Kriegsfeld LJ, Silver R. Two antiphase oscillations occur in each suprachiasmatic nucleus of behaviorally split hamsters. J Neurosci. 2005;25:9017–9026. doi: 10.1523/JNEUROSCI.2538-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Silver R, Gorman M. Reorganization of suprachiasmatic nucleus networks under 24-h LDLD conditions. J Biol Rhythms. 2010;25:19–27. doi: 10.1177/0748730409352054. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.