Abstract
We report the discovery of light organs (photophores) adjacent to the dorsal defensive spines of a small deep-sea lanternshark (Etmopterus spinax). Using a visual modeling based on in vivo luminescence recordings we show that this unusual light display would be detectable by the shark's potential predators from several meters away. We also demonstrate that the luminescence from the spine-associated photophores (SAPs) can be seen through the mineralized spines, which are partially translucent. These results suggest that the SAPs function, either by mimicking the spines' shape or by shining through them, as a unique visual deterrent for predators. This conspicuous dorsal warning display is a surprising complement to the ventral luminous camouflage (counterillumination) of the shark.
In the permanent darkness of the deep-sea a surprising diversity of organisms, from bacteria to fish, have evolved the capability to produce a visible light1,2. This bioluminescence comes from a chemical reaction1,2,3 that occurs either in the exterior environment or within cells; these photogenic cells (photocytes) are often embedded in organs called photophores that can reach a high degree of sophistication, including reflectors, light guides, wavelength-specific filters and optical lenses4,5,6. Numerous adaptive benefits have been suggested for bioluminescent glows and flashes (light emission < 2 s), all falling in the three functional categories essential to an organism's survival: predator evasion, food acquisition and reproduction1,2,3,7.
In the mesopelagic zone (200–1000 m), many animals produce a continuous ventral glow to match down-welling sunlight and conceal their silhouette from organisms swimming beneath them, a process called counterillumination2,8,9. Conversely, dorsal glows are rarely observed in this environment, probably because they are highly detectable against the darker background of deeper waters when viewed from above. As a consequence, such conspicuous displays are usually of a predatory nature, produced by ‘anatomical fishing lures’ that attract prey from a distance2,7. Probably because of the stark difference in the apparent roles of dorsal and ventral luminescence, dorsal and ventral photogenic organs are rarely observed to glow concomitantly in the same mesopelagic organism10. Here, however, we report a particular exception where the dorsal glows of a deepwater shark might actually complement the counterilluminating luminescence produced by its ventral photophores.
The velvet belly lanternshark, Etmopterus spinax, is a diminutive shark (≤ 60 cm in total length) that spends most of its time at mesopelagic depths11. Like other members of the Etmopteridae family, E. spinax harbours defensive mineralized spines in front of its dorsal fins and has a ventral surface covered by thousands of tiny (< 200 μm in diameter) photophores11,12. Stimulated by melatonin or prolactin, these organs produce a long lasting blue-green luminescence13. This self-produced light, the chemistry of which remains unknown, provides this lanternshark with a counterilluminating camouflage14 and perhaps aids in intraspecific communication15,16.
When investigating this species' bioluminescence, we were surprised to observe glows from the frontal edges of the dorsal fins, forming conspicuous arcs immediately behind the spines (Fig. 1). This dorsal luminescent display seems in direct conflict with the camouflage role of the ventral photophores. Here we document the structural basis of this intriguing display and investigate its functional significance. We examined the histology and light emission of the dorsal photophores and determined the 3D structure and spectral transmittance of their associated spines. Using these structural and in vivo luminescence data, ocular measurements from local fishes and marine mammals and a recent theory for visual detection in pelagic habitats17, we then determined the detection range of the luminescence produced by the spine-associated photophores (SAPs) in the shark's environment, characterizing their in situ performance and suggesting their ecological role.
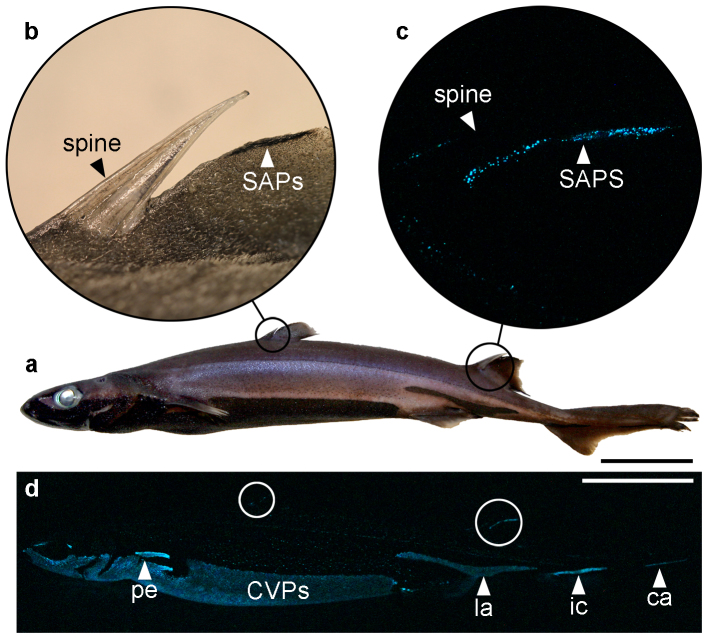
Figure 1. Spine-associated bioluminescent display.
(a) Location of mineralized finspines in an adult Etmopterus spinax specimen. (b) Close-up of first dorsal spine with SAPs in natural light. (c) Close-up of second dorsal spine with spontaneously glowing SAPs in darkness. (d) Spontaneous luminescence from another specimen of this species showing the glowing dorsal SAPs (white circles), counterilluminating ventral photophores14 (CVPs) and lateral photophore areas believed to be involved in intraspecific communication15,16: ca, caudal; ic, infracaudal; la, lateral; pe, pectoral. Scale bars, 5 cm.
Results
Structural basis
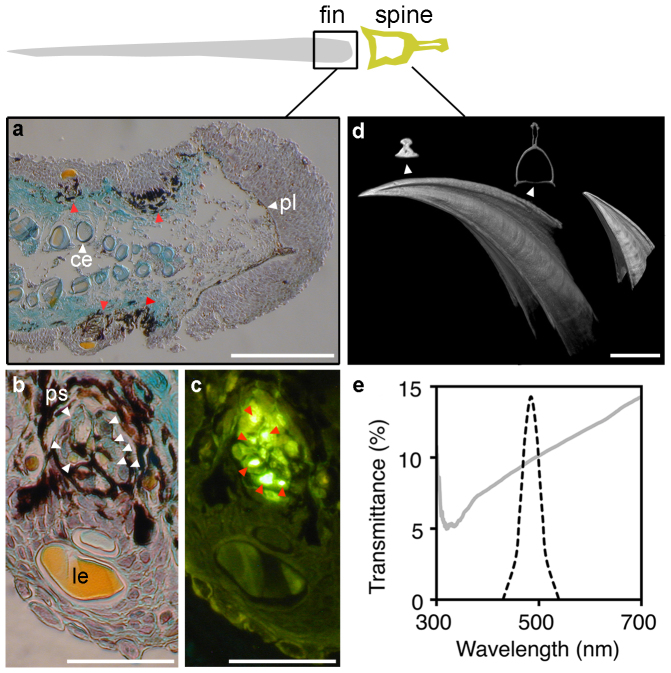
SAPs form two to four vertical rows on both sides of the dorsal fins, at a distance of about 1–3 mm behind the finspines (Fig. 2a). These organs appear structurally similar to ventral photophores18, each organ comprising a pigmented sheath covered by one or several lens cells, and enclosing a cluster of photogenic cells (photocytes) containing fluorescent vesicles (Fig. 2b,c). Considering the relative orientation of lenses and photocytes, SAPs emit most of their light laterally, forming sickle-shaped glows on the sides of the leading edges of the dorsal fins, mimicking the curve of the spines anterior to them. Given this intimate association of photophores and spines (Fig. 2d) and that dorsal fins have been shown to rotate during swimming in some shark species19, we examined the potential interaction of SAP luminescence and spines by measuring the spectral transmittance of these structures. The spines could hypothetically transmit about 10% of the SAP luminescence if the dorsal fins were to rotate laterally during swimming (Fig. 2e).
Figure 2. Structural basis and spatial organization of spine-associated bioluminescent display.
(a) Frontal edge of the second dorsal fin in transversal section (TS; 7 μm) showing the position of SAP rows (red arrows). ce, ceratotrichia; pl, pigmented layer. (b) Close-up of a SAP (TS; 7 μm) showing the position of photogenic cells (photocytes; white arrows). le, lens; ps, pigmented sheath. (c) Same SAP (TS; 7 μm) showing the autofluorescence of the photocyte vesicles (red arrows) under U.V. application. (d) Micro-CT of first and second dorsal finspines. Top inserts are transversal sections situated close to the tip and the base. Note how the spine cross-section in 2d have been rotated 90° anticlockwise relative to the cross-section at the top of the figure. Scale bars correspond to 500 μm in (a), (d) and to 150 μm in (b), (c). (e) Spectral transmittance of the spine (grey line) with a superimposed Gaussian spectrum (dashed line) of shark luminescence14. The spine can hypothetically transmit ~ 10% of SAP luminescence frontally.
Functional significance
Ventral photophore and SAP luminescences are long-lasting and their intensities are significantly correlated (Fig. 3a), suggesting a common hormonal control for these photogenic structures. This information, combined with local environmental data and the visual theory developed by Nilsson et al.17 allowed us to calculate the depth at which the ventral photophores provide an optimal counterilluminating camouflage for the brightest of our specimens (Fig. 3b). This theoretical depth should reflect the preferred depth of the shark in its natural habitat. Using Nilsson et al. 's theory again as well as the spine transmittance data and ocular anatomy of local fishes and marine mammals, we determined that, at theoretical counterillumination depth, SAPs can emit a close range luminescent signal, both around and through the spines, that would be detectable by most of the shark's potential predators—but not its main prey—from several meters away (Fig. 3c).
Figure 3. In vivo recordings and visual modeling of Etmopterus spinax luminescence.
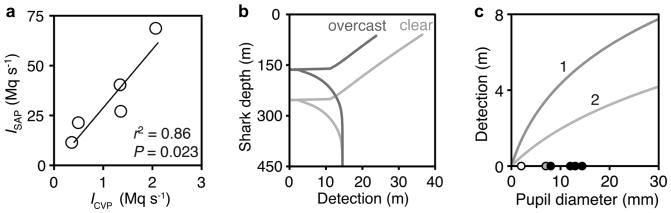
(a) Correlation between SAP (from the second dorsal fin) and counterilluminating ventral photophore (CVP) luminescence intensity (I). Mq s−1, megaquanta (106 photons) per second. (b) Detection of CVP luminescence as a function of the shark's occurrence depth for heavy overcast and clear skies (sun at 45°). Counterillumination occurs when detection = 0. (c) Detection distance of SAP luminescence at counterillumination depth as a function of the pupil diameter of the observer. This distance is calculated (1) around or (2) through the spine, assuming 10% transmission. Dots represent pupil diameter of the shark (grey), its main prey (the pearlside fish Maurolicus muelleri; white) and its potential predators (piscivorous fishes and marine mammals; black).
Discussion
In the mesopelagic zone, where most animals—including E. spinax—have converged on camouflage strategies20, the dorsal long-lasting luminescence produced by the shark's SAPs appears to be a paradoxical and risky display. As a consequence, a strong adaptive benefit of these intriguing photogenic structures might be expected.
We explored the functional significance of SAP luminescence using an integrative method involving structural and performance data and a recent theoretical visual modeling17. This novel approach appears particularly useful to test performance hypotheses involving deep-sea animals like E. spinax, which live in a largely inaccessible environment and demonstrate a physiological fragility that considerably restricts their use in behavioral experiments. The validity of our modeling was confirmed by the similarity between the theoretical counterillumination depth (164–254 m deep, depending on surface light conditions), which suggests the occurrence depth of the shark, and the actual capture depth of our specimens (170–230 m). At this theoretical counterillumination depth, the particular window of detection distances of SAP luminescence would not be effective in prey attraction: our calculations predict that the main prey of the shark (the pearlside fish Maurolicus muelleri)21 can only detect SAPs from short distance away (~ 1.5 m); conversely potential predators can detect them at several meters. This strongly suggests that dorsal bioluminescence in the lanternshark serves as a “predators-eyes-only” signal and therefore somehow functions as an aposematic (warning) beacon to deter approaching predators (or conspecifics), albeit without jeopardizing prey capture.
The exact message of this aposematic signal remains unclear and intriguing. Given the particular spatial organisation and close association of SAPs with the defensive dorsal finspines, and the fact that the finspines are capable of transmitting a portion of SAP luminescence (probably thanks to a reduced enameloid covering compared to the spines of other non-bioluminescent squalid sharks12), we propose that SAPs evolved as a means of highlighting the spines themselves.
Dorsal mineralized finspines, sometimes linked to venomous glands, are found in chimaeras and a number of sharks9,22,23. While stingray barbs can be easily whipped into defensive position in the event of a threat, the dorsal anchoring of the finspines largely restricts their use to risky post-capture defensive events as a means of wounding the soft tissue in the mouth of a biting predator.
SAP “advertisement” of these spines—by luminescent strips mimicking spine shape and/or shining through the spines when the fins rotate—would be particularly advantageous, since it would allow the shark to signal to potential predators from a distance the danger of the stinging appendages, maybe preventing attacks before they happen. The high density of E. spinax individuals present in Norwegian fjords24 limit the time between encounters with this species, perhaps facilitating a predator's avoidance learning through a succession of unsuccessful predation events.
Bioluminescent aposematism has been experimentally demonstrated in some terrestrial invertebrates25,26, and suggested for a number of marine animals including cnidarians27, molluscs28, annelids29, crustaceans30, echinoderms31 and teleost fish32; this, however, is the first suggestion of this behaviour in a cartilaginous fish.
Transmission of bioluminescent signals by mineralized structures, such as the spines of the velvet belly lanternshark, are rare in nature, having only been observed in several organisms including opisthoproctid fishes33 and a marine snail (Hinea brasiliana)27. Further examination of E. spinax's spines may provide insights into evolutionary co-opting of structural biomaterials for optic functions.
Although SAPs appear structurally and physiologically similar to the other photophores harbored by the velvet belly lanternshark, their dorsal glow represents a unique bioluminescent display. This ‘abnormal’ luminescence might also be found in other lanternsharks (Etmopteridae), which harbor similar dorsal finspines and ventral photophores11. Even though the function of these organs remains hypothetical, they clearly demonstrate that warning luminescence and counterillumination, two apparently contradictory strategies, could surprisingly operate in a single organism at the same time.
Methods
Sharks
Adult sharks were collected using longlines in the Raunefjord (Norway) between December 2009 and October 2012, transferred to Espeland Marine Station and housed in tanks placed in a dark cold room. Our protocol, including fish sacrifice, was in accordance with national guidelines for experimental fish care (fish handling approval #1664 was given by the National Committees for Research Ethics of Norway).
Photographs of spontaneous luminescence were taken in complete darkness with a Canon 7D camera (whole shark, sensitivity 6400 ISO, objective 20 mm; dorsal fins, 12800 ISO, 60 mm; both with an aperture of 2.8 and an exposure time of 30 s). For visibility purpose, a post adjustment of brightness and contrast was applied to the entire pictures using Adobe Photoshop®. Other pictures were taken with the same camera in natural light.
Luminescence recording
A Berthold FB12 luminometer coupled to an optical fibre was used to record in vivo intensity of dorsal and ventral continuous luminescence from several live specimens according to Claes et al.14. Values were corrected for fiber absorption and angular losses. Ventral photophore spacing/density and mean intensity of specimens was estimated according to Claes and Mallefet15, by counting the photophores present in standard (0.25 cm2) ventral skin patches under a binocular microscope.
Spine structure
Dorsal finspines were removed from dead specimens wrapped in plastic wrap with a small amount of fluid for hydration, and packed tightly with paper towels into plastic tubes for micro-CT scanning (1174 scanner, SkyScan®, Kontich, Belgium). The X-ray source was set at 50kVp (peak kilovoltage) and 800 μA; 220 projections were acquired over an angular range of 360degrees. Samples were scanned with an isotropic voxel size of 16.8 μm, an integration time of 4500ms, and a 0.25mm aluminum filter to decrease beam-hardening effects. Scans were reconstructed using commercial software (NRecon® SkyScan® software, version 1.6.1.2) then visualized in 3d and segmented virtually in multiple planes using Amira software (Mercury Computer Systems) to examine spine structure and morphology.
Histology
Skin tissues containing SAPs from dorsal fins of freshly dead specimens were excised and fixed in a 3.5% formaldehyde solution for two weeks. Tissues were progressively dehydrated (50, 70, 2 × 90%, 1 hour each), placed 1 h in 100% butanol and left overnight in 100% butanol at 60°C. Tissues were then submerged in paraffin wax for different periods (12 h, 1 h and 3 h) at melting temperature (58°C). Sections were performed with a metal knife microtome, stained using Masson's trichrome, and photographed under a light/fluorescence microscope (Leitz Diaplan, Leitz, Wetzlar, Germany).
Spine transmission
The second dorsal finspines of freshly dead specimens were removed and their anterior-posterior spectral transmittance was measured near the tip, at 75% of the exterior spine length, using an Ocean Optics S2000 spectroradiometer. Broadband white light from an Ocean Optics PX-2 pulsed xenon lamp was delivered to the caudal surface of the spine via an optical fibre terminated with a quartz lens. Light transmitted anteriorly by the spine was collected via a second quartz lens coupled to an optical fibre connected to the spectroradiometer.
Visual modeling
The visibility ranges of both ventral and dorsal glows were calculated according to the theory developed by Nilsson et al.17. This range depends on the intensity of down-welling daylight and thus on depth in the sea. Etmopterus spinax was assumed to occur at ‘counterillumination depth’ where its silhouette, concealed by ventral photophores, is not detectable from below14. This depth was determined using the ventral photophore spacing (0.234 mm) and mean intensity (2.1 × 106 photons s−1) of the brightest shark in our dataset as inputs in the equation 7 in Supplemental Information from Nilsson et al.17. We adapted Nilsson et al. 's theory, originally developed for clear oceanic waters, by setting the beam attenuation and back-scattering coefficients to 0.3 m−1 and 0.0385 m−1, respectively, to agree with the turbid green waters of the fjords. The visibility range of SAP luminescence, modeled as a point source seen against a black background (the dorsal part of the shark's body), was then calculated at this counterillumination depth. The SAP luminescence intensity of the brightest shark (6.87 × 107 photons s−1) was used as input value in the equation 13 in Supplemental Information from Nilsson et al.17. This equation relates the maximum detection distance to the observer's pupil diameter. Possible observers include conspecifics, the shark's main prey (the pearlside fish Maurolicus muelleri)21, and potential predators such as piscivorous fishes (Galeus melastomus, Molva molva) and marine mammals (Phocoena phoecoena, Phoca vitulina). Pupil diameters of these animals were set to 7, 2, 12, 13, 8 and 14.4 mm, respectively, according to direct measurements (fishes) or published values (marine mammals34,35). Photoreceptor cell diameter, which plays a negligible role in the modeling17, was set to 3 μm.
Author Contributions
J.M.C. and J.M. collected experimental animals, took photographs and recorded in vivo luminescence measurements. J.M.C. performed the histological sections of the dorsal fins. The micro-CT scans were performed by M.N.D. while N.S.H. made the finspine spectral transmittance measurements. D.E.N. conducted the visual modeling. J.M.C. and M.N.D. wrote the main manuscript. J.M.C. prepared the figures. All authors reviewed the manuscript.
Acknowledgments
We thank T. Sørlie and A. Aadnesen for help in specimen collection and maintenance. We also thank A. Atkins, N. Kalish and R. Shahar for use of and assistance with the microCT machine. JMC is a postdoctoral researcher at Fonds National de la Recherche Scientifique (FNRS, Belgium). Open access fee was covered by a FNRS grant. This work was supported in part by a Alexander von Humboldt post-doctoral fellowship to MND. NSH acknowledges the support of S. Collin (UWA). JM is a research associate FNRS. This is a contribution to the Biodiversity Research Center (BDIV) and to the Centre Interuniversitaire de Biologie Marine (CIBIM).
References
- Shimomura O. Bioluminescence: Chemical Principles and Methods (World Scientific Publishing Company, Singapore, 2006). [Google Scholar]
- Haddock S. H. D., Moline M. A. & Case J. F. Bioluminescence in the sea. Annu. Rev. Mar. Sci. 2, 443–493 (2010). [DOI] [PubMed] [Google Scholar]
- Herring P. J. How to survive in the dark: bioluminescence in the deep-sea. Symp. Soc. Exp. Biol. 39, 323–350 (1985). [PubMed] [Google Scholar]
- Widder E. A. Bioluminescence in the ocean: origins of biological, chemical and ecological diversity. Science 328, 704–708 (2010). [DOI] [PubMed] [Google Scholar]
- Denton E. J., Herring P. J., Widder E. A., Latz M. F. & Case J. F. The role of filters and photophores of oceanic animals and their relation to vision in the oceanic environment. Proc. Roy. Soc. B 225, 63–97 (1985). [Google Scholar]
- Herring P. J. Bioluminescent signals and the role of reflectors. J. Opt. A Pure Appl. Opt. 2, R29–R38 (2000). [Google Scholar]
- Buck J. B. in Bioluminescence in Action (ed P. J. Herring) 419–460 (Academic Press, New York, 1978). [Google Scholar]
- Clarke W. D. Function of bioluminescence in mesopelagic organisms. Nature 198, 1244–1246 (1960). [Google Scholar]
- Young R. E., Kampa E. M., Maynard S. D., Mencher F. M. & Roper C. F. E. Counterillumination and the upper depth limit of midwater animals. Deep-sea Res. A 27, 671–691 (1980). [Google Scholar]
- McAllister D. E. The significance of ventral bioluminescence in fishes. J. Fish. Res. Board Can. 24, 537–554 (1967). [Google Scholar]
- Compagno L., Dando M. & Fowler S. A field guide to the sharks of the world (HarperCollins, London, 2005). [Google Scholar]
- Maisey J. G. Finspine morphogenesis in squalid and heterodontid sharks. Zool. J. Linn. Soc. 66, 161–183 (1979). [Google Scholar]
- Claes J. M. & Mallefet J. Hormonal control of luminescence from lanternshark (Etmopterus spinax) photophores. J. Exp. Biol. 212, 3684–3692 (2009). [DOI] [PubMed] [Google Scholar]
- Claes J. M., Aksnes D. L. & Mallefet J. Phantom hunter of the fjords: counterillumination in a shark, Etmopterus spinax. J. Exp. Mar. Biol. Ecol. 388, 28–32 (2010). [Google Scholar]
- Claes J. M. & Mallefet J. Ontogeny of photophore pattern in the velvet belly lantern shark, Etmopterus spinax. Zoology 112, 433–441 (2009). [DOI] [PubMed] [Google Scholar]
- Claes J. M. & Mallefet J. Functional physiology of lanternshark (Etmopterus spinax) luminous pattern: differential hormonal regulation of luminous zones. J. Exp. Biol. 112, 433–441 (2009). [DOI] [PubMed] [Google Scholar]
- Nilsson D. E., Warrant E. J., Johnsen S., Hanlon R. & Shashar N. A unique advantage for giant eyes of giant squid. Curr. Biol. 22, 683–688 (2012). [DOI] [PubMed] [Google Scholar]
- Claes J. M. & Mallefet J. Early development of bioluminescence suggests camouflage by counter-illumination in the velvet belly lanternshark Etmopterus spinax (Squaloidea: Etmopteridae). J. Fish Biol. 73, 1337–1350 (2008). [Google Scholar]
- Maia A. M. R. Functional morphology of the dorsal fins in sharks during steady swimming and maneuvering. Ph.D. Thesis. The University of Rhode Island (U.S.). Paper AAI3464746 (2011). [Google Scholar]
- Zylinski S. & Johnsen S. Cephalopod switch between transparency and pigmentation to optimize camouflage in the deep. Curr. Biol. 21, 1937–1941 (2011). [DOI] [PubMed] [Google Scholar]
- Klimpel S., Palm H. W. & Seehagen A. Metazoan parasites and food composition of juvenile Etmopterus spinax (L., 1758) (Dalatiidae, Squaliformes) from the Norwegian deep. Parasitol. Res. 89, 245–251 (2003). [DOI] [PubMed] [Google Scholar]
- Halstead B. W. & Bunker N. C. The venom apparatus of the ratfish,. Hydrolagus colliei. Copeia 1952, 128–138 (1952). [Google Scholar]
- Haddad V. Jr & Gadig O. B. F. The spiny dogfish (“cacao-bagre”): description of an envenoming in a fisherman, with taxonomic and toxinologic comments on the Squalus gender. Toxicon 46, 108–110 (2005). [DOI] [PubMed] [Google Scholar]
- Claes J. M. Function and control of luminescence from lanternshark (Etmopterus spinax) photophores. Ph.D. Thesis. Université catholique de Louvain (Belgium). [Google Scholar]
- De Cock R. & Matthysen E. Glow-worm larvae bioluminescence (Coleoptera: Lampyridae) operates as an aposematic signal upon toads (Bufo bufo). Behav. Ecol. 14, 103–108 (2003). [Google Scholar]
- Marek P., Papaj D., Yeager J., Molina S. & Moore W. Bioluminescent aposematism in millipedes. Curr. Biol. 21, 680–681 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring P. J. & Widder E. A. Bioluminescence of deep-sea coronate medusae (Cnidaria: Scyphozoa). Mar. Biol. 146, 39–51 (2004). [Google Scholar]
- Deheyn D. D. & Wilson N. G. Bioluminescent signals spatially amplified by wavelength-specific diffusion through the shell of a marine snail. Proc. Roy. Soc. B 278, 2112–2121 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassot J. M. & Nicolas M. T. Bioluminescence in scale-worm photosomes: the photoprotein polynoidin is specific for the detection of superoxide radicals. Histochem. Cell Biol. 104,199–210 (1995). [DOI] [PubMed] [Google Scholar]
- Rivers T. J. & Morin J. G. The relative cost of using luminescence for sex and defense: light budgets in cypridinid ostracods. J. Exp. Biol. 215, 2860–2868 (2012). [DOI] [PubMed] [Google Scholar]
- Grober M. S. Brittle-star bioluminescence functions as an aposematic signal to deter crustacean predators. Anim. Behav. 36, 493–501 (1988). [Google Scholar]
- Lane E. D. A study of the Atlantic midshipman, Porichthys porosissimus, in the vicinity of Port Aransas, Texas. Contr. Mar. Sci. Univ. Tex. 12, 1–53 (1967). [Google Scholar]
- Herring P. J. & Morin J. G. in Bioluminescence in Action (ed Herring P. J., ed. ) 273–329 (Academic Press, New York, 1978). [Google Scholar]
- Kroger R. H. H. & Kirschfield K. Optics of the harbor porpoise eye in water. J. Opt. Soc. Am. 10, 1481–1489 (1993). [DOI] [PubMed] [Google Scholar]
- Levenson D. H. & Schusterman R. J. Pupillometry in seals and sea lions: ecological implications. Can. J. Zool. s75, 2050–2057 (1997). [Google Scholar]