Abstract
Phthalates and bisphenol A are environmental endocrine-disrupting chemicals used widely in common consumer products. There is increasing concern about human exposure to phthalates and bisphenol A due to the potential adverse effects related to the anti-androgenic activity of phthalates and estrogenic activity of bisphenol A. In assessing environmental exposure to phthalates and bisphenol A, it is essential to have a validated analytical method that can quantify trace concentrations of phthalate metabolites and bisphenol A in humans. In this study, we developed and validated an accurate, sensitive, and robust LC-MS/MS method to simultaneously quantify 5 phthalate monoester metabolites, including mono-methyl phthalate, mono-ethyl phthalate, mono-butyl phthalate, mono-benzyl phthalate, mono-2-ethylhexyl phthalate, and bisphenol A in human urine. In this method, the phthalate metabolites and bisphenol A, along with their isotope labeled internal standards, were extracted from 200 μl of human urine using automated off-line solid phase extraction. The analytes were quantitatively determined using LC-MS/MS operated in negative electrospray ionization multiple reaction-monitoring mode. The limit of quantification was 0.3 ng/ml for mono-methyl phthalate, mono-ethyl phthalate, mono-benzyl phthalate and bisphenol A, and 1 ng/ml for mono-butyl phthalate and mono-2-ethylhexyl phthalate. The precision and accuracy were well within the acceptable 15% range. This validated method has been used successfully in assessing exposure to phthalates and bisphenol A in humans.
Keywords: phthalate metabolite, Bisphenol A, SPE, LC-MS/MS
Introduction
Phthalates and Bisphenol A (BPA) are high-production chemicals commonly used in plastic products [1]. Phthalates are used in many household and personal care products, toys, and medical supplies, as well as in plastics to improve flexibility. BPA is a main ingredient in plastics and resins, and is used in a variety of consumer products including water bottles, the inside lining of cans, and in cash register receipt paper. The ubiquitous use of phthalates and BPA results in widespread human exposure. The U.S. Centers for Disease Control and Prevention (CDC) has reported measurable concentrations of phthalate metabolites such as mono-ethyl phthalate (MEP), mono-butyl phthalate (MBP), and mono-benzyl phthalate (MBzP) in > 97%, and mono-2-ethylhexyl phthalate (MEHP) and mono-methyl phthalate (MMP) in > 75% of the US population [2,3]. Detectable levels of BPA have also found in urine in 92% of Americans [4].
Phthalates and BPA are known to be endocrine disruptors, man-made chemicals that disrupt the endocrine system by competing with endogenous hormones by binding to receptors or by altering the synthesis and metabolism of these hormones. Numerous laboratory animal studies and some human epidemiological studies have demonstrated that phthalates and BPA can interfere with human hormones, potentially creating problems with health and normal growth and development. Exposure to phthalates and BPA has been associated with altered hormone levels, reproductive effects (male fertility), precocious puberty in pubertal girls, increased incidence of chronic disease, and a possible role in the development of cancer [5-9].
Once in the body, phthalates are rapidly metabolized by hydrolysis to their corresponding monoester metabolites, which are then further metabolized or eliminated in the urine as glucuronides. Phthalates are ubiquitous both in the environment and in the analytical laboratory, where they are present in analytical instruments, gloves, plastic tubing, solvents and air. To prevent potential sample contamination from phthalates in the environment, phthalate exposure assessment in humans has been chiefly conducted by biomonitoring of phthalate metabolites in urine. The ingested BPA is mainly conjugated with glucuronic acid and eliminated in the urine [2,3]. Urinary levels of BPA, including both conjugated and unconjugated forms, are often monitored to reflect exposure to this chemical in humans [4].
Various analytical methods have been developed for measuring BPA or phthalate monoester metabolites separately in urine, mostly using gas chromatography coupled with mass spectrometry (GC-MS) [10, 11] and liquid chromatography coupled with mass spectrometry (LC-MS) or tandem MS (LC-MS/MS) [11-18]. LC-MS/MS methods, which combine high selectivity and sensitivity, show an advantage over GC-MS methods because of simple sample preparation without the need for derivatization. The earlier methods for the determination of phthalate metabolites or BPA in human urine by LC-MS/MS combined with manual or automated offline solid phase extraction (SPE) usually required 1ml of urine [13-15]. The on-line SPE LC-MS/MS methods using smaller sample volume (0.1 ml) demonstrated high sensitivity with limit of detection (LOD) value of 0.4 ng/ml for BPA and ranging from 0.15-1.1, 0.4-0.7, 0.4-0.6, 0.11-0.3, 0.9-1.1 ng/ml for MMP, MEP, MBP, MBzP and MEHP, respectively [16-18]. Although these on-line SPE LC-MS/MS methods are considered the most advanced for urine biomonitoring studies, they are limited by their high cost and therefore not practical for many studies. Due to the increasing concern on the endocrine disrupting chemicals, such as phthalates and BPA, and the needs to assess their exposure to individuals, it is essential to have a sensitive, low cost and efficient analytical method that can quantify these chemicals or their metabolite levels simultaneously in human urine. However, to our knowledge, there is no single analytical method reported utilizing LC-MS/MS for the simultaneous determination of BPA and phthalate monoester metabolites in human urine. Instead, studies on assessing human exposure to BPA and phthalate have been using GC-MS for BPA and LC-MS/MS for phthalate metabolites or two different LC-MS/MS methods to quantify phthalate metabolites or BPA separately [19-24]
In this paper, we have developed a simple, sensitive and low-cost LC-MS/MS method combined with automated off-line SPE to measure the concentrations of five phthalate monoester metabolites: MMP, MEP, MBP, MBzP and MEHP, and BPA simultaneously in human urine samples. The method is validated in terms of linearity, limit of quantification (LOQ), accuracy, precision, stability, and matrix effect. After successful development and validation of this method, it was utilized in analyzing a set of human urine samples collected for evaluating potential BPA and phthalates exposure. The accuracy, reproducibility, and rigor of this assay have been demonstrated in this analysis.
1. Experimental
1.1 Materials
MMP, MEP, MBP, MBzP and MEHP were purchased from Accustandard (New Haven, CT, USA). BPA, 4-methylumbelliferone, 4-methylumbelliferyl glucuronide and β-glucuronidase/sulfatase (Helix pomatia, H1), LC-MS grade ammonium acetate, acetic acid, and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). The isotope labeled internal standards 13C12-BPA (99%), 13C4-4-methylumbelliferone, 13C4-mono-methyl phthalate (13C4-MMP), 13C4-mono-ethyl phthalate (13C4-MEP), 13C4-mono-butyl phthalate (13C4-MBP) and 13C4-mono-benzyl phthalate (13C4-MBzP) solutions were purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA, USA). D4-mono-2-ethylhexyl phthalate (D4-MEHP) was purchased from C/D/N Isotopes, Inc. (Quebec, Canada). HPLC-grade reagents, including acetonitrile, methanol, and water were purchased from JT Baker (Center Valley, PA, USA). Frozen human urine (pooled from 20 individuals) were shipped from Bioreclamation (Hicksville, NY, USA) under dry ice and stored at −20 °C until use. All the glassware used in the experiments was methanol-rinsed and dried.
2.2 Instrument
The HPLC system consisted of a Shimadzu LC SCL-10AVP solvent delivery unit, an online solvent degasser, a gradient mixer and a system controller (Shimadzu Scientific, Columbia, MD, USA). A CTC-PAL autosampler (LEAP Technologies, Carrboro, NC, USA) was used to inject samples. The mass spectrometer was API 4000 LC-MS/MS system (AB SCIEX, Framingham, MA, USA) equipped with a Turbo V IonSpray ionization source. Analyst software version 1.4.1 from AB SCIEX was used for data acquisition and processing.
2.3 LC conditions
The chromatographic separation was performed on a Thermo Betasil phenyl column (50 mm × 2.1 mm, 3 μm particle size, Thermo Electron, Bellefonte, PA, USA) maintained at the ambient temperature with a precolumn inline filter (0.5 μm, Sigma-Aldrich, St. Louis, MO, USA). The CTC-PAL Leap cooling unit was set at 4 °C, and the sample injection volume was 10 μl. The mobile phase was 0.1% acetic acid in water (A) and 0.1% acetic acid in acetonitrile (B) at a flow rate of 220 μl/min. The mobile phase gradient was as follows: 5% B for 2.0 min; linear increased to 100% B from 2.0 to 7.0 min, and then maintained at 100% B from 7.0 to 9.0 min, went back to 5% B from 9.0 to 10.0 min and maintained at this proportion from 10.0 to 12.0 min.
2.4 Mass spectrometer conditions
The electrospray probe was operated in the negative ion mode. Ultra pure nitrogen (N2, 99.995%, Airgas, Radnor, PA, USA) was used as nebulizer, curtain, and collision gas. The optimum operating conditions of the electrospray ionization (ESI) were as follows: nebulizing gas (GS1), turbo gas (GS2), curtain gas (CUR), collision activated dissociation gas (CAD), turbo-spray voltage and turbo temperature were set to 20, 18, 10, 10 psi, −4.5 kV and 440 °C, respectively. All analytes were quantified using multiple reaction-monitoring (MRM) mode. The mass spectrometer was operated in unit resolution for both Q1 and Q3 in MRM mode, with a dwell time of 150 ms per MRM channel with a 5 ms pause between scans. The transitions monitored were as follows: MMP (precursor ion → product ion, m/z 179 →107), 13C4-MMP (m/z 183 →79), MEP (m/z 193 →121), 13C4-MEP (m/z 197 →79), MBP (m/z 221 →77), 13C4-MBP (m/z 225 →151), MBzP (m/z 255 →107), 13C4-MBzP (m/z 259 →107), MEHP (m/z 277 →134), d4-MEHP (m/z 281 →138), BPA (m/z 227 →133), 13C12 BPA (m/z 239 →224). To monitor the extent of the enzymatic reaction, 4-methylumbelliferone, 13C4-4-methylumbelliferone and 4-methylumbelliferyl glucuronide were acquired at m/z 175 →133, m/z 179→135 and m/z 351→175, respectively.
The peak areas of analytes and their IS’s were determined using analyst version 1.4.1. Identification of each analyte was based on its retention time and mass spectrum. Additionally, the stable isotope ISs served as a tool for the confirmation of the analytes in unknown samples by providing a chromatographic reference for each peak selection. For each analytical batch, a calibration curve containing 5 phthalate monoesters and BPA with slope, intercept and correlation coefficient (r) was derived from weighted (1/x) linear least squares regression of the peak area ratio (analyte: IS) versus the concentration of the standards. The regression equation from the calibration curve was used to back-calculate the measured concentration of each standard and quality control (QC). The results were compared to the theoretical concentrations to obtain the accuracy, expressed as a percentage of the theoretical value, for each standard and QC measured. The standard calibration was injected before and after all samples, including blanks, QCs and unknowns, to monitor sensitivity changes.
Due to the lack of a phthalate-and BPA-free urine matrix, levels in QCs were blank-corrected. Each batch of 30 samples included 3 low QC (QCL) at 10 ng/ml, 3 high QC (QCH) at 100 ng/ml and 3 reagent blanks. Trace concentrations of MBP and MEHP were detected in reagent blanks, and sample concentrations were subtracted from blank values for these 2 compounds. Samples with concentrations above the highest calibrator were diluted with water into calibration range and re-extracted. Concentrations below the limit of quantitation (LOQ) were substituted with a value equal to the LOQ divided by 2 or by the square root of 2 for the calculation of mean and geometric mean, respectively. All the reported urinary metabolite values were not adjusted for creatinine.
2.5 Preparation of standard and QC samples
The initial stock standard solutions of BPA were prepared by dissolving accurately weighed standard compounds in methanol and quantitatively transferring this solution to a 10-ml volumetric flask. The stock standard solutions of the 5 phthalate monoesters were prepared in acetonitrile. The initial stock standard solutions were stored in amber glass vials at −20 °C until use. The intermediate standard solutions for BPA and 5 phthalate monoesters were prepared by serial dilutions of the initial stock standard solution with methanol. The intermediate IS solution of 13C12-BPA, 13C4-labeled phthalate monoester standards and D4-MEHP was prepared by aliquoting the commercial standard solution (100 μg/ml in acetonitrile) to each 10 ml glass volumetric flask and diluting by volume with methanol to give a final concentration of 5 μg/ml for 13C12-BPA and 2 μg/ml for 13C4-labeled phthalate monoesters and D4-MEHP . All standard solutions were prepared in methanol-rinsed and dried glassware.
The calibration curves for BPA and 5 phthalate monoesters at ten levels ranged from 0.3 to 200 ng/ml and were prepared by adding aliquots of intermediate standard solutions to water. The QC samples at two concentration levels 10 (QCL) and 100ng/ml (QCH) were prepared the same way in pooled human urine. The standards and QCs were stored frozen at −20 °C. The enzyme solution was prepared fresh daily for each run by dissolving 3.6 mg of β-glucuronidase/sulfatase (H. pomatia, 1926, 000 units/g of solid) in 1 ml of 1.0 mol/l ammonium acetate buffer solution (pH 5.0), and mixing gently to prevent denaturation of the enzyme.
2.6 Sample preparation
Urine samples were thawed to room temperature and vortexed to ensure homogeneity. Each of the 200 μl urine sample was then transferred into a glass test tube and mixed with 10 μl intermediate IS solution (1000 ng/ml of 13C12-BPA and 500 ng/ml of 13C4 labeled phthalate monoesters), 150 μl ammonium acetate buffer solution (1 mol/l, pH 5.0), 100 μl water, and 20 μl of enzyme solution of β-glucuronidase. The samples were mixed and then incubated at 37 °C for 3 hr in a shaking water bath. Calibration standards, 4 QCL, 4 QCH, and blanks were also prepared by adding 10 μl intermediate IS solution, 150 μl ammonium acetate buffer solution and 20 μl of enzyme solution, but 20 μl of acetate buffer was added in QCs instead of enzyme solution. The samples were then incubated under the same conditions as the urine samples. After incubation, 10 μl of formic acid was added to each deconjugated urine samples to stop the enzyme activity, and then 400 μl of water was added. The calibration standards and QCs were processed in the same way as the deconjugated urine samples by adding 10 μl of formic acid and diluted with 400 μl of water.
A RapidTrace SPE Workstation (Biotage, Charlotte, NC, USA) and SampliQ SPE cartridges packed with 200 mg of silica-based bonded C18 material (3 ml) (Agilent Technology, Santa Clara, CA, USA) were used for extraction. The SPE cartridges were conditioned by successive washes of 2 ml of methanol and 2 ml of water, followed by the loading of deconjugated urine samples, calibration standards, or QCs. The cartridges were washed with 1.5 ml of water and eluted with 1 ml of acetonitrile. The eluent was collected and concentrated to dryness using a TurboVap LV Evaporator (Zymark, Framingham, MA) at 40°C under a gentle nitrogen stream. The dry residue was reconstituted by 150 μl 0.1% acetic acid in acetonitrile/water (1:9, v/v), and 10 μl of this mixture was injected into the LC-MS/MS for analysis. The concentration of 4-methylumbelliferone formed from the enzymatic hydrolysis of 4-methylumbelliferone glucuronide was run in parallel with the urine samples for the purpose of monitoring the completion of the deglucuronidation reaction by β-glucuronidase. Reagent blanks, standards, and QCs were analyzed with the urine samples to ensure accuracy of the data.
2.7 Assay validation
The analytical method was validated to demonstrate the recovery, LOQ, accuracy, and precision of the measurements. All calibration samples were prepared as described above using a double blank (extracted water samples without IS), blank (extracted sample with IS) and water spiked with respective amounts of reference standards.
The linearity of the calibration curve was evaluated from three consecutively prepared batches at the concentration range 0.3-200 ng/ml. The peak area ratios of analytes and the corresponding IS were used to calculate the correlation coefficient, intercept and slope.
Fifteen replicates of QC samples generated at concentrations of 10, and 100 ng/ml from runs on three consecutive days were used to evaluate precision and accuracy at each concentration level to determine the intra- and inter-day validation. Precision was calculated as the relative standard deviation (RSD) for both intra-day and inter-day variability and accuracy as the degree of closeness of the determination value to the true value. The limits of detection (LODs) were calculated as 3S0, where S0 is the value of the standard deviation as the concentration approaches zero, and the limit of quantitation (LOQ) was calculated as 3LOD. Acceptable precision was defined by a RSD <15% for the standards, and <20% at LOQ. Accuracy was acceptable if the deviation for the standard <15%, and LOQ <20% compared to the nominal values.
Matrix effect (ME) occurs when the co-elutes from the same sample matrix affects (either attenuates or enhances) the response of the analyte during quantitation by LC-MS/MS. To determine the matrix effect of urine on the ESI process, blank urine samples were extracted, dried, reconstituted and spiked with phthalate monoester metabolites and BPA (post-extraction spiked sample), then compared to the same level of concentrations of phthalate monoester metabolites and BPA in neat standard solutions injected directly into LC-MS/MS using the following equation:
ME% = (Mean post-extraction peak area/Mean neat solution peak area)×100 The values of ME indicate either ion enhancement (>100%) or ion suppression (<100%). A value of 100% indicates that there is no matrix effect.
The extraction process recovery was calculated by dividing the area counts of individual extracted urine samples by the mean area counts of neat standard solutions injected directly into LC-MS/MS at 2 concentration level (10 and 100 ng/ml) of phthalate metabolites and BPA.
Stability of standard stock solutions of phthalate monoesters and BPA and their ISs in methanol used in the preparation of standards and QCs was investigated at both room temperature and at −20 °C. The stabilities were calculated by comparing mean response ratios (area of response per unit of concentration) of stability solutions to mean response ratios of freshly prepared control solutions.
The stabilities of the analytes in spiked urine samples at room temperature (22 °C) for 4 hr, −20 °C for 90 days and after three freeze/thaw cycles (frozen for 24 h and thawed at room temperature as one cycle) were evaluated at two concentration levels (10 and 100 ng/ml) in six replicates. The stability of extracted samples in reconstitution solution at 4 °C (the temperature of the autosampler) for 2 days was also evaluated. The mean analyte concentration at each level was compared to each mean concentration determined in the initial testing. The analytes were considered to be stable if degradation was <10% of the concentration at day 0.
2.8 Determination of phthalate monoester metabolites and BPA concentrations in human urine
A total of 50 anonymous adult human urine samples were randomly selected and analyzed using this LC-MS/MS method. These samples were collected from volunteers participating in a study aiming to assess their occupational exposures to phthalates. These samples were collected with approval from Institutional Review Board. We used these materials after removing all identifiable labels with demographic information. Samples were stored at −20 °C prior to the analysis.
3. Results and Discussion
3.1 Optimization of SPE protocol
Due to the different chemical characteristics of BPA and phthalate monoester metabolites, the analytical methods developed so far can only monitor either BPA or phthalate monoester metabolites in human urine using separate analytical methods [11-18]. There were challenges in both sample preparation and detection of analytes in developing the LCMS/MS method to detect BPA and phthalate monoester metabolites in human biological matrix simultaneously. The first challenge was to maintain acceptable recoveries for both BPA and phthalate monoester metabolites (especially MMP) while simultaneously cleaning up interference from urine as much as possible during the SPE step of sample preparation. This is especially important for BPA, as its concentrations in the general population are usually low [3]. However, MMP was easily washed out if excess water or solvents were present in the wash step during SPE, resulting in low recoveries.
We previously reported [25] a validated off-line SPE/LC-MS method for quantifying BPA levels in human aminiotic fluid. Based on this method, we further optimized the SPE procedures under different pHs of loading sample, wash steps, and elution solvents. Since both phthalate monoester and the phenolic compound of BPA are weak acids, acidifying samples prior to SPE extraction will promote equilibrium to the unionized form and increase the extraction efficiency. Therefore, 10 μl of formic acid was added to the samples prior to SPE extraction. The addition of water in the acidified samples reduced the viscosity of the sample, thus a better flow rate was achieved during the SPE sample loading step. After the sample was loaded, the SPE cartridge was washed with 1.5 ml of water. No solvent was added in this wash step to minimize any loss of MMP and/or MEP. After testing different amounts of water to clean up the samples, it was determined that 1.5 ml of water was ideal to wash out the interference of the sample matrix without sacrificing the recovery. Since there are trace levels of MBP and MEHP detected in solvents, solvents from different vendors were compared. Methanol from JT Baker and Sigma, acetonitrile from JT Baker, Sigma, Alfa Aesar (Ward Hill, MA, USA) and Honeywell Burdick & Jackson (Muskegon, MI, USA) and ethyl acetate from JT Baker were compared as eluent solvents. For each solvent, 2 ml was dried under N2 and peak areas of MBP and MEHP were compared. Acetonitrile from JT Baker gave the smallest peak, so it was chosen as the “elution solvent”. Acetonitrile as an elution solvent was applied in volumes of 1, 1.5 and 2 ml, and recoveries were similar. In order to reduce the use of organic solvents and the contamination from the solvents, 1 ml of acetonitrile was chosen. We used 0.1% acetic acid in water with 10% acetonitrile as the reconstituting solution because acetonitrile increases the signal of BPA and won’t cause early elution of MMP in chromatograms.
3.2 Optimization of LC-MS conditions
In addition to the recoveries from the SPE steps, detection of both BPA and phthalate monoester metabolites under the same LC and MS conditions with good sensitivity was a challenge during method development. We first optimized the MS conditions. The negative-ion ESI MS/MS product-ion spectra of each analyte and its IS were obtained by direct infusion of the analyte solutions via a tee connection between the LC and the mass spectrometer. After the mass spectrometer operating parameters were optimized, methods using the most abundant product ion of each analyte were selected to build the MRM method. Final MRM transitions were selected on the basis of signal to noise ratio (S/N) with on-column injection analysis.
After the detection conditions were optimized, experiments were conducted to optimize the chromatographic conditions. We investigated several reversed phase columns, including Water’s XTerra MS C18 (100 mm × 2.1 mm, 3.5 μm), YMC pro-C18 (100 mm × 2.1 mm, 3 μm), Zorbax Eclipse XDB-C8 (150 mm × 4.6 mm, 5 μm), Phenomenex Synergi polar RP (100 mm × 2.0 mm, 4 μm), Thermo Betasil C18 (100 mm × 2.1 mm, 3.5 μm) and Thermo Betasil Phenyl (50 mm × 2.1 mm, 3.5 μm). The retention time, analyte response, peak shapes, resolution, and background interference of these columns were compared using the same mobile phase with optimized gradients. Thermo Betasil Phenyl (50 mm × 2.1 mm, 3.5 μm) showed superior peak shape with adequate retention time for each analyte compared to other columns tested.
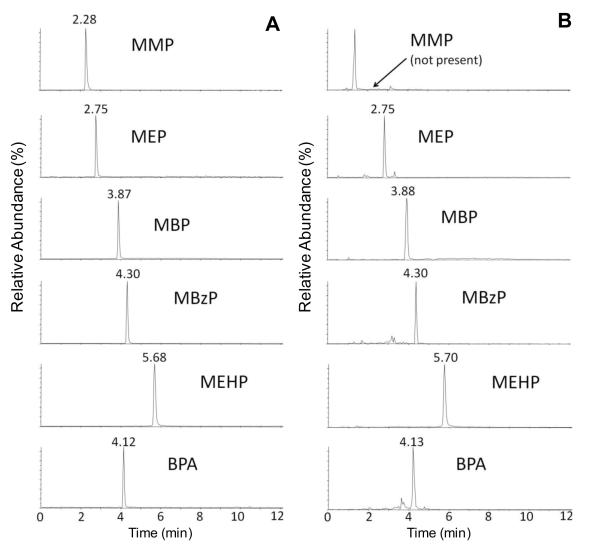
The LCMS/MS methods reported so far for analyzing BPA contain no organic acid in the mobile phase since they can suppress the signal of BPA [15,16,26]. Similarly, no mobile phase additives, such as formic acid, acetic acid, ammonium acetate or ammonia were added in our previous method [25] because we also found that they can cause severe signal suppression for BPA analysis. However, these methods could not be applied to analyze phthalate metabolites under the same analytical conditions since without organic acid (such as acetic acid) added in the mobile phase, the phthalate monoesters, especially MMP, MEP, MBP and MBzP, wouldn’t bind effectively to the stationary phase of the analytical column and eluted out in 2 mins. After testing HPLC mobile phase with different additives, the mobile phase containing 0.1% acetic acid resulted in adequate retention time and good separation for phthalate metabolites and BPA. Acetonitrile and methanol were compared as the organic part of the mobile phase. Acetonitrile was chosen because it had slightly higher resolution and better peak shapes for phthalate monoesters than methanol. Since the instrument used in the current method is more sensitive than the LC-MS system used in our previous method [25], and sample clean up by SPE also effectively reduced the interference from matrix, we were able to achieve a good sensitivity for BPA using mobile phase containing 0.1% acetic acid. The HPLC elution gradients and the flow rate were also optimized. The best chromatographic conditions were obtained using acetonitrile with 0.1% acetic acid as mobile phase B and 0.1% acetic acid in water as mobile phase A starting the gradient from 5% to 100% at a flow rate of 220 μl/min. This elution gradient can separate analytes from potential interferences with less background noise and gives the best and most robust chromatographic peaks, as shown in Fig. 1. The total run time was 12 min.
Fig. 1.
Representative LC-MS/MS chromatograms of a standard solution containing 5 phthalate metabolites and BPA at 10 ng/ml (A) and a non-spiked human urine extract (B) with detected concentrations (in ng/ml) of below LOQ (MMP), 186.0 (MEP), 10.3 (MBP), 9.44 (MEzP), 6.0 (MEHP) and 0.9 (BPA).
Stable isotopic labeled standards are internal standards, as they have the same physical and chemical properties as the analyte. We chose 13C4-labeled phthalate monoester metabolites except MEHP (its IS is D4-MEHP) and 13C12-BPA as ISs for the quantitation experiments and added them at the beginning of the sample preparation. The addition of IS reduced variations in sample preparation and injection, minimized matrix effects on quantitation, and improved peak identification by providing a chromatographic reference (retention time reference) for peak selection of the analytes, especially at trace level analysis.
3.3 Assay validation
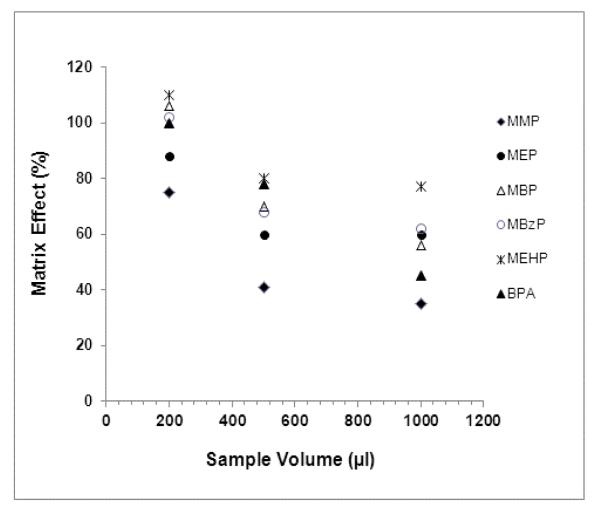
Because it is readily available in sufficient amounts for analysis, urine is an important matrix for biological monitoring of phthalates and BPA exposure in humans. The metabolite concentrations are also higher in urine than in blood due to their relatively rapid metabolism and excretion. However, matrix effects from biological matrices such as urine may impair the accuracy and the precision of the method. During our initial method development based on the previous method [25], we had difficulty in detecting low levels of MMP, MEP, BPA and their ISs using 500-μl sample volume. Increasing the urine volume to 1ml decreased the sensitivity and therefore did not solve this problem. Matrix effects on analyte responses were therefore investigated using different urine sample volumes (200, 500 and 1000μl). Results indicated severe ME as the urine sample volume increased (Fig. 2). Decreasing the volume of reconstituted solution and increasing the injection volume also resulted in substantial ME. In order to reduce ME, 200μl urine was selected as the sample volume in this study. Dilution of extracts by increasing the volume of reconstituted solution from 110 to 150μl, and reducing the volume of sample injected from 20 to 10μl were found to reduce the ME significantly and resulted in quantifiable low levels of MMP, MEP and BPA signals.
Fig. 2.
Influence of human urine sample volume used during SPE extractions on the matrix effect (%) of 5 phthalate monoester metabolites and BPA. The volumes investigated were 200, 500 and 1000 μl.
Matrix effect was further assessed at 10 and 100 ng/ml phthalate metabolites and BPA in urine. Ion suppression of the analyte responses of MMP (75.2-77.2%) and MEP (85.6-88.6%) was observed. Minor ion enhancement, which may come from improved ionization, was observed for MBP, MBzP and MEHP with less than 10% the average of intensity of the analyte ions enhanced at both levels. Because isotope-labeled internal standards were used for each analyte in this study and the same degree of ion suppression/enhancement was also observed in ISs at each of the corresponding analyte level, the quantification based on the analyte/IS response ratios should not be influenced by the matrix effect. The results indicate that the current method, including sample clean up and HPLC separation, is adequate to minimize matrix effect on the MS ionization, and the remaining matrix effect can be corrected by IS.
Table 1 shows the mean extraction process recoveries in urine at two concentration levels. The recoveries of MBP, MBzP, MEHP and BPA were completed with values ranging from 94.6% to 105.3%, but were relatively lower for MMP and MEP, with mean recoveries of 69.8% and 85.2%, respectively. After subtracting the mean loss from matrix effects for MMP (23.8%) and MET (12.9%), the remaining loss from the extraction was less than 10%. Therefore, the loss in the recoveries of MMP and MEP during the sample analysis process is mainly due to the matrix effect during ESI, while the loss of MMP and MEP during the SPE extraction was less significant. Since the same degree of ion suppression was observed in each IS at both concentration levels, the recovery using internal standardization by calculating the ratio of analyte/IS brought the recovery values close to 100% for each analyte. The results demonstrated that the extraction recovery was adequate to achieve the accuracy and precision needed.
Table 1.
The mean extraction process recovery of five phthalate metabolites and BPA in fortified human urine (n=5)
| Analytes | Recovery (%)a | ME (%)b | ||
|---|---|---|---|---|
|
| ||||
| 10ng/ml | 100ng/ml | 10ng/ml | 100ng/ml | |
| MMP | 70.8 ± 10.5 | 68.7 ± 9.4 | 75.2 ± 9.3 | 77.2 ± 8.0 |
| M EP | 84.3 ± 5.8 | 86.1 ± 10.5 | 88.6 ± 6.0 | 85.6 ± 9.1 |
| MBP | 101.9 ± 8.0 | 95.3 ± 2.2 | 105.3 ± 7.0 | 103.5 ± 5.9 |
| MBzP | 103.2 ± 3.3 | 98.9 ± 5.1 | 106.9 ± 4.7 | 108.9 ± 6.8 |
| MEHP | 104.9 ± 6.3 | 105.3 ± 7.5 | 107.7 ± 11.3 | 110.0 ± 8.8 |
| BPA | 94.6 ± 6.1 | 100.3 ± 5.9 | 99.1 ± 4.4 | 100.8 ± 5.0 |
Recovery (%) expressed as mean ± standard deviation
Matrix effect (%) expressed as mean ± standard deviation
Calibrations were prepared in water based on the results of ME and recovery described above. Any urinary matrix effect on the calibration curve was evaluated by analyzing the standards prepared in water versus those spiked into human urine. The slopes of calibration curves containing matrix-matched standards prepared in human urine showed no significant difference from the slopes of calibration curves in water. Therefore, matrix effect was sufficiently eliminated for the range of analyte concentrations measured, and subsequent calibration curves used in this study were all prepared in water. The calibration curves showed good linearity within the given concentration range 0.3-200 ng/ml for MMP, MEP, MBzP and BPA, and 1-200 ng/ml for MBP and MEHP, with the correlation coefficient (r) exceeding 0.99 (Table 2). The mean back-calculated concentrations of the standards were between 94.2 and 106.1% of the theoretic values of analytes. The retention times and linearity of 5 phthalate metabolites and BPA are shown in Table 1.
Table 2.
Retention times, quantitation ranges, and correlation coefficients for five phthalate metabolites and BPA.
| Analytes | tR (min) | Quantitation range (ng/ml) | Correlation coefficient (r) |
|---|---|---|---|
| MMP | 2.28 | 0.3-200 | 0.996 |
| MEP | 2.75 | 0.3-200 | 0.993 |
| MBP | 3.87 | 1.0-200 | 0.997 |
| MBzP | 4.30 | 0.3-200 | 0.997 |
| MEHP | 5.68 | 1-200 | 0.997 |
| BPA | 4.12 | 0.3-200 | 0.998 |
The LOD and LOQ for MMP, MEP, MBzP and BPA in a 200μl urine sample were 0.1 and 0.3 ng/ml, respectively. The LOD and LOQ for MBP and MEHP were 0.3 and 1.0 ng/ml, respectively. Laboratory blanks were prepared in water to capture possible environmental contamination of analytes from water or solvents, as well as contaminants released from material used for sample preparation (including tubes, pipette tips and autosampler vials). We found trace levels of MBP and MEHP in tested laboratory blank samples. Background subtraction was used to correct for this minor contamination. Twelve replicates of lower limit of quantification (LLOQ) samples were used to evaluate the precision and accuracy at the low end of the assay range from three separate runs. The precisions were 4.2-11.1% and the accuracies were 95-114%. This indicates acceptable precision and accuracy at LLOQ levels for all analytes.
The intra- and inter-day precision and accuracy were evaluated using urine spiked with phthalate metabolites and BPA at two different concentrations. The intra-day precision was between 4.0 and 10.2%, and the inter-assay precision was between 3.7 and 9.5%. The intra-assay and inter-assay mean accuracies, expressed as percents of theoretical, were between 98.1-110.1 % and 95.1-112.2% (Table 3). These values show the good accuracy and reproducibility of this method and that it is therefore adequate for biomonitoring studies.
Table 3.
Intra- and inter-day precision and accuracy for five phthalate metabolites and BPA.
| Intra-day (ng/ml) (n=5) |
Inter-day (ng/ml) (n=15) |
||||
|---|---|---|---|---|---|
| Analysis | 10.0 | 100.0 | 10.0 | 100.0 | |
| MMP | Mean | 10.6 | 98.1 | 10.2 | 96.9 |
| SD | 1.0 | 9.6 | 0.8 | 7.9 | |
| Precision (%) | 9.4 | 9.8 | 6.9 | 8.2 | |
| Accuracy (%) | 106.2 | 98.1 | 102.3 | 96.9 | |
| MEP | Mean | 9.8 | 106.6 | 9.5 | 107.0 |
| SD | 1.0 | 9.2 | 0.9 | 10.1 | |
| Precision (%) | 10.2 | 8.6 | 9.5 | 9.4 | |
| Accuracy (%) | 98.1 | 106.6 | 95.1 | 107.0 | |
| MBP | Mean | 10.7 | 105.5 | 10.3 | 104.9 |
| SD | 0.7 | 6.3 | 0.5 | 4.7 | |
| Precision (%) | 6.5 | 6.0 | 4.8 | 4.2 | |
| Accuracy (%) | 107.3 | 105.5 | 103.2 | 104.9 | |
| MBzP | Mean | 9.9 | 98.3 | 9.9 | 97.5 |
| SD | 0.5 | 3.9 | 0.6 | 3.6 | |
| Precision (%) | 5.1 | 4.0 | 6.0 | 3.7 | |
| Accuracy (%) | 99.0 | 98.3 | 99.3 | 97.5 | |
| MEHP | Mean | 11.0 | 108.1 | 11.2 | 106.3 |
| SD | 0.8 | 7.2 | 0.7 | 5.9 | |
| Precision (%) | 7.3 | 6.9 | 6.2 | 5.6 | |
| Accuracy (%) | 110.1 | 108.1 | 112.2 | 106.3 | |
| BPA | Mean | 10.1 | 99.2 | 10.4 | 99.5 |
| SD | 0.6 | 5.4 | 0.5 | 4.5 | |
| Precision (%) | 5.9 | 5.4 | 4.8 | 4.5 | |
| Accuracy (%) | 101.2 | 99.2 | 104.4 | 99.5 | |
The stability of the standard stock solutions at room temperature and − 20°C was established for at least 6 hours and 90 days, respectively. The differences between the stored and fresh solutions were ≤ 5%, indicating acceptable stability for these storage durations. The phthalate monoester metabolites and BPA in urine under long-term (− 20 °C for 90 days) and short-term (room temperature for 4 hr) storage conditions following three freeze-thraw cycles were also investigated. There were no significant differences (≤ 8%) in the mean analyte concentrations at each level of 10 and 100 ng/ml compared with initial mean values, indicating analyte stability. Additionally, the stability of the extracted samples in reconstituted solution in an autosampler was also evaluated, and samples were found to be stable at 4 °C at least for 24 hr. These results showed that degradation of analytes in urine is negligible within the time required for analyzing the samples. Phthalate metabolites and BPA also had acceptable stability under these test conditions.
3.4 Application of the assay
The LC-MS/MS method described above has been applied to the analysis of 50 human urine samples collected from anonymous adult volunteers with possible exposure to phthalates in their workplaces. Table 4 presents the summary of five phthalate monoester metabolites and BPA levels found in these samples. Overall, the detectable rates of MMP, MEP, MBP, MBzP, MEHP and BPA were 88, 100, 86, 100, 100 and 87%, respectively. The median levels of MMP, MEP, MBP, MBzP, MEHP and BPA in all urine samples were 2.8, 43.7, 10.2, 4.7, 5.9 and 1.9 ng/ml, respectively. The geometric mean without creatinine adjustments for MEP and MBzP levels in human urine were lower, and MBP and MEHP levels were a little higher than the reported levels in urine [3]. The geometric mean of MMP and BPA levels were comparable to the reported levels. This study confirmed detectable levels of 5 phthalate metabolites and BPA in human urine samples.
Table 4.
Urinary concentrations of 5 phthalate metabolites and BPA detected in adult human spot urine samples (n=50)
| Analytes | Concentrations (ng/ml) | |||
|---|---|---|---|---|
| Range | Median | Mean | Geometric Mean | |
| MMP MEP MBP MBzP MEHP BPA |
<LOQ – 14.2 5.3 – 906 <LOQ – 156 0.4 −23.3 2.3 – 34.1 <LOQ – 11.2 |
2.8 43.7 10.2 4.7 5.9 1.9 |
3.6 118.8 22.5 7.0 7.5 2.5 |
1.8 56.5 9.1 4.0 6.5 2.2 |
<LOQ: below limit of quantitation
4. Conclusions
We have developed an LC-MS/MS method for simultaneously analyzing 5 phthalate monoester metabolites and BPA in human urine. The method has proven to be sensitive, accurate and precise using a small sample volume of 200-μl, with a relatively short chromatographic run time of 12 min. These results have demonstrated the value of this method in assessing human exposure to phthalates and BPA in future studies.
Highlights.
A LC-MS/MS method for simultaneously analyzing 5 phthalate monoester metabolites and BPA in human urine is developed.
The method was validated and demonstrated to be sensitive, accurate and precise using 200 μl sample volume with a run time of 12 min.
This method has been used successfully in assessing the exposure of phthalate and BPA in 50 humans.
Acknowledgments
This project was supported by the Harvard-NIEHS Center for Environmental Health (ES000002). Authors would like to thank Dr. Syam Sundar Andra and Ms. Michaela Kapp for their assistance in the lab, as well as in the preparation of this manuscript. Authors declare no conflict of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- [1].Halden RU. Annu. Rev. Public Health. 2010;31:179. doi: 10.1146/annurev.publhealth.012809.103714. [DOI] [PubMed] [Google Scholar]
- [2].Silva MJ, Slakman AR, Reidy JA, Preau JL, Jr, Herbert AR, Samandar E, Needham LL, Calafat AM. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2004;805:161. doi: 10.1016/j.jchromb.2004.02.038. [DOI] [PubMed] [Google Scholar]
- [3].The Fourth National Report on Human Exposure to Environmental Chemicals, Updated Tables, February 2012. Centers for Disease Control and Prevention; National Center for Environmental Health; Division of Laboratory Sciences; Atlanta, GA: 2012. [Google Scholar]
- [4].Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Environ. Health Perspect. 2008;116:39. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Meeker JD, Ferguson KK. Environ. Health Perspect. 2011;119:1396. doi: 10.1289/ehp.1103582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Foste PM. Crit. Rev. Toxicol. 2005;35:713. doi: 10.1080/10408440591007395. [DOI] [PubMed] [Google Scholar]
- [7].Roy JR, Chakraborty S, Chakraborty TR. Med. Sci. Monit. 2009;15:RA137. [PubMed] [Google Scholar]
- [8].Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. J.A.M.A. 2008;300:1303. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- [9].Takashima K, Ito Y, Gonzalez FJ, Nakajima T. Occup. Health. 2008;50:169. doi: 10.1539/joh.l7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Geens T, Neels H, Covaci A. J. Chromatogr B Analyt. Technol. Biomed. Life Sci. 2009;877:4042. doi: 10.1016/j.jchromb.2009.10.017. [DOI] [PubMed] [Google Scholar]
- [11].Kondo F, Ikai Y, Hayashi R, Okumura M, Takatori S, Nakazawa H, Izumi S, Makino T. Bull. Environ. Contam. Toxicol. 2010;85:92. doi: 10.1007/s00128-010-0051-8. [DOI] [PubMed] [Google Scholar]
- [12].Inoue K, Kawaguchi M, Funakoshi Y, Nakazawa H. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;798:17. doi: 10.1016/j.jchromb.2003.08.042. [DOI] [PubMed] [Google Scholar]
- [13].Blount BC, Milgram KE, Silva MJ, Malek NA, Reidy JA, Needham LL, Brock JW. Anal. Chem. 2000;72:4127. doi: 10.1021/ac000422r. [DOI] [PubMed] [Google Scholar]
- [14].Silva MJ, Malek NA, Hodge CC, Reidy JA, Kato K, Barr DB, Needham LL, Brock JW. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2003;789:393. doi: 10.1016/s1570-0232(03)00164-8. [DOI] [PubMed] [Google Scholar]
- [15].Völkel W, Bittner N, Dekant W. Drug Metab Dispos. 2005;33:1748. doi: 10.1124/dmd.105.005454. [DOI] [PubMed] [Google Scholar]
- [16].Ye X, Kuklenyik Z, Needham LL, Calafat AM. Anal. Chem. 2005;77:5407. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- [17].Kato K, Silva MJ, Needham LL, Calafat AM. Anal. Chem. 2005;77:2985. doi: 10.1021/ac0481248. [DOI] [PubMed] [Google Scholar]
- [18].Silva MJ, Samandar E, Preau JL, Jr., Reidy JA, Needham LL, Calafat AM. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2007;860:106. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- [19].krotz SP, Carson SA, Tomey C, Buster JE. J Assist Reprod Genet. 2012 Apr 27; doi: 10.1007/s10815-012-9775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Vandentorren S, Zeman F, Morin L, Sarter H, Bidondo ML, Oleko A, Leridon H. Environ Res. 2011;111:761. doi: 10.1016/j.envres.2011.05.018. [DOI] [PubMed] [Google Scholar]
- [21].Casas L, Fernández MF, Llop S, Guxens M, Ballester F, Olea N, Irurzun MB, Rodríguez LS, Riaño I, Tardón A, Vrijheid M, Calafat AM, Sunyer J. Environ Int. 2011;37:858. doi: 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- [22].Silva MJ, Preau JL, Jr., Needham LL, Calafat AM. J Chromatogr B. 2008;873:180. doi: 10.1016/j.jchromb.2008.08.017. [DOI] [PubMed] [Google Scholar]
- [23].Yolton K, Strauss Y. Xu, D., Altaye M, Calafat AM, Khoury J. Neurotoxicol Teratol. 2011;33:558. doi: 10.1016/j.ntt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Braun JM, Smith KM, Williams PL, Calafat AM, Berry K, Ehrlich S, Hauser R. Environ Health Perspect. 2012;120:739. doi: 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen M, Edlow AG, Lin T, Smith NA, McElrath TF, Lu C. J. Sep. Sci. 2011;34:1648. doi: 10.1002/jssc.201100152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Markham DA, Waechter JM, Jr., Wimber M, Rao N, Connolly P, Chuang JC, Hentges S, Shiotsuka RN, Dimond S, Chappelle AH. J Anal Toxicol. 2010;34:293. doi: 10.1093/jat/34.6.293. [DOI] [PubMed] [Google Scholar]