Abstract
Preeclampsia is a heterogeneous syndrome affecting 3–5% of all pregnancies. An imbalance of the anti and pro-angiogenic factors, soluble receptor fms-like tyrosine kinase 1 (sFLT1) and placental growth factor (PGF), are thought to contribute to the pathophysiology of preeclampsia. Maternal plasma PGF and sFLT1 were quantified by specific immunoassays in cross-sectional samples from 130 preeclamptic subjects and 342 normotensive controls at delivery, and longitudinally in samples from 50 women who developed preeclampsia and 250 normotensive controls. Among women who developed preeclampsia, 46% (n=23) evidenced a pattern of consistently low maternal PGF across pregnancy below the lower 95%CI of controls from 15 weeks gestation to term. In contrast, the remaining 54% (n=27) women who developed preeclampsia had maternal PGF concentrations similar to or above (n=7) those of normotensive controls. Subjects with low PGF across pregnancy who developed preeclampsia evidenced significantly higher blood pressure in early pregnancy (p<0.05), and after diagnosis, earlier gestational age at delivery (p<0.05), and more preterm birth (p<0.05) compared to preeclamptic patients with high PGF. A significant subset of women who develop preeclampsia evidence consistently low PGF across pregnancy. Low PGF with preeclampsia was associated with preterm delivery compared to preeclamptic patients with high PGF. Identifying women with consistently low plasma PGF during pregnancy may provide a greater understanding of preeclampsia pathophysiology, and may provide more focused research and clinical activities.
Keywords: pregnancy, preeclampsia, preterm birth, placental growth factor, soluble vascular endothelial growth factor receptor-1
Introduction
Preeclampsia is a pregnancy-specific syndrome affecting 3 to 5% of all pregnancies and is a leading cause of maternal and fetal morbidity and mortality worldwide. 1–3 Preeclampsia is diagnosed clinically by the presence of new onset gestational hypertension and proteinuria after 20 weeks gestation. Historically, these diagnostic criteria identify a subset of women at high risk for adverse maternal and fetal outcomes. 4 However, preeclampsia is recognized as a complex and heterogeneous syndrome, and far more than the diagnostic criteria of hypertension and proteinuria. 5
The pathophysiology of preeclampsia remains incompletely elucidated, however increased attention has been directed toward the role of angiogenic and anti-angiogenic factors including elevated soluble fms-like tyrosine kinase 1 receptor (sFLT1; also known as soluble VEGF receptor 1) and lower placental growth factor (PGF). 6–8 Concentrations of these factors are significantly different in women who develop preeclampsia several weeks before clinical manifestations of the disorder compared to women who have uncomplicated normotensive pregnancies. 7–9 However, the biologic variability in sFLT1 and PGF concentrations among subjects who later develop preeclampsia compared to normotensive controls appears to limit their clinical utility as predictive markers. 10–13 Conversely, the biologic variability in sFLT1 and PGF concentrations observed in subjects who later develop preeclampsia may be useful in further differentiating the heterogeneous nature of preeclampsia and provide further insight into the pathophysiology of the syndrome.
The goal of this study was to investigate the heterogeneous nature of PGF and sFLT1 longitudinally among subjects who later develop preeclampsia compared to normotensive controls, and to investigate if longitudinal patterns of these factors may identify subsets of preeclamptic subjects with differences in demographics and pregnancy outcome.
Material and Methods
A detailed method description is provided in the accompanying online-only Data Supplement.
Results
PGF and sFLT1 in Term and Preterm Preeclampsia
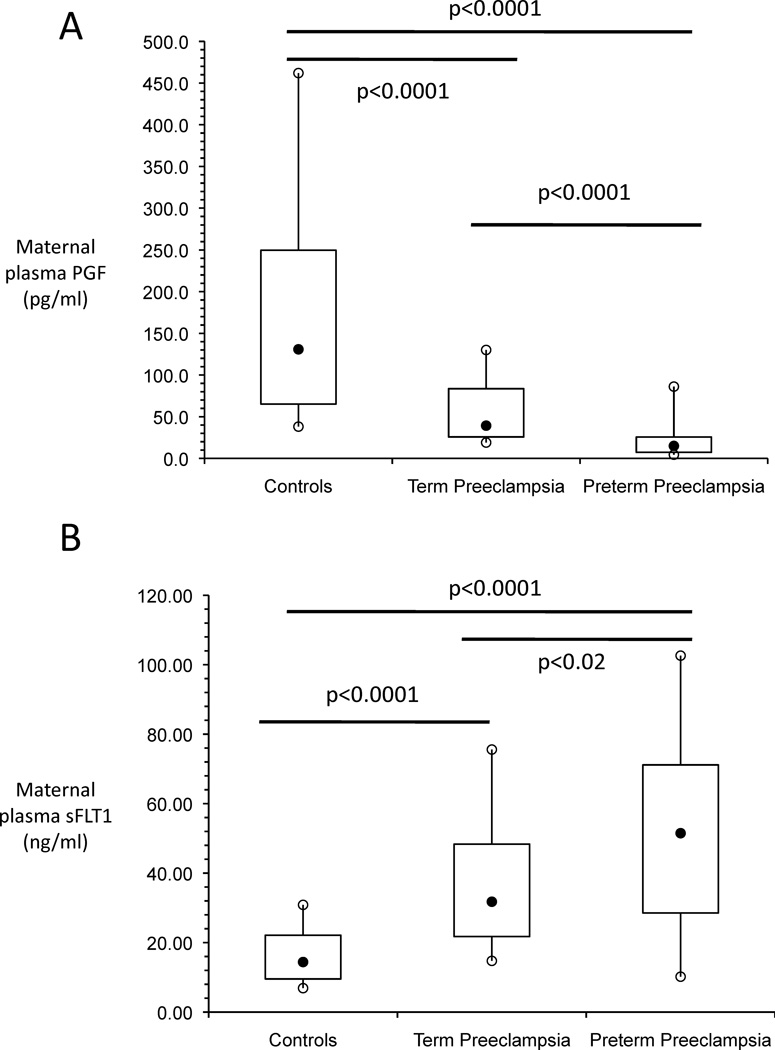
Similar to previous reports 7–9, maternal plasma PGF was significantly lower and sFLT1 was significantly higher in women with preeclampsia compared to normotensive controls (Figures 1A & 1B). PGF was significantly lower and sFLT1 was significantly higher in women with term preeclampsia compared to controls (both p<0.0001). In addition, PGF was significantly lower and sFLT1 was significantly higher in women with preterm preeclampsia compared to women with term preeclampsia (p<0.0001 and p<0.02, respectively) as well as normotensive controls (p<0.0001 and p<0.0001, respectively).
Figure 1. Box and whisker plots of maternal plasma PGF (A) and maternal plasma sFLT1 (B) at delivery.
The filled black circles are the median, the open circles are the 90th and 10th percentiles, and the top and bottom lines of the box are the 75th and 25th percentiles of the data for each group. Statistical significance is indicated by p values.
Longitudinal differences in PGF and sFLT1 between Preeclampsia and Normotensive Controls
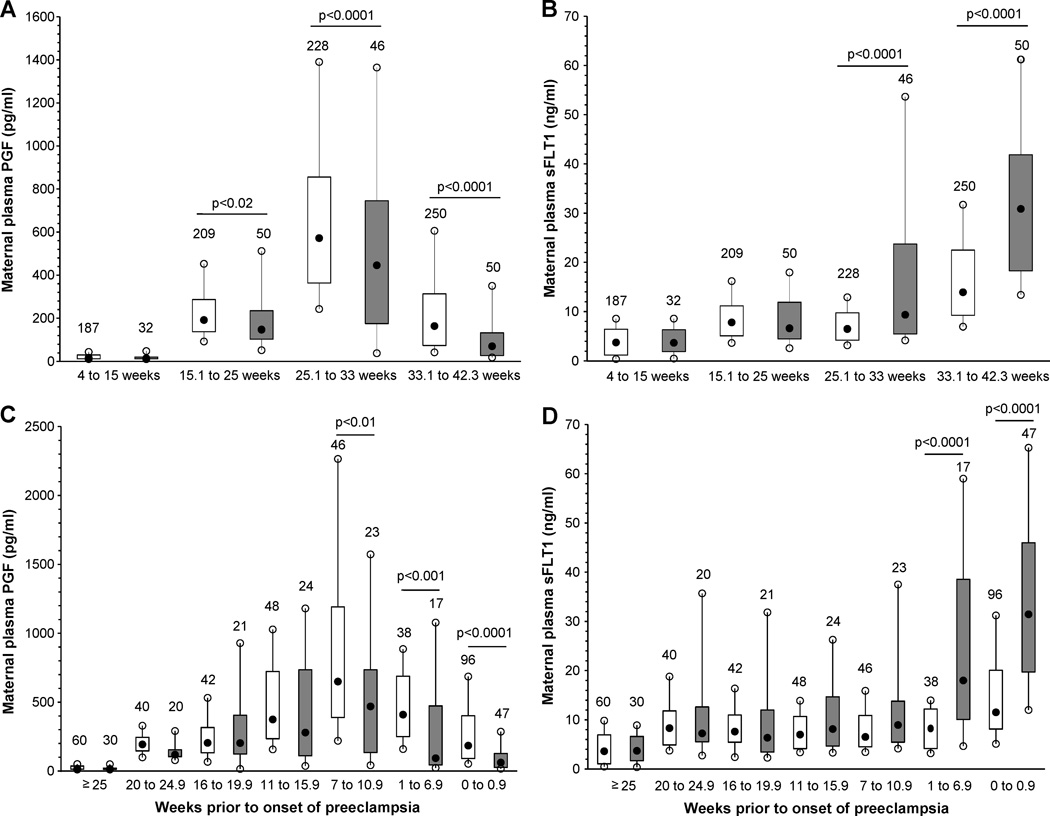
We next investigated differences in PGF and sFLT1 in maternal plasma samples collected longitudinally during pregnancy. Maternal plasma PGF concentrations were not different between 4 to 15 weeks gestation in women who later developed preeclampsia compared to uncomplicated controls (Figure 2A). However, plasma PGF concentrations were significantly lower between 15.1 to 25 weeks gestation in women who later developed preeclampsia (p<0.02, Figure 2A), and maternal plasma PGF remained significantly lower in preeclampsia cases compared to normotensive controls between 25.1 weeks gestation to term delivery (Figure 2A).
Figure 2. Box and whisker plots of maternal plasma PGF (A, C) and maternal plasma sFLT1 (B, D) across gestation (A, B) or by clinical onset of preeclampsia with gestational age matched control samples (C, D).
Open boxes are controls and shaded boxes are subjects who developed preeclampsia. The number above each box indicates the number of samples. The filled black circles are the median, the open circles are the 90th and 10th percentiles, and the top and bottom lines of the box are the 75th and 25th percentiles of the data for each group. Statistical significance is indicated by p values.
In contrast, maternal plasma sFLT1 concentrations were not different between 4 to 15 weeks or at 15.1 to 25 weeks gestation between women who later developed preeclampsia compared to normotensive controls. However, plasma sFLT1 concentrations were significantly higher in preeclampsia cases compared to normotensive controls from 25.1 to 33 weeks and 33.1 weeks gestation to term (p<0.0001 both, Figure 2B).
ROC curves were constructed using maternal concentrations of PGF, sFLT1 and the PGF/sFLT1 ratio as predictors of preeclampsia and preterm preeclampsia. ROC curves were constructed using samples obtained between 15 and 23 weeks gestation before the onset of preeclampsia. The area under the ROC curve for PGF predicting preeclampsia and preterm preeclampsia was 0.68 and 0.73 respectively. Similarly, the area under the ROC curve for maternal sFLT1 between 15 and 23 weeks was 0.51 for predicting preeclampsia, and 0.56 for predicting preterm preeclampsia. The area under the ROC curve predicting preeclampsia using the PGF/sFLT1 ratio was 0.58, and 0.59 for predicting preterm preeclampsia. Using the best ROC curve and its optimal cut-off point the positive predictive value of PGF to predict preterm preeclampsia assuming a 1% occurrence rate would be 5%. These data are consistent with the heterogeneity of maternal plasma PGF and sFLT1 observed among women who later develop preeclampsia compared to normotensive controls as shown in Figures 2A and B.
We next investigated when the maternal concentration of PGF and sFLT1 were different between women who developed preeclampsia compared with normotensive controls in relation to the clinical onset of the syndrome. Box and whisker plots of maternal plasma PGF concentrations according to the clinical onset of preeclampsia (time 0) show significantly lower PGF as early as 7 to 10.9 weeks before the onset of the syndrome in women who developed preeclampsia compared to controls matched for gestational age at sample collection (p<0.01) and remained significantly lower including at the onset of the syndrome (p<0.0001, Figure 2C). Similarly, maternal plasma sFLT1 concentrations were significantly higher between 1 to 6.9 weeks before the onset of preeclampsia (p<0.0001) and remained significantly higher including at the onset of the syndrome (p<0.0001, Figure 2D). These data are consistent with previous studies, and also further demonstrate the heterogeneous nature of these factors in pregnancy and preeclampsia.
Low Maternal PGF Throughout Pregnancy Identifies a Subset of Preeclampsia Patients
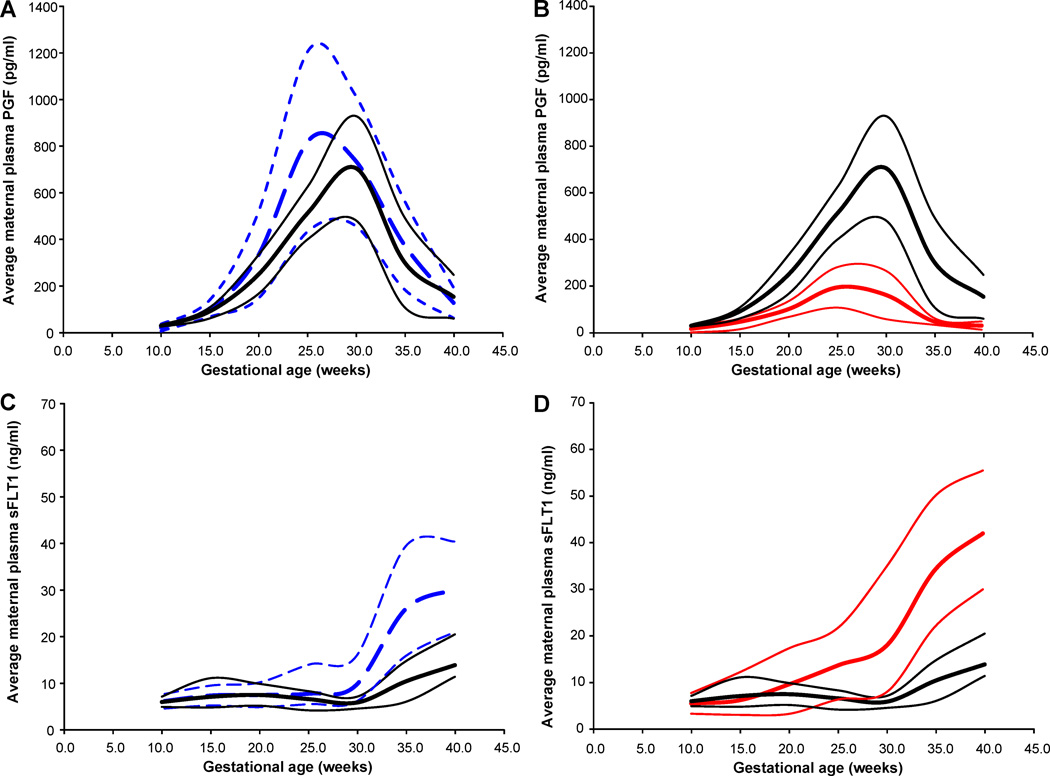
While differences in maternal plasma PGF and sFLT1 during gestation between women who develop preeclampsia and normotensive controls were statistically significant, it was evident that there was considerable variability in the concentration of these factors among subjects who developed preeclampsia. Therefore, we asked if this heterogeneity might resolve into identifiable longitudinal patterns suggestive of subsets of subjects. This investigation identified two distinct patterns of maternal plasma PGF during pregnancy among women who later developed preeclampsia. The first pattern of PGF among subjects who developed preeclampsia was similar to maternal plasma PGF concentrations observed among normotensive control subjects, in which plasma PGF concentrations rise sharply in early pregnancy, peak around 25 to 28 weeks gestation and after which PGF concentrations decrease sharply to term. The concentration of maternal plasma PGF in these preeclampsia patients, although lower at the time of the clinical presentation of the syndrome, was within (or greater than, n=7 subjects) the 95% confidence interval of plasma PGF in normotensive control subjects (Figure 3A). Among the 50 preeclampsia subjects in this study, 27 (54%) exhibited this pattern of PGF across gestation. The second pattern of PGF was present in the remaining 23 women who developed preeclampsia (46%). In these subjects PGF concentrations were consistently below the lower 95% confidence interval of maternal PGF concentrations observed in normotensive control subjects from 15 weeks gestation to the end of pregnancy (Figure 3B). Interestingly, the pattern of plasma sFLT1 was also different across pregnancy between these two groups of preeclamptic subjects with differing patterns of PGF. The concentration of sFLT1 was not different between normotensive controls and preeclampsia subjects with the high PGF pattern until after 35 weeks gestation (p<0.05)(Figure 3C). In contrast, as shown in Figure 3D, women who developed preeclampsia and exhibited the low PGF pattern exhibited a significant and earlier rise in plasma sFLT1 after 30 weeks gestation as well as higher concentrations of sFLT1 in late pregnancy compared to normotensive controls (p<0.01). The PGF/sFLT1 ratios for the low and high PGF preeclampsia groups compared to controls follow the pattern of maternal PGF and are shown in supplemental figure 1 (S1).
Figure 3. Median ± 95% CI of plasma PGF concentrations (A, B) and sFLT1 concentrations (C, D) from 10 to 40 weeks gestation.
Women with uncomplicated pregnancies (solid black line), women who developed preeclampsia and had plasma PGF concentrations within the 95%CI of control uncomplicated pregnant women (dashed blue line, A & C), and women who developed preeclampsia and had plasma PGF concentrations below the 95%CI of control uncomplicated pregnant women from 15 weeks gestation to delivery (solid red line, B & D).
We next investigated if these markedly different patterns of PGF might identify subsets of preeclamptic women. To address this question we examined clinical data for differences between these two groups as well as differences compared to normotensive control subjects. Compared to preeclampsia subjects with the high PGF pattern, preeclampsia subjects with the low PGF pattern delivered significantly earlier (37.2±3.8 vs. 38.6±1.9 weeks gestation, p<0.05), had more preterm births (39% vs. 11%, p<0.05), higher average blood pressures before 20 weeks gestation (p<0.001), more SGA infants (22% vs. 11%, p=0.30), and lower average infant birth weight centiles (34.9±24.8% vs. 41.6±32.8%, p=0.42) but not statistically different. We also examined outcomes in normotensive control subjects with the lowest 5% of PGF concentrations across pregnancy (n=12) similar to the low PGF preeclampsia group, and these subjects did not deliver earlier or have smaller babies compared to controls without low PGF (Table 3). Interestingly, normotensive controls with low PGF evidenced higher average pre-delivery systolic blood pressures compared to normotensive controls without low PGF (p<0.03) and a trend toward significantly higher pre-delivery diastolic blood pressure (p=0.08). In addition, there were significantly fewer smokers among the preeclamptic and normotensive control subjects with low PGF compared to controls without low PGF, however the pattern of low PGF persisted after controlling for smoking among the preeclampsia subjects. Overall, there are two distinct patterns of maternal circulating PGF concentrations during pregnancy among women who later develop preeclampsia in the absence of differences in maternal adiposity or race, and these PGF patterns are associated with differences in maternal and infant outcomes among preeclamptic subjects.
Table 3.
Characteristics of Control and Preeclampsia Subjects and their Infants According to Maternal PGF Profile during Pregnancy.
| variable | Controls (n=238) |
Controls, low PGF (n=12) |
Preeclampsia, high PGF (n=27) |
Preeclampsia, low PGF (n=23) |
|---|---|---|---|---|
| Maternal age (years) | 22.3±4.4 | 26.7±6.5* | 23.4±5.4 | 23.1±6.0 |
| Pre-pregnancy BMI (kg/m2) |
25.2±6.4 | 28.9±7.4 | 25.2±5.6 | 27.4±6.4 |
| Gestational age at delivery (weeks) |
40.1±1.0 | 40.6±1.1 | 38.6±1.9* | 37.2±3.8*† |
| Preterm birth n (%) |
0 (0%) | 0 (0%) | 3 (11%) | 10 (43%)† |
| Pre-delivery uric acid (mg/dl) |
4.9±1.1 | 4.6±0.8 | 5.8±1.3* | 5.5±1.4* |
| Average blood pressure before 20 weeks gestation (mmHg) |
112.8±7.5/ 68.4±5.5 |
113.6±9.1/ 70.2±5.1 |
113.3±6.5/ 67.0±6.8 |
118.7±9.1*†/ 71.4±4.7*† |
| Average blood pressure pre-delivery (mmHg) |
119.9±10.9/ 72.7±7.9 |
127.5±16.5*/ 76.7±5.3 |
143.6±13.0*/ 87.6±7.3* |
148.3±13.1*/ 91.2±8.7* |
| Race (% African American) |
78 (33%) | 3 (25%) | 9 (33%) | 9 (39%) |
| Smokers n (%) |
145 (61%) | 2 (17%)* | 13 (48%) | 8 (35%)* |
| Placental weight (grams) (n) |
466.2±88.9 (45) |
400.0±70.7 (3) |
436.7±98.4 (15) |
396.1±140.8* (15) |
| Infant birth weight (grams) |
3431.9±429.4 | 3585.7±349.6 | 3066.2±666.6* | 2601.9±825.2*† |
| Infant length (cm) |
50.6±3.8 | 51.1±2.1 | 49.7±3.6 | 47.9±3.5* |
| Infant head circumference (cm) |
34.2±1.6 | 34.9±1.3 | 32.7±2.2* | 32.8±2.1* |
| Birth weight centile (%) |
51.9±26.0 | 60.2±25.1 | 41.6±32.8 | 34.9±24.8* |
| SGA infants n (%) |
7 (3%) | 0 (0%) | 3 (11%)* | 5 (22%)* |
Data are mean±SD;
p<0.05 compared to controls;
p<0.05 compared to preeclampsia.
Discussion
In this study we observed two distinct patterns of maternal plasma PGF across pregnancy. One PGF pattern resembled that observed among uncomplicated normotensive pregnant women, while the other maternal plasma PGF pattern was consistently below the lower 95%CI of plasma PGF observed among uncomplicated pregnant women after 15 weeks gestation to delivery. This low pattern of maternal PGF was present in nearly half (46%, 23/50) of the women who developed preeclampsia in this study. It is obvious that subjects with this low PGF pattern prior to clinically evident preeclampsia account for the statistically significant differences in PGF observed among the entire longitudinal cohort of women who develop preeclampsia. Finally, we observed that preeclamptic subjects with the consistent low PGF pattern across pregnancy exhibited differences in clinical outcome data compared to preeclamptic subjects with the high PGF pattern including: significantly elevated blood pressure before 20 weeks gestation, significantly earlier gestational age at delivery, more preterm births, and more SGA infants. Low PGF alone was not sufficient to account for these findings in the absence of other components of preeclampsia pathophysiology since these clinical differences were not present in the 5% of normotensive control women with similarly low PGF concentrations across pregnancy (although blood pressure was slightly higher at delivery in this group). However, these data support the heterogenous nature of preeclampsia, and suggest that these strikingly different patterns of maternal PGF across pregnancy among women who later develop preeclampsia may identify important subsets of preeclamptic patients.
In addition, we have confirmed that women with preeclampsia have significantly lower plasma PGF and higher sFLT1 concentrations compared to women with uncomplicated pregnancies, and that these differences are greatest among women with preterm preeclampsia. We also observed that overall maternal plasma PGF concentrations were significantly lower by mid-pregnancy (15 to 25 weeks gestation) and remained lower among women who later developed preeclampsia, and that maternal sFLT1 concentrations were significantly elevated later in pregnancy (25 to 33 weeks gestation) and remained elevated among women who developed preeclampsia compared to women with uncomplicated pregnancies. These differences in PGF and sFLT1 preceded the clinical onset of preeclampsia by 7 to 11 weeks and 1 to 7 weeks respectively. These data are consistent with other published studies that have suggested these analytes, alone or in combination, as potential predictive markers of preeclampsia. 10, 12, 14, 15 However, the heterogeneity of PGF and sFLT1 concentrations among women who develop preeclampsia limits their practical use as predictive markers of the syndrome as evidenced by the poor ROC curves obtained using PGF, sFLT1 and the PGF/sFLT1 ratio values from samples obtained between 15 and 23 weeks gestation before the clinical onset of the syndrome.
The variability of PGF and sFLT1 among pregnant women and women who develop preeclampsia is evident in many studies. 8, 16–18 PGF is a member of the vascular endothelial growth factor subfamily, it is expressed by trophoblast cells, and has both vasculogenetic and angiogenetic functions. PGF’s angiogenic abilities have been speculated to play a role in normal pregnancy, and changes in the concentrations of PGF and sFLT1 have been proposed in the pathophysiology of preeclampsia. 7, 9, 19 However, given the heterogeneous nature of PGF and sFLT1 among women who develop preeclampsia it seems unlikely that a single pattern or mechanism can explain all cases of preeclampsia. Additionally, these data indicate that these angiogenic markers are not necessarily causative for the incomplete or failed uterine spiral artery remodeling reported in preeclampsia, since their concentrations are similar to those observed among uncomplicated pregnant women in early pregnancy when these placental abnormalities begin to develop. 20
Several studies have reported that the circulating concentration of maternal PGF is significantly lower in early pregnancy among women who later develop preeclampsia compared to women with uncomplicated pregnancies, and that low maternal PGF concentrations are more pronounced among women who develop early onset preeclampsia and/or deliver SGA infants. 12, 18, 21–25 As shown in this study, the observation of low PGF in early pregnancy among all women who later develop preeclampsia was driven by an identifiable subset of women who exhibited consistently low PGF across pregnancy, below the 95% CI of uncomplicated pregnant women. Importantly, the subset of preeclamptic women who exhibited consistently low PGF across pregnancy accounted for almost half of the preeclampsia cases in this longitudinal investigation. In addition, these subjects were more likely to deliver preterm and have SGA babies compared to preeclamptic women with PGF concentrations similar to normotensive controls. These women also exhibited elevated blood pressures in early pregnancy compared to women who developed preeclampsia and had PGF concentrations similar to uncomplicated controls.
The observation of low and high maternal PGF concentrations across pregnancy among women who later develop preeclampsia may be an important observation in relation to the pathophysiology and study of preeclampsia. First, the presence of these different patterns of maternal PGF across pregnancy may indicate that at least two different pathophysiological processes underlie this heterogeneous syndrome. Perhaps these different patterns of maternal PGF indicate that half of the cases of preeclampsia are the result of insufficient angiogenic signaling (low PGF) and half of the cases of preeclampsia are the result of insensitivity to angiogenic signaling (high PGF) or are determined by a different pathogenic factor. Based on these two different patterns of maternal PGF we speculate that this may represent a relevant division of preeclampsia, low vs. high PGF or type 1 vs. type 2 preeclampsia. This finding may be similar to that of diabetes with its well-defined and understood type 1 (low insulin) vs. type 2 (high insulin) classification. In addition, the presence of low and high patterns of maternal PGF in preeclampsia explains previous published data regarding the poor predictive nature of PGF and sFLT1 for preeclampsia. If low PGF in preeclampsia is present in only half of all cases, then it is obvious that many cases of preeclampsia would be missed if only using this marker. Conversely, these longitudinal data suggest women with persistent PGF concentrations below the lowest 5% of concentrations observed in uncomplicated pregnant women after 15 weeks gestation have a 30% chance of developing preeclampsia, and this group of women appears to represent half of the cases of preeclampsia. It is important for this observation to be duplicated in another independent longitudinal study.
Despite the large number of normotensive control subjects investigated in the longitudinal portion of this study, this group is limited in that none of these subjects had preterm birth and relatively few had SGA infants (3%). Therefore differences in preterm birth and SGA infants between controls and the preeclampsia subjects are more pronounced and are likely elevated compared to more heterogeneous control populations. However, because of this limitation, this study focused its attention primarily on differences between the low vs. high PGF preeclampsia groups, as well as comparisons between the low PGF normotensive control group and the low PGF preeclampsia group indicating that the low maternal PGF pattern is not just a consequence of SGA.
Perspectives
It is well accepted that preeclampsia is a heterogeneous syndrome. 5, 26 Most studies address the heterogeneity of preeclampsia by dividing subjects according to severity of the syndrome and clinically relevant outcomes such as early onset/preterm delivery and small for gestational age infants. In many cases these studies also investigate differences in biological markers, such as PGF and sFLT1, according to the previously defined clinical outcomes. 5, 26 Identifying such subsets might aid in the understanding of pathophysiology and perhaps allow individual preventive therapy. 27 In this study we have identified two different patterns of PGF among women who develop preeclampsia, and investigated how women who develop preeclampsia based on significant differences in the patterns of PGF are similar and different in their clinical outcome. Using this approach we have found that a significant proportion of women (46%) who develop preeclampsia evidence a consistent pattern of low PGF at or below the 95% CI of normotensive controls from 15 weeks gestation onward, and that this group of preeclamptic women have elevated blood pressures in early pregnancy, more preterm births and SGA infants compared to preeclamptic women with PGF concentrations similar to normotensive controls. Based on these findings, we propose that dividing preeclampsia cases by differences in patterns of pathophysiologically relevant analytes may be useful for future research studies, as well as provide insights into pathophysiologic and clinically relevant subtypes, and if analyte patterns can be predicted by demographic or preexisting clinical factors. 28
Supplementary Material
Table 1.
Maternal and Newborn characteristics of cross-sectional study subjects
| variable | Controls (n=342) |
Term Preeclampsia (n=97) |
Preterm Preeclampsia (n=33) |
|---|---|---|---|
| Maternal age (years) | 23.9±5.7 | 26.0±6.1* | 24.5±5.0 |
| Pre-pregnancy BMI (kg/m2) | 26.2±6.8 | 26.3±6.8 | 27.2±8.5 |
| Average blood pressure before 20 weeks gestation (mmHg) |
113.6±8.1/ 69.1±5.6 |
115.6±10.6/ 70.1±6.9 |
115.5±10.4/ 70.6±7.5 |
| Average blood pressure at delivery (mmHg) |
121.5±11.4/ 73.3±7.6 |
147.6±12.4*/ 88.2±7.2* |
159.1±18.9*†/ 95.7±9.4*† |
| Gestational age at delivery (weeks) | 40.0±1.0 | 39.4±1.1* | 31.7±2.8*† |
| Race (% African American) | 98 (28.6%) | 20 (20.6%) | 6 (18.2%) |
| Smokers (n) (%) | 185 (54.1%) | 34 (35.0%)* | 7 (21.2%)* |
| Gestational age at sample collection (weeks) |
38.3±3.6 | 39.3±1.1* | 31.6±2.8*† |
| Pre-delivery uric acid (mg/dl) |
5.0±1.2 | 5.8±1.3* | 6.2±1.5* |
| Placenta weight (grams) (n) | 461.5±91.6 (n=58) |
471.6±94.4 (n=52) |
267.9±97.9*† (n=29) |
| Infant birth weight (grams) | 3454.5±444.4 | 3198.4±494.6* | 1586.5±664.6*† |
| Birth weight centile (%) |
54.0±26.2 | 41.4±28.3* | 28.6±22.5*† |
| SGA infants n (%) |
9 (2.6%) | 14 (14.4%)* | 7 (21.2%)* |
Data are mean ± SD.
p<0.05 compared to controls;
p<0.01 compared to term preeclampsia
Table 2.
Maternal and Newborn characteristics of longitudinal study subjects
| variable | Controls (n=250) |
Preeclampsia (n=50) |
|---|---|---|
| Maternal age (years) | 22.5±4.6 | 23.2±5.6 |
| Pre-pregnancy BMI (kg/m2) | 25.4±6.5 | 26.3±6.0 |
| Gestational age at delivery (weeks) | 40.2±1.0 | 37.9±2.9‡ |
| Preterm birth (n)(%) |
0 (0%) | 13 (26%) |
| Pre-delivery uric acid (mg/dl) | 4.8±1.1 | 5.7±1.3* |
| Average blood pressure before 20 weeks gestation (mmHg) |
112.8±7.6/ 68.5±5.5 |
115.8±8.2*/ 69.1±6.2 |
| Average blood pressure pre-delivery (mmHg) |
120.3±11.4/ 72.8±7.9 |
145.8±13.2‡/ 89.2±8.1‡ |
| Race (% African American) |
82 (32.8%) | 19 (38.0%) |
| Smokers (n)(%) |
147 (58.8%) | 21 (42.0%)* |
| Placenta weight (grams) (n) | 463.4±88.7 (n=48) |
416.4±121.1* (n=30) |
| Infant birth weight (grams) | 3439.3±426.6 | 2852.6±772.1* |
| Birth weight centile (%) | 52.5±26.1 | 38.6±29.3† |
| SGA infants (%) | 7 (2.8%) | 8 (16.0%)* |
Data are mean±SD;
p<0.05 compared to controls;
p<0.01 compared to controls;
p<0.001 compared to controls.
Novelty and Significance.
What Is New?
In a longitudinal analysis 46% of patients who later developed preeclampsia evidenced maternal PGF consistently below the lower 95%CI of uncomplicated pregnant women across pregnancy.
Preeclampsia patients with consistently low PGF have significantly higher blood pressures, delivery earlier and have more preterm births compared to preeclampsia patients without low PGF.
What Is Relevant?
Preeclampsia is a significant hypertensive complication of pregnancy affecting 3 to 5% of all pregnancies and is a leading cause of maternal and fetal morbidity and mortality worldwide.
Summary
A significant proportion of women (46%) who develop preeclampsia evidence a pattern of consistently low PGF at or below the 95% CI of normotensive pregnant patients from 15 weeks gestation onward.
Investigating differences in patterns of pathophysiologically relevant analytes in preeclampsia cases may be useful for future research studies, as well as provide insights into pathophysiologic and clinically relevant subtypes.
Acknowledgments
Sources of Funding
This project supported by National Institutes of Health grant number P01-HD30367, NCRR CTSA Grant 1 UL1 RR024153, and funding from Abbott Laboratories.
Footnotes
Presented at the 57th Annual meeting of the Society for Gynecologic Investigation, Orlando, Florida, March 2010.
Disclosures
None.
References
- 1.Duley L. Maternal mortality associated with hypertensive disorders of pregnancy in Africa, Asia, Latin America and the Caribbean. British Journal of Obstetrics & Gynaecology. 1992;99:547–553. doi: 10.1111/j.1471-0528.1992.tb13818.x. [DOI] [PubMed] [Google Scholar]
- 2.Goldenberg RL, Rouse DJ. Prevention of premature birth. New England Journal of Medicine. 1998;339:313–320. doi: 10.1056/NEJM199807303390506. [DOI] [PubMed] [Google Scholar]
- 3.Roberts JM, Funai EF. Pregnancy-Related Hypertension. In: Creasy RK, Resnik R, Iams JD, Lockwood CJ, Moore TR, editors. Maternal Fetal Medicine. Sixth Edition. Philadelphia: W. B. Saunders; 2009. pp. 651–688. [Google Scholar]
- 4.Chesley LC. Hypertensive disorders of pregnancy. New York: Appleton-Century-Crofts; 1978. [Google Scholar]
- 5.Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. American Journal of Obstetrics and Gynecology. 1996;175:1365–1370. doi: 10.1016/s0002-9378(96)70056-x. [DOI] [PubMed] [Google Scholar]
- 6.Maynard SE, Min JY, Merchan J, Lim KH, Li J, Mondal S, Libermann TA, Morgan JP, Sellke FW, Stillman IE, Epstein FH, Sukhatme VP, Karumanchi SA. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. Journal of Clinical Investigation. 2003;111:649–658. doi: 10.1172/JCI17189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levine RJ, Maynard SE, Qian C, Lim K-H, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. New England Journal of Medicine. 2004;350:672–683. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 8.Powers RW, Roberts JM, Cooper KM, Gallaher MJ, Frank MP, Harger GF, Ness RB. Maternal serum soluble fms-like tyrosine kinase 1 concentrations are not increased in early pregnancy and decrease more slowly postpartum in women who develop preeclampsia. American Journal of Obstetrics & Gynecology. 2005;193:185–191. doi: 10.1016/j.ajog.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 9.Levine RJ, Lam C, Qian C, Yu KF, Maynard SE, Sachs BP, Sibai BM, Epstein FH, Romero R, Thadhani R, Karumanchi SA. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. New England Journal of Medicine. 2006;355:992–1005. doi: 10.1056/NEJMoa055352. [DOI] [PubMed] [Google Scholar]
- 10.Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenatal Diagnosis. 2011;31:66–74. doi: 10.1002/pd.2660. [DOI] [PubMed] [Google Scholar]
- 11.Woodham PC, Brittain JE, Baker AM, Long DL, Haeri S, Camargo CA, Jr, Boggess KA, Stuebe AM. Midgestation maternal serum 25-hydroxyvitamin D level and soluble fms-like tyrosine kinase 1/placental growth factor ratio as predictors of severe preeclampsia. Hypertension. 2011;58:1120–1125. doi: 10.1161/HYPERTENSIONAHA.111.179069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kusanovic JP, Romero R, Chaiworapongsa T, Erez O, Mittal P, Vaisbuch E, Mazaki-Tovi S, Gotsch F, Edwin SS, Gomez R, Yeo L, Conde-Agudelo A, Hassan SS. A prospective cohort study of the value of maternal plasma concentrations of angiogenic and anti-angiogenic factors in early pregnancy and midtrimester in the identification of patients destined to develop preeclampsia. J Matern Fetal Neonatal Med. 2009;22:1021–1038. doi: 10.3109/14767050902994754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim JH, Kim SY, Park SY, Yang JH, Kim MY, Ryu HM. Effective prediction of preeclampsia by a combined ratio of angiogenesis-related factors. Obstetrics & Gynecology. 2008;111:1403–1409. doi: 10.1097/AOG.0b013e3181719b7a. [DOI] [PubMed] [Google Scholar]
- 14.Verlohren S, Galindo A, Schlembach D, Zeisler H, Herraiz I, Moertl MG, Pape J, Dudenhausen JW, Denk B, Holgeret S. An automated method for the determination of the sFlt- 1/PIGF ratio in the assessment of preeclampsia. American Journal of Obstetrics & Gynecology. 2010;202:161.e1–161.e11. doi: 10.1016/j.ajog.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Yu J, Shixia CZ, Wu Y, Duan T. Inhibin A, activin A, placental growth factor and uterine artery Doppler pulsatility index in the prediction of pre-eclampsia. Ultrasound Obstet Gynecol. 2011;37:528–533. doi: 10.1002/uog.8800. [DOI] [PubMed] [Google Scholar]
- 16.Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertension in Pregnancy. 2004;23:101–111. doi: 10.1081/PRG-120028286. [DOI] [PubMed] [Google Scholar]
- 17.Unal ER, Robinson CJ, Johnson DD, Chang EY. Second-trimester angiogenic factors as biomarkers for future-onset preeclampsia. American Journal of Obstetrics & Gynecology. 2007;197:211.e1–211.e4. doi: 10.1016/j.ajog.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 18.Romero R, Nien JK, Espinoza J, Todem D, Fu W, Chung H, Kusanovic JP, Gotsch F, Erez O, Mazaki-Tovi S, Gomez R, Edwin S, Chaiworapongsa T, Levine RJ, Karumanchi SA. A longitudinal study of angiogenic (placental growth factor) and anti-angiogenic (soluble endoglin and soluble vascular endothelial growth factor receptor-1) factors in normal pregnancy and patients destined to develop preeclampsia and deliver a small for gestational age neonate. J Matern Fetal Neonatal Med. 2008;21:9–23. doi: 10.1080/14767050701830480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Widmer M, Villar J, Benigni A, Conde-Agudelo A, Karumanchi SA, Lindheimer M. Mapping the theories of preeclampsia and the role of angiogenic factors: a systematic review. Obstetrics & Gynecology. 2007;109:168–180. doi: 10.1097/01.AOG.0000249609.04831.7c. [DOI] [PubMed] [Google Scholar]
- 20.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–958. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Chaiworapongsa T, Espinoza J, Gotsch F, Kim YM, Kim GJ, Goncalves LF, Edwin S, Kusanovic JP, Erez O, Than NG, Hassan SS, Romero R. The maternal plasma soluble vascular endothelial growth factor receptor-1 concentration is elevated in SGA and the magnitude of the increase relates to Doppler abnormalities in the maternal and fetal circulation. J Matern Fetal Neonatal Med. 2008;21:25–40. doi: 10.1080/14767050701832833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poon LCY, Zaragoza E, Akolekar R, Anagnostopoulos E, Nicolaides KH. Maternal serum placental growth factor (PlGF) in small for gestational age pregnancy at 11(+0) to 13(+6) weeks of gestation. Prenatal Diagnosis. 2008;28:1110–1115. doi: 10.1002/pd.2143. [DOI] [PubMed] [Google Scholar]
- 23.Wikstrom A-K, Larsson A, Eriksson UJ, Nash P, Norden-Lindeberg S, Olovsson M. Placental growth factor and soluble FMS-like tyrosine kinase-1 in early-onset and late-onset preeclampsia. Obstetrics & Gynecology. 2007;109:1368–1374. doi: 10.1097/01.AOG.0000264552.85436.a1. [DOI] [PubMed] [Google Scholar]
- 24.Smith GCS, Crossley JA, Aitken DA, Jenkins N, Lyall F, Cameron AD, Connor JM, Dobbie R. Circulating angiogenic factors in early pregnancy and the risk of preeclampsia, intrauterine growth restriction, spontaneous preterm birth, and stillbirth. Obstetrics & Gynecology. 2007;109:1316–1324. doi: 10.1097/01.AOG.0000265804.09161.0d. [DOI] [PubMed] [Google Scholar]
- 25.Ohkuchi A, Hirashima C, Matsubara S, Suzuki H, Takahashi K, Arai F, Watanabe T, Kario K, Suzuki M. Alterations in placental growth factor levels before and after the onset of preeclampsia are more pronounced in women with early onset severe preeclampsia. Hypertension Research - Clinical & Experimental. 2007;30:151–159. doi: 10.1291/hypres.30.151. [DOI] [PubMed] [Google Scholar]
- 26.von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertension in Pregnancy. 2003;22:143–148. doi: 10.1081/PRG-120021060. [DOI] [PubMed] [Google Scholar]
- 27.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32–S37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts JM. Preeclampsia: new approaches but the same old problems. American Journal of Obstetrics & Gynecology. 2008;199:443–444. doi: 10.1016/j.ajog.2008.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.