Abstract
In mammals, light entrains the central pacemaker within the suprachiasmatic nucleus (SCN) via both a direct neuronal projection from the retina and an indirect projection from the intergeniculate leaflet (IGL) of the thalamus. Although light comparable in intensity to moonlight is minimally effective at resetting the phase of the circadian clock, dimly lit and completely dark nights are nevertheless perceived differentially by the circadian system, even when nighttime illumination is below putative thresholds for phase resetting. Under a variety of experimental paradigms, dim nighttime illumination exerts effects that may be characterized as enhancing the plasticity of circadian entrainment. For example, relative to completely dark nights, dimly lit nights accelerate development of photoperiodic responses of Siberian hamsters transferred from summer to winter day lengths. Here we assess the neural pathways underlying this response by testing whether IGL lesions eliminate the effects of dim nighttime illumination under short day lengths. Consistent with previous work, dimly lit nights facilitated the expansion of activity duration under short day lengths. Ablation of the IGL, moreover, did not influence photoperiodic responses in animals held under completely dark nights. However, among animals provided dimly lit nights, IGL lesions prevented the short-day typical expansion of activity duration as well as the seasonally-appropriate gonadal regression and reduction in body weight. Thus, the present data indicate that the IGL plays a central role in mediating the facilitative effects of dim nighttime illumination under short day lengths, but in the absence of the IGL, dim light at night influences photoperiodic responses via residual photic pathways.
Keywords: circadian, intergeniculate leaflet, dim nighttime illumination, short day photoperiod, Siberian hamster
Introduction
The mammalian circadian pacemaker within the suprachiasmatic nucleus (SCN) is entrained to the 24 h environment primarily by light. The circadian system’s best-characterized responses to light (e.g., phase resetting and melatonin suppression) rely on mechanisms that are functionally and anatomically distinct from those of the image-forming visual system. Specifically, the former responses are characterized by higher intensity thresholds, a unique action spectrum with peak sensitivity to short wavelength light, and the capacity for photon integration over several hours (Brainard et al., 1982, Takahashi et al., 1984, Nelson and Takahashi, 1991a, b). These classic circadian responses to light are mediated in large part by intrinsically photosensitive retinal ganglion cells (ipRGCs) that contain the photopigment melanopsin (Berson, 2003, Gooley et al., 2003). In addition to intrinsic photosensitivity, ipRGCs relay signals from rods and cones, which can influence both ipRGC and SCN function (Belenky et al., 2003, Hattar et al., 2003, Bullough et al., 2005, Guler et al., 2008).
Photic entrainment in mammals is mediated exclusively by input from the retina and is conveyed to the SCN by the retino-hypothalamic (RHT) and geniculo-hypothalamic tracts (GHT) (Meijer and Schwartz, 2003). The RHT is formed by axon collaterals of ipRGCs (Morin et al., 2003, Hattar et al., 2006), and this tract is both necessary and sufficient for circadian photoentrainment (Johnson et al., 1988a). The GHT, in contrast, arises from the intergeniculate leaflet (IGL) within the lateral geniculate nucleus of the thalamus, a structure that also receives ipRGC input (Harrington and Rusak, 1989, Morin et al., 2003, Hattar et al., 2006). Although the IGL is not required for photo-entrainment to standard light:dark cycles, it nevertheless modulates a variety of circadian responses to light (Harrington and Rusak, 1986, Pickard et al., 1987, Edelstein and Amir, 1999, Redlin et al., 1999, Morin and Pace, 2002). In particular, IGL lesions influence circadian entrainment under seasonally changing and skeleton photoperiods, suggesting that this structure is important for entrainment under conditions that would be experienced by animals in nature (Harrington and Rusak, 1986, Pickard et al., 1987, Pickard, 1989, Shinohara et al., 1993a, Edelstein and Amir, 1999, Freeman et al., 2004). The IGL also mediates nonphotic inputs to the SCN (Johnson et al., 1988b, Janik and Mrosovsky, 1994, Wickland and Turek, 1994).
Light below the intensity of moonlight (~0.3 lux at full moon; (Biberman et al., 1966, Thorington, 1980, Brainard et al., 1984) has been demonstrated to be only minimally effective at resetting circadian phase or suppressing melatonin secretion (Brainard et al., 1982, Nelson and Takahashi, 1991a, b). Nevertheless, across a wide array of circadian entrainment paradigms, dim nighttime illumination below this intensity (0.004 - 0.1 lux) alters entrainment of activity rhythms when compared to entrainment under identical LD cycles with complete darkness at night (Gorman et al., 2006, Evans et al., 2009). For example, after transfer from long day to short day photoperiods, Siberian hamsters exposed to dimly lit nights display accelerated photoperiodic responses (i.e., expansion of nocturnal activity (α), gonadal regression, and weight loss; Gorman and Elliott, 2004). Additional effects of dim illumination in hamsters include enhanced re-entrainment to simulated jetlag protocols (Evans et al., 2009, Frank et al., 2010), elevated incidence of bifurcated rhythm entrainment under 24 h light:dark:light:dark (LDLD) cycles (Gorman et al., 2003, Gorman and Elliott, 2004, Evans et al., 2005), and an increase in the upper limit of entrainment to non 24-h days (Gorman et al., 2005). Taking the larger corpus of published results, the collective effects of dim light are consistent with the hypothesis that dim nighttime illumination enhances circadian plasticity with respect to both changes in phase and in waveform. The potent and pervasive effects of dim light appear to differ both qualitatively and quantitatively from classical circadian responses to brighter light (Evans et al., 2007), raising the possibility that dim light responses are mediated by physiological mechanisms categorically distinct from those underlying phase shifting and melatonin suppression.
The present study is the first to assess the photic pathway that transmits dim nighttime illumination to the central pacemaker by determining whether its influence persists following lesions of the IGL. If the IGL is the primary conduit of this signal, then IGL-lesioned animals housed under dimly lit nights should respond like animals held under completely dark nights. If the IGL plays no role in the facilitative effects of dim light, then ablation of the IGL would not compromise the dim light effect. The results demonstrate that the IGL mediates the facilitative effects of dim nighttime illumination on expansion of activity duration in short photoperiods. Moreover, the data suggest that other photic pathways are capable of transmitting dim nighttime illumination to the central pacemaker with results opposite of those under the intact condition.
Experimental Procedures
Breeding and Initial Husbandry
Male Siberian hamsters (Phodopus sungorus) were selected from a colony established at UCSD since 1994 and maintained under a 24 h light:dark cycle with 14 h light and 10 h darkness (LD 14:10, lights on: 0600 PST, lights off: 2000 PST; photophase: ~100 lux, scotophase: 0 lux). After weaning, hamsters were group-housed inside polypropylene cages (48 × 27 × 20 cm) on open racks. Ambient temperature was maintained at 22 ± 2° C with ad libitum access to water and food (Purina Rodent Chow #5001, St Louis, MO). Experimental procedures were approved by the Institutional Animal Care and Use Committee, University of California, San Diego and were conducted in compliance with all the rules and regulations of this committee.
Surgical Procedures
At 30 days of age, animals were positioned in a stereotaxic apparatus (Leica Microsystems, Bannockburn, IL) under deep sodium pentobarbital anesthesia (80 mg/kg, i.p.). Bilateral radiofrequency lesions (1 mA, 15 secs) were produced with a high voltage stimulus isolator (A360D; WPI, Berlin, Germany) controlled by a Pro4 timer (WPI, Berlin, Germany) using coordinates determined in preliminary studies using age-matched animals (AP: -2.2 mm, ML: ±2.75 mm from bregma, and DV: -4.2 mm below dura; skull level). Successful lesions typically damaged at least some portions of the surrounding dorsal and ventral lateral geniculate nuclei in addition to the IGL (Figure 1). For sham lesions, the electrode was lowered to the same coordinates for the same amount of time but no current was passed. For both IGL- and sham-lesioned hamsters, the microelectrode was withdrawn after an additional 4 secs, and the head was cleaned, sutured and salved with nitrofurazone before animals were injected i.p. with buprenorphine (0.05 mg/kg) and returned to a clean cage. Hamsters remained group-housed for at least four weeks post-operatively.
Figure 1.
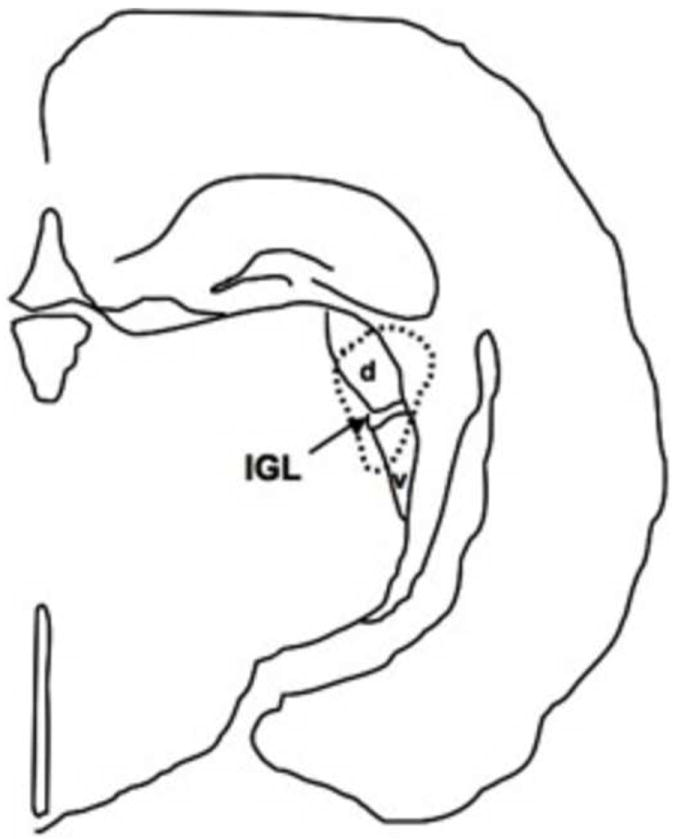
Line drawing illustrating the lesion of a representative hamster with a complete ablation of the IGL (area of lesion indicated by dotted line). d = dorsal lateral geniculate nuclei, v = ventral lateral geniculate nuclei.
Short Day Photoperiod Entrainment
IGL- and sham-lesioned hamsters were weighed and transferred to individual cages housed within experimental chambers (photophase intensity: 500 lux provided by broad-spectrum, cool white fluorescent bulbs). Each cage was equipped with a passive infrared motion detector (PIR, Coral Plus, Visonic, Bloomfield, CT) positioned ~16 cm above the cage floor for continuous monitoring of locomotor activity rhythms. Hamsters were maintained under LD 14:10 for one week to assess baseline entrainment (Figure 2). The lighting cycle was then changed to a short day photoperiod (LD10:14; lights on 0800 PST) with either dimly lit (DIM-IGLx, DIM-Intact) or completely dark nights (DARK-IGLx, DARK-Intact). Dim nighttime illumination (0.03 ± 0.002 lux, mean intensity equivalent to 5.4 × 10-9 W/cm2 and 1.5 × 1010 photons/cm2sec) was provided by narrowband, green light-emitting diodes (LEDs, 0.03 W, λ = 560 ± 23 nm). DIM and DARK groups did not differ in photophase light intensity (p > 0.5). Animals remained under the short day photoperiod with one of these two different scotophase conditions for eight weeks.
Figure 2.
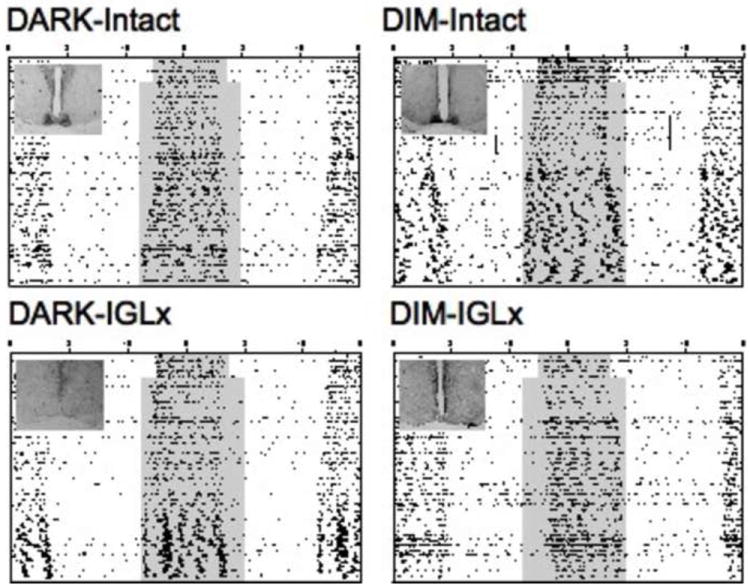
Representative double-plotted actograms depicting general locomotor activity rhythms of animals transferred from long day (LD 14:10) to short day photoperiods (LD 10:14), with the inset illustrating the results of histological analyses of NPY-ir within the SCN of each animal. Internal shading within center of each actogram indicates the timing of the scotophase (either dimly lit or completely dark). Activity counts were recorded with passive infrared motion detectors (actograms scaled 0 to 5 counts/ 6 min bin).
During the eighth week under LD10:14, body weight and testis size were measured. Hamsters were lightly anesthetized with isoflurane vapors (Aerane, Fort Dodge, Iowa, USA) for external measurement of the length and width of the left testis using calipers. The product of testis length and the squared testis width was used to estimate testis volume (estimated testes volume, ETV), which yields a reliable index of testis size (Gorman and Zucker, 1997).
Histology
Under deep sodium pentobarbital anesthesia, animals were perfused transcardially with a phosphate buffered solution, followed by 4% paraformaldehyde. Brains were postfixed overnight, cryoprotected in 20% sucrose solution for two days, and then frozen. Coronal sections (30 μm) were prepared for immunohistochemical analysis using the avidin biotin technique. Free-floating sections were incubated for 48 h with NPY antibody (1:5K dilution, Chemicon, Cat.#AB1583). After incubation, the sections were immunostained using an ABC elite kit (antiperoxidase rabbit IgG; Vectastain). Lesions were assessed by a researcher blind to the experimental treatment of the animals via examination of the stained sections for the presence of NPY immunoreactivity (NPY-ir) within the SCN (Figure 2), with the absence of NPY-ir indicating that IGL input was functionally destroyed (DIM-IGLx: n = 19; DARK-IGLx: n = 17) and dense NPY-ir indicating IGL input was intact due either to sham surgery (DIM-Intact: n = 9; DARK-Intact: n = 9) or a missed lesion (DIM-Intact: n = 3; DARK-Intact: n = 6). Behavioral responses of animals with sham and missed lesions did not differ from one another (p > 0.18) and data from these two groups were combined for statistical analyses. Importantly, inclusion of animals with missed lesions did not alter the results of statistical analyses or the overall conclusions of this study.
Data collection and analyses
Activity counts were collected and compiled into 6 min bins by Vital View software (Mini-Mitter, Bend, OR). Actograms were prepared and analyzed with Clocklab software (Actimetrics, 8 Evanston, IL). For each day under LD 14:10 and LD 10:14, the time of activity onset was identified as the first bin after 1700 PST above the daily mean that was followed within 30 min by at least two bins likewise above the daily mean and preceded by at least 6 h of low activity levels. The time of activity offset was likewise defined as the last time point preceded by a bin exceeding the daily mean and followed by at least 6 h of low activity levels. The temporal difference between activity offset and onset was used to calculate activity duration (α), and the weekly average α was calculated for each animal. Additionally, the temporal difference between lights-off and activity onset was used to calculate phase angle of entrainment, and the weekly average was calculated for each animal. Lastly, total activity counts were summed on a daily basis, and the weekly average was calculated for each animal. Because motion detector positioning and animal activity patterns can conceivably influence detector sensitivity, activity counts should be considered a semi-quantitative measure of general locomotor levels (Larkin et al., 2001).
Statistical analyses were performed with JMP software (SAS Institute, Cary, NC). Group differences in α and total activity levels were assessed initially with a full-factorial repeated-measures ANOVA (Factors: Scotophase Condition (SC), IGL status, SC*IGL Status, Week Under LD 10:14, Week* SC, Week*IGL Status, Week*SC*IGL Status), where Week 0 is the last five days under LD 14:10 and Weeks 1-8 are under LD 10:14. In the event of a significant interaction between Week and a between-subjects factor, subsequent analyses were conducted using a full-factorial repeated-measures ANOVA partitioned by SC or IGL Status. For changes in α, post hoc tests were conducted with full-factorial ANOVA and planned least squared means (LSM) contrasts to test whether DIM-IGLx animals differed in predicted ways from the other three groups. For total activity levels, post hoc tests were conducted weekly after transfer to short days using ANOVA and Tukey-Kramer HSD to determine whether activity levels over the course of the experiment differed between groups. Group differences in body weight and ETV measured after eight weeks under short day photoperiods were assessed with a full-factorial ANOVA and planned LSM contrasts for post hoc tests. Group differences in phase angle of entrainment were assessed with Kruskal-Wallis Rank Sums Test. Figures illustrate mean ± SEM.
Results
Overall, activity duration (α) lengthened progressively during the eight week exposure to the short day photoperiod (Week: F(8,52) = 12.51, p < 0.0001; Figures 2, 3A). Scotophase condition significantly influenced changes in α under the short day photoperiod (Week*SC: F(8,52) = 2.97, p < 0.01), in a manner that tended to differ between IGL-Intact and IGL-Lesioned animals (Week*SC*IGL Status: F(8,52) = 2.07, p < 0.06). When partitioned by IGL Status, scotophase condition significantly influenced changes in activity duration of both IGL-Intact (SC: F(1,25) = 4.43, p < 0.05; Week*SC: F(8,18) = 3.39, p < 0.05) and IGL-Lesioned animals (SC: F(1,34) = 7.46, p < 0.01; Week*SC: F(8,27) = 2.93, p < 0.05). When partitioned by scotophase condition, IGL Status significantly influenced changes in activity duration of DIM animals (IGL Status: F(1,29) = 18.65, p < 0.001; Week* IGL Status: F(8,22) = 2.49, p < 0.05), but not DARK animals (IGL Status: F(1,30) = 0.16, p = 0.69; Week* IGL Status: F(8,23) = 0.42, p = 0.90).
Figure 3.
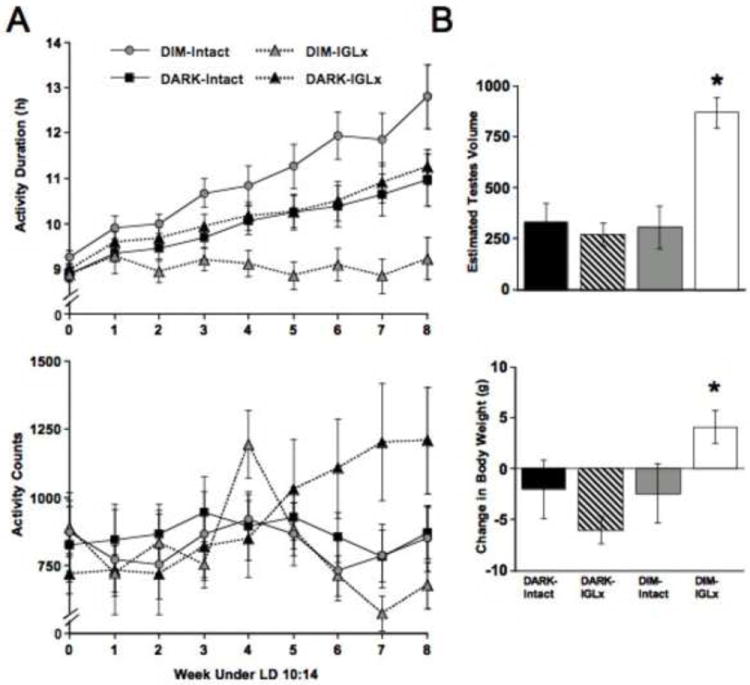
Short day induced changes in behavioral and physiological measures. A) Weekly measures of activity duration (top) and activity counts (bottom). Week 0 is the last five days under LD 14:10 and Weeks1-8 are under LD 10:14. B) Measures of estimated testes volume (top) and body weight loss (bottom) collected during the eighth week of short day photoperiod.
Replicating the results of a previous study, post hoc tests revealed that DIM-Intact animals displayed significantly longer α than DARK-Intact animals starting the sixth week after transfer to short day lengths (Figure 3A; LSM Contrasts, p < 0.05). Consistent with the hypothesis that the IGL transmits dim light information to the circadian pacemaker, DIM-Intact animals also displayed larger α than DIM-IGLx animals starting the third week after transfer to short days (Figure 3A; LSM Contrasts, p < 0.01). However, DIM-IGLx animals did not behave like DARK animals. Rather, DIM-IGLx animals displayed shorter α than both DARK-Intact and DARK-IGLx animals, with significant differences manifesting five weeks after transfer to short day lengths (Figure 3A; LSM Contrasts, p < 0.01). As illustrated in Figure 3B, DIM-IGLx animals also failed to display the short day-induced physical changes displayed by the three other groups (Body weight loss: SC*IGL Status- F(1, 59) = 5.96, p < 0.05; Gonadal regression: SC*IGL Status- F(1, 59) = 14.54, p < 0.0005; LSM Contrasts, p < 0.05).
Inspection of individual activity records supports these findings, although there was variability in the pattern of entrainment displayed by DIM-IGLx animals. In contrast to subjects within the other groups, the vast majority of DIM-IGLx animals displayed a short α entrained to either lights-off (Figure 4A, n = 7/19) or lights-on (Figure 4B, n = 9/19). Only three DIM-IGLx animals displayed the seasonally-appropriate α expansion over the eight weeks exposure to the short day photoperiod (Figure 4C, average increase for these three animals: 3.67 h). DIM-IGLx animals that displayed expansion of α exhibited gonadal regression and decreased body weight, whereas all DIM-IGLx animals with short α failed to display these responses. Phase angle of entrainment did not differ significantly between groups (e.g., Week 8: χ2(3) = 6.1, p = 0.11), which is not unexpected given the heterogeneous entrainment responses of DIM-IGLx hamsters.
Figure 4.
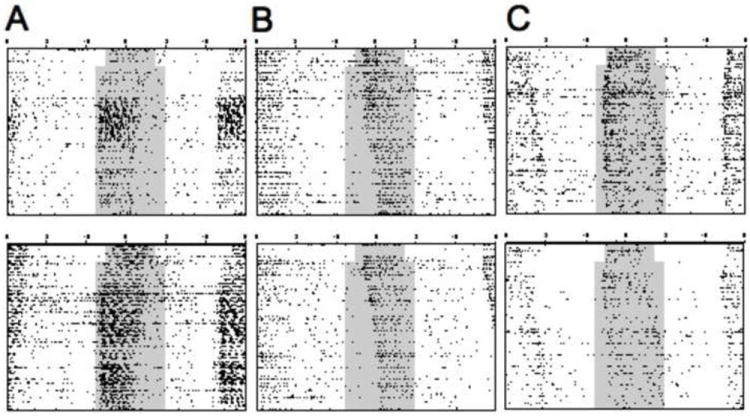
Variability in the pattern of entrainment displayed by IGL-lesioned animals provided LD 10:14 with dim nighttime illumination. Unlike other groups, the majority of DIM-IGLx animals displayed a short active phase entrained unambiguously to either lights-off (A, 7/19 animals) or lights-on (B, 9/19 animals). Only three DIM-IGLx animals displayed an expansion of activity duration under LD 10:14 (C).
Total activity levels changed over time (Week: (F(8,52) = 2.87, p < 0.05), and scotophase condition significantly influenced changes in total activity levels (Week*SC: F(8,52) = 2.86, p < 0.05) in a manner that differed between IGL-Intact and IGL-Lesioned animals (Week*SC*IGL Status: F(8,52) = 2.72, p < 0.05). When partitioned by scotophase condition, IGL status did not influence changes in activity levels of either DIM animals (Week*IGL Status: F(8,22) = 1.85, p = 0.12) or DARK animals (Week*IGL Status: F(8,23) = 1.66, p = 0.16). When partitioned by IGL status, scotophase condition significantly influenced changes in activity levels of IGL-Lesioned animals (Week*SC: F(8,27) = 4.66, p < 0.05) but not IGL-Intact animals (Week*SC: F(8,18) = 1.21, p = 0.34). Post hoc tests indicated that DARK-IGLx animals displayed higher levels of activity than DIM-IGLx animals during the last two weeks under the short day photoperiod (Figure 3A), although this was largely attributable to one DARK-IGLx animal that doubled its activity during this time.
Discussion
Dim nighttime illumination in IGL-intact animals accelerated expansion of α under short day lengths, as previously demonstrated (Gorman and Elliott, 2004). Extending prior research establishing that the IGL is not necessary for photo-entrainment (Pickard et al., 1987, Johnson et al., 1989), ablating the IGL did not influence circadian entrainment with completely dark nights in short day photoperiods. Most notably, IGL-lesioned hamsters provided with dimly lit nights failed to display the accelerated responses to short day photoperiods displayed by cohorts with an intact IGL, which supports the hypothesis that the IGL plays a key role in mediating the facilitative effects of dim nighttime illumination in the expression of short photoperiod responses. Unexpectedly, the vast majority of DIM-IGLx animals failed to display expansion of activity duration, body weight loss, and gonadal regression under short day photoperiods, which is the species-typical short day phenotype that was exhibited by the three other groups during the eight weeks of this study. Thus, dim nighttime illumination accelerates photoperiodic responses in the intact animal through an IGL-dependent pathway, but in the absence of the IGL, dim light input actually prevents (or markedly delays) the behavioral and physiological response to short day photoperiods. This suggests that dim nighttime illumination was transmitted to the central pacemaker in the absence of the IGL, but this input prevented most animals from responding appropriately to the short day photoperiods.
The SCN controls photoperiodic responses by regulating the duration of melatonin release from the pineal gland, which in turn regulates the hypothalamic-pituitary-gonadal axis (Goldman, 2001). In rats, IGL lesions reduce the amplitude of rhythms in melatonin precursors (Cipolla-Neto et al., 1995). In the present study, the photoperiodic response was clearly intact in IGL-lesioned animals since gonadal regression occurred in the IGLx hamsters exposed to completely dark nights. This result confirms previous findings that IGL lesions do not abolish photoperiodic responses per se (Smale and Morin, 1990, Menet et al., 2001, Freeman et al., 2006). Rather, the gonadal and somatic responses are well predicted by the change in activity duration, which closely tracks the duration of elevated melatonin secretion in hamsters (Elliott and Tamarkin, 1994). The most parsimonious interpretation is that IGL lesions and scotopic illumination exert their primary effect on entrainment of the central circadian pacemaker.
While the IGL is not necessary for photic entrainment, it was deemed a candidate for transmitting dim nighttime illumination to the central pacemaker for several reasons. First, as measured electrophysiologically, IGL neurons are characterized by lower photic thresholds than are SCN neurons (Meijer et al., 1986, Harrington and Rusak, 1989, Kornhauser et al., 1990, Warren et al., 2003, Muscat and Morin, 2006). Moreover, photic responses in the IGL are not gated in a circadian fashion like those of the SCN (Park et al., 1993), which is consistent with data suggesting that dim light can alter circadian function even when provided with other stimuli during subjective day (Evans et al., 2005). Lastly and most important, IGL lesions are reported to alter circadian behavior in ways that appear to be opposite from the effects of dim nighttime illumination. Specifically, IGL lesions delay re-entrainment in jetlag protocols and under short day photoperiods and also decrease the incidence of bifurcated rhythms under “splitting” protocols (Dark and Asdourian, 1975, Rusak, 1977, Rusak and Boulos, 1981, Harrington and Rusak, 1986, Pickard et al., 1987, Harrington and Rusak, 1988, Johnson et al., 1988b, Johnson et al., 1989, Jacob et al., 1999, Morin and Pace, 2002, Freeman et al., 2004, Freeman et al., 2006). In contrast, dim nighttime illumination accelerates re-entrainment under jetlag and short day paradigms and increases the incidence of bifurcated entrainment under 24 h light:dark:light:dark cycles (Gorman et al., 2006, Evans et al., 2009). It remains to be tested whether the IGL mediates the other entrainment effects of dim nighttime illumination demonstrated in previous studies (Gorman et al., 2006, Evans et al., 2009). Future studies should also assess whether other light-responsive structures connected to the IGL play a role in processing dim nighttime illumination (Morin and Pace, 2002, Zhao and Rusak, 2005).
Besides communicating light information to the SCN, the IGL is also a key structure for transmitting nonphotic stimuli to the SCN (Janik and Mrosovsky, 1992, Harrington, 1997, Hastings et al., 1997, Mikkelsen et al., 1998), which can influence short day photoperiodic responses (Freeman and Goldman, 1997, Freeman et al., 2006). Specifically, among an artificially-selected Siberian hamster line that fails to expand activity duration in short day lengths, addition of a running wheel restores both a typical pattern of circadian entrainment to short day lengths as well as photoperiodic responsiveness, which are effects that require the IGL. In the present study, motion detectors were selected over running wheels to monitor activity rhythms with the specific purpose of limiting the possible confound of nonphotic cues differentially affecting the responses of animals with and without IGL lesions (Mrosovsky, 1995, Redlin et al., 1999, Lewandowski and Usarek, 2002). Activity levels of intact animals in dimly lit and completely dark nights did not differ, and the status of the IGL did not influence activity levels in animals provided dimly lit nights, despite marked differences in the response of these two groups. While we observed a decrease in the activity levels of DIM-IGLx animals relative to DARK-IGLx animals, it is unlikely to be a causal factor determining the differences in activity duration since the difference in activity levels appeared two weeks after the activity duration first differed between these two groups. Thus, there is little reason to expect that the circadian effects of IGL lesions under dimly lit nights observed in the present study are attributable to differences in feedback effects of locomotor activity. This conclusion is consistent with the results of previous studies that indicate dim nighttime illumination does not act merely by increasing nonphotic feedback to the central pacemaker, but instead operates as a photic stimulus to affect circadian function in a novel manner (Evans et al., 2005, 2007).
If dim light effects on circadian rhythmicity were mediated exclusively by the IGL, then DIM-IGLx animals should resemble DARK animals, which they did not. Instead, dim nighttime illumination in the absence of the IGL prevented (or markedly delayed) the species-typical response to the short photoperiod. DARK animals bearing IGL lesions were clearly able to display the full suite of short day responses, which demonstrates that the lack of response in DIM-IGLx animals must be due to the presence of dim nighttime illumination and not due to unintended consequences of IGL lesions (e.g., (Pickard, 1985, Smale and Morin, 1990). This suggests that dim nighttime illumination was transmitted to the central pacemaker in the absence of the IGL, but this input prevented most animals from responding appropriately to the short day photoperiods. Although the residual pathway mediating this effect of dim light cannot be determined from the present experiment, the RHT is an obvious candidate. This result establishes the potential for opposing actions of dim light mediated by at least two distinct neural pathways to the central pacemaker, which can be addressed in future studies. Preliminary results of fluence response studies also suggest that there are multiple photic mechanisms through which dim nighttime illumination influences the response to short photoperiods. In IGL-intact hamsters, activity duration in short days increases monotonically over a limited range of increasing nighttime irradiances; however, activity duration is shortened as irradiance is increased further (Gorman and Elliott, unpublished observations), similar to the effects of constant bright light as described by Aschoff’s second “rule” (Aschoff, 1960, Pittendrigh, 1960, Aschoff, 1979). IGL lesions may increase the sensitivity to nighttime light and thus would be predicted to alter the shape of this fluence-response curve.
In IGL-intact animals, dim nighttime illumination can prevent a form of short day “non-responsiveness” that has been well characterized in Siberian hamsters (Gorman and Elliott, 2004). This previously documented form of short day “non-responsiveness” is subject to artificial selection (Puchalski and Lynch, 1986, Kliman and Lynch, 1992), and in both unselected and selected lines of hamsters, requires prior exposure to day lengths above a critical value (~LD15:9) for the trait to manifest (Gorman and Zucker, 1997, Goldman and Goldman, 2003). Animals that display this traditional non-responder phenotype almost exclusively entrain to lights-on under short days (i.e., display a large negative phase angle of entrainment), which suggests that these animals fail to expand activity duration due to a lengthening of the central pacemaker’s free-running period (Freeman and Goldman, 1997, Gorman and Elliott, 2004, Freeman et al., 2006). This traditionally observed entrainment pattern differs from that detected in the present sample of DIM-IGLx animals, in that animals were equally likely to entrain to either lights-on or lights-off. This mixed pattern of entrainment suggests that the failure to expand activity duration in the present study is not due to a systematic lengthening of free-running period by dim light or a systematic change in the photic phase response curve, but is instead due to an overall decrease in the plasticity of circadian waveform. Because animals in the present study were never exposed the long day lengths typically required to induce the short day “non-responder” phenotype, it is not clear whether there is a mechanistic relationship between that reported in previous studies and that observed here.
The effects of dimly lit nights on the expansion of activity duration in IGL-intact animals found within the present study closely mirror those of a previous study using this species (Gorman and Elliott, 2004). This earlier study also reported that dim nighttime illumination produced greater gonadal regression and body weight loss after 8 weeks of short photoperiods, whereas there was no difference in testis size or body weight in IGL-intact animals held under dimly lit and completely dark nights in the present study. This discrepancy likely reflects the manner in which the short photoperiod was introduced, since the relative phasing of the LD14:10 and LD10:14 photocycles differed across these studies and this variable is known to affect entrainment and gonadal regression rates (Illnerova et al., 1986, Gorman et al., 1997). The longer winter scotophase was achieved in the present study through symmetric changes in the time of lights-on and lights-off, whereas the earlier study only advanced lights-off. The latter method of introducing the short photoperiod retards the photoperiod response and would thus be a more sensitive assay. Nevertheless, the current results support the hypothesis that the IGL plays a key role in mediating the effects of dim nighttime illumination under short photoperiods.
In conclusion, the present study adds to a growing body of evidence that highlights the importance of IGL input under a variety of conditions that incorporate naturalistic lighting elements. In the wild, day lengths change gradually with the seasons; nocturnal rodents commonly minimize daytime light exposure by resting in darkened burrows; and nocturnal activity occurs under dim illumination from the moon and stars (Biberman et al., 1966, Thorington, 1980, Brainard et al., 1984, but see Daan et al., 2011). Laboratory conditions simulating some aspects of a naturalistic environment reveal that the IGL does contribute to photo-entrainment (Shinohara et al., 1993a, Shinohara et al., 1993b, Edelstein and Amir, 1999). Outside the laboratory, nocturnal rodents likely never encounter square-wave lighting cycles that are static or change abruptly. Likewise, IGL lesions modulate entrainment of Syrian hamsters held under sinusoidal light:dark cycles (Pickard, 1989) and influence short day responses of Siberian hamsters exposed to decreasing simulated natural photoperiods (Freeman et al., 2004). Thus, while the IGL is not necessary for entrainment under standard laboratory photocycles, the IGL does appear to influence entrainment in functionally significant ways under photic conditions like those experienced in nature.
Highlights.
Siberian hamsters adjust more quickly to winter photoperiods with dimly lit nights.
Ablating the intergeniculate leaflet (IGL) prevents this dim-light effect.
Unexpectedly, IGLx prevents the expression of photoperiod responses with dim nights.
This supports the hypothesis that the IGL transmits dim light input to the clock.
But this also suggests that dim light was transmitted through a non-IGL pathway.
Acknowledgments
This work was supported by NSF grant IBN- 0346391 and NIH grant NICHD-36460. We thank Antonio Mora and Robert Sundberg for providing excellent animal care. We are also grateful to our research assistants Lindsey Stewart and Yasasvi Vasili.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Circadian rhythms: Influences of internal and external factors on the period measured in constant conditions. Z Tierpsychol. 1979;49:225–249. doi: 10.1111/j.1439-0310.1979.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Belenky MA, Smeraski CA, Provencio I, Sollars PJ, Pickard GE. Melanopsin retinal ganglion cells receive bipolar and amacrine cell synapses. J Comp Neurol. 2003;460:380–393. doi: 10.1002/cne.10652. [DOI] [PubMed] [Google Scholar]
- Berson DM. Strange vision: Ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- Biberman LM, Dunkelman L, Fickett ML, Finke RG. Levels of Nocturnal Illumination. Washington, D.C: Institute for Defense Analyses, Research, and Engineering Support Division; 1966. [Google Scholar]
- Brainard GC, Richardson BA, Hurlbut EC, Steinlechner S, Matthews SA, Reiter RJ. The influence of various irradiances of artificial light, twilight, and moonlight on the suppression of pineal melatonin content in the Syrian hamster. J Pineal Res. 1984;1:105–119. doi: 10.1111/j.1600-079x.1984.tb00202.x. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Richardson BA, Petterborg LJ, Reiter RJ. The effect of different light intensities on pineal melatonin content. Brain Res. 1982;233:75–81. doi: 10.1016/0006-8993(82)90931-3. [DOI] [PubMed] [Google Scholar]
- Bullough JD, Figueiro MG, Possidente BP, Parsons RH, Rea MS. Additivity in murine circadian phototransduction. Zoolog Sci. 2005;22:223–227. doi: 10.2108/zsj.22.223. [DOI] [PubMed] [Google Scholar]
- Cipolla-Neto J, Bartol I, Seraphim PM, Afeche SC, Scialfa JH, Peracoli AM. The effects of lesions of the thalamic intergeniculate leaflet on the pineal metabolism. Brain Res. 1995;691:133–141. doi: 10.1016/0006-8993(95)00654-9. [DOI] [PubMed] [Google Scholar]
- Daan S, Spoelstra K, Albrecht U, Schmutz I, Daan M, Daan B, Rienks F, Poletaeva I, Dell’omo G, Vyssotski A, Lipp HP. Lab mice in the field: Unorthodox daily activity and effects of a dysfunctional circadian clock allele. J Biol Rhythms. 2011;26:118–129. doi: 10.1177/0748730410397645. [DOI] [PubMed] [Google Scholar]
- Dark JG, Asdourian D. Entrainment of the rat’s activity rhythm by cyclic light following lateral geniculate nucleus lesions. Physiol Behav. 1975;15:295–301. doi: 10.1016/0031-9384(75)90097-9. [DOI] [PubMed] [Google Scholar]
- Edelstein K, Amir S. The role of the intergeniculate leaflet in entrainment of circadian rhythms to a skeleton photoperiod. J Neurosci. 1999;19:372–380. doi: 10.1523/JNEUROSCI.19-01-00372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J, Tamarkin L. Complex circadian regulation of pineal melatonin and wheel-running in Syrian hamsters. J Comp Physiol A. 1994;174:469–484. doi: 10.1007/BF00191713. [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Circadian entrainment and phase resetting differ markedly under dimly illuminated versus completely dark nights. Behav Brain Res. 2005;162:116–126. doi: 10.1016/j.bbr.2005.03.014. [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Circadian effects of light no brighter than moonlight. J Biol Rhythms. 2007;22:356–367. doi: 10.1177/0748730407301988. [DOI] [PubMed] [Google Scholar]
- Evans JA, Elliott JA, Gorman MR. Dim nighttime illumination accelerates adjustment to timezone travel in an animal model. Curr Biol. 2009;19:R156–157. doi: 10.1016/j.cub.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Frank DW, Evans JA, Gorman MR. Time-dependent effects of dim light at night on re-entrainment and masking of hamster activity rhythms. J Biol Rhythms. 2010;25:103–112. doi: 10.1177/0748730409360890. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Dhandepani K, Goldman BD. The thalamic intergeniculate leaflet modulates photoperiod responsiveness in Siberian hamsters. Brain Res. 2004;1028:31–38. doi: 10.1016/j.brainres.2004.08.049. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Goldman BD. Evidence that the circadian system mediates photoperiodic nonresponsiveness in Siberian hamsters: The effect of running wheel access on photoperiodic responsiveness. J Biol Rhythms. 1997;12:100–109. doi: 10.1177/074873049701200202. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Teubner BJ, Goldman BD. The thalamic intergeniculate leaflet mediates locomotor activity-induced reversal of phenotype in photoperiod nonresponsive Siberian hamsters. J Biol Rhythms. 2006;21:206–213. doi: 10.1177/0748730406287996. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: Formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Goldman SL, Goldman BD. Early photoperiod history and short-day responsiveness in Siberian hamsters. J Exp Zoolog A Comp Exp Biol. 2003;296:38–45. doi: 10.1002/jez.a.10202. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Lu J, Fischer D, Saper CB. A broad role for melanopsin in nonvisual photoreception. J Neurosci. 2003;23:7093–7106. doi: 10.1523/JNEUROSCI.23-18-07093.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman MR, Elliott JA. Dim nocturnal illumination alters coupling of circadian pacemakers in Siberian hamsters, Phodopus sungorus. J Comp Physiol [A] 2004;190:631–639. doi: 10.1007/s00359-004-0522-7. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Elliott JA, Evans JA. Plasticity of hamster circadian entrainment patterns depends on light intensity. Chronobiol Int. 2003;20:233–248. doi: 10.1081/cbi-120018576. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Evans JA, Elliott JA. Potent circadian effects of dim illumination at night in hamsters. Chronobiol Int. 2006;23:245–250. doi: 10.1080/07420520500521905. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Freeman DA, Zucker I. Photoperiodism in hamsters: Abrupt versus gradual changes in day length differentially entrain morning and evening circadian oscillators. J Biol Rhythms. 1997;12:122–135. doi: 10.1177/074873049701200204. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Kendall ME, Elliott JA. Scotopic illumination enhances entrainment of circadian rhythms to lengthening light:dark cycles. J Biol Rhythms. 2005;20:38–48. doi: 10.1177/0748730404271573. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Environmental induction of photononresponsiveness in the Siberian hamster, Phodopus sungorus. Am J Physiol. 1997;272:R887–895. doi: 10.1152/ajpregu.1997.272.3.R887. [DOI] [PubMed] [Google Scholar]
- Guler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington ME. The ventral lateral geniculate nucleus and the intergeniculate leaflet: Interrelated structures in the visual and circadian systems. Neurosci Biobehav Rev. 1997;21:705–727. doi: 10.1016/s0149-7634(96)00019-x. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Rusak B. Lesions of the thalamic intergeniculate leaflet alter hamster circadian rhythms. J Biol Rhythms. 1986;1:309–325. doi: 10.1177/074873048600100405. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Rusak B. Ablation of the geniculo-hypothalamic tract alters circadian activity rhythms of hamsters housed under constant light. Physiol Behav. 1988;42:183–189. doi: 10.1016/0031-9384(88)90296-x. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Rusak B. Photic responses of geniculo-hypothalamic tract neurons in the Syrian hamster. Vis Neurosci. 1989;2:367–375. doi: 10.1017/s0952523800002170. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Duffield GE, Ebling FJ, Kidd A, Maywood ES, Schurov I. Non-photic signalling in the suprachiasmatic nucleus. Biol Cell. 1997;89:495–503. doi: 10.1016/s0248-4900(98)80005-1. [DOI] [PubMed] [Google Scholar]
- Hattar S, Kumar M, Park A, Tong P, Tung J, Yau KW, Berson DM. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497:326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau KW. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illnerova H, Hoffman K, Vanecek J. Adjustment of the rat pineal N-acetyltransferase rhythm to change from long to short photoperiod depends on the direction of the extension of the dark period. Brain Res. 1986;362:403–408. doi: 10.1016/0006-8993(86)90473-7. [DOI] [PubMed] [Google Scholar]
- Jacob N, Vuillez P, Lakdhar-Ghazal N, Pevet P. Does the intergeniculate leaflet play a role in the integration of the photoperiod by the suprachiasmatic nucleus? Brain Res. 1999;828:83–90. doi: 10.1016/s0006-8993(99)01324-4. [DOI] [PubMed] [Google Scholar]
- Janik D, Mrosovsky N. Gene expression in the geniculate induced by a nonphotic circadian phase shifting stimulus. Neuroreport. 1992;3:575–578. doi: 10.1097/00001756-199207000-00007. [DOI] [PubMed] [Google Scholar]
- Janik D, Mrosovsky N. Intergeniculate leaflet lesions and behaviorally-induced shifts of circadian rhythms. Brain Res. 1994;651:174–182. doi: 10.1016/0006-8993(94)90695-5. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Loss of entrainment and anatomical plasticity after lesions of the hamster retinohypothalamic tract. Brain Res. 1988a;460:297–313. doi: 10.1016/0006-8993(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Moore RY, Morin LP. Lateral geniculate lesions alter circadian activity rhythms in the hamster. Brain Res Bull. 1989;22:411–422. doi: 10.1016/0361-9230(89)90068-3. [DOI] [PubMed] [Google Scholar]
- Johnson RF, Smale L, Moore RY, Morin LP. Lateral geniculate lesions block circadian phase-shift responses to a benzodiazepine. Proc Natl Acad Sci U S A. 1988b;85:5301–5304. doi: 10.1073/pnas.85.14.5301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliman RM, Lynch GR. Evidence for genetic variation in the occurrence of the photoresponse of the Djungarian hamster, Phodopus sungorus. J Biol Rhythms. 1992;7:161–173. doi: 10.1177/074873049200700207. [DOI] [PubMed] [Google Scholar]
- Kornhauser JM, Nelson DE, Mayo KE, Takahashi JS. Photic and circadian regulation of c-fos gene expression in the hamster suprachiasmatic nucleus. Neuron. 1990;5:127–134. doi: 10.1016/0896-6273(90)90303-w. [DOI] [PubMed] [Google Scholar]
- Larkin JE, Freeman DA, Zucker I. Low ambient temperature accelerates short-day responses in Siberian hamsters by altering responsiveness to melatonin. J Biol Rhythms. 2001;16:76–86. doi: 10.1177/074873040101600109. [DOI] [PubMed] [Google Scholar]
- Lewandowski MH, Usarek A. Effects of intergeniculate leaflet lesions on circadian rhythms in the mouse. Behav Brain Res. 2002;128:13–17. doi: 10.1016/s0166-4328(01)00264-9. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Groos GA, Rusak B. Luminance coding in a circadian pacemaker: The suprachiasmatic nucleus of the rat and the hamster. Brain Res. 1986;382:109–118. doi: 10.1016/0006-8993(86)90117-4. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Schwartz WJ. In search of the pathways for light-induced pacemaker resetting in the suprachiasmatic nucleus. J Biol Rhythms. 2003;18:235–249. doi: 10.1177/0748730403018003006. [DOI] [PubMed] [Google Scholar]
- Menet J, Vuillez P, Jacob N, Pevet P. Intergeniculate leaflets lesion delays but does not prevent the integration of photoperiodic change by the suprachiasmatic nuclei. Brain Res. 2001;906:176–179. doi: 10.1016/s0006-8993(01)02518-5. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD, Vrang N, Mrosovsky N. Expression of Fos in the circadian system following nonphotic stimulation. Brain Res Bull. 1998;47:367–376. doi: 10.1016/s0361-9230(98)00121-x. [DOI] [PubMed] [Google Scholar]
- Morin LP, Blanchard JH, Provencio I. Retinal ganglion cell projections to the hamster suprachiasmatic nucleus, intergeniculate leaflet, and visual midbrain: Bifurcation and melanopsin immunoreactivity. J Comp Neurol. 2003;465:401–416. doi: 10.1002/cne.10881. [DOI] [PubMed] [Google Scholar]
- Morin LP, Pace L. The intergeniculate leaflet, but not the visual midbrain, mediates hamster circadian rhythm response to constant light. J Biol Rhythms. 2002;17:217–226. doi: 10.1177/07430402017003005. [DOI] [PubMed] [Google Scholar]
- Mrosovsky N. A non-photic gateway to the circadian clock of hamsters. Ciba Found Symp. 1995;183:154–167. doi: 10.1002/9780470514597.ch9. [DOI] [PubMed] [Google Scholar]
- Muscat L, Morin LP. Intergeniculate leaflet: Contributions to photic and non-photic responsiveness of the hamster circadian system. Neuroscience. 2006;140:305–320. doi: 10.1016/j.neuroscience.2006.01.050. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Comparison of visual sensitivity for suppression of pineal melatonin and circadian phase-shifting in the golden hamster. Brain Res. 1991a;554:272–277. doi: 10.1016/0006-8993(91)90200-f. [DOI] [PubMed] [Google Scholar]
- Nelson DE, Takahashi JS. Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus) J Physiol. 1991b;439:115–145. doi: 10.1113/jphysiol.1991.sp018660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HT, Baek SY, Kim BS, Kim JB, Kim JJ. Profile of Fos-like immunoreactivity induction by light stimuli in the intergeniculate leaflet is different from that of the suprachiasmatic nucleus. Brain Res. 1993;610:334–339. doi: 10.1016/0006-8993(93)91419-s. [DOI] [PubMed] [Google Scholar]
- Pickard GE. Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neurosci Lett. 1985;55:211–217. doi: 10.1016/0304-3940(85)90022-9. [DOI] [PubMed] [Google Scholar]
- Pickard GE. Entrainment of the circadian rhythm of wheel-running activity is phase shifted by ablation of the intergeniculate leaflet. Brain Res. 1989;494:151–154. doi: 10.1016/0006-8993(89)90154-6. [DOI] [PubMed] [Google Scholar]
- Pickard GE, Ralph MR, Menaker M. The intergeniculate leaflet partially mediates effects of light on circadian rhythms. J Biol Rhythms. 1987;2:35–56. doi: 10.1177/074873048700200104. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- Puchalski W, Lynch GR. Evidence for differences in the circadian organization of hamsters exposed to short day photoperiod. J Comp Physiol [A] 1986;159:7–11. doi: 10.1007/BF00612490. [DOI] [PubMed] [Google Scholar]
- Redlin U, Vrang N, Mrosovsky N. Enhanced masking response to light in hamsters with IGL lesions. J Comp Physiol [A] 1999;184:449–456. doi: 10.1007/s003590050344. [DOI] [PubMed] [Google Scholar]
- Rusak B. Involvement of the primary optic tracts in mediation of light effects on hamster circadian rhythms. J Comp Physiol. 1977;118:165–172. [Google Scholar]
- Rusak B, Boulos Z. Pathways for photic entrainment of mammalian circadian rhythms. Photochem Photobiol. 1981;34:267–273. doi: 10.1111/j.1751-1097.1981.tb08996.x. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Fukuhara C, Otori Y, Inouye SI. Processing of photic information within the intergeniculate leaflet of the lateral geniculate body: Assessed by neuropeptide Y immunoreactivity in the suprachiasmatic nucleus of rats. Neuroscience. 1993a;56:813–822. doi: 10.1016/0306-4522(93)90129-4. [DOI] [PubMed] [Google Scholar]
- Shinohara K, Tominaga K, Isobe Y, Inouye ST. Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: Daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide Y. J Neurosci. 1993b;13:793–800. doi: 10.1523/JNEUROSCI.13-02-00793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale L, Morin LP. Photoperiodic responsiveness of hamsters with lesions of the lateral geniculate nucleus is related to hippocampal damage. Brain Res Bull. 1990;24:185–190. doi: 10.1016/0361-9230(90)90204-d. [DOI] [PubMed] [Google Scholar]
- Takahashi JS, DeCoursey PJ, Bauman L, Menaker M. Spectral sensitivity of a novel photoreceptive system mediating entrainment of mammalian circadian rhythms. Nature. 1984;308:186–188. doi: 10.1038/308186a0. [DOI] [PubMed] [Google Scholar]
- Thorington L. Actinic effects of light and biological implications. Photochem Photobiol. 1980;32:117–129. doi: 10.1111/j.1751-1097.1980.tb03998.x. [DOI] [PubMed] [Google Scholar]
- Warren EJ, Allen CN, Brown RL, Robinson DW. Intrinsic light responses of retinal ganglion cells projecting to the circadian system. Eur J Neurosci. 2003;17:1727–1735. doi: 10.1046/j.1460-9568.2003.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickland C, Turek FW. Lesions of the thalamic intergeniculate leaflet block activity-induced phase shifts in the circadian activity rhythm of the golden hamster. Brain Res. 1994;660:293–300. doi: 10.1016/0006-8993(94)91302-1. [DOI] [PubMed] [Google Scholar]
- Zhao H, Rusak B. Circadian firing-rate rhythms and light responses of rat habenular nucleus neurons in vivo and in vitro. Neuroscience. 2005;132:519–528. doi: 10.1016/j.neuroscience.2005.01.012. [DOI] [PubMed] [Google Scholar]


