Abstract
Introduction
There are often multiple anatomic factors that contribute to nasal obstruction, creating difficulty in deciding which components to address for a successful outcome. The purpose of this pilot study is to demonstrate the effect of individual components of functional nasal airway surgery in a patient with multifactorial obstruction and discuss the potential benefit of computational fluid dynamics (CFD)-aided virtual surgery.
Methods
A 53 year old female underwent septoplasty, turbinate reduction, and nasal valve repair. Pre- and post-operative digital nasal models were created from CT images and nasal resistance was calculated using CFD techniques. The digital models were then manipulated to isolate the effects of the components of the surgery, creating a nasal valve repair alone model and a septoplasty/turbinate reduction alone model.
Results
Bilateral nasal resistance on the post-operative model was approximately 25% less than pre-operative values. Similarly, CFD analysis showed reductions in nasal resistance of the virtual models: 19% reduction with intranasal surgery alone and 6% reduction with nasal valve repair alone.
Conclusions
Most of the reduction in nasal resistance was accomplished with performance of septoplasty and inferior turbinate reduction. The contribution from nasal valve repair was less in comparison but not insignificant. This pilot study implies that CFD-aided virtual surgery may be useful as part of pre-operative planning in patients with multifactorial anatomic nasal airway obstruction.
Introduction
Nasal airway obstruction (NAO) is a common occurrence that is concerning to patients and is associated with a poor quality of life1. This leads to numerous clinician visits and medical and surgical treatments. Among the leading causes of NAO are inflammatory conditions and anatomic abnormalities, such as septal deviation, turbinate hypertrophy, and nasal valve compromise (NVC). While the management of inflammatory nasal conditions is primarily medical, surgery is the mainstay for treatment of anatomic abnormalities. However, the results of surgical treatment of NAO are variable. For example, after performance of septoplasty with or without turbinectomy, about 25% of patients may be unsatisfied with surgical results, with less than half completely satisfied2.
There are potential explanations for this patient dissatisfaction. One is the poor correlation between subjective and objective methods of measuring NAO3–5, which creates difficulty in analyzing and reporting the results of functional nasal surgery. Another potential reason for surgical failure is inappropriate procedure selection or failing to target the appropriate problematic components of the nasal airway6. Patients can have multifactorial anatomic obstruction due to co-existing problems and there can be difficulty in deciding which component(s) to address for the best functional outcome. While there is evidence for the efficacy of septoplasty7, turbinate reduction8, and nasal valve repair9 individually in the treatment of nasal obstruction, data on procedures performed in conjunction is less clear. Studies involving patients with both septal deviation and turbinate hypertrophy have demonstrated no additional benefit from performing both turbinate reduction and septoplasty compared to either procedure in isolation10,11.
Inappropriate surgical procedures, such as a septoplasty in a patient whose main problem was, in fact, NVC, exposes patients to unnecessary risk while providing little or no benefit. Undertreatment, such as treating a patient with both septal deviation and NVC with septoplasty alone, provides incomplete benefit and may result in a poor outcome and patient dissatisfaction with surgery. Surgically overtreating patients with NAO (i.e. a “shotgun” approach consisting of septoplasty, turbinate reduction, and nasal valve repair for every patient) might expose patients to unnecessary risk while not providing additional benefit. There are also financial ramifications to overtreatment, with additional cost to patients, insurance providers, and an already overburdened health care system.
In today’s evidence-based medicine environment there is an emphasis on demonstrating efficacy for treatments or interventions using validated outcome measures. In addition, insurance companies are more commonly requiring pre-authorization for surgery and denying compensation for procedures with questionable benefit or that are not clearly indicated. These factors place additional pressure on providers to offer safe, effective, and patient-centered care in a cost-efficient manner. In an ideal situation, a nasal surgeon would have technologically-advanced tools at his disposal to assist in the pre-operative evaluation of patients with NAO in order to better assess the nasal airway, identify problematic areas, and select the optimal surgical intervention in order to produce the best possible outcome.
At present such tools do not exist. The current methods of objectively assessing the nasal airway, such as acoustic rhinometry and rhinomanometry, have limitations that prevent them from being widely accepted12 and do not provide much insight into pre-operative planning. Newer technology adopted from engineering fields has the potential to fill this gap. Computational fluid dynamics (CFD) is a technique that can be used to study nasal airflow and resistance, as well as other aspects of nasal physiology. Using anatomically-accurate three-dimensional (3-D) digital models created from CT or MRI scans, CFD simulation software can calculate nasal resistance, airflow velocity, air conditioning, and wall shear stress. Furthermore, the computational models can be digitally modified to simulate the changes created by surgery and new simulations can be performed to determine what effect these changes produce with respect to resistance and other parameters of interest13.
This article presents the results of a study that is part of a larger prospective project to correlate CFD data with patient-reported subjective measures of nasal obstruction and examine the role of virtual surgery in predicting post-operative outcomes. The aims of this particular study are to demonstrate the effect of individual components of functional nasal airway surgery in a patient with multifactorial obstruction and discuss the potential benefit of computational fluid dynamics (CFD)-aided virtual surgery.
Methods
Patient Recruitment and Treatment
Research methods were approved by the Institutional Review board at the Medical College of Wisconsin and informed consent was obtained. For this study, a 53 year old female with a long-standing bilateral nasal obstruction and no prior history of nasal trauma or surgery was selected. Physical examination revealed the presence of rightward septal deviation, bilateral inferior turbinate hypertrophy, and nasal valve compromise. Modified contiguous CT scans in the axial plane of the entire nasal cavity and external nasal soft tissues were performed pre-operatively and 6 months post-operatively (both with 6.0-mm increments and 0.313-mm resolution). Surgical treatment decisions were made by the surgeon (J.S.R.) based on the clinical presentation and standard of medical care. For this patient septoplasty, nasal valve repair, and inferior turbinate reduction were performed. The standard external rhinoplasty approach was used to gain access to the septum and lower 2/3 of the external nose. Submucosal resection of the deviated mid-portion of the septum was performed. Septal cartilage was then used to create a butterfly graft, bridging the nasal dorsum and positioned beneath the lower lateral cartilages. Inferior turbinate reductions were accomplished via submucosal resection of the bone and soft tissue of the anterior half of the turbinates. Standard post-operative cares were performed and the patient’s recovery and healing were uneventful.
Modeling and Simulation
The pre-surgery and post-surgery CT scans were used to create digital 3-D models of the patient’s nasal cavity (excluding the paranasal sinuses), as well as the soft tissues surrounding the nasal cavity, using the image analysis software Mimics™ 14.0 (Materialise Inc., Plymouth, MI) (Fig. 1). Pre- and post-surgery models were co-registered using 3D reconstructions of the skull in each scan. In order to isolate the effects of the separate components of the surgery, two hybrid nasal cavity models using portions of the pre- and post-operative CT scans were created. To allow investigation of the intranasal component of the surgery (septoplasty and turbinate reduction) alone, a model was created using the external nose of the pre-operative model fused to the nasal cavity of the post-operative model. Another model was created using the external nose of the post-operative model fused to the nasal cavity of the pre-operative model to allow investigation of the nasal valve repair component of the surgery alone. Any minor mismatches at the interfaces where the portions of the models were fused were smoothed using the image analysis software in order to minimize their effect on the results of the simulation.
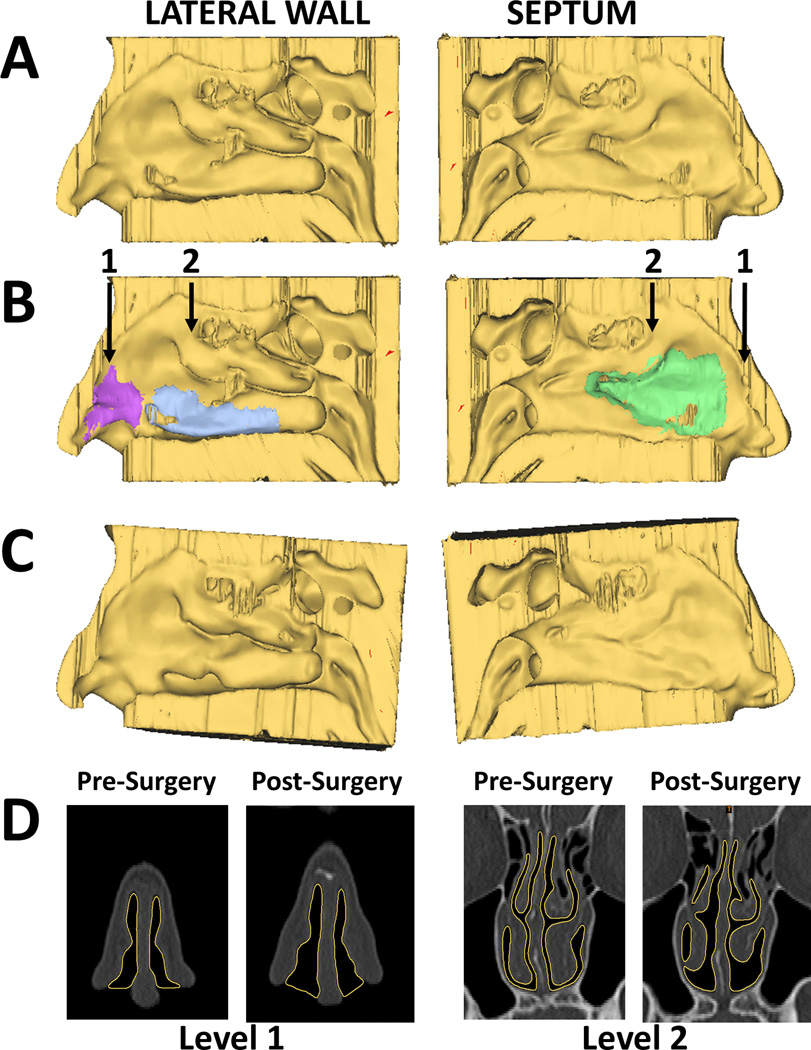
Figure 1.
(A) Reconstructions of right lateral wall and right side of septum from pre-surgery CT scan. (B) Same views with surgical sites marked. Purple=nasal valve repair; blue=right inferior turbinate reduction; green=septoplasty. Arrows indicate coronal levels shown in (D). (C) Reconstructions of right lateral wall and right septum from post-surgery CT scan, coregistered with pre-surgery scan. (D) Coronal views from pre- and post-surgery CT scans. Yellow outline highlights border between soft tissue and nasal air space.
The CFD modeling methods used here have been described in detail elsewhere14. Briefly, planar nostril and outlet surfaces were created and the digital models were meshed with approximately 4 million tetrahedral cells using ICEM-CFD™ 11.0 (ANSYS Inc, Canonsburg, PA). Steady-state inspiratory airflow simulations were conducted using Fluent™ 12.0 (ANSYS Inc, Canonsburg, PA) with the following boundary conditions: (1) a “wall” condition (in which velocity was set to zero at airway walls which were assumed to be stationary); (2) a “pressure-inlet” condition at the nostrils in which gauge pressure was set to zero; and (3) a “pressure-outlet” condition at the outlet in which gauge pressure was set to a negative value in Pascals (Pa) corresponding to a target steady-state flow rate. The target airflow rate was 14.9 L/min, twice the patient’s estimated minute volume (amount of air exhaled in 1 min), which was obtained from body weight15. Values used for the density and dynamic viscosity of air were 1.204 kg/m3 and 1.825 × 10−5 kg/m-sec, respectively. Fluent™ and the post-processing software Fieldview™ 12.0 (Intelligent Light, Lyndhurst, NJ) were used for simulation analysis and visualization of results.
Outcome Measures
The outcome measures calculated by CFD analysis were bilateral and unilateral nasal resistance and airflow allocation. Resistance was calculated as ∆p/Q, where ∆p was the transnasal pressure drop in Pascals (Pa) between the nostrils and the posterior end of the septum, and Q was the volumetric airflow rate (ml/sec). Airflow allocation was determined as the percentage of the total airflow that traveled through each side of the nasal cavity. Additionally, the patient was administered the Nasal Obstruction Symptom Evaluation (NOSE) Scale to collect information on patient-reported symptoms before and after surgery16. The NOSE Scale is a disease-specific quality of life instrument for NAO that has been validated for septoplasty11 and nasal valve repair, for which it has also been used to measure surgical success1,17. Finally, as part of the research protocol, unilateral Visual Analog Scale (VAS) scores for nasal airflow were determined pre- and post-operatively. The patient was asked to cover one nostril and rate their ability to breathe through the uncovered nostril on a scale from 0 (completely obstructed) to 10 (no obstruction). The process was then repeated for the opposite nostril.
Results
Before surgery, bilateral nasal resistance was estimated from CFD analysis to be 0.0979 Pa/(ml/s). In the post-operative model, bilateral nasal resistance was 0.0742 Pa/(ml/s), a 24.2% reduction from pre-surgical resistance. In the model with nasal valve repair alone, bilateral nasal resistance was 0.0922 Pa/(ml/s), a 5.8% reduction from pre-surgical resistance. In the model with septoplasty and inferior turbinate reduction alone, bilateral nasal resistance was 0.0788 Pa/(ml/s), a 19.5% reduction from pre-surgical resistance (Table 1). When examined unilaterally, right-sided nasal resistance decreased from 0.2604 Pa/(ml/s) pre-operatively to 0.1217 Pa/(ml/s) post-operatively, a 53.2% reduction. Conversely, left-sided nasal resistance was mildly increased from 0.1577 Pa/(ml/s) pre-operatively to 0.1904 Pa/(ml/s) post-operatively, an increase of 20.7%. In the nasal valve repair alone model, nasal resistance was mildly decreased in both sides compared to pre-surgery, while in the septoplasty and turbinate reduction model right-sided nasal resistance was reduced by about 50% but left-sided nasal resistance was increased by approximately 27% compared to pre-surgery. The unilateral nasal resistance results of the septoplasty and turbinate reduction alone model approximated the results of the post-surgery model (Table 2).
Table 1.
Changes in bilateral nasal resistance by model
| Model | Bilateral Nasal Resistance (Pa/(mL/s)) | % Change |
|---|---|---|
| Pre-surgery | 0.0979 | Reference |
| Nasal valve repair alone | 0.0922 | −5.8 |
| Septoplasty, turbinate reduction alone | 0.0788 | −19.5 |
| Post-surgery | 0.0742 | −24.2 |
Table 2.
Changes in unilateral nasal resistance by model
| Unilateral nasal resistance (Pa/(mL/s)) | ||||
|---|---|---|---|---|
| Model | Right | % change | Left | % change |
| Pre-surgery | 0.2604 | reference | 0.1577 | reference |
| Nasal valve repair alone | 0.2476 | −4.9 | 0.1479 | −6.2 |
| Septoplasty, turbinate reduction alone | 0.1298 | −50.2 | 0.2010 | +27.5 |
| Post-surgery | 0.1217 | −53.2 | 0.1904 | +20.7 |
Allocation of airflow between the nasal cavities was altered by surgery as well (Table 3). Prior to surgery 62.9% of airflow passed through the left side of the nose, while after surgery 38.4% passed through the left side. The nasal valve repair alone model showed an allocation pattern similar to pre-surgery (63.3% through the left side), while the septoplasty/inferior turbinate reduction model had a pattern similar to the post-surgery pattern (39.0% through the left side). Airstream flow through the post-operative nasal passage was noted to be qualitatively improved as exemplified by more laminar flow (Videos 1, 2). Symptomatically the patient reported improvement after surgery, with a reduction in NOSE score from 60 pre-surgery to 20 post-surgery. VAS scores improved as well, with improvements on the right side from 2 pre-operatively to 7 post-operatively and on the left side from 3 pre-operatively to 10 post-operatively. Comparison of the appearance of the external nose pre- and post-operatively shows a qualitative improvement in the area of the nasal valves (Video 3), and comparison of the appearance between the actual photos and digital soft tissue models shows excellent correlation (Figs. 2,3). Virtual endoscopic view demonstrates improvement in the nasal valve area as viewed from the vestibule (Fig. 4).
Table 3.
Airflow allocation by model
| Airflow Allocation | ||
|---|---|---|
| Model | Right (%) | Left (%) |
| Pre-surgery | 37.1 | 62.9 |
| Nasal valve repair alone | 36.7 | 63.3 |
| Septoplasty, turbinate reduction alone | 61.0 | 39.0 |
| Post-surgery | 61.6 | 38.4 |
Figure 2.
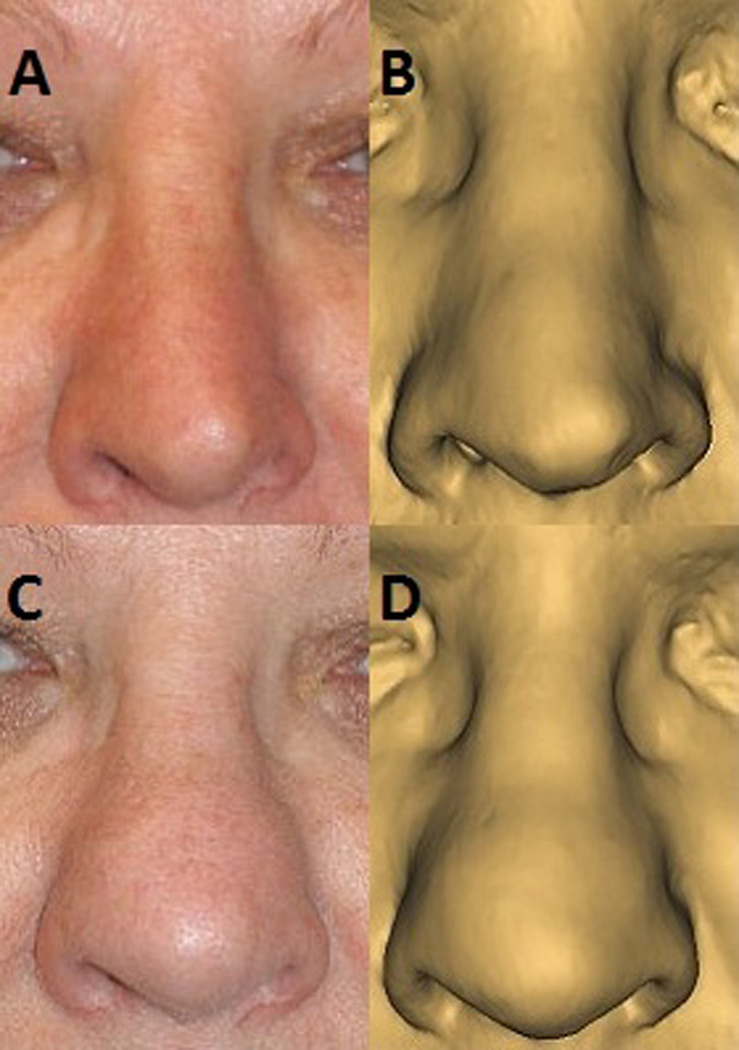
Pre-operative frontal view (A), compared to appearance on digital model of soft tissue of face (B). 6 month post-operative frontal view (C), compared to appearance on digital model of soft tissue of face (D).
Figure 3.
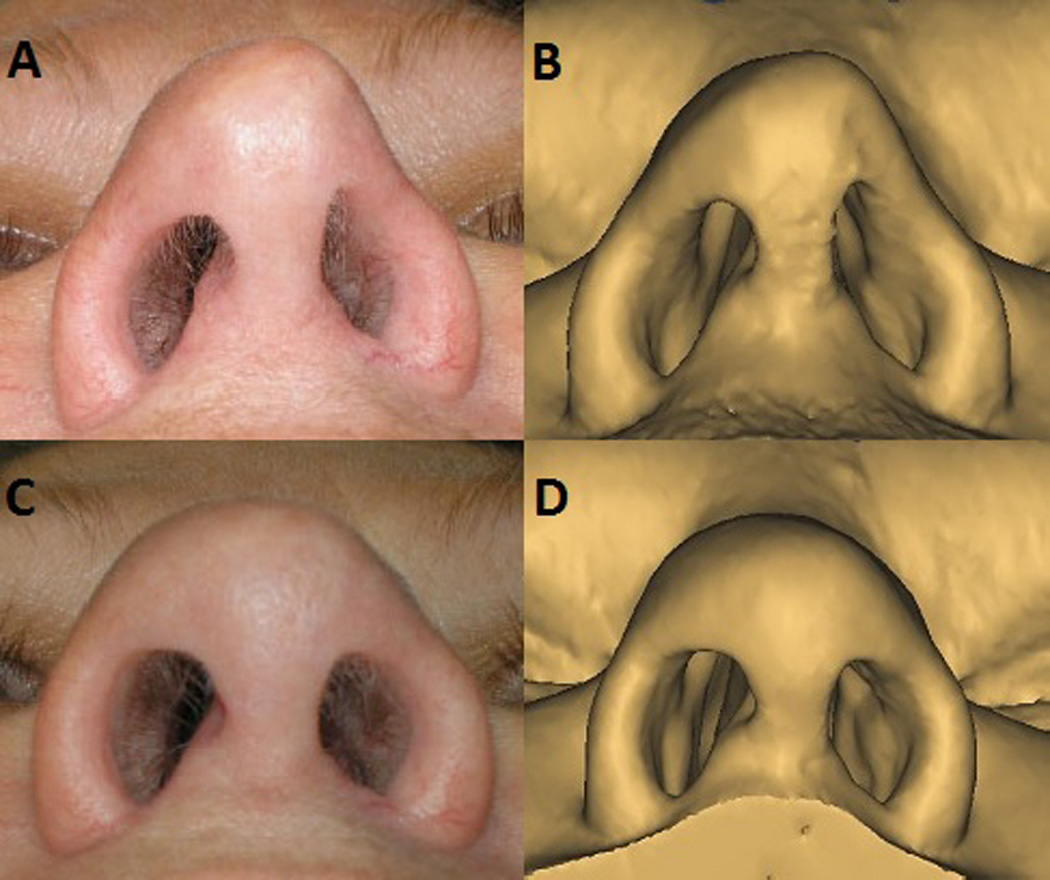
Pre-operative base view (A), compared to appearance on digital model of soft tissue of face (B). 6 month post-operative base view (C), compared to appearance on digital model of soft tissue of face (D).
Figure 4.
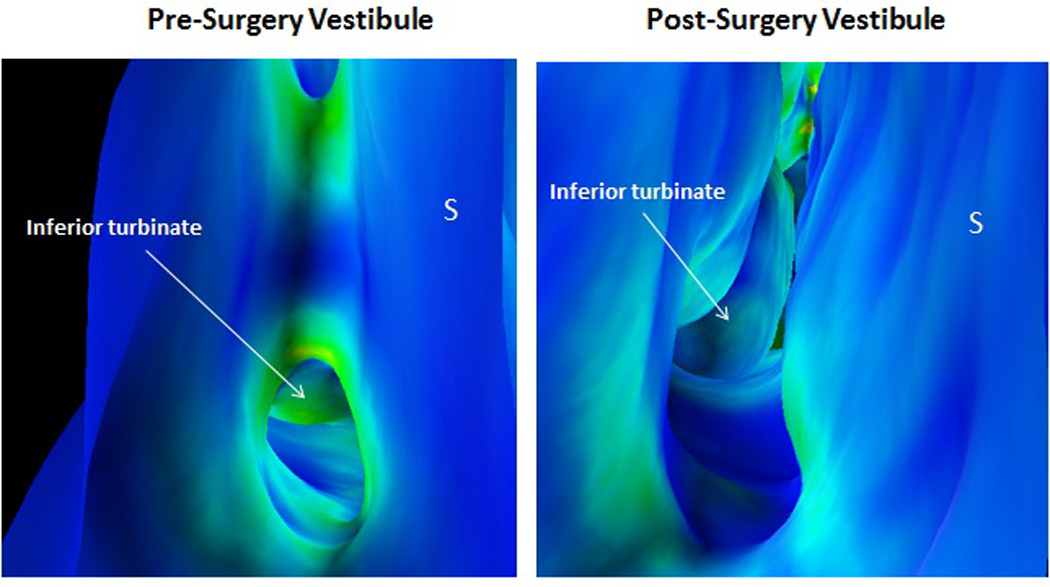
Virtual endoscopic view from right nasal vestibule comparing pre- and post-operative appearance. Images are color-coded for wall shear, with blue signifying areas of low shear stress and green-yellow signifying areas of higher shear stress. “S” denotes nasal septum. Note contact between septum and lateral wall in pre-operative image with associated higher shear stress above and below this area.
Comment
Nasal obstruction is a complex phenomenon that likely has many contributing factors. Even within the domain of anatomic nasal obstruction there can be several potential problematic areas, creating a dilemma for the nasal surgeon in deciding which components of the nasal airway to target surgically in order to produce the best possible outcome for the patient. While experience and clinical judgment are essential, it would be useful to have additional assistance with pre-operative planning.
In this study we have examined a patient with multiple anatomic causes of nasal obstruction treated surgically in an effort to determine how much benefit could be attributed to each of the components. In this particular patient, the majority of the benefit with respect to bilateral nasal resistance was a result of the combined septoplasty and inferior turbinate reduction. However, additional benefit in overall nasal resistance was added by the performance of nasal valve repair. The right side, which was most affected by the septal deviation, showed substantial decrease in nasal resistance following septoplasty and turbinate reduction when examined unilaterally, while the left side showed a moderate increase, as would be expected following correction of the septal deviation. The effect of surgery on airflow allocation was exclusively a result of the septoplasty and turbinate reduction as well. Nasal valve repair alone produced mild decreases in unilateral nasal resistance for both sides of the nose.
To our knowledge, this is the first study that simulates individual virtual surgery scenarios (nasal valve surgery vs. internal nasal surgery) with comparison to a known outcome. A previous article published by our group13 used a virtual surgery model and CFD techniques to examine the effects of septoplasty and turbinate reduction on nasal resistance and airflow. One of the best attempts at quantifying the nasal valve component (a.k.a. functional rhinoplasty) separate from other surgical manipulations was performed by Constantian and Clardy18, in which they investigated the relative importance of septal surgery and nasal valve repair in correcting NAO. They prospectively evaluated a group of patients with NAO using rhinomanometry as their primary outcome measure. Patients were stratified according to the site of their obstruction as determined by clinical exam: internal nasal valves, external nasal valves, septum, or any combination of these three; patients with turbinate hypertrophy were excluded. Surgery was then performed with targeting of problematic areas identified pre-operatively. The authors concluded that the effects of NVC may equal or even surpass septal deviation as the primary cause of NAO, in contrast to what was discovered in the present simulation.
The question of what constitutes a successful nasal surgery is a key consideration. As of yet a correlation between CFD data and subjective, patient-reported measures has not been reported. In this particular case, the patient did report symptomatic improvement in nasal symptoms, as represented by her improvements in NOSE and VAS scores. Accordingly, she also exhibited improvement in bilateral nasal resistance as well in unilateral resistance on the side affected by septal deviation as determined with CFD techniques. Interestingly, however, the patient reported an improvement in VAS scores on the contralateral side even though the nasal resistance actually increased on the left side (less symptomatic side) as one would expect when the septum is placed more in the midline. This potentially paradoxical finding does highlight the seemingly inconsistent relationship between objective measures such as nasal resistance and patient reported measures and the need for a more sophisticated model of understanding perceptions of nasal airflow with objective measures of nasal patency.
In this particular instance, this paradoxical finding may be explained by the following possibilities: surgical placebo effect, “halo” effect (i.e. improvement of the symptomatic side overrides all), and/or threshold effect (i.e. nasal resistance increased but not enough for a perceptual change). The underlying assumption of a “threshold effect” is that there is a certain objective point at which patients will experience a subjective improvement in their obstructive symptoms. Therefore, one need only select the procedure or combination of procedures that would meet this cutoff point, and additional procedures above and beyond this would be unlikely to provide additional benefit. This concept of “threshold effect” is an intriguing one that is highlighted by breaking down the individual components of our example of functional airway surgery. Though the nasal valve surgery effect on nasal resistance was less than the internal nasal surgery (septoplasty and turbinate reduction), the question remains whether the patient would have subjectively felt improved without the additional nasal valve component. Furthermore, if the left turbinate (the less symptomatic side pre-operatively) was not addressed, would the resultant nasal resistance on the left side been too high producing a sensation of nasal obstruction on that side? Would the “threshold” have been breached? The underlying rationale for the left turbinate reduction pre-operatively was to mitigate the potential reverse nasal obstruction sensation that may result when performing a septoplasty with a compensatory hypertrophied turbinate on the less symptomatic side.
Moreover, it is unclear whether the absolute versus relative values of nasal resistance between sides of the nasal cavity and between pre- and post-operative conditions are more paramount for measuring patient outcomes. There is a “normal” range of nasal resistances and certainly marked outliers would likely be correlated with patient-perceived nasal obstruction. However, this normal range is quite large and it may be that the relative values between sides of the nasal cavity and change from the pre-operative state dictate a patient-perceived improvement.
Another area of uncertainty is the importance of balanced airflow allocation between sides of the nose. In the pre-operative model more airflow occurred through the left side, while this pattern was essentially reversed in the post-operative state. The airflow allocation in the rhinoplasty alone model closely mirrored that of the pre-operative model, while that of the septoplasty/inferior turbinate reduction model closely mirrored the post-operative model. This suggests that in this patient surgery on the internal portion of the nasal airway had a greater effect on airflow allocation than that of external nasal surgery. The clinical significance of this is unclear. Whether imbalance in airflow allocation is an important contributing factor in the sensation of nasal obstruction has not been well studied. Additional objective measures, such as wall shear, mucosal temperature changes, or specific airflow allocation patterns within a nasal passage, may be contributors to patient-perceived improvement and successful outcomes. In essence, the objective measures that correlate best with patient-perceived improvement may be multifactorial and we are just beginning to model and understand these components.
There are several limitations to this study which should be acknowledged. The dynamic nature of nasal valve compromise was not accounted for in the CFD modeling presented here as the walls of the nasal cavity were fixed. Additional assumptions of laminarity and that steady-state airflow was a useful approximation for cyclic flow are reasonable in many cases but may not always hold true. From a practical standpoint, at present a great deal of time, technical expertise, and computing capability is needed to construct the digital models and run the simulations which precludes its routine use at this time. Finally, differences among patients in healing, tissue properties, nasal sensation, and psychological factors may limit the ability to precisely predict successful surgical outcomes.
A potential criticism of this particular study is the fact that the changes in both unilateral and bilateral nasal resistance of the post-operative model do not match the sum of the hybrid models. As mentioned in the methods section, there was not an exact match in the transition point when the model portions were fused together, with some minor modifications necessary which may account for some of the difference. Another possibility is that there is some subtle interaction between the surgical procedures such that their effect when combined is different than the sum of their individual effects. The Constantian study18 supports this, as their results showed that the effects of individual surgical components are not simply additive when performed in conjunction. Regardless of the cause, the calculated differences were relatively small compared to the overall large percentage changes.
Much of the literature on simulation in surgery involves its utility in the acquisition of surgical skills, not as a tool to guide pre-operative planning. However, the complexity of nasal obstruction lends itself well to such an endeavor. Furthermore, the advent of CFD technology provides a powerful tool that greatly aids the study of nasal physiology in both normal and pathological conditions and its potential can be extended to the use of virtual surgery in pre-operative planning. At this point CFD-aided virtual nasal surgery is still in its infancy and routine use is not currently practical. However, one can envision a future in which a nasal surgeon would have the ability to use validated CFD-aided virtual surgery tools using easily obtainable imaging data and packaged in a user-friendly configuration that would allow rapid, sophisticated in-office analysis to identify problematic areas and simulate the effects of surgical techniques in a way that would allow the surgeon to tailor an individualized surgical approach. Such a tool would help provide safe, cost-effective, and patient-centered care in a manner that could lead to the best possible surgical outcomes.
Supplementary Material
Airstreams of right nasal cavity prior to surgery.
Airstreams of right nasal cavity after surgery.
Appearance of nasal valve and nasal base before and after surgery.
Acknowledgments
Financial support:
This research was funded by grant R01EB009557 from the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering to the Medical College of Wisconsin (MCW) and by subcontract from MCW to the University of North Carolina at Chapel Hill.
Footnotes
Financial disclosures/conflicts of interest:
The authors have nothing to disclose.
Presentation:
Accepted for presentation at the American Academy of Facial Plastic and Reconstructive Surgery meeting at the Combined Otolaryngology Spring Meeting, April 18–19, 2012 in San Diego, California.
References
- 1.Rhee JS, Poetker DM, Smith TL, Bustillo A, Burzynski M, Davis RE. Nasal valve surgery improves disease-specific quality of life. Laryngoscope. 2005;115(3):437–440. doi: 10.1097/01.mlg.0000157831.46250.ad. [DOI] [PubMed] [Google Scholar]
- 2.Illum P. Septoplasty and compensatory inferior turbinate hypertrophy: Long-term results after randomized turbinoplasty. Eur Arch Otorhinolaryngol. 1997;254(Suppl 1):S89–S92. doi: 10.1007/BF02439733. [DOI] [PubMed] [Google Scholar]
- 3.Andre RF, Vuyk HD, Ahmed A, Graamans K, Nolst Trenite GJ. Correlation between subjective and objective evaluation of the nasal airway. A systematic review of the highest level of evidence. Clin Otolaryngol. 2009;34(6):518–525. doi: 10.1111/j.1749-4486.2009.02042.x. [DOI] [PubMed] [Google Scholar]
- 4.Lam DJ, James KT, Weaver EM. Comparison of anatomic, physiological, and subjective measures of the nasal airway. Am J Rhinol. 2006;20(5):463–470. doi: 10.2500/ajr.2006.20.2940. [DOI] [PubMed] [Google Scholar]
- 5.Stewart MG, Smith TL. Objective versus subjective outcomes assessment in rhinology. Am J Rhinol. 2005;19(5):529–535. [PubMed] [Google Scholar]
- 6.Becker SS, Dobratz EJ, Stowell N, Barker D, Park SS. Revision septoplasty: Review of sources of persistent nasal obstruction. Am J Rhinol. 2008;22(4):440–444. doi: 10.2500/ajr.2008.22.3200. [DOI] [PubMed] [Google Scholar]
- 7.Moore M, Eccles R. Objective evidence for the efficacy of surgical management of the deviated septum as a treatment for chronic nasal obstruction: A systematic review. Clin Otolaryngol. 2011;36(2):106–113. doi: 10.1111/j.1749-4486.2011.02279.x. [DOI] [PubMed] [Google Scholar]
- 8.Batra PS, Seiden AM, Smith TL. Surgical management of adult inferior turbinate hypertrophy: A systematic review of the evidence. Laryngoscope. 2009;119(9):1819–1827. doi: 10.1002/lary.20544. [DOI] [PubMed] [Google Scholar]
- 9.Rhee JS, Arganbright JM, McMullin BT, Hannley M. Evidence supporting functional rhinoplasty or nasal valve repair: A 25-year systematic review. Otolaryngol Head Neck Surg. 2008;139(1):10–20. doi: 10.1016/j.otohns.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Harrill WC, Pillsbury HC, 3rd, McGuirt WF, Stewart MG. Radiofrequency turbinate reduction: A NOSE evaluation. Laryngoscope. 2007;117(11):1912–1919. doi: 10.1097/MLG.0b013e3181271414. [DOI] [PubMed] [Google Scholar]
- 11.Stewart MG, Smith TL, Weaver EM, et al. Outcomes after nasal septoplasty: Results from the nasal obstruction septoplasty effectiveness (NOSE) study. Otolaryngol Head Neck Surg. 2004;130(3):283–290. doi: 10.1016/j.otohns.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Pawar SS, Garcia GJ, Kimbell JS, Rhee JS. Objective measures in aesthetic and functional nasal surgery: Perspectives on nasal form and function. Facial Plast Surg. 2010;26(4):320–327. doi: 10.1055/s-0030-1262314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rhee JS, Pawar SS, Garcia GJ, Kimbell JS. Toward personalized nasal surgery using computational fluid dynamics. Arch Facial Plast Surg. 2011;13(5):305–310. doi: 10.1001/archfacial.2011.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia GJ, Bailie N, Martins DA, Kimbell JS. Atrophic rhinitis: A CFD study of air conditioning in the nasal cavity. J Appl Physiol. 2007;103(3):1082–1092. doi: 10.1152/japplphysiol.01118.2006. [DOI] [PubMed] [Google Scholar]
- 15.Garcia GJ, Schroeter JD, Segal RA, Stanek J, Foureman GL, Kimbell JS. Dosimetry of nasal uptake of water-soluble and reactive gases: A first study of interhuman variability. Inhal Toxicol. 2009;21(7):607–618. doi: 10.1080/08958370802320186. [DOI] [PubMed] [Google Scholar]
- 16.Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the nasal obstruction symptom evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130(2):157–163. doi: 10.1016/j.otohns.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 17.Most SP. Analysis of outcomes after functional rhinoplasty using a disease-specific quality-of-life instrument. Arch Facial Plast Surg. 2006;8(5):306–309. doi: 10.1001/archfaci.8.5.306. [DOI] [PubMed] [Google Scholar]
- 18.Constantian MB, Clardy RB. The relative importance of septal and nasal valvular surgery in correcting airway obstruction in primary and secondary rhinoplasty. Plast Reconstr Surg. 1996;98(1):38–54. doi: 10.1097/00006534-199607000-00007. discussion 55-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Airstreams of right nasal cavity prior to surgery.
Airstreams of right nasal cavity after surgery.
Appearance of nasal valve and nasal base before and after surgery.