Abstract
Aims
The ECOST trial examined prospectively the long-term safety and effectiveness of home monitoring (HM) of implantable cardioverter defibrillators (ICD).
Methods and results
The trial's primary objective was to randomly compare the proportions of patients experiencing ≥1 major adverse event (MAE), including deaths from all causes, and cardiovascular, procedure-related, and device-related MAE associated with HM (active group) vs. ambulatory follow-ups (control group) in a sample of 433 patients. The 221 patients assigned to the active group were seen once a year, unless HM reported an ICD dysfunction or a clinical event requiring an ambulatory visit, while the 212 patients in the control group underwent ambulatory visits every 6 months. The characteristics of the study groups were similar. Over a follow-up of 24.2 months, 38.5% of patients in the active and 41.5% in the control group experienced ≥1 MAE (P < 0.05 for non-inferiority). The overall number of shocks delivered was significantly lower in the active (n = 193) than in the control (n = 657) group (P < 0.05) and the proportion of patients who received inappropriate shocks was 52% lower in the active (n = 11) than in the control (n = 22) group (P < 0.05). At the end of the follow-up, the battery longevity was longer in the active group because of a lower number of capacitor charges (499 vs. 2081).
Conclusion
Our observations indicate that long-term HM of ICD is at least as safe as standard ambulatory follow-ups with respect to a broad spectrum of MAE. It also lowered significantly the number of appropriate and inappropriate shocks delivered, and spared the device battery.
Clinical trials registration
Keywords: Telemedicine, Remote monitoring, Implantable cardioverter defibrillator
Introduction
The application of information and communication technology is expected to markedly improve the worldwide delivery of healthcare in this decade.1 eHealth addresses a wide variety of critical medical issues, including risk of sudden cardiac death, particularly in recipients of implantable cardioverter defibrillators (ICD). It is, therefore, critical to verify the safety of eHealth. Thus far, remote monitoring of ICD has mostly been used as a supplement to direct ambulatory contacts between patients and caregivers, recommended at 6-month intervals.2 The goals of ICD follow-ups include the review of data stored in the device memory, including recordings of ventricular and supraventricular tachyarrhythmias, therapy delivered, and various indicators of system status and lead integrity. This monitoring can now be performed remotely, using long-distance telemetry, which allows the transmission of data to a service centre, where they can be reviewed online. Previous studies have shown that remote monitoring of ICD, compared with standard ambulatory follow-up at 3- or 6-month intervals, reduces the number of ambulatory visits and facilitates the early detection of arrhythmic events and technical malfunctions.3–10 Consequently, remote monitoring, if completely safe, could replace ambulatory visits. In an earlier study, it reduced the number of ambulatory follow-ups without jeopardizing safety, though its endpoint was limited to death, strokes, and procedures, such as device explantations or lead revisions, up to only 1 year.11
This trial was designed to compare the safety of remote monitoring vs. ambulatory follow-ups of ICD, using major adverse events (MAE) as a primary composite endpoint, including death from any cause, and major cardiovascular, procedure-related, and device-related adverse events.
Methods
Trial design and monitoring
The protocol of ECOST has been described previously.12 Patients scheduled to undergo implantation of ICD were randomly assigned, in a 1:1 design, to remote monitoring (active group) vs. ambulatory follow-ups (control group), before implantation of a single- or dual-chamber ICD. Cross-over from one study group to the other was not allowed.
The trial protocol was reviewed and approved by the pertinent Ethics Committees and all patients granted their written consent to participate in the study. All patient information was treated confidentially by the sponsor and all other parties involved in the trial, and all clinical centres were regularly monitored by the study team. Major adverse events were monitored and adjudicated by an Endpoint Adjudication Committee composed of three cardiologists who did not participate in the trial (see Supplementary material online, Appendix). An Electrogram Analysis Core Laboratory (see Supplementary material online, Appendix) reviewed all transmitted intracardiac electrograms (IEGM) independently.
Trial participants
The patients enrolled in this trial had an approved indication for a first implant or replacement of an ICD (Lumos® or Lumax®, Biotronik), as defined in professional practice guidelines.2 Patients in New York Heart Association heart failure functional class IV at the time of ICD implantation were excluded from enrolment.
Trial randomization and set-up of home monitoring
The patients were randomly assigned to remote monitoring vs. ambulatory follow-up over a dedicated telephone line. The date of randomization was that of enrolment in the trial. The patients underwent implantation of commercially available ICD equipped with Biotronik Home Monitoring® (HM; Biotronik SE and Co. KG, Berlin, Germany), according to each enrolling centre's standard operating procedures. The technical characteristics of HM have been detailed previously.6,11,12
After registration of the patients with the service centre and instructions on the use of the system, HM was activated in all study participants. However, access by the physicians was limited to the data transmitted by the active group. The use of HM was verified daily. In the absence of transmission for >7 days, the cause was immediately addressed.
For the duration of the trial, the data transmitted by HM were automatically processed daily. Clinical or technical events triggered notifications that were reviewed during working hours on the Internet site of the service centre. The investigator was notified by e-mail, and additional follow-up visits were scheduled, if appropriate. Level I events prompted the mandatory scheduling of an interim follow-up as soon as possible. Level II events prompted an ambulatory visit, if deemed necessary by the physician, after online analysis of IEGM (IIa) or system data (IIb) (Table 1). Home monitoring was not used as a substitute for medical emergency response systems. The decision to hospitalize a patient following an HM alert was left to the investigators' discretion.
Table 1.
Programming of home monitoring and levels of notification for clinical and technical events
| Study group |
|||
|---|---|---|---|
| Notification levela | Active | Control | |
| Technical | |||
| >7 days absence of transmission not explained remotely | I | ON | ON |
| System integrity | |||
| Impedance (ohm) | |||
| Lead | |||
| Ventricular <250 or >1500 | I | ON | OFF |
| Atrial <250 or >1500 | I | ON | OFF |
| Last shock <25 or >110 | I | ON | OFF |
| Special implant statusb | I | ON | ON |
| Elective replacement indicator (low battery) | I | ON | ON |
| Clinical | |||
| Therapy | |||
| Ineffective 30 J shock | I | ON | OFF |
| Ventricular tachycardia 1 (slow) detected | IIa | ON | OFF |
| Ventricular tachycardia 2 (fast) detected | IIa | ON | OFF |
| Ventricular fibrillation detected | IIa | ON | OFF |
| Supraventricular tachycardia detected | Iib | ON | OFF |
| Diagnostic | |||
| <90% intrinsic ventricular rhythm | IIb | OFF/ONc | OFF |
| First mode switch since last follow-up | IIb | OFF/ONc | OFF |
| >75% (18 h) spent in automatic mode switch | IIb | ON | OFF |
| Mean number of premature ventricular complexes >50/h | IIb | OFF/ONc | OFF |
ON/OFF, the investigator is/is not notified of the event.
aLevel I mandates an ambulatory visit as soon as possible; level II may prompt an ambulatory visit after online analysis of intracardiac electrograms (IIa) or system data (IIb).
bInactive ventricular tachycardia/fibrillation detection.
cAt the discretion of the investigator.
Device programming and management
The ICD Holter function was programmed to record IEGM for 30 s before, and 15 s after the detection of an arrhythmic event. Long-term RR memory and automatic impedance measurements were programmed ‘ON’. In dual-chamber ICD, the SMARTTM algorithm (Biotronik) was systematically activated for the detection and re-detection of tachyarrhythmias, to discriminate ventricular (VT) from supraventricular tachycardia. Ventricular tachycardia and ventricular fibrillation (VF) therapies were programmed at the physician's discretion. Ultimately, the lower limit of the first VT zone was programmed <150 b.p.m. in 4% of patients (in whom a slow VT had been documented), between 150 and 176 b.p.m. in 71%, and >176 b.p.m. in 12% of patients. The lower limit of the VF zone was ≥214 b.p.m. in 71% of patients. In the VT zones, several sequences of antitachycardia pacing (ATP) were programmed before the delivery of shocks.
Patient follow-ups
Patients in the active group were seen by a cardiac electrophysiologist in the ambulatory department within 1–3 months of ICD implantation, and at months 15 and 27 of follow-up, thereafter. At any time, additional ambulatory visits could be triggered by HM or at the request of the patient or a physician. In the control group, patients were seen in the ambulatory department at 1–3 months after ICD implantation for a first follow-up, and at 9, 15, 21, and 27 months of follow-up, thereafter. Additional visits could be scheduled if requested by the patient or physician. Furthermore, if needed, the study participants were followed by a cardiologist who managed their underlying heart disease, though was not authorized to interrogate or reprogram the ICD.
Study endpoints
The primary study endpoint was the proportion of patients who experienced ≥1 MAE, including death from any cause, cardiovascular, and procedure- or device-related MAE. A cardiovascular, procedure- or device-related adverse event was classified as major if it: (i) was fatal or life-threatening; (ii) prompted or prolonged a hospitalization; (iii) caused major or permanent disability or injury; or (iv) required an intervention to prevent permanent disability or injury. Device-related adverse events included: (i) delivery of ≥1 inappropriate shock(s); (ii) delivery of ≥2 symptomatic inappropriate ATP; (iii) symptomatic defibrillator-mediated tachycardia; (iv) infection or extrusion of the ICD; (v) ineffective pacing; (vi) intermittent or permanent undersensing; (vii) clinical phrenic stimulation; and (viii) lead or pulse generator dysfunction. The endpoints were ascertained by the investigators. All adverse events were documented on dedicated case report forms and, when major, were reported to the study team within two working days.
Test of non-inferiority and sample-size calculations
The primary study hypothesis was non-inferiority of HM compared with standard follow-ups, ascertained by the proportion of patients who suffered ≥1 MAE in each group. The primary analysis consisted of a test of non-inferiority, applied in the per-protocol population, which included all patients who completed the scheduled 27-month follow-up or experienced an earlier MAE (Figure 1). The test of non-inferiority was also applied to the intention-to-treat population. The estimated sample size was based on the comparison of the dichotomous primary endpoint between the two study groups, using a non-inferiority test with a 5% significance level, a non-inferiority margin equal to 20% of the control group and an 80% power to reject the null hypothesis. Non-inferiority was declared if the proportion of patients who experienced ≥1 MAE in the active group was ≤20% greater than the proportion of patients in the control group. It was assumed that 26% of patients in the control and 20% in the active group would experience an MAE. These assumptions were based on data published up to 2006,3,13 when the protocol of ECOST was developed. Further assuming a 15% dropout rate, the trial had to randomly assign 400 patients in a 1:1 ratio to the active vs. the control group. The sample size was re-assessed at 15 months and, based on the interim results, remained unchanged.
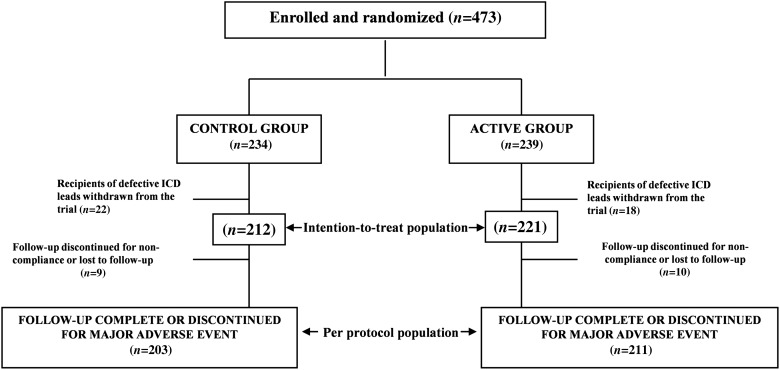
Figure 1.
Flow of patients between enrolment and end of follow-up.
Statistical analysis
The baseline nominal characteristics of the study groups were compared by χ2 test. The normal distribution of variables was verified, using the Kolmogorov–Smirnov and Shapiro–Wilk tests. Normally distributed variables were compared, using Student’t-test, after confirmation of the equality of variances by Levene's test. MAE-free survival was calculated by the Kaplan–Meier method and compared between groups, using the log-rank test. Cox's proportional hazard regression analysis was used to estimate the dependency of survival with respect to groups, after verifying the proportional hazard assumption. Hazard ratios (HR) for the occurrence of events in patient subgroups were calculated, using a stratified single variable Cox model. The components of the composite primary study endpoint were examined separately, modelling the probability of each type of MAE on the condition of a MAE occurring, and using a dichotomous logistic model, comparing the two study groups with odd ratio (OR) and 95% confidence intervals (CI). Since statistical significance was reached for the primary study endpoint, the appropriate and inappropriate shock deliveries and ICD capacitor charges, identified as secondary endpoints, were analysed, and their impact on the ICD battery longevity was evaluated by the slope of battery depletion over time, using a linear regression model.
All tests were performed at a P = 0.05 significance level. The SPSS, version 18.0 (SPSS Institute Inc., Chicago, IL, USA) and SAS, version 9.0 (SAS Institute Inc., Cary, NC, USA) statistical software was used for the analyses.
Results
Study population
Between January 2007 and April 2008, 473 patients were enrolled in ECOST at 43 French medical centres (see Supplementary material online, Appendix). On 15 October 2007, an urgent advisory was issued regarding a defective defibrillator lead, which had a nearly 17% fracture rate at 5 years.14 By that time, 40 recipients of that lead, who were advised to undergo a special surveillance program, had been enrolled in ECOST. While their continued participation in the trial might have considerably increased the adverse events rate, several ethical and methodological considerations weighed in favour of their exclusion. Regardless of their initial random assignment, these patients were (i) offered HM, (ii) systematically followed at 3-month intervals in the ambulatory department, (iii) entered in a registry,12 and (iv) withdrawn from the study upon the recommendation of the ECOST Endpoint Adjudication Committee, to optimize their safety and eliminate a possibly important bias in favour of the HM group. Since these patients were replaced, the final sample was greater than initially calculated. Ultimately, 221 patients were assigned to the active group, 212 were assigned to the control group, and 19 patients either discontinued their participation in, or were withdrawn from the study, or were lost to follow-up (Figure 1). The mean follow-up duration was 24.2 ± 7.3 months. The baseline clinical characteristics of the study groups were similar (Table 2).
Table 2.
Baseline characteristics of the intention-to-treat population
| All patients (n = 433) | Study group | ||
|---|---|---|---|
| Active (n = 221) | Control (n = 212) | ||
| Age, years | 61.6 ± 12.5 | 62.0 ± 13.0 | 61.2 ± 12.0 |
| Men/women | 382 (88.2)/51 (11.8) | 193 (87.3)/28 (12.7) | 189 (89.2)/23 (10.8) |
| Left-ventricular ejection fraction, % | 34.9 ± 13.3 | 34.7 ± 13.0 | 35.1 ± 13.6 |
| Indication for implantable cardioverter defibrillator | |||
| Primary prevention | 232 (53.6) | 119 (53.8) | 113 (53.3) |
| Secondary prevention | 201 (46.4) | 102 (46.2) | 99 (46.7) |
| Implanted device | |||
| Single chamber | 302 (69.7) | 161 (72.9) | 141 (66.5) |
| Dual chamber | 131 (30.3) | 60 (27.1) | 71 (33.5) |
| Device implant | |||
| First implantation | 369 (85.2) | 186 (84.2) | 183 (86.3) |
| Replacement | 64 (14.8) | 35 (15.8) | 29 (13.7) |
| New York Heart Association functional class | |||
| I | 113 (26.1) | 60 (27.1) | 53 (25.0) |
| II | 268 (61.9) | 139 (62.9) | 129 (60.8) |
| III | 38 (8.8) | 15 (6.4) | 25 (11.8) |
| Underlying heart disease | |||
| Coronary heart disease | 283 (65.4) | 143 (64.7) | 140 (66.5) |
| Non-ischaemic dilated cardiomyopathy | 76 (17.6) | 39 (17.6) | 37 (17.5) |
| Primary electric diseasea | 21 (4.8) | 11 (4.7) | 10 (4.7) |
| Hypertrophic cardiomyopathy | 15 (3.5) | 5 (2.3) | 10 (4.7) |
| Valvular heart disease | 12 (2.8) | 3 (1.4) | 9 (4.2) |
| Hypertension | 6 (1.4) | 3 (1.4) | 3 (1.4) |
| Other cardiomyopathy | 11 (2.5) | 8 (3.6) | 3 (1.4) |
| Undetermined | 8 (1.8) | 4 (1.8) | 4 (1.9) |
| None | 16 (3.7) | 11 (5.0) | 5 (2.6) |
| History of | |||
| Sustained ventricular tachycardia | 109 (25.2) | 54 (24.4) | 55 (25.9) |
| Ventricular fibrillation | 57 (13.2) | 30 (13.6) | 27 (12.7) |
| Torsade de pointes | 4 (0.9) | 2 (0.9) | 2 (0.9) |
| Atrial arrhythmia | 68 (15.7) | 38 (17.2) | 30 (14.2) |
Values are means ± SD, or numbers (%) of observations. Between-groups differences are all statistically non-significant.
aBrugada syndrome, long-QT syndrome.
Primary endpoint analysis
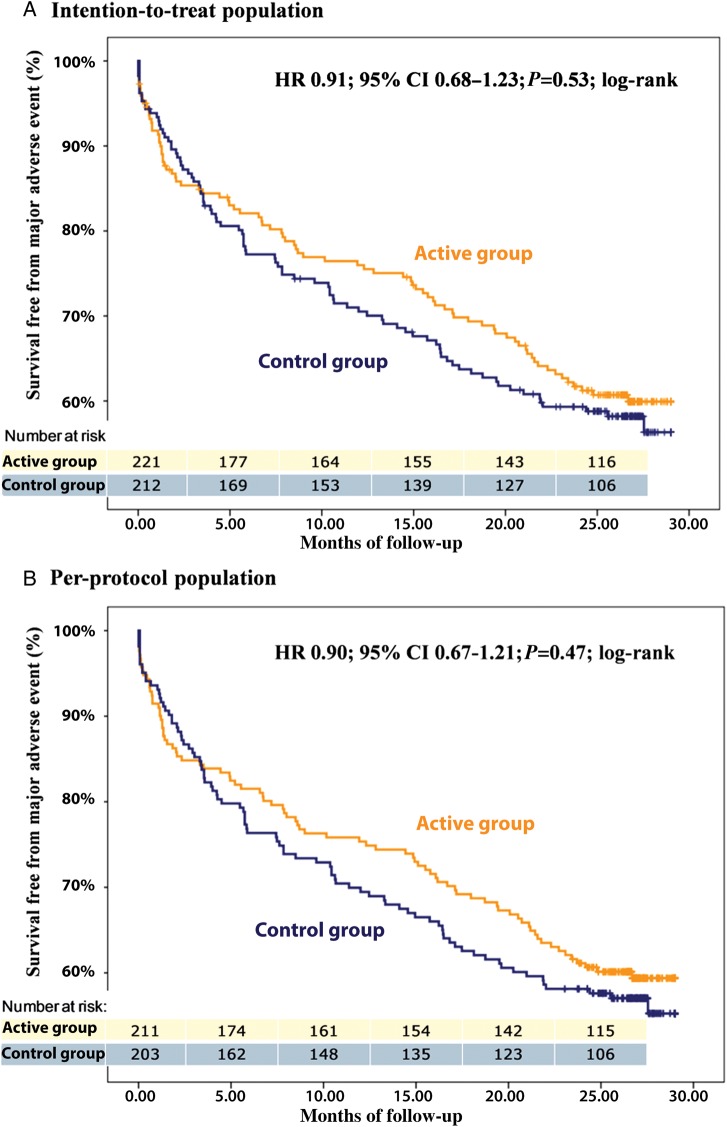
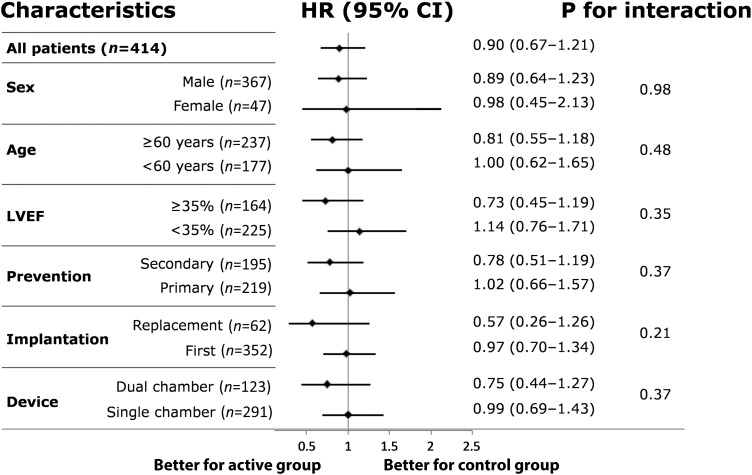
In the per-protocol analysis, ≥1 MAE occurred in 173 patients (41.8%), of whom 85 (40.3%) were in the active and 88 (43.3%) were in the control group (HR 0.90; 95% CI 0.67–1.21; P = 0.04 for non-inferiority). The first or only MAE experienced by individual patients included 12 deaths (5.7%), 50 cardiovascular (23.7%), 12 implant procedure-related (5.7%), and 11 device-related (5.2%) MAE in the active group, vs. 11 deaths (5.4%), 53 cardiovascular (26.1%), 10 implant procedure-related (4.9%), and 14 device-related (6.9%) MAE in the control group. Kaplan–Meier estimates of MAE-free survivals in the two groups are shown in Figure 2B, and the HR calculated in selected patient subgroups are shown in Figure 3. In the intention-to-treat analysis, 85 MAE (38.5%) occurred in the active, and 88 (41.5%) in the control group (HR 0.91; 95% CI 0.68–1.23; P = 0.04 for non-inferiority; Figure 2A).
Figure 2.
Cumulative survival free from major adverse events in the per-protocol (A) and in the intention-to-treat (B) populations.
Figure 3.
Risk of major adverse events according to selected characteristics. LVEF, left-ventricular ejection fraction. Prevention pertains to the implantable cardioverter defibrillator implantation indication.
No between-groups difference was observed in separate analyses of each component of the composite MAE (Table 3).
Table 3.
Rates of major adverse events in the per-protocol population
| Study group |
|||
|---|---|---|---|
| Active (n = 211) | Control (n = 203) | ||
| Causes of deatha | |||
| Stroke | 1 (0.5) | 0 (0) | |
| Heart failure | 7 (3.3) | 8 (3.9) | |
| Myocardial infarction | 0 (0) | 1 (0.5) | |
| Ventricular tachyarrhythmia | 3 (1.4) | 1 (0.5) | |
| Non-cardiac | 7 (3.3) | 8 (3.9) | |
| Undetermined | 2 (0.9) | 2 (0.9) | |
| All deaths | 20 (9.5) | 20 (9.9) | |
| Cardiovascular MAEa | |||
| Ventricular tachyarrhythmia | |||
| Without shock | 8 (3.8) | 4 (2.0) | |
| With shock | 9 (4.3) | 5 (2.5) | |
| Electrical storm | 11 (5.2) | 12 (5.9) | |
| Myocardial infarction | 0 (0) | 1 (0.5) | |
| Supraventricular arrhythmia | 5 (2.4) | 1 (0.5) | |
| Stroke | 4 (1.9) | 0 (0) | |
| Heart failure | 25 (11.8) | 32 (15.8) | |
| Acute coronary syndrome | 6 (2.8) | 10 (4.9) | |
| Other | 1 (0.5) | 6 (2.9) | |
| All cardiovascular MAE | 59 (28.0) | 63 (31.0) | |
| Procedure-related MAEa | |||
| Haematoma | 2 (0.9) | 1 (0.5) | |
| Infection | 4 (1.9) | 2 (0.9) | |
| Venous thrombosis | 0 (0) | 2 (0.9) | |
| Pneumothorax | 3 (1.4) | 0 (0) | |
| <1-month-old lead dislodgement | 5 (2.4) | 1 (0.5) | |
| Induction test failure | 1 (0.5) | 3 (1.5) | |
| Other | 0 (0) | 2 (0.9) | |
| All implant procedure-related MAE | 14 (6.6) | 11 (5.4) | |
| Device-related MAEa | |||
| Inappropriate shocks due to: | |||
| Supraventricular arrhythmia | 2 (0.9) | 6 (2.9) | |
| T-wave oversensing | 1 (0.5) | 1 (0.5) | |
| Lead dysfunction | 1 (0.5) | 4 (2.0) | |
| Lead dysfunction without inappropriate shock | 5 (2.4) | 1 (0.5) | |
| Other | 3 (1.4) | 4 (2.0) | |
| All device-related MAE | 12 (5.7) | 14 (6.9) | |
| All MAE | 85 (40.3) | 88 (43.3) | |
Values are numbers (%) of observations.
MAE, major adverse events.
aIncludes all first events of this component, whether preceded by another MAE or not.
Implantable cardioverter defibrillator therapies
The proportion of appropriate and inappropriate shocks delivered was 71% lower in the active than in the control group (P = 0.02; Table 4). In the control group, 22 recipients (10.4%) of 15 (10.6%) single-chamber and seven (9.9%) dual-chamber ICD, received 283 inappropriate shocks, while in the active group, 11 recipients (5.0%) of 10 single-chamber (6.2%) and one (1.7%) dual-chamber ICD, received 28 inappropriate shocks, representing a 52% lower rate of inappropriate shocks (P =0.03). Among these patients, 11 were hospitalized in the control vs. three in the active group, corresponding to a 72% lower rate of hospitalizations (P = 0.02). The 76% lower proportion of delivered or undelivered capacitor charges increased the battery longevity significantly since, among patients whose ICD experienced >1 capacitor charge, the mean battery depletion was −0.18 ± 0.59% daily in the control, vs. -0.07 ± 0.02% daily in the active group (P = 0.02), representing a 7.9 months saving in longevity (95% CI: 2.6–13.2, P = 0.005). At the end of the study, three batteries in the active group had a <50% remaining longevity, vs. 11 batteries in the control group (P = 0.02). Among these, three batteries had reached elective replacement indicator in the control, vs. no battery in the active group.
Table 4.
All shocks, inappropriate shocks, and capacitor charges observed in the intention-to-treat population
| Study groups |
P | ||
|---|---|---|---|
| Active (n = 221) | Control (n = 212) | ||
| Appropriate and inappropriate shocks delivered | 193 [0–33] | 657 [0–116] | |
| Patients with ≥1 delivered shock | 47 (21.3) | 56 (26.4) | 0.21 |
| Mean per patient-month | 0.04 ± 0.27 | 0.20 ± 1.13 | 0.02 |
| Inappropriate shocks delivered | 28 [1–8] | 283 [1–82] | |
| Patients with ≥1 inappropriate shock | 11 (5.0) | 22 (10.4) | 0.03 |
| Mean per patient-month | 0.13 ± 0.15 | 0.83 ± 1.86 | 0.28 |
| Capacitor charges | 499 [0–58] | 2081 [0–760] | |
| Patients with ≥1 capacitor charge | 69 (31.2) | 72 (34.0) | 0.54 |
| Mean per patient-month | 0.11 ± 0.38 | 1.65 ± 18.81 | 0.11 |
Values are number of observations [ranges], numbers (%) of observations, or means ± SD.
Scheduled and unscheduled patient follow-ups
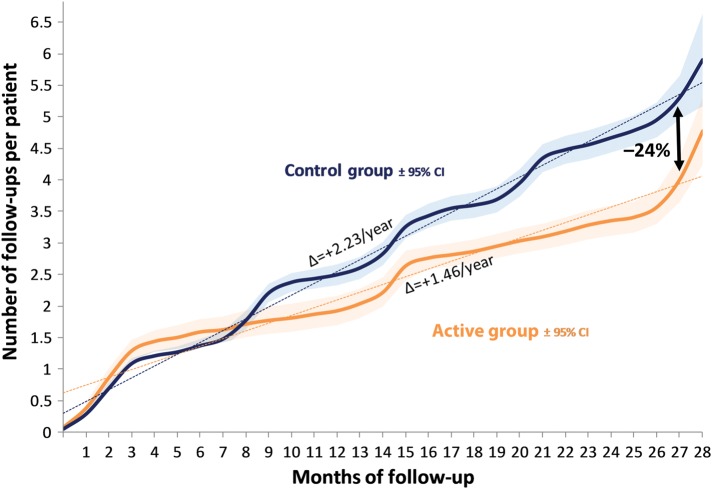
In part by study design, the number of patient encounters, including ambulatory visits and in-hospital ICD interrogations (Figure 4, Table 5), was significantly lower in the active (887 = 1.46/patient/year) than in the control (1064 = 2.23/patient/year) group (P < 0.001). Overall, 292 ambulatory visits (180 in the active and 112 in the control group) were unscheduled and prompted by HM alerts or by a patient or a caregiver. These unscheduled visits prompted major interventions (such as ICD reprogramming, changes in drug therapy, or radiofrequency ablation) in 62 and 60% of instances in the active and control groups, respectively.
Figure 4.
Cumulative incidence of ambulatory visits up to 27 months of follow-up. The blue and orange curves show the number of follow-ups per patient in the control vs. the active group, with the respective 95% confidence intervals (CI) represented by the shaded areas. At 27 months, the incidence of follow-up was 24% lower in the active than in the control group. The slope of each curve represents the number of visits per patient per year (P < 0.001).
Table 5.
Scheduled and unscheduled ambulatory visits, and interventions prompted by these ambulatory visits in each study group
| Study groups |
||
|---|---|---|
| Active | Control | |
| All visits | 887 | 1064 |
| Visits/patient/year | 1.46 | 2.23* |
| Scheduled ambulatory visits | 624 | 880 |
| Interventions prompted by scheduled ambulatory visits | 100 (16) | 132 (15) |
| Unscheduled ambulatory visits | 180 | 112 |
| Interventions prompted by unscheduled ambulatory visits | 110 (61) | 67 (60) |
| ICD interrogations during hospitalizations | 83 | 72 |
Values are numbers (%) of observations.
ICD, implantable cardioverter defibrillator.
*P < 0.001.
Discussion
In this randomized comparison, HM was non-inferior to ambulatory follow-ups with respect to the associated risks of death from any cause, as well as of cardiovascular, procedure-related, and device-related MAE. Similar observations were made in the per-protocol and the intention-to-treat analyses. Moreover, HM was associated with a prominently lower number of capacitor charges, and appropriate and inappropriate shocks delivered. In a previous study in ICD recipients, HM safely lowered the number of scheduled office visits over 12 months of follow-up.11 Our results are consistent with, and extend these observations to a broader spectrum of adverse events, over a considerably longer follow-up.
While ICD are highly effective in lowering the rate of arrhythmic deaths in high-risk patients, the delivery of shocks, especially when repetitive, remains a major cause of discomfort, anxiety, depression, and poor quality of life.15,16 Remote monitoring enables the very early detection of causes of appropriate and inappropriate ICD interventions, and rapid implementation of preventive measures. This may confer important advantages from the standpoint of patient quality of life and device longevity. In ECOST, the proportion of patients whose battery was depleted at the end of the study was >50% lower in the active than in the control group. The longer battery lives and need for fewer device replacements are associated with a lower risk of infectious complications.17,18 The outcome of the SF36 quality of life questionnaire revealed no difference between the two study groups, perhaps because its scope was too broad. It is, nevertheless, noteworthy that the rate of hospitalizations prompted by inappropriate shocks was reduced by 72%.
The practice guidelines for device-based therapy of arrhythmias, issued by ACC/AHA/HRS in 2008, suggested that HM was in need of further research and development.2 Furthermore, based on the consensus issued by HRS/EHRA in 2008, remote ICD monitoring can now replace some ambulatory follow-ups.19 Our randomized study strengthens the evidence supporting the safety of HM of ICD as an alternative to ambulatory follow-ups, as well as its ability to decrease the risk of shocks.
Compared with recently published data,16,20,21 the 10.4% incidence of inappropriate shocks delivered in our control group was relatively low. It is also noteworthy that EVATEL, another large and similar trial conducted contemporaneously, found no significant difference in its composite primary study endpoint.22
A considerable proportion of MAE cannot be prevented by HM. Therefore, with regard to safety, ECOST was found ‘non-inferior’ instead of ‘superior’ to standard follow-ups. From an efficacy standpoint, the fewer shocks delivered in the active group is likely explained by the early suppression of inappropriate therapies prompted by recurrent supraventricular tachyarrhythmias or by oversensing, and prevention of appropriate therapies by the early introduction of antiarrhythmic measures.
Home monitoring and burden on the health care system
A major factor intervening in the implementation of HM is the amount of additional work it represents for the caregivers.23 Therefore, the temporal burden imposed by the system and the cost-effectiveness of HM were thoroughly studied and will be published separately.
Study limitations
While the Endpoint Adjudication Committee was unaware of the random patient assignment, the investigators who made decisions regarding hospitalizations, which was a criterion to classify MAE, were aware of the assignments. One might hypothesize that this awareness contributed to lowering the rate of hospital admission for adverse events in the active group. This hypothesis is supported by the known ability to remotely monitor the effects of changes in device programming or drug therapy in response to an adverse event, which might have prevented a proportion of hospitalizations in the active group.
One other limitation of our study pertains to the application of its results, which were obtained in recipients of ICD implanted for primary or secondary prevention indications, without resynchronization therapy. Compared with our study population, the clinical characteristics of CRT-D recipients are different, as they usually suffer from more severe heart disease. The development of new instrumentation, especially the incorporation of sensors of congestive heart failure in upcoming implantable devices, is likely to broaden the benefits conferred by HM.24
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
The authors thank Nicolas Canot, Clinical Study Manager, Xavier Laroche, Home-Monitoring Manager, and Sophie Fauquembergue, Clinical Study Engineer, Biotronik France SAS, for their assistance in the conduct of the ECOST trial, and Rodolphe Ruffy for reviewing the manuscript.
Funding
This study was supported by an unrestricted grant from Biotronik SE & Co. KG.
Conflict of interest: L.G.-M. receives consulting fees from Biotronik, Saint Jude Medical, and Sorin Group; N.S. receives consulting fees from Biotronik, Boston Scientific, Medtronic Inc., Saint Jude Medical, and Sorin Group; E.A. receives consulting fees from Biotronik, Medtronic Inc., Saint Jude Medical; S.K. is the recipient of institutional grants and of consulting and speaker fees from Biotronik, Boston Scientific, Medtronic Inc., Saint Jude Medical, and Sorin Group; O.B. receives consulting fees from Biotronik, Boston Scientific, and Saint Jude Medical.
References
- 1.ICT for better healthcare in Europe. http://ec.europa.eu/information_society/activities/health/index_en.htm. 18 June 2012.
- 2.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:2820–2840. doi: 10.1161/CIRCUALTIONAHA.108.189742. doi:10.1161/CIRCUALTIONAHA.108.189741. [DOI] [PubMed] [Google Scholar]
- 3.Brugada P. What evidence do we have to replace in-hospital implantable cardioverter defibrillator follow-up? Clin Res Cardiol. 2006;95(Suppl. 3):III/3–9. doi: 10.1007/s00392-006-1302-x. [DOI] [PubMed] [Google Scholar]
- 4.Heidbüchel H, Lioen P, Foulon S, Huybrechts W, Ector J, Willems R, Ector H. Potential role of remote monitoring for scheduled and unscheduled evaluations of patients with an implantable defibrillator. Europace. 2008;10:351–357. doi: 10.1093/europace/eun010. doi:10.1093/europace/eun010. [DOI] [PubMed] [Google Scholar]
- 5.Nielsen JC, Kottkamp H, Zabel M, Aliot E, Kreutzer U, Bauer A, Schuchert A, Neuser H, Schumacher B, Schmidinger H, Stix G, Clémenty J, Danilovic D, Hindricks G. Automatic home monitoring of implantable cardioverter defibrillators. Europace. 2008;10:729–735. doi: 10.1093/europace/eun099. doi:10.1093/europace/eun099. [DOI] [PubMed] [Google Scholar]
- 6.Lazarus A. Remote, wireless, ambulatory monitoring of implantable pacemakers, cardioverter defibrillators, and cardiac resynchronization therapy systems: analysis of a worldwide database. Pacing Clin Electrophysiol. 2007;30(Suppl. 1):S2–S12. doi: 10.1111/j.1540-8159.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- 7.Ricci RP, Morichelli L, Santini M. Home monitoring remote control of pacemaker and implantable cardioverter defibrillator patients in clinical practice: impact on medical management and health-care resource utilization. Europace. 2008;10:164–170. doi: 10.1093/europace/eum289. doi:10.1093/europace/eum289. [DOI] [PubMed] [Google Scholar]
- 8.Crossley GH, Boyle A, Vitense H, Chang Y, Mead RH CONNECT Investigators. The CONNECT (Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision) trial: the value of wireless remote monitoring with automatic clinician alerts. J Am Coll Cardiol. 2011;57:1181–1189. doi: 10.1016/j.jacc.2010.12.012. doi:10.1016/j.jacc.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Spencker S, Coban N, Koch L, Schirdewan A, Müller D. Potential role of home monitoring to reduce inappropriate shocks in implantable cardioverter-defibrillator patients due to lead failure. Europace. 2009;11:483–488. doi: 10.1093/europace/eun350. doi:10.1093/europace/eun350. [DOI] [PubMed] [Google Scholar]
- 10.Sacher F, Probst V, Bessouet M, Wright M, Maluski A, Abbey S, Bordachar P, Deplagne A, Ploux S, Lande G, Jaïs P, Hocini M, Haïssaguerre M, Le Marec H, Clémenty J. Remote implantable cardioverter defibrillator monitoring in a Brugada syndrome population. Europace. 2009;11:489–494. doi: 10.1093/europace/eup034. doi:10.1093/europace/eup034. [DOI] [PubMed] [Google Scholar]
- 11.Varma N, Epstein AE, Irimpen A, Schweikert R, Love C TRUST Investigators. Efficacy and safety of automatic remote monitoring for implantable cardioverter-defibrillator follow-up: The Lumos-T Safely Reduces Routine Office Device Follow-Up (TRUST) trial. Circulation. 2010;122:325–332. doi: 10.1161/CIRCULATIONAHA.110.937409. doi:10.1161/CIRCULATIONAHA.110.937409. [DOI] [PubMed] [Google Scholar]
- 12.Guédon-Moreau L, Chevalier P, Marquié C, Kouakam C, Klug D, Lacroix D, Brigadeau F, Kacet S. Contributions of remote monitoring to the follow-up of implantable cardioverter-defibrillator leads under advisory. Eur Heart J. 2010;31:2246–2252. doi: 10.1093/eurheartj/ehq203. doi:10.1093/eurheartj/ehq203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alter P, Waldhans S, Plachta E, Moosdorf R, Grimm W. Complications of implantable cardioverter defibrillator therapy in 440 consecutive patients. Pacing Clin Electrophysiol. 2005;28:926–932. doi: 10.1111/j.1540-8159.2005.00195.x. doi:10.1111/j.1540-8159.2005.00195.x. [DOI] [PubMed] [Google Scholar]
- 14.Groves R. Urgent medical device information: Sprint Fidelis® lead patient management recommendations. October 15, 2007 Letter to physicians http://www.medtronic.com/product-advisories/physician/sprint-fidelis/PROD-ADV-PHYS-OCT. 17 July 2012. [Google Scholar]
- 15.Schron EB, Exner DV, Yao Q, Yao Q, Jenkins LS, Steinberg JS, Cook JR, Kutalek SP, Friedman PL, Bubien RS, Page RL, Powell J. Quality of life in the Antiarrhythmics Versus Implantable Defibrillators trial: impact of therapy and influence of adverse symptoms and defibrillator shocks. Circulation. 2002;105:589–594. doi: 10.1161/hc0502.103330. doi:10.1161/hc0502.103330. [DOI] [PubMed] [Google Scholar]
- 16.van Rees JB, Borleffs CJ, de Bie MK, Stijnen T, van Erven L, Bax JJ, Schalij MJ. Inappropriate implantable cardioverter-defibrillator shocks: incidence, predictors, and impact on mortality. J Am Coll Cardiol. 2011;57:556–562. doi: 10.1016/j.jacc.2010.06.059. doi:10.1016/j.jacc.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 17.Klug D, Balde M, Pavin D, Hidden-Lucet F, Clémenty J, Sadoul N, Rey JL, Lande G, Lazarus A, Victor J, Barnay C, Grandbastien B, Kacet S PEOPLE Study Group. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation. 2007;116:1349–1355. doi: 10.1161/CIRCULATIONAHA.106.678664. doi:10.1161/CIRCULATIONAHA.106.678664. [DOI] [PubMed] [Google Scholar]
- 18.Poole JE, Gleva MJ, Mela T, Chung MK, Uslan DZ, Borge R, Gottipaty V, Shinn T, Dan D, Feldman LA, Seide H, Winston SA, Gallagher JJ, Langberg JJ, Mitchell K, Holcomb R for the REPLACE Registry Investigators. Complication rates associated with pacemaker or implantable cardioverter-defibrillator generator replacements and upgrade procedures: results from the REPLACE registry. Circulation. 2010;122:1553–1561. doi: 10.1161/CIRCULATIONAHA.110.976076. doi:10.1161/CIRCULATIONAHA.110.976076. [DOI] [PubMed] [Google Scholar]
- 19.Wilkoff BL, Auricchio A, Brugada J, Cowie M, Ellenbogen KA, Gillis AM, Hayes DL, Howlett JG, Kautzner J, Love CJ, Morgan JM, Priori SG, Reynolds DW, Schoenfeld MH, Vardas PE. HRS/EHRA expert consensuson the monitoring of cardiovascular implantable electronic devices(CIEDs): description of techniques, indications, personnel, frequencyand ethical considerations. Europace. 2008;10:707–725. doi: 10.1093/europace/eun122. doi:10.1093/europace/eun122. [DOI] [PubMed] [Google Scholar]
- 20.Daubert JP, Zareba W, Cannom D, McNitt S, Rosero SZ, Wang P, Schuger C, Steinberg JS, Higgins SL, Wilber DJ, Klein H, Andrews ML, Hall WJ, Moss AJ MADIT II investigators. Inappropriate implantable cardioverter-defibrillator shocks in MADIT II: frequency, mechanisms, predictors, and survival impact. J Am Coll Cardiol. 2008;51:1357–1365. doi: 10.1016/j.jacc.2007.09.073. doi:10.1016/j.jacc.2007.09.073. [DOI] [PubMed] [Google Scholar]
- 21.Saxon LA, Hayes DL, Gilliam FR, Heidenreich PA, Day J, Seth M, Meyer TE, Jones PW, Boehmer JP. Long-term outcome after ICD and CRT implantation and influence of remote device follow-up: the ALTITUDE survival study. Circulation. 2010;122:2359–2367. doi: 10.1161/CIRCULATIONAHA.110.960633. doi:10.1161/CIRCULATIONAHA.110.960633. [DOI] [PubMed] [Google Scholar]
- 22.Mabo P, Defaye P, Sadoul N, Davy J-M, Deharo J-C, Kacet S, Bellissant E, Daubert J-C. EVATEL: Remote follow-up of patients implanted with an ICD: the prospective randomized EVATEL study. ESC Congress report. Hot Line II - Frontiers in interventional and device treatments http://www.escardio.org/congresses/esc-2011/congress-reports/Pages/707-1-EVATEL.aspx. last accessed 17 July 2012.
- 23.Papavasileiou LP, Forleo GB, Panattoni G, Schirripa V, Minni V, Magliano G, Bellos K, Santini L, Romeo F. Work burden with remote monitoring of implantable cardioverter defibrillator: is it time for reimbursement policies? J Cardiovasc Med (Hagerstown) 2012 doi: 10.2459/JCM.0b013e328354e3e1. (24 May 2012) [DOI] [PubMed] [Google Scholar]
- 24.Auricchio A, Moccetti T. Electronic cardiac medicine: present and future opportunities. Swiss Med Wkly. 2010;140:w13052. doi: 10.4414/smw.2010.13052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.