Abstract
Background
Sudden cardiac death is common and accounts largely for the excess mortality of patients on maintenance dialysis. It is unknown whether aldosterone and cortisol increase the incidence of sudden cardiac death in dialysis patients.
Methods and results
We analysed data from 1255 diabetic haemodialysis patients participating in the German Diabetes and Dialysis Study (4D Study). Categories of aldosterone and cortisol were determined at baseline and patients were followed for a median of 4 years. By Cox regression analyses, hazard ratios (HRs) were determined for the effect of aldosterone, cortisol, and their combination on sudden death and other adjudicated cardiovascular outcomes. The mean age of the patients was 66 ± 8 years (54% male). Median aldosterone was <15 pg/mL (detection limit) and cortisol 16.8 µg/dL. Patients with aldosterone levels >200 pg/mL had a significantly higher risk of sudden death (HR: 1.69; 95% CI: 1.06–2.69) compared with those with an aldosterone <15 pg/mL. The combined presence of high aldosterone (>200 pg/mL) and high cortisol (>21.1 µg/dL) levels increased the risk of sudden death in striking contrast to patients with low aldosterone (<15 pg/mL) and low cortisol (<13.2 µg/dL) levels (HR: 2.86, 95% CI: 1.32–6.21). Furthermore, all-cause mortality was significantly increased in the patients with high levels of both hormones (HR: 1.62, 95% CI: 1.01–2.62).
Conclusions
The joint presence of high aldosterone and high cortisol levels is strongly associated with sudden cardiac death as well as all-cause mortality in haemodialysed type 2 diabetic patients. Whether a blockade of the mineralocorticoid receptor decreases the risk of sudden death in these patients must be examined in future trials.
Keywords: Aldosterone, Cortisol, Sudden cardiac death, Cardiovascular events, Mortality, Kidney disease
Introduction
Chronic kidney disease is a strong risk factor for cardiovascular morbidity and mortality.1 Even patients with moderately impaired kidney function have an increased risk of cardiovascular complications.2 When end-stage renal disease is reached, the prevalence of cardiac comorbidities is excessively high. Coronary heart disease, left ventricular hypertrophy, cardiac fibrosis, and heart failure may contribute to the high incidence of sudden cardiac death, which is the most common cause of death in dialysis patients.3,4
In an effort to identify pathways potentially involved, we evaluated the correlation between aldosterone and cortisol and the risk of sudden death. Recent investigations have documented that—apart from the classical effect on sodium reabsorption—aldosterone exerts a variety of other effects that are potentially important for renal and cardiovascular injury. Aldosterone increases oxidative stress through induction of NADPH oxidase5–7and promotes vascular inflammation.5,8 Furthermore, aldosterone impairs endothelial function by reducing the bioavailability of nitric oxide.9,10 Finally, high aldosterone concentrations in the presence of salt overload cause cardiac hypertrophy and fibrosis, which are prevented by the administration of the mineralocorticoid receptor antagonist eplerenone.11The mineralocorticoid receptor binds aldosterone and cortisol with comparably high affinity;12 and under normal circumstances cortisol fails to activate the mineralocorticoid receptor because it is converted into the inactive metabolite cortisone by the 11β hydroxysteroid dehydrogenase type 2 (11βHSD2).13,14 However, the efficacy of this enzyme is reduced in advanced age, inflammation, and potentially also in uraemia.15 Furthermore, a number of tissues (e.g. cardiomyocytes) do not express 11βHSD2, suggesting that the mineralocorticoid receptor is activated mainly by cortisol, which circulates in much higher concentrations.12 Thus, the beneficial effects of spironolactone and eplerenone may result from blocking the action of both aldosterone and cortisol at the mineralocorticoid receptor.
We, therefore, hypothesized that increased concentrations of both aldosterone and cortisol synergistically activate the mineralocorticoid receptor in end-stage renal disease, thus potentially increasing the incidence of sudden cardiac death. Therefore, we assessed whether the concentrations of serum aldosterone and cortisol were correlated with the cardiovascular outcomes in 1255 haemodialysis patients participating in the German Diabetes Dialysis Study (4D Study: Die Deutsche Diabetes Dialyse Studie).
Methods
Study design and participants
The 4D study design has previously been reported in detail.16 Briefly, this was a prospective randomized controlled trial investigating the effect of atorvastatin on cardiovascular outcomes in 1255 patients aged 18–80 years with type 2 diabetes mellitus and on haemodialysis for <2 years. Between March 1998 and October 2002, patients were recruited in 178 dialysis centres in Germany. Study visits took place at baseline and regularly after randomization until the date of death, censoring, or end of the study in March 2004. At each follow-up visit, an electrocardiogram and clinical information (including adverse cardiovascular events) were recorded. Baseline blood samples were kept frozen at −80°C.
Definition of endpoints
The primary endpoint of the 4D study was defined as a composite of death from cardiac causes, stroke, and myocardial infarction (MI), whichever occurred first. Death from cardiac causes comprised fatal MI (death within 28 days after an MI), sudden cardiac death (SCD), death due to congestive heart failure (CHF), death due to coronary heart disease during or within 28 days after an intervention, and all other deaths ascribed to coronary heart disease. Patients who died unexpectedly and did not present with serum potassium >7.5 mmol/L before the start of the three most recent sessions of haemodialysis were considered to have had sudden death from cardiac causes. 4D Study endpoints were centrally adjudicated by three members of the endpoint committee blinded to study treatment and according to pre-defined criteria.4
For the present analysis, sudden cardiac death, MI, stroke, combined cardiovascular events (CVE) and all-cause mortality were chosen as separate outcomes. The study was approved by the medical ethical committee, and all the patients gave their written informed consent before inclusion.
Data collection
Information on age and smoking status was obtained through patient interviews. Comorbidities, including the presence of coronary artery disease (CAD), CHF, duration of diabetes mellitus, and dialysis treatment, were provided by the patients’ nephrologist. Coronary artery disease was defined by a history of MI, coronary artery bypass grafting surgery, percutaneous coronary intervention, or angiography. Congestive heart failure was defined according to the classification system of the New York Heart Association.
Biochemical measurements
All general laboratory measurements of the 4D Study were performed centrally at the Department of Clinical Chemistry, University of Freiburg, Germany. C-reactive protein and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were measured in blood samples taken at baseline, i.e. at study visit 3 (1 week before randomization), by turbidimetry on a Modular PP analyser (Roche Diagnostics, Mannheim, Germany).
Aldosterone was determined by a validated immunoassay as described previously.17,18 Cortisol was measured using a commercially available immunoassay (Immulite 2000, Siemens, Germany).
For both assays, the intra- and inter-assay coefficients of variation were <8 and <12%, respectively. All blood samples were taken before the start of dialysis sessions and administration of drugs.
Statistical analysis
Patient characteristics are presented according to categories of aldosterone and cortisol concentrations. The groups were selected post hoc and on the basis of the distribution of aldosterone and cortisol concentrations at baseline, respectively. We aimed for equal groups and formed quartiles for cortisol (<13.2, 13.2–16.8, 16.8–21.1, >21.1 µg/dL). Regarding aldosterone, the majority of patients had aldosterone concentrations below the detection limit. Therefore, we divided the remaining population with aldosterone concentrations above the detection limit into three groups aiming for similar numbers of patients in each subgroup. By such procedure, we obtained the following groups: patients with aldosterone levels <15 pg/mL (detection limit, Group 1), levels between 15 and 100 pg/mL (Group 2), levels between 100 and 200 pg/mL (Group 3), and levels >200 pg/mL (Group 4). Continuous variables were expressed as mean ± SD or median with interquartile range as appropriate; categorical variables were expressed as percentages. We first assessed the association between aldosterone status and SCD. Kaplan–Meier curves were performed in each group and the log-rank test was computed to compare the curves. Using Cox regression analyses, hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) were calculated and adjusted for the confounders age, sex, atorvastatin treatment, systolic blood pressure, smoking status, duration of dialysis, BMI, levels of HDL and LDL cholesterol, calcium, phosphate, potassium, and haemoglobin (Model 1). Additional adjustments were made for medication use, including ACE-inhibitors, AT2 receptor antagonists, beta blockers, and diuretics (Model 2). To investigate potential intermediate conditions, we performed additional Cox regression analyses with the inclusion of CAD, CHF, arrhythmia, left ventricular hypertrophy, C-reactive protein, and NT-proBNP, which may lie in the causal pathway of the effect of aldosterone on sudden death (Model 3).
We also determined the relation between aldosterone status and further adverse outcomes, including MI, stroke, combined cardiovascular events, death due to infection, and all-cause mortality. We further analysed the effect of cortisol on all adverse outcomes, using the same approach and statistical procedures as mentioned earlier for aldosterone. Finally, we investigated whether both hormones interacted to increase the incidence of sudden cardiac death and other cardiac outcomes. For this purpose, patients were grouped according to their combined aldosterone and cortisol status at baseline. The patients with both high aldosterone and high cortisol concentrations were compared with the patients with low concentrations of both hormones. In this article, all the P-values are reported two-sided. Analyses were performed using SPSS version 16.0.
Results
Patient characteristics
The mean follow-up period was 4 years. Of all the patients, 617 patients died during follow-up; 160 patients died of SCD and a total of 469 patients reached the combined cardiovascular event with MI and stroke occurring in 200 and 103 patients, respectively. Regarding fatal events, one-quarter of all deaths (26%) could be attributed to sudden cardiac death, 11% of the deaths to acute MI and coronary heart disease, 7% to CHF, and 6% to stroke. About one-half of deaths were due to non-cardiovascular causes, including infection and cancer as well as other causes.
The baseline characteristics of the patients are presented in Table 1. Baseline serum aldosterone concentration (median: <15 pg/mL; IQR: 15–108 pg/mL) was measured in 1180 and cortisol (median: 16.8 µg/dL; IQR: 13.2–21.1 µg/dL) in 1156 patients. The patients of the highest category of aldosterone concentration more often had CAD and arrhythmia and were less often on ACE-inhibitors or AT-2 antagonists (Supplementary material online, Table S1a). In addition, they had higher levels of NT-proBNP and C-reactive protein. There were no meaningful differences in potassium levels across the categories of aldosterone. The correlation between aldosterone and NT-proBNP and C-reactive protein was highly significant (P < 0.001, respectively). Furthermore, the patients with higher cortisol concentrations at baseline had a higher burden of arrhythmia and lower concentrations of potassium. The patients with both high aldosterone and high cortisol concentrations more often had CAD and CHF, higher levels of C-reactive protein, and NT-proBNP, and less often used ACE-inhibitors and AT-2 antagonists than the patients with low aldosterone and low cortisol concentrations. Potassium levels were similar in the patients with both high aldosterone and cortisol levels than the patients with low aldosterone and cortisol levels (Supplementary material online, Table S1b).
Table 1.
Baseline patient characteristics, study population n = 1255
| Characteristic | Whole sample |
|---|---|
| Age, years | 66 (8) |
| Gender, % men | 53.9 |
| Systolic BP, mmHg | 145 (22) |
| Diastolic BP, mmHg | 76 (11) |
| BMI, kg/m2 | 27.5 (4.8) |
| Duration of diabetes, years | 18.1 (8.8) |
| Time on dialysis, months | 8.2 (6.9) |
| Arrhythmia | 18.8 |
| History of | |
| CAD, % | 29.4 |
| CHF, % | 35.4 |
| PVD, % | 44.6 |
| Smoker/Ex-smoker, % | 40.4 |
| Laboratory parameters | |
| LDL cholesterol, mg/dL | 126 (30) |
| HDL cholesterol, mg/dL | 36 (13) |
| Triglycerides, mg/dL | 264 (167) |
| Haemoglobin, g/dL | 10.9 (1.3) |
| Albumin, g/dL | 3.8 (0.3) |
| C-reactive protein, mg/L | 5.8 (2.3–12.4) |
| Calcium, mmol/L | 2.3 (0.2) |
| Phosphate, mmol/L | 6.0 (1.6) |
| Potassium, mmol/L | 5.2 (0.9) |
| HbA1c, % | 6.7 (1.3) |
| NT-proBNP, pg/mL | 3361 (1433–9271) |
CAD, coronary artery disease; CHF, congestive heart failure; PVD, peripheral vascular disease.
Aldosterone status and adverse clinical outcomes
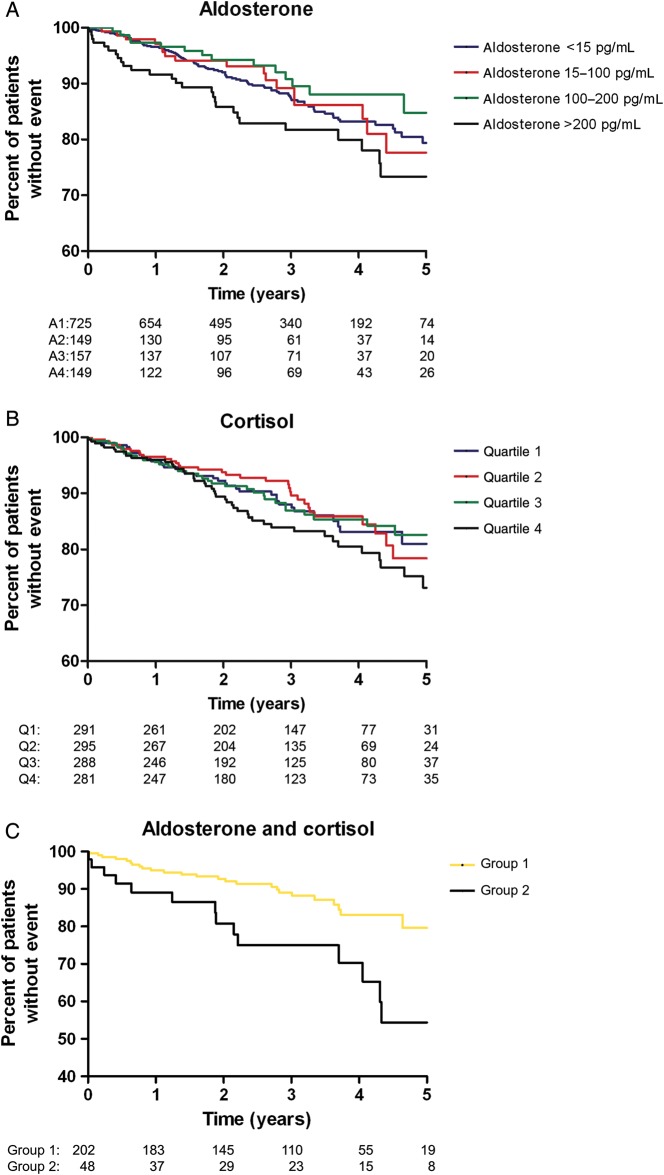
High aldosterone concentrations at baseline were significantly associated with sudden cardiac death during follow-up (see Figure 1A). By adjusted Cox regression analysis, the risk of sudden death was 69% higher in the group of patients with the highest aldosterone concentrations (>200 pg/mL) compared with the lowest concentrations (<15 pg/mL): HR: 1.69, 95% CI: 1.06–2.69 (Table 2). The additional adjustments for potential intermediate variables largely attenuated the association [HR: 1.35 (0.84–2.17)], suggesting that these mechanisms considerably explain the association between aldosterone and sudden death. Compared with the patients with the lowest aldosterone concentrations, those with concentrations between 15 and 100 pg/mL (Group 2) or between 100 and 200 pg/mL (Group 3) were not at an increased risk of sudden death (Table 2).
Figure 1.
(A–C) Kaplan–Meier curves for the time to sudden cardiac death according to groups of (A) aldosterone concentration, (B) cortisol concentration, and (C) combined aldosterone and cortisol status at baseline. The categories are: (A) aldosterone: Group 1: <15 pg/mL, Group 2: 15–100 pg/mL, Group 3: 100–200 pg/mL, Group 4: >200 pg/mL; log-rank test Group 1 vs. Group 4: P = 0.06. (B) Cortisol: quartile 1: <13.2 µg/dL, quartile 2: 13.2–16.8 µg/dL, quartile 3: 16.8–21.1 µg/dL, quartile 4: >21.1 µg/dL; log-rank test quartile 1 vs. quartile 4: P = 0.16. (C) Aldosterone and cortisol combined: Group 1: low aldosterone <15 pg/mL and low cortisol <13.2 µg/dL, Group2: high aldosterone >200 pg/mL and high cortisol >21.1 µg/dL log-rank test Group 1 vs. Group 2: P = 0.003.
Table 2.
Hazard ratios with 95% confidence intervals (HR: 95% CI) for sudden cardiac death, death due to heart failure, stroke, myocardial infarction, combined cardiovascular events, and all-cause mortality according to the aldosterone status (pg/mL) at baseline
| Outcome | Aldosterone category |
|||
|---|---|---|---|---|
| <15 (n = 725) | 15–100 (n = 149) | 100–200 (n = 157) | >200 (n = 149) | |
| Sudden death | ||||
| Crude HR (95% CI) | 1 | 0.96 (0.57–1.61) | 0.76 (0.44–1.31) | 1.48 (0.96–2.27) |
| Adj.a HR (95% CI) | 1 | 0.95 (0.56–1.60) | 0.79 (0.46–1.39) | 1.68 (1.08–2.62) |
| Adj.b HR (95% CI) | 1 | 0.94 (0.56–1.59) | 0.80 (0.45–1.40) | 1.69 (1.06–2.69) |
| Adj.c HR (95% CI) | 1 | 1.00 (0.59–1.69) | 0.81 (0.46–1.43) | 1.35 (0.84–2.17) |
| Deaths due to heart failure | ||||
| Crude HR (95% CI) | 1 | 0.70 (0.21–2.35) | 1.48 (0.63–3.47) | 1.37 (0.56–3.39) |
| Adj.a HR (95% CI) | 1 | 0.78 (0.23–2.62) | 1.54 (0.64–3.73) | 1.85 (0.71–4.84) |
| Adj.b HR (95% CI) | 1 | 0.85 (0.25–2.88) | 1.66 (0.67–4.09) | 2.11 (0.76–5.84) |
| Adj.c HR (95% CI) | 1 | 0.71 (0.21–2.46) | 1.36 (0.54–3.42) | 1.42 (0.49–4.12) |
| Stroke | ||||
| Crude HR (95% CI) | 1 | 1.08 (0.60–1.91) | 0.62 (0.31–1.23) | 0.50 (0.23–1.10) |
| Adj.a HR (95% CI) | 1 | 1.07 (0.59–1.97) | 0.69 (0.34–1.40) | 0.59 (0.27–1.31) |
| Adj.b HR (95% CI) | 1 | 1.10 (0.60–2.02) | 0.73 (0.36–1.49) | 0.61 (0.27–1.38) |
| Adj.c HR (95% CI) | 1 | 1.17 (0.63–2.17) | 0.76 (0.37–1.55) | 0.47 (0.21–1.09) |
| Myocardial infarction | ||||
| Crude HR (95% CI) | 1 | 0.87 (0.54–1.40) | 1.09 (0.72–1.65) | 0.82 (0.51–1.33) |
| Adj.a HR (95% CI) | 1 | 0.94 (0.58–1.52) | 1.02 (0.67–1.55) | 0.82 (0.51–1.34) |
| Adj.b HR (95% CI) | 1 | 0.94 (0.58–1.53) | 1.03 (0.67–1.59) | 0.80 (0.48–1.33) |
| Adj.c HR (95% CI) | 1 | 0.91 (0.56–1.48) | 1.00 (0.65–1.55) | 0.71 (0.43–1.19) |
| Cardiovascular events | ||||
| Crude HR (95% CI) | 1 | 0.92 (0.68–1.24) | 0.83 (0.62–1.12) | 0.99 (0.74–1.32) |
| Adj.a HR (95% CI) | 1 | 0.95 (0.70–1.28) | 0.85 (0.63–1.16) | 1.09 (0.81–1.46) |
| Adj.b HR (95% CI) | 1 | 0.96 (0.71–1.31) | 0.87 (0.64–1.19) | 1.09 (0.80–1.48) |
| Adj.c HR (95% CI) | 1 | 0.98 (0.72–1.33) | 0.85 (0.62–1.16) | 0.89 (0.65–1.22) |
| All-cause mortality | ||||
| Crude HR (95% CI) | 1 | 1.06 (0.82–1.36) | 0.91 (0.71–1.18) | 1.03 (0.80–1.32) |
| Adj.a HR (95% CI) | 1 | 1.10 (0.85–1.42) | 0.93 (0.72–1.21) | 1.23 (0.96–1.60) |
| Adj.b HR (95% CI) | 1 | 1.08 (0.84–1.40) | 0.91 (0.70–1.18) | 1.16 (0.89–1.51) |
| Adj.c HR (95% CI) | 1 | 1.08 (0.83–1.39) | 0.89 (0.68–1.16) | 0.95 (0.73–1.25) |
aModel 1: adjusted for age; sex; atorvastatin treatment; systolic blood pressure; smoking status; duration of dialysis; BMI; and levels of HDL and LDL-cholesterol, calcium, phosphate, potassium, and haemoglobin.
bModel 2: additional adjustments for medication use including ACE-inhibitors, AT2-receptor-antagonists, beta blockers, and diuretics.
cModel 3: additional adjustments for intermediate variables including coronary artery disease, congestive heart failure, arrhythmia, left ventricular hypertrophy, C-reactive protein, and NT-proBNP.
The risk of dying from heart failure was two-fold increased in the patients with the highest compared with those with lowest aldosterone concentrations, although not significant (HR: 2.11, 95% CI: 0.76–5.84). Of note, this negative impact for the group of patients with high aldosterone derives almost exclusively from the patients with aldosterone levels of >300 pg/mL [HR: 3.38 (1.06–10.81), Supplementary material online, Table S2a].
Similarly, the endpoints of combined CVE and all-cause mortality were not significantly related to aldosterone status except in the subgroup of patients with very high aldosterone concentrations of >300 pg/mL [HR: 1.47 (1.01–2.13) for CVE and 1.66 (1.21–2.29) for mortality, Supplementary material online, Table S2a]. In contrast, we found no significant association between aldosterone status and MI and stroke in any aldosterone category. Stroke consists of various pathophysiologies; it could not be subdivided according to its origin (ischaemic, haemorrhagic) because of relatively small patient groups.
Cortisol status and adverse clinical outcomes
Cortisol concentrations at baseline were not significantly associated with sudden cardiac death during follow-up (see Figure 1B and Supplementary material online, Table S2b). Compared with the lowest cortisol quartile, the adjusted HR for sudden cardiac death was 1.31 (95% CI: 0.84–2.06) for the patients in the highest cortisol quartile, 1.00 (0.62–1.61) for the third-quartile, and 0.98 (0.61–1.58) for the second-quartile. There was no association with death because of heart failure.
There was an increase in MI in patients in the highest vs. the lowest cortisol quartile (unadjusted HR: 1.56, 95% CI: 1.02–2.39), which lost its significance after adjustments (adjusted HR: 1.47, 95% CI: 0.94–2.28). A similar non-significant association was seen between cortisol status and stroke (adjusted HR: 1.50, 95% CI: 0.80–2.79, Supplementary material online, Table S2b).
There was no significant association between cortisol status and combined cardiovascular events and all-cause mortality.
Synergism between aldosterone and cortisol in relation to sudden cardiac death
The patients with high aldosterone concentrations (>200 pg/mL) plus high cortisol concentrations (>21.1 µg/dL) were compared with the patients with both low aldosterone (<15 pg/mL) and low cortisol (<13.2 µg/dL) concentrations. In the patients with high concentrations of both hormones, the risk of sudden cardiac death was increased >2.5-fold (HR: 2.59, 95% CI: 1.34–4.98) compared with the patients with low concentrations of both hormones. This association was even stronger after adjustment for confounders (HR: 2.86, 95% CI: 1.32–6.21) (Table 3). All-cause mortality was significantly higher by 62% in the patients with both high aldosterone and cortisol concentrations (HR: 1.62, 95% CI: 1.01–2.62) compared with the patients with both low concentrations. Additional analysis showed that this relation is explained mainly by the synergistic impact of both hormones on sudden cardiac death; no association was found for the other adverse CV outcomes and an additional endpoint comprising all-cause mortality except SCD.
Table 3.
Hazard ratios with 95% confidence intervals (HR: 95% CI) for sudden cardiac death, stroke, myocardial infarction, combined cardiovascular events, and all-cause mortality according to the combined aldosterone (pg/mL) and cortisol (µg/dL) status at baseline
| Outcome | Combined aldosterone (<15) and cortisol (<13.2), n = 202 | Combined aldosterone (>200) and cortisol (>21.1), n = 48 |
|---|---|---|
| Sudden death | ||
| Crude HR (95% CI) | 1 | 2.59 (1.34–4.98) |
| Adj.a HR (95% CI) | 1 | 3.34 (1.60–6.94) |
| Adj.b HR (95% CI) | 1 | 2.86 (1.32–6.21) |
| Adj.c HR (95% CI) | 1 | 1.56 (0.68–3.57) |
| Stroke | ||
| Crude HR (95% CI) | 1 | 0.96 (0.28–3.31) |
| Adj.a HR (95% CI) | 1 | 0.85 (0.20–3.58) |
| Adj.b HR (95% CI) | 1 | 0.86 (0.18–4.11) |
| Adj.c HR (95% CI) | 1 | 0.51 (0.10–2.71) |
| Myocardial infarction | ||
| Crude HR (95% CI) | 1 | 0.83 (0.32–2.17) |
| Adj.a HR (95% CI) | 1 | 0.99 (0.35–2.78) |
| Adj.b HR (95% CI) | 1 | 1.00 (0.33–3.05) |
| Adj.c HR (95% CI) | 1 | 0.70 (0.22–2.22) |
| Cardiovascular events | ||
| Crude HR (95% CI) | 1 | 1.44 (0.90–2.30) |
| Adj.a HR (95% CI) | 1 | 1.63 (0.98–2.70) |
| Adj.b HR (95% CI) | 1 | 1.57 (0.92–2.70) |
| Adj.c HR (95% CI) | 1 | 0.98 (0.55–1.76) |
| All-cause mortality | ||
| Crude HR (95% CI) | 1 | 1.53 (1.01–2.32) |
| Adj.a HR (95% CI) | 1 | 1.89 (1.20–2.98) |
| Adj.b HR (95% CI) | 1 | 1.62 (1.01–2.62) |
| Adj.c HR (95% CI) | 1 | 0.85 (0.50–1.43) |
High aldosterone and cortisol levels were present in a subgroup of 48 patients, and the event numbers concerning heart failure deaths were too small to study the effect in these subgroup analyses.
aModel 1: adjusted for age; sex; atorvastatin treatment; systolic blood pressure; smoking status; duration of dialysis; BMI; and levels of HDL and LDL-cholesterol, calcium, phosphate, potassium, and haemoglobin.
bModel 2: additional adjustments for medication use including ACE-inhibitors, AT2-receptor-antagonists, beta blockers, and diuretics.
cModel 3: additional adjustments for intermediate variables including coronary artery disease, congestive heart failure, arrhythmia, left ventricular hypertrophy, C-reactive protein, and NT-proBNP.
The patients of the intermediary groups [aldosterone between 15 and 200 pg/mL (Groups 2 and 3), cortisol quartiles 2 and 3] did not show an increased risk of sudden death [HR: 0.89 (0.45–1.75) or mortality (HR: 1.13 (0.81–1.58)]. Compared with the patients with low levels of both hormones, the patients with any other combination of aldosterone and cortisol (low-intermediary or low-high, high-intermediary or high-low) did not show an increased risk of adverse events except those with the highest levels of both hormones as already mentioned.
Atorvastatin treatment
To rule out any influence by atorvastatin treatment, we have corrected for the use of atorvastatin in all analyses. In addition, we have investigated potential interaction by study treatment. The included interaction term was not significant in any of the analyses, indicating no interaction by atorvastatin treatment.
Discussion
This study investigated the impact of aldosterone and cortisol concentrations on cardiac and vascular events and all-cause mortality in patients with type 2 diabetes mellitus undergoing maintenance haemodialysis. The major finding of this study is that high aldosterone concentrations in these patients are associated with a significant increase in the incidence of sudden cardiac death. The combined presence of high serum aldosterone and high cortisol concentrations increased the risk of sudden cardiac death further by a factor of almost three and this accounted largely for the significantly increased all-cause mortality. In addition, a very high aldosterone level is associated with deaths due to heart failure, CVE, and mortality, while there is no significant association between cortisol and adverse events in adjusted analyses.
In the general population, MI is the most common cause of cardiovascular death.19,20 In contrast, registry data indicate that in dialysis patients sudden cardiac death accounts for 47% of cardiovascular deaths.21,22 The mechanisms causing sudden death are largely unknown;23,24 the current finding is therefore of interest that—at least in diabetic dialysis patients—increased levels of aldosterone are significantly associated with the risk of sudden cardiac death. This finding is in line with many studies indicating that aldosterone is an important mediator of cardiovascular damage in non-renal patients and also in patients with early chronic kidney disease and CV damage.25,26 Furthermore, the mineralocorticoid receptor blockade in heart failure patients has been shown to reduce sudden cardiac death and all-cause mortality.27,28 The underlying mechanisms are unclear but the following considerations may be of relevance. Aldosterone is known to cause electrical instability. For instance, in the study by Lendeckel et al.29 aldosterone was shown to promote development of atrial fibrillation. This was attributed to the action of aldosterone to promote oxidative stress, inflammation, and fibrosis with subsequent remodelling-induced electrical abnormalities.30
It is of interest that aldosterone causes electrical remodelling independent of hypertension.31 Aldosterone was also correlated with atrial fibrillation in the LURIC study.32 The effect of aldosterone on electrical stability and sudden death has recently also been commented upon by Luft.33
Cardiac interstitial fibrosis is a feature of cardiomyopathy in renal failure as shown by Amann and Ritz 34 and many other authors. Aldosterone has pro-hypertrophic and pro-fibrotic effects.35 In a rat study, hypertensive aldosterone-treated animals exhibited increased left ventricular end-diastolic pressure, increased cardiomyocyte size, collagen deposition, and inflammation; these findings were reversed by spironolactone.36 For the adverse hypertrophic and fibrotic effects, there is cross-talk between aldosterone and angiotensinII.37 It is, therefore, of interest that our finding of increased sudden death was adjusted for the concomitant use of ACE-inhibitors/angiotensin-receptor blockers. According to Waanders et al.,38 blood-pressure-independent effects play a role in situations of low circulating and high tissue aldosterone levels, high sodium intake possibly aggravating the effect of aldosterone. We have no information on sodium intake, but ultrafiltration volume as an indirect measure of inter-dialytic salt intake was not significantly correlated with the risk of sudden death.
Oxidative stress is increased by aldosterone through induction of NADPH oxidase5–7 contributing to vascular inflammation.5,8 Aldosterone-dependent inflammation has been well documented in vascular smooth muscle cells and other tissues as a culprit causing inflammatory cell infiltration and adhesion as well as activation of NFκB, thus provoking an inflammatory phenotype.39 This prompted us to analyse the correlation between C-reactive protein and plasma aldosterone concentration in the aforementioned dialysed patients, which was significant (P < 0.001). We found a meaningful increase in median C-reactive protein with increasing categories of aldosterone concentration, supporting the aforementioned concept. Krug et al.40 found that elevated mineralocorticoid receptor activity promotes and amplifies a pro-inflammatory phenotype via extracellular signal-regulated kinase and epidermal growth factor-dependent pathways, particularly in aged rats.
The enhancement of the adverse effect of aldosterone by the coexistence of elevated cortisol concentrations deserves comment. It has been shown that aldosterone and cortisol were associated with medium-term left ventricular remodelling when measured early after acute MI.41 In a recent study by Yamaji et al.,42 high serum cortisol was an independent predictor of cardiac events in patients with chronic heart failure, particularly in the presence of oxidative stress as measured by oxLDL. Similarly, elevated cortisol concentrations have been shown to provide an incremental mortality risk in patients with systolic heart failure.43 This mutual enforcement of adverse effects could also be demonstrated in this study of haemodialysed diabetic patients. It has been shown by N'Gankam et al.14 that the activity of 11βHSD2 is reduced in haemodialysed patients, with consecutive increased cortisol metabolites. This may contribute to explanation of our finding that the cardiac risk induced by aldosterone is amplified in the presence of elevated cortisol. Furthermore, cortisol concentrations may be particularly important and decisive of outcomes in the cells not expressing 11βHSD2. While the enzyme is present mainly in the kidney and the blood vessels, it is not abundant in the heart. Under conditions when the efficacy of this enzyme is additionally reduced such as in advanced age, inflammation, and uraemia,15 cardiac mineralocorticoid receptors, therefore, may be activated mainly by cortisol, which circulates in concentrations 100-fold to 1000-fold higher than aldosterone.12
Our finding that high aldosterone and high cortisol levels are associated with sudden cardiac death and all-cause mortality provides a strong rationale that the pharmacological blockade of the mineralocorticoid receptor may improve outcomes in dialysis patients. Given that mineralocorticoid receptor antagonists can cause dangerous hyperkalaemia particularly when combined with other drugs interfering with the renin angiotensin aldosterone system (RAAS), both spironolactone and eplerenone are contraindicated in patients with moderately to severely impaired kidney function. This is also reflected by our study, in which there were eight patients only using spironolactone. This number was too small to allow adjustments regarding MR antagonist use. The 4D study took place in the years 1998 to 2004, when MR antagonist use was not part of clinical practice. We also investigated potassium levels in our study. Potassium levels were not meaningfully different across the categories of aldosterone. Furthermore, the patients with both high aldosterone and high cortisol concentrations had potassium levels similar to those in the patients with low aldosterone and low cortisol. To strengthen our results, we still adjusted our outcome analyses for potassium levels to rule out potential residual influences. Beyond this, encouraging data from several smaller clinical studies suggest that low-dose spironolactone treatment in dialysis patients appears to be safe.44 The largest of these trials found only a mild increase in pre-dialysis potassium levels of 0.2 mmol/L in 61 dialysis patients treated with 25 mg spironolactone daily.45 Most importantly, during the 4-month treatment, dangerous hyperkalaemia as defined by a potassium >6.8 mmol/L was not reported. These data suggest that dialysis patients appear to be protected from spironolactone-induced potassium accumulation (no functioning kidney, regular electrolyte equilibration during dialysis) and thus, spironolactone appears to be safe in these patients. Efficacy data of spironolactone on cardiovascular parameters in dialysis patients are scarce. Vukusich et al.46 reported a beneficial effect on carotid intima media thickness, whereas data on cardiac hypertrophy are controversial.47,48 Currently, the randomized clinical trial ‘Mineralocorticoid Receptor Antagonists in end stage renal disease’ (MiREnDa, NCT No.: NCT01691053) investigates the effect of spironolactone on left ventricular mass in dialysis patients. Further trials will address the effect of low-dose spironolactone on hard endpoints. The novel randomized studies will provide important insights into both safety and efficacy of mineralocorticoid receptor antagonists in dialysis patients.
Potential limitations of the study need to be acknowledged. It was a post hoc analysis within a selected cohort of German patients with type 2 diabetes mellitus undergoing haemodialysis. Therefore, the relationship between aldosterone, cortisol and adverse outcome may not be generalizable to other patient populations. In addition, more than half of our patients had an aldosterone level below the detection limit. This is possibly related to the phenomenon of hyporeninemic hypoaldosteronism, which is particularly relevant in diabetic patients.49 However, this might be partly explained also by the assay used for aldosterone measurement. It has been shown that some (but not all) commercially available aldosterone assays report higher circulating aldosterone concentrations compared with our in house assay.50 Another fact that has to be acknowledged is the low percentage of patients with high aldosterone that were treated with drugs targeting the RAAS. First, this can be responsible—in part—for the higher aldosterone levels and second this might impact on the clinical outcome. Although we adjusted during the statistical analysis for drugs, we cannot fully exclude that the lack of RAAS inhibition influenced our results. Furthermore, we did not have other detailed parameters of the RAAS available and cannot distinguish the patients with low aldosterone levels. In addition, measurements of oxidative stress were not available to study potential interaction with aldosterone concentrations. Despite careful adjustments for possible confounders, we cannot rule out residual confounding. However, since the known important confounders were considered, the effect of potential residual confounding is likely to be small. We do not have echocardiographic measurements for feasibility reasons (1255 dialysis patients from 178 centres). The main strengths of this study were the specific outcomes, which were verified by a blinded independent endpoint committee. In this context, the long-term follow-up, adequate sample size, and high incidence of pre-specified endpoints are of further major relevance.
Conclusions
In conclusion, the combination of high aldosterone with high cortisol was highly correlated with the incidence of sudden cardiac death and all-cause mortality in haemodialysed type 2 diabetic patients. The effect on sudden cardiac death thereby largely accounted for the mortality risk being associated with the combination of high aldosterone and cortisol levels. Whether the blockade of the mineralocorticoid receptor decreases the risk of sudden death in haemodialysis patients without causing side effects must be examined in future trials.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
We express our gratitude to all the patients who participated in the 4D study. We thank all the investigators and study nurses who took part and contributed to data collection in the 4D study.
Funding
This work was supported by a research grant from the Deutsche Forschungsgemeinschaft (DR 804/1-1) to C.D. and by a fellowship grant from the Medical Faculty of the University of Würzburg (Habilitationsstipendium) to C.D. The work was furthermore supported by the German Ministry of Education and Research (project 01GL0304: Comprehensive Heart Failure Center). Funding for open access charge: University Hospital Wuerzburg (project 01GL0304: Comprehensive Heart Failure Center).
Conflict of interest: A.T. is partially funded by the EU Project ‘MASCARA’ (‘Markers for Sub-Clinical Cardiovascular Risk Assessment’; THEME HEALTH.2011.2.4.2-2; Grant agreement no: 278249). W.M. is partially funded by Synlab Services GmbH and Shareholder of Synlab services GmbH. The other authors report no conflict of interest.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 3.US Renal Data System. Bethesda, MD: 2008. National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases. [Google Scholar]
- 4.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 5.Iwashima F, Yoshimoto T, Minami I, Sakurada M, Hirono Y, Hirata Y. Aldosterone induces superoxide generation via Rac1 activation in endothelial cells. Endocrinology. 2008;149:1009–1014. doi: 10.1210/en.2007-0864. [DOI] [PubMed] [Google Scholar]
- 6.Leopold JA, Dam A, Maron BA, Scribner AW, Liao R, Handy DE, Stanton RC, Pitt B, Loscalzo J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13:189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakamura T, Kataoka K, Fukuda M, Nako H, Tokutomi Y, Dong YF, Ichijo H, Ogawa H, Kim-Mitsuyama S. Critical role of apoptosis signal-regulating kinase 1 in aldosterone/salt-induced cardiac inflammation and fibrosis. Hypertension. 2009;54:544–551. doi: 10.1161/HYPERTENSIONAHA.109.135392. [DOI] [PubMed] [Google Scholar]
- 8.Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol. 2002;283:H1802–H1810. doi: 10.1152/ajpheart.01096.2001. [DOI] [PubMed] [Google Scholar]
- 9.Blanco-Rivero J, Cachofeiro V, Lahera V, ras-Lopez R, Marquez-Rodas I, Salaices M, Xavier FE, Ferrer M, Balfagon G. Participation of prostacyclin in endothelial dysfunction induced by aldosterone in normotensive and hypertensive rats. Hypertension. 2005;46:107–112. doi: 10.1161/01.HYP.0000171479.36880.17. [DOI] [PubMed] [Google Scholar]
- 10.Garnier A, Bendall JK, Fuchs S, Escoubet B, Rochais F, Hoerter J, Nehme J, Ambroisine ML, De AN, Morineau G, d'Estienne P, Fischmeister R, Heymes C, Pinet F, Delcayre C. Cardiac specific increase in aldosterone production induces coronary dysfunction in aldosterone synthase-transgenic mice. Circulation. 2004;110:1819–1825. doi: 10.1161/01.CIR.0000142858.44680.27. [DOI] [PubMed] [Google Scholar]
- 11.Endemann DH, Touyz RM, Iglarz M, Savoia C, Schiffrin EL. Eplerenone prevents salt-induced vascular remodeling and cardiac fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension. 2004;43:1252–1257. doi: 10.1161/01.HYP.0000128031.31572.a3. [DOI] [PubMed] [Google Scholar]
- 12.Funder JW. Reconsidering the roles of the mineralocorticoid receptor. Hypertension. 2009;53:286–290. doi: 10.1161/HYPERTENSIONAHA.108.119966. [DOI] [PubMed] [Google Scholar]
- 13.Gekle M, Grossmann C. Actions of aldosterone in the cardiovascular system: the good, the bad, and the ugly? Pflugers Arch. 2009;458:231–246. doi: 10.1007/s00424-008-0616-0. [DOI] [PubMed] [Google Scholar]
- 14.N'Gankam V, Uehlinger D, Dick B, Frey BM, Frey FJ. Increased cortisol metabolites and reduced activity of 11beta-hydroxysteroid dehydrogenase in patients on hemodialysis. Kidney Int. 2002;61:1859–1866. doi: 10.1046/j.1523-1755.2002.00308.x. [DOI] [PubMed] [Google Scholar]
- 15.Henschkowski J, Stuck AE, Frey BM, Gillmann G, Dick B, Frey FJ, Mohaupt MG. Age-dependent decrease in 11beta-hydroxysteroid dehydrogenase type 2 (11beta-HSD2) activity in hypertensive patients. Am J Hypertens. 2008;21:644–649. doi: 10.1038/ajh.2008.152. [DOI] [PubMed] [Google Scholar]
- 16.Wanner C, Krane V, Marz W, Olschewski M, Asmus HG, Kramer W, Kuhn KW, Kutemeyer H, Mann JF, Ruf G, Ritz E. Randomized controlled trial on the efficacy and safety of atorvastatin in patients with type 2 diabetes on hemodialysis (4D study): demographic and baseline characteristics. Kidney Blood Press Res. 2004;27:259–266. doi: 10.1159/000080241. [DOI] [PubMed] [Google Scholar]
- 17.Hannemann A, Meisinger C, Bidlingmaier M, Doring A, Thorand B, Heier M, Belcredi P, Ladwig KH, Wallaschofski H, Friedrich N, Schipf S, Ludemann J, Rettig R, Peters J, Volzke H, Seissler J, Beuschlein F, Nauck M, Reincke M. Association of plasma aldosterone with the metabolic syndrome in two German populations. Eur J Endocrinol. 2011;164:751–758. doi: 10.1530/EJE-10-1074. [DOI] [PubMed] [Google Scholar]
- 18.Manolopoulou J, Bielohuby M, Caton SJ, Gomez-Sanchez CE, Renner-Mueller I, Wolf E, Lichtenauer UD, Beuschlein F, Hoeflich A, Bidlingmaier M. A highly sensitive immunofluorometric assay for the measurement of aldosterone in small sample volumes: validation in mouse serum. J Endocrinol. 2008;196:215–224. doi: 10.1677/JOE-07-0134. [DOI] [PubMed] [Google Scholar]
- 19.McGovern PG, Pankow JS, Shahar E, Doliszny KM, Folsom AR, Blackburn H, Luepker RV. Recent trends in acute coronary heart disease—mortality, morbidity, medical care, and risk factors. The Minnesota Heart Survey Investigators. N Engl J Med. 1996;334:884–890. doi: 10.1056/NEJM199604043341403. [DOI] [PubMed] [Google Scholar]
- 20.Yeh RW, Sidney S, Chandra M, Sorel M, Selby JV, Go AS. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362:2155–2165. doi: 10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 21.Collins AJ, Foley RN, Herzog C, Chavers BM, Gilbertson D, Ishani A, Kasiske BL, Liu J, Mau LW, McBean M, Murray A, St PW, Guo H, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers PW, Agodoa L. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidny Dis. 2010;55(1 Suppl 1):S1–420. doi: 10.1053/j.ajkd.2009.10.009. A426–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzog CA. Cardiac arrest in dialysis patients: approaches to alter an abysmal outcome. Kidney Int Suppl. 2003;84:S197–S200. doi: 10.1046/j.1523-1755.63.s84.17.x. [DOI] [PubMed] [Google Scholar]
- 23.Herzog CA, Asinger RW, Berger AK, Charytan DM, Diez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80:572–586. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]
- 24.Ritz E, Wanner C. The challenge of sudden death in dialysis patients. Clin J Am Soc Nephrol. 2008;3:920–929. doi: 10.2215/CJN.04571007. [DOI] [PubMed] [Google Scholar]
- 25.Edwards NC, Steeds RP, Stewart PM, Ferro CJ, Townend JN. Effect of spironolactone on left ventricular mass and aortic stiffness in early-stage chronic kidney disease: a randomized controlled trial. J Am Coll Cardiol. 2009;54:505–512. doi: 10.1016/j.jacc.2009.03.066. [DOI] [PubMed] [Google Scholar]
- 26.Tomaschitz A, Pilz S, Ritz E, Grammer T, Drechsler C, Boehm BO, Marz W. Association of plasma aldosterone with cardiovascular mortality in patients with low estimated GFR: the Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Am J Kidny Dis. 2011;57:403–414. doi: 10.1053/j.ajkd.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 27.Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 28.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 29.Lendeckel U, Dobrev D, Goette A. Aldosterone-receptor antagonism as a potential therapeutic option for atrial fibrillation. Br J Pharmacol. 2010;159:1581–1583. doi: 10.1111/j.1476-5381.2010.00675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raschi E, Boriani G, De Ponti F. Targeting the arrhythmogenic substrate in atrial fibrillation: focus on structural remodeling. Curr Drug Targets. 2011;12:263–286. doi: 10.2174/138945011794182728. [DOI] [PubMed] [Google Scholar]
- 31.Dartsch T, Fischer R, Gapelyuk A, Weiergraeber M, Ladage D, Schneider T, Schirdewan A, Reuter H, Mueller-Ehmsen J, Zobel C. Aldosterone induces electrical remodeling independent of hypertension. Int J Cardiol. doi: 10.1016/j.ijcard.2011.06.100. Advance Access published July 18, 2011, doi:10.1016/j.ijcard.2011.06.100. [DOI] [PubMed] [Google Scholar]
- 32.Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, Marz W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Eur Heart J. 2010;31:1237–1247. doi: 10.1093/eurheartj/ehq019. [DOI] [PubMed] [Google Scholar]
- 33.Luft FC. Connecting the renin-angiotensin-aldosterone system with sudden death. J Mol Med (Berl) 2011;89:631–633. doi: 10.1007/s00109-011-0772-0. [DOI] [PubMed] [Google Scholar]
- 34.Amann K, Ritz E. Cardiac disease in chronic uremia: pathophysiology. Adv Ren Replace Ther. 1997;4:212–224. doi: 10.1016/s1073-4449(97)70030-x. [DOI] [PubMed] [Google Scholar]
- 35.Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol. 2010;6:261–273. doi: 10.1038/nrneph.2010.30. [DOI] [PubMed] [Google Scholar]
- 36.Young MJ, Rickard AJ. Mechanisms of mineralocorticoid salt-induced hypertension and cardiac fibrosis. Mol Cell Endocrinol. 2012;350:248–255. doi: 10.1016/j.mce.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Rautureau Y, Paradis P, Schiffrin EL. Cross-talk between aldosterone and angiotensin signaling in vascular smooth muscle cells. Steroids. 2011;76:834–839. doi: 10.1016/j.steroids.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Waanders F, de Vries LV, van Goor H, Hillebrands JL, Laverman GD, Bakker SJ, Navis G. Aldosterone, from (patho)physiology to treatment in cardiovascular and renal damage. Curr Vasc Pharmacol. 2011;9:594–605. doi: 10.2174/157016111796642689. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert KC, Brown NJ. Aldosterone and inflammation. Curr Opin Endocrinol Diabetes Obes. 2010;17:199–204. doi: 10.1097/med.0b013e3283391989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krug AW, Allenhofer L, Monticone R, Spinetti G, Gekle M, Wang M, Lakatta EG. Elevated mineralocorticoid receptor activity in aged rat vascular smooth muscle cells promotes a proinflammatory phenotype via extracellular signal-regulated kinase 1/2 mitogen-activated protein kinase and epidermal growth factor receptor-dependent pathways. Hypertension. 2010;55:1476–1483. doi: 10.1161/HYPERTENSIONAHA.109.148783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weir RA, Tsorlalis IK, Steedman T, Dargie HJ, Fraser R, McMurray JJ, Connell JM. Aldosterone and cortisol predict medium-term left ventricular remodelling following myocardial infarction. Eur J Heart Fail. 2011;13:1305–1313. doi: 10.1093/eurjhf/hfr129. [DOI] [PubMed] [Google Scholar]
- 42.Yamaji M, Tsutamoto T, Kawahara C, Nishiyama K, Yamamoto T, Fujii M, Horie M. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: the impact of oxidative stress. Circ Heart Fail. 2009;2:608–615. doi: 10.1161/CIRCHEARTFAILURE.109.868513. [DOI] [PubMed] [Google Scholar]
- 43.Guder G, Bauersachs J, Frantz S, Weismann D, Allolio B, Ertl G, Angermann CE, Stork S. Complementary and incremental mortality risk prediction by cortisol and aldosterone in chronic heart failure. Circulation. 2007;115:1754–1761. doi: 10.1161/CIRCULATIONAHA.106.653964. [DOI] [PubMed] [Google Scholar]
- 44.Chua D, Lo A, Lo C. Spironolactone use in heart failure patients with end-stage renal disease on hemodialysis: is it safe? Clin Cardiol. 2010;33:604–608. doi: 10.1002/clc.20838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsumoto Y, Kageyama S, Yakushigawa T, Arihara K, Sugiyama T, Mori Y, Sugiyama H, Ohmura H, Shio N. Long-term low-dose spironolactone therapy is safe in oligoanuric hemodialysis patients. Cardiology. 2009;114:32–38. doi: 10.1159/000210553. [DOI] [PubMed] [Google Scholar]
- 46.Vukusich A, Kunstmann S, Varela C, Gainza D, Bravo S, Sepulveda D, Cavada G, Michea L, Marusic ET. A randomized, double-blind, placebo-controlled trial of spironolactone on carotid intima-media thickness in nondiabetic hemodialysis patients. Clin J Am Soc Nephrol. 2010;5:1380–1387. doi: 10.2215/CJN.09421209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McGill RL, Biederman RW, Getts RT, Hazlett SM, Sharma SB, Duran J, Brandys DE, Sysak JC, Sureshkumar KK, Sandroni SE, Marcus RJ. Cardiac magnetic resonance imaging in hemodialysis patients. J Nephrol. 2009;22:367–372. [PubMed] [Google Scholar]
- 48.Taheri S, Mortazavi M, Shahidi S, Pourmoghadas A, Garakyaraghi M, Seirafian S, Eshaghian A, Ghassami M. Spironolactone in chronic hemodialysis patients improves cardiac function. Saudi J Kidney Dis Transpl. 2009;20:392–397. [PubMed] [Google Scholar]
- 49.Grande Villoria J, Macias Nunez JF, Miralles JM, De Castro del Pozo S, Tabernero Romo JM. Hyporeninemic hypoaldosteronism in diabetic patients with chronic renal failure. Am J Nephrol. 1988;8:127–137. doi: 10.1159/000167571. [DOI] [PubMed] [Google Scholar]
- 50.Schirpenbach C, Seiler L, Maser-Gluth C, Beuschlein F, Reincke M, Bidlingmaier M. Automated chemiluminescence-immunoassay for aldosterone during dynamic testing: comparison to radioimmunoassays with and without extraction steps. Clin Chem. 2006;52:1749–1755. doi: 10.1373/clinchem.2006.068502. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.