Abstract
Objective.
The main purpose of the study was to examine whether emotion impairs associative memory for previously seen items in older adults, as previously observed in younger adults.
Method.
Thirty-two younger adults and 32 older adults participated. The experiment consisted of 2 parts. In Part 1, participants learned picture–object associations for negative and neutral pictures. In Part 2, they learned picture–location associations for negative and neutral pictures; half of these pictures were seen in Part 1 whereas the other half were new. The dependent measure was how many locations of negative versus neutral items in the new versus old categories participants remembered in Part 2.
Results.
Both groups had more difficulty learning the locations of old negative pictures than of new negative pictures. However, this pattern was not observed for neutral items.
Discussion.
Despite the fact that older adults showed overall decline in associative memory, the impairing effect of emotion on updating associative memory was similar between younger and older adults.
Keywords: Aging, Emotion, Memory updating, Associative memory
Associative memory is the ability to remember various features of an object, a person, or an event together as a coherent whole, which is an essential component of episodic memory. The associative deficit hypothesis (ADH; Naveh-Benjamin, 2000) posits that age-related decline in episodic memory is, in part, due to impairments in associative memory. This is supported by a number of behavioral studies showing that older adults have an associative memory deficit compared with their younger counterparts (Chalfonte & Johnson, 1996). Neuroimaging studies provide further evidence for an age-related associative memory deficit. The prefrontal cortex and the hippocampal/medial-temporal region are critical for associative memory (Achim & Lepage, 2005), and these regions show structural decline in normal aging (Raz et al., 2005). With respect to functional decline, older adults (relative to younger adults) show differences in hippocampal (Mitchell, Johnson, Raye, & D’Esposito, 2000) and parahippocampal (Chee et al., 2006) activity during stimuli presentation conditions that elicit or require associative learning. Furthermore, younger adults show greater left lateral prefrontal cortex activation when given a memory test about the previous format and location of items than when given an old–new memory test for the items themselves, whereas older adults do not show this increased prefrontal activity during the source judgment task (Mitchell, Raye, Johnson, & Greene, 2006).
The emotional salience of stimuli can also affect associative memory. Arousal-biased competition theory (Mather & Sutherland, 2011) suggests that an emotional item captures attention when there is no other competing high priority information. This arousal-induced attention increases associative feature binding, leading to deeper encoding and thus better retention of bound information. Previous research suggests that the theory applies to both younger and older adults. For example, the ability to detect emotionally arousing stimuli is relatively stable with normal aging (Leclerc & Kensinger, 2008). Furthermore, emotional arousal improves associative memory in older adults, as well as in younger adults (Nashiro & Mather, 2011). Taken together, although there is clear evidence suggesting age-related deficits in associative memory, emotional arousal seems to have enhancing affects on associative memory even in older adults.
In contrast with these emotion-enhancing effects for learning about novel stimuli, recent research with younger adults suggests that emotional arousal impairs forming new associations to old information. One hypothesis is that while the amygdala facilitates emotional memories for new items, it opposes updating of old emotional associations (Sakaki, Niki, & Mather, 2011). This hypothesis is consistent with animal research showing that amygdala lesions facilitate updating stimulus-punishment associations (Izquierdo & Murray, 2005). In addition, younger adults have more difficulty updating emotional than neutral associative memories (Mather & Knight, 2008; Novak & Mather, 2009) due perhaps to the amygdala’s protection of old memories.
The main purpose of the current study was to test whether emotion would also impair memory updating in older adults. Based on the associative memory deficits observed in the past studies (Naveh-Benjamin, 2000), we predicted a main effect of age group, such that overall, older adults would have poorer associative memory than younger adults, irrespective of item’s emotionality or novelty. Given relatively good structural and functional preservation of the amygdala in normal aging (for a review see Nashiro, Sakaki, & Mather, 2011), the amygdala should still facilitate learning new emotional associations and prevent updating of old emotional associations in older adults. Thus, we hypothesized that like younger adults, older adults would show more difficulty learning associations to old emotional items than to new emotional items.
Method
Participants
Thirty-two younger adults (M age = 20.78, age range = 18–29, 7 male, 25 females, M education = 14.71) and 32 older adults (M age = 74.00, age range = 65–93, 15 males, 17 females, M education = 15.97) participated. There were no significant effects of participant sex in any of the analyses. We recruited older adults from local communities and retirement homes who had not been diagnosed with dementia or other cognitive disorders.
Materials
The stimuli were 64 matched-pair pictures (e.g., Novak & Mather, 2009), for which each negative picture was yoked with a visually similar but less arousing neutral picture. Participants saw either the negative or neutral version from a pair; which version was shown was counterbalanced. For each participant, 16 negative arousing and 16 neutral pictures were used as new pictures without prior associations, whereas the other 16 negative arousing and 16 neutral pictures were used as old pictures (the old/new sets were counterbalanced across participants). Each old picture was randomly paired with a drawing of a neutral object (e.g., apple).
Procedure
The experiment consisted of two parts: Part 1 was a picture–object association phase and Part 2 was a picture–location association phase.
In Part 1, participants first studied 32 picture–object pairs. During the learning session, participants saw a picture at the center of the screen (a location not used in the study phase in Part 2). After 3 s, an object appeared in one of the bottom two corners for 2 s while the picture remained on the screen. Participants were asked to remember the pair while indicating whether the object appeared on the right or left bottom corner by pressing a key.
The pair memory test was a two-alternative forced choice design in which participants saw a picture in the center of the screen and two previously seen objects in the top two corners. They were asked to choose the object associated with the picture by pressing a key. Because the main purpose of the current study was to examine how initial learning affects later learning, the task was made easy to promote initial learning. To facilitate learning, the study-test cycle was repeated once, and feedback was provided by the correct object remaining on the screen for 1 s after each response.
In Part 2, participants viewed the 64 pictures (half seen in Part 1) in a randomized order, each for 2 s at one of the six locations (the top three and bottom three cells of a 3 × 3 grid partitioning the screen). Participants were instructed to remember picture–location combinations while indicating whether the picture appeared higher or lower than the center of the screen by pressing a key. In the subsequent memory test, each of the 64 pictures was presented at the center of the screen. Participants indicated each picture’s previous location by pressing a key.
At the end of the experiment, participants provided arousal ratings for the pictures using a 9-point scale (1 = not at all arousing to 9 = extremely arousing) and valence ratings using a 9-point scale (1 = extremely negative to 9 = extremely positive).
Results
Picture Ratings
Older adults rated two negative arousing pictures as very low in arousal (M < 3.5); thus, those pictures were excluded from all the analyses for the older group. Both groups rated the rest of the pictures in the expected direction on arousal (M negative = 6.31, standard error [SE] = .17; M neutral = 2.56, SE = .15), F(1, 62) = 538.94, mean square error [MSE] = 0.83, p < .001, = .90, and on valence (M negative = 2.58, SE = .10; M neutral = 5.56, SE = .11), F(1, 62) = 308.95, MSE = 0.92, p < .001, = .83. In both ratings, there were no interactions between group and valence category.
Picture–Object Association Memory
A 2 (group: younger, older) × 2 (emotion: neutral, negative arousing) × 2 (test cycle: Test 1, Test 2) mixed analysis of variance (ANOVA) was performed on the correct response rates in the Part 1 memory tests. The ANOVA revealed that younger adults (M = .97, SE = .02) performed significantly better than older adults (M = .87, SE = .02), F(1, 62) = 19.12, MSE = 0.03, p < .001, = .24, and that Test 2 (M = .96, SE = .01) yielded better performance than Test 1 (M = .89, SE = .02), F(1,62) = 42.04, MSE = 0.01, p < .001, = .40. In addition, there was an interaction between group and test cycle, F (1, 62) = 11.68, MSE = 0.01, p = .001, = .16, suggesting that older adults (Mtest1 = .82, SE = .02; Mtest2 = .92, SE = .01) showed a greater improvement in Test 2 than did younger adults (M Test1 = .96, SE = .02; M Test 2 = .99, SE = .01). As a result, both groups learned most pairs by the second test. This initial learning was intentionally made easy (as discussed earlier), which resulted in ceiling effects, which may have obscured potential differences in pair memory for neutral and negative arousing items.
Picture–Location Association Memory
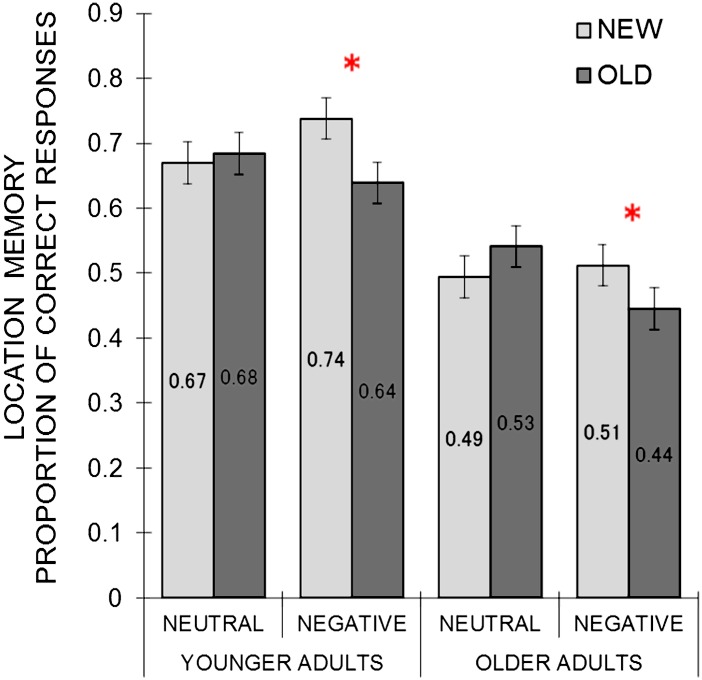
Because the purpose of the study was to examine the effects of initial learning on subsequent memory performance, we excluded items for which participants did not show correct memory performance by Test 2 in the initial learning phase (Part 1). A 2 (group) × 2 (emotion) × 2 (novelty: new, old) mixed ANOVA was performed on the proportion of correct location memory responses in Part 2. There was a significant effect of group (M younger = .68, SE = .03; M older = .49, SE = .03), F(1, 62) = 25.21, MSE = 0.09, p <.001, = .29. In addition, the ANOVA revealed a significant interaction between emotion and novelty, F(1,62) = 16.13, MSE = 0.01, p < .001, = .21 (see Figure 1). There was no significant interaction between group, emotion type, and novelty (p = .99), indicating the age-independent nature of the interaction. Separate 2 (emotion) × 2 (novelty) ANOVAs for each age group also confirmed a significant interaction between emotion and novelty for both younger adults, F (1, 31) = 9.86, MSE = 0.01, p = .004, = .24, and older adults, F (1, 31) = 6.80, MSE = 0.02, p = .014, = .18. When pictures were negative, location memory was worse for old than new pictures in younger adults, t(31) = 3.49, p = .001, and in older adults, t(31) = 2.40, p = .023. In contrast, location memory did not differ between old neutral and new neutral pictures in younger adults, t(31) = 0.37, p = .714, or in older adults, t(31) = 1.01, p = .320.
Figure 1.
Picture–Location Association Memory. Younger and older adults showed similar interactions between emotion (neutral vs. negative) and novelty (new vs. old). Both groups had worse location memory for old negative than new negative pictures, whereas no significant differences were observed between old neutral and new neutral items. Means are shown inside the bars. Error bars represent standard errors. Chance performance is one sixth (0.17).
Discussion
Consistent with previous findings on age-related declines in associative memory (e.g., Naveh-Benjamin, 2000), older adults learned picture locations less well than did younger adults. However, older and younger adults showed similar interactions of picture novelty and emotion. For negative pictures, participants remembered more locations of novel pictures than locations of old pictures. In contrast, for neutral pictures, location memory did not significantly differ for old and new pictures. Importantly, younger and older adults showed a similar pattern of interactions, indicating that the effects of emotion on associative memory updating remain stable with age. This is consistent with previous findings that the amygdala is relatively well preserved in aging structurally (e.g., Allen, Bruss, Brown, & Damasio, 2005) and functionally (Wright et al., 2008). Although older adults sometimes show reduced amygdala activity to negative stimuli but not positive stimuli (e.g., Leclerc & Kensinger, 2011; Mather et al., 2004), this may be due to their emotion regulation efforts, rather than functional or structural impairment of the amygdala (see Nashiro et al., 2012, for a review). These results together suggest that emotion continues to modulate memory updating in normal aging.
Some limitations of the study need to be mentioned. Because this is a cross-sectional study, we cannot make strong conclusions about developmental changes or stability in the effects of emotion on associative memory. Another limitation is that we did not use positive arousing pictures and thus cannot address whether the observed effects of emotion on location memory is due to arousal or valance of the stimuli. Given older adults’ preference for positive information relative to negative information in attention and memory (compared with younger adults; Mather & Carstensen, 2005), it is possible that positive arousing items differentially affect younger and older adults. Future research should also use a larger sample size to investigate potential age differences in the magnitude of the observed emotion effects on subsequent memory.
Despite these limitations, the current study provides evidence suggesting that emotion interacts with associative memory similarly across the two age groups. Given age-related declines in associative memory, this line of research is important for understanding how emotional arousal affects older adults’ memory performance. Future research may help us further identify the costs and benefits of emotion on associative learning and develop strategies for improving associative memory.
Funding
This work was supported by grants from the National Institute on Aging (R01AG025340, K02AG032309, and 5T32AG000037). We thank Chris Peterka for assistance with recruitment and data collection.
References
- Achim AM, Lepage M. Neural correlates of memory for items and for associations: An event-related functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2005;17:652–667. doi: 10.1162/0898929053467578. doi:10.1162/0898929053467578. [DOI] [PubMed] [Google Scholar]
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. doi:10.1016/j.neurobiolaging.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Chalfonte BL, Johnson MK. Feature memory and binding in young and older adults. Memory & Cognition. 1996;24:403–416. doi: 10.3758/bf03200930. doi:10.3758/BF03200930. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Goh JOS, Venkatraman V, Tan JC, Gutchess A, Sutton B, Hebrank A, Park D. Age-related changes in object processing and contextual binding revealed using fMR adaptation. Journal of Cognitive Neuroscience. 2006;18:495–507. doi: 10.1162/jocn.2006.18.4.495. doi:10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. European Journal of Neuroscience. 2005;22:2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. doi:10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Effects of age on detection of emotional information. Psychology and Aging. 2008;23:209–215. doi: 10.1037/0882-7974.23.1.209. doi:10.1037/0882-7974.23.1.209. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Neural processing of emotional pictures and words: A comparison of young and older adults. Developmental Neuropsychology. 2011;36:519–538. doi:10.1080/87565641.2010.549864. [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, Carstensen LL. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. doi:10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. doi:10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. The emotional harbinger effect: Poor context memory for cues that previously predicted something arousing. Emotion. 2008;8:850–860. doi: 10.1037/a0014087. doi:10.1037/a0014087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Sutherland MR. Arousal-biased competition in perception and memory. Perspectives on Psychological Science. 2011;6:114–133. doi: 10.1177/1745691611400234. doi:10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK, Raye CL, D’Esposito M. fMRI evidence of age-related hippocampal dysfunction in feature binding in working memory. Cognitive Brain Research. 2000;10:197–206. doi: 10.1016/s0926-6410(00)00029-x. doi:10.1016/S0926-6410(00)00029-X. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Raye CL, Johnson MK, Greene EJ. An fMRI investigation of short-term source memory in young and older adults. Neuroimage. 2006;30:627–633. doi: 10.1016/j.neuroimage.2005.09.039. doi:10.1016/j.neuroimage.2005.09.039. [DOI] [PubMed] [Google Scholar]
- Nashiro K, Mather M. The effect of emotional arousal on memory binding in normal aging and Alzheimer's Disease. American Journal of Psychology. 2011;124:301–312. doi: 10.5406/amerjpsyc.124.3.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashiro K, Sakaki M, Mather M. Age differences in brain activity during emotion processing: Reflections of age-related decline or increased emotion regulation? Gerontology. 2012;58:156–163. doi: 10.1159/000328465. doi:10.1159/000328465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naveh-Benjamin M. Adult age differences in memory performance: Tests of an associative deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26:1170–1187. doi: 10.1037//0278-7393.26.5.1170. doi:10.1037/0278-7393.26.5.1170. [DOI] [PubMed] [Google Scholar]
- Novak DL, Mather M. The tenacious nature of memory binding for arousing negative items. Memory & Cognition. 2009;37:945–952. doi: 10.3758/MC.37.7.945. doi:10.3758/MC.37.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1679–1689. doi: 10.1093/cercor/bhi044. doi:10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Sakaki M, Niki K, Mather M. Updating existing emotional memories involves the frontopolar/orbitofrontal cortex in ways that acquiring new emotional memories does not. Journal of Cognitive Neuroscience. 2011;23:3498–3514. doi: 10.1162/jocn_a_00057. doi:10.1162/jocn_a_00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Negeira A, Gold AL, Britton JC, Williams D, Barrett LF. Neural correlates of novelty and face-age effects in young and elderly adults. Neuroimage. 2008;42:956–968. doi: 10.1016/j.neuroimage.2008.05.015. doi:10.1016/j.neuroimage.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]