Abstract
We designed this community-based participatory research (CBPR) project aiming to generate evidence-based research results in order to encourage residents living in urban low-income public housing dwellings engaging in a community-wide integrated pest management (IPM) program with the intention to improve their health and quality of life, as well as household conditions.
We enrolled 20 families and their children in this study in which we utilized environmental exposure assessment (surface wipe and indoor air) tools to quantitatively assessing residential pesticide exposure in young children before the implementation of an IPM program. We analyzed those samples for 19 organophosphate (OP) and pyrethroid pesticides.
The most commonly detected pesticides were pyrethroids, particularly permethrin and cypermethrin with average concentrations of 2.47 and 3.87 µg/m2, respectively. In many dwellings, we detected OPs, which are no longer available on the market, however, their levels are significantly lower than those of pyrethroids. None of the 20 families was free from pesticide contamination in their households, and pesticides were commonly detected in living room and children’s bedroom.
The correlation among household hygienic conditions, the sighting of live pests/pest debris, and the degree of indoor pesticide contamination highlights the failure of conventional chemical-based applications for pest controls. The results from the current study, as well as other recent studies, conducted in low-income public housing, child care centers, and randomly selected homes in the U.S. should accentuate the need for alternative pest management programs that incorporate safer and more sustainable protocols for pest controls.
Keywords: Community-based participatory research, CBPR, pesticide exposure, pyrethroids, organophosphate pesticides, public housing, indoor pesticide application
Introduction
Several survey and observational studies have indicated that urban low-income, multifamily, public housing dwellings are prone to have severe pest infestation mainly due to the presence of abundant food sources (both indoor and outdoor), persistent moisture problem, household cluttering, and cracks and crevices in the walls, windows, and doors (1–3). Despite the evidence in documenting the link between pesticide uses either in agricultural or residential environment and the exposure to children living in the same households (4–8), very few studies have focused on quantifying residential pesticide exposure for children living in urban low-income public housing developments. Two separate studies conducted in New York City’s low-income housing dwellings filled the gap of the paucity of quantitative data in regard to the benefits of implementing the integrated pest management (IPM) in urban low-income dwellings. Williams et al. (9) have suggested that the IPM program could be effective in reducing pest infestation levels and subsequently lowering maternal exposure to common insecticides used for indoor pest control applications. Brenner et al. (10) have demonstrated that individually tailored IPM program can be successful and cost-effective among public housing residents in East Harlem.
The residential pesticide contamination problem in urban low-income multifamily dwellings has been demonstrated in recent studies conducted in New York City (7) and in Boston Massachusetts (11, 12). Personal ambient air samples collected from 72 pregnant women living in Manhattan and south Bronx contained detectable organophosphate (OP) pesticides (chlorpyrifos and diazinon), fungicides (phenylphenol) and carbamates (propoxur) at 100% frequency of detection, and pyrethroids (permethrin) and organochlorine (DDT and chlordane) in the range of 47–78% frequency of detection. Julien et al. (11, 12) collected house dust and floor wipe samples from 42 families living in 3 different Boston public housing developments and showed a wide range of 12 pesticide levels with permethrin (pyrethroids) and chlorpyrifos (OP) detected in every home, and cyfluthrin (a restricted-use pyrethroids) detected in a majority of homes. Those findings clearly and quantitatively showed the high degree of pesticide contaminations in those low-income multifamily dwellings. Earlier studies have concluded that children living in urban and suburban environment with higher social economic status (SES) are predominantly exposed to pesticides through their daily dietary intakes; however, such low level but chronic pesticide exposure scenario is likely to be modified upward by periodic residential pesticide exposures resulting from indoor pest control applications (13, 14). Hence, we would anticipate an elevated overall pesticide exposure among children living in urban low-income public housing compared to those with higher SES. Therefore, it is warranted to further assess pesticide exposures among children living in urban low SES households, and more importantly to develop and institutionalize a safe, sustainable, and practical pest control program in urban public housing developments to mitigate the excessive residential pesticide exposures in those households.
The instigation of this community-based participatory research (CBPR) project stemmed from an earlier multi-institution collaboration under the Healthy Public Housing Initiative (11), which included the Committee for Boston Public Housing, Boston Housing Authority, and research partners at Harvard School of Public Health, Tufts School of Medicine and the Boston University School of Public Health. In this CBPR project, we aimed to provide evidence-based research results in order to encourage residents living in Boston public housing developments engaging in a community-wide IPM program with the intention to improve their health and quality of life, as well as household conditions. We utilized environmental exposure assessment and biomarker tools in this study to quantitatively assessing the changes in residential pesticide exposure in young children before and after the IPM program, similar to an approach reported earlier (9). In this paper, we reported results of characterizing important exposure pathways for pesticides used in the pest control application in selected households prior to the implementation of a community-wide IPM program in this specific low-income public housing development in Boston.
Method
Subject Recruitment
We recruited 20 families with young children living in one of the Boston public housing developments in which conventional pesticide-based pest control application was being employed as of March 2011. The eligibilities for participating in this study included: (1) family with a child ages of 3 to 11 at the beginning of the study, (2) the child was not currently on regular medications, (3) the child spent greater than 80% nights per week at this home, and (4) families and their children were willing to participate in the study for a period of 3 years. Families were recruited through letters, flyers, and by direct personal contact of community management personnel. Adult family members and their child were consented prior to the initiation of data/sample collection. Families were reimbursed for their time and effort with monthly gift cards for 6 consecutive months to local supermarkets of their choosing. The use of human subjects in this study has been approved by IRB at Harvard School of Public Health (Protocol #18075).
Subject Participation
We collected environmental (indoor air and surface wipes) and biological (urine and saliva) samples from participants and their households before and after the implementation of an Integrated Pest Management (IPM) program. During the first month of the 6-month pre-IPM sampling phase, we collected surface wipes and indoor air on Day 1. We also collected spot urine, saliva, and 24-hr duplicate foods (fresh produce and juices) samples in which parents or caregivers of the children were asked to assist in collection. We only report results from questionnaire and the environmental samples (air and wipe) in this paper.
Wipe and Indoor Air Sample Collection and Preparation
We collected wipe samples of the living room floor, child’s bedroom floor, and kitchen countertop from an area (30cm × 30cm) uncovered by furniture or rugs and likely to be encountered by a child. All floor coverings in the households were vinyl tile. Two 3”× 3” sterile cotton gauze pads wetted with a mist of isopropanol (about 2 ml) were used to wipe the designated area with three sequential vertical and horizontal strokes, respectively. After wiping, gauze pads were placed in the same jar, transported in a chilled cooler, and upon return to the lab were stored at −20°C until analysis.
We also collected one 24-hour indoor air sample from each family (set up in the living room) by using a high-volume sampling pump (drawing 70 liter/min of air) connected to a glass cartridge with 3 3.5-cm diameter polyurethane foam (PUF) plugs. Upon the completion of air sampling, the glass cartridges with PUFs in them were capped, transported in chilled coolers, and then stored at 4°C until processing in the lab. The first two PUFs were placed in a jar, supplemented with 2 ml of acetyl nitrite and stored at −20°C until extraction. The third PUF, used as the breakthrough sample, was placed in a separated jar and then stored at −20°C. The third PUF samples would not be analyzed unless the corresponding samples (the 1st and 2nd PUFs) contained excessive amount of pesticides.
Laboratory Analysis
Wipe (gauze pad) and air (PUF) samples were thawed to room temperature and then known amounts of mixed internal standards were added, including D6-dichlorvos, D10-diazinon, and D6-trans-permethrin before extraction. Sixty-milliliter (mL) of ethyl acetate was added into each sample bottle and the bottle was shaken for 30 minutes on a shaker table at high speed. This procedure was repeated with an additional 60mL of ethyl acetate. Ethyl acetate from the extractions were combined (approximately 120 mL) and evaporated to near dryness at 40°C under gentle nitrogen in a TurboVap evaporator. The extract of the gauze pad sample was reconstituted with 0.4 mL of ethyl acetate and then transferred to an auto-sampler vial for GC-MS analysis. The extract of PUF samples was reconstituted with 0.8 mL of ethyl acetate and toluene (3:1 by volume), and then transferred to a 2-mL dispersive-SPE centrifuge tube (Agilent Technologies, Santa Clara, CA), which contained 150mg of MgSO4, 50 mg of primary and secondary amine exchange materials (PSA), 50 mg of C18, and 7.5 mg of graphite carbon (GCB) for cleanup. The d-SPE tube was vortexed for 1 minute, and then centrifuged at 7500 rpm for 5 minutes. Four hundred-microliter (µL) of supernatant was transferred into an auto-sampler vial for GC-MS analysis.
A total of 10 pyrethroids and 8 organophosphates (OP) were separated and identified using an Agilent 6890 gas chromatograph coupled with an Agilent 5973 mass selective detector (MSD) (Table 1). One microliter of sample was injected in splitless mode. The injection inlet temperature was kept at 270°C. Pesticides were separated through a 30 m × 0.25 mm DB-5ms column. The oven temperature program was started and held at 60°C for two minutes, ramped to 180°C at 25 °C/min and held at 180°C for 4 minutes, then ramped to 250 °C at 10 °C/min and held for 4 minutes, then to 270°C at 3 °C/min and held for 4 minutes, and finally to 300°C at 20 °C/min, plus 3 minutes post-run at 300°C. The carrier gas was helium and the flow rate was constantly at 1.2 mL/min. The MSD was kept at 230 °C for source, 150°C for quad, and 280 °C for Aux-2 temperatures. One quantitation ion and two to three confirmation ions were monitored for each chemical using selected ion mode (SIM). Targeted pesticides were identified based on the quantitation ion, confirmation ions and retention time, while the concentrations were quantified based on the quantitation ion only. Blank quality control (QC) samples were prepared by adding internal standards into brand new gauze pads and PUF, and then followed the extraction steps described above. Blanks contained trace level (<1 ng per analysis) of bifenthrin, allethrin, tefluthrin, and chlorpyrifos, and 19 ng of resmethrin per analysis. The quality assurance (QA) samples were prepared by fortifying blank gauze pad and PUF samples at two levels, 50 and 500 ng/sample, along with the internal standards to determine the overall recoveries of pesticides (Table 2). The final concentrations were calculated using linear regression generated from a 10-point external calibration curve, and were corrected by blank levels and the recoveries generated from matrix-spiked calibration standards.
Table 1.
The quality assurance data for pyrethroids and organophosphate (OP) pesticides that are detected in the floor wipe and indoor-air (PUF) samples.
| Pesticide | PUF | Wipe | |||
|---|---|---|---|---|---|
| Recovery Efficiency (%) | Recovery Efficiency (%) | ||||
| Name | Vapor Pressure (Pa)1 |
50 ng/sample (n=4) |
500 ng/sample (n=4) |
50 ng/sample (n=4) |
500 ng/sample (n=4) |
| Pyrethroids | |||||
| Allethrin | 0.16 × 10−2 | 100 | 103 | 139 | 60 |
| Bifenthrin | 2.4 × 10−5 | 103 | 102 | 129 | 91 |
| Cyfluthrin | 1 –8.5 × 10−8 2 | 94 | 116 | ||
| Cyhalothrin | 1 × 10−6 | 98 | 100 | 112 | |
| Cypermethrin | 1.9 × 10−7 | 117 | 123 | ||
| Fenvalerate | 1.9 × 10−5 | 97 | 124 | ||
| Permethrin-cis | 2.5 × 10−6 | 125 | 114 | 136 | 105 |
| Permethrin-trans | 1.5 × 10−6 | 140 | 117 | 109 | |
| Resmethrin | < 1 × 10−5 | 128 | 107 | 90 | |
| Tefluthrin | 8 × 10−3 | 97 | 97 | 110 | 81 |
| Tetramethrin | 9.4 × 10−4 | 112 | 102 | 91 | |
| OPs | |||||
| Chlorpyrifos | 2.5 × 10−3 | 102 | 101 | 117 | 86 |
| Diazinon | 1.9 × 10−2 | 104 | 97 | 127 | 84 |
| Fenthion | 5.3 × 10−3 | 112 | 106 | 144 | 86 |
| Malathion | 5.3 × 10−3 | 144 | 109 | 107 | |
| Mevinphos | 0.4 | 130 | 107 | 110 | |
| Phosalone | 6.7 × 10 −5 | 133 | 138 | ||
| Pirimphos-ethyl | 3.9 × 10−2 | 100 | 99 | 115 | 83 |
| Pirimphos-methyl | 1.5 × 10−2 | 104 | 100 | 112 | 80 |
Vapor pressures were determined at temperatures ranging from 20 – 30°C (International Programme on Chemical Safety. Available at: http://www.inchem.org/).
Table 2.
The demographics of families living in one of the Boston’s public housing developments in Boston, Massachusetts.
| RACE | Hispanic | NH Black | NH White | Asian | Am. Indian | Other |
|---|---|---|---|---|---|---|
| No. of Families1 | 1,002 (53%) | 784 (42%) | 60 (3%) | 30 (2%) | 2 (0.1%) | 2 (0.1%) |
| AGE | 0–6 | 7–13 | 14–17 | 18–24 | 25–50 | > 50 |
| No. of Families1 | 265 (14%) | 343 (18%) | 175 (9%) | 226 (12%) | 496 (26%) | 375 (20%) |
| HOUSEHOLD INCOME | <$4,999 | <$9,999 | <$14,999 | <$19,999 | <$24,999 | >$25,000 |
| No. of Families1 | 47 (7%) | 219 (30%) | 157 (22%) | 103 (14%) | 66 (9%) | 128 (18%) |
| INCOME SOURCE | Child care | Employment | S.S.2 | TANF3 | Unemployment benefits | Other |
| No. of Families1 | 68 (9%) | 286 (40%) | 486 (68%) | 138 (19%) | 26 (4%) | 59 (8%) |
| LANGUAGE SPOKEN | Spanish | English | Chinese | Other | Unknown | |
| No. of Families1 | 263 (37%) | 258 (36%) | 5 (1%) | 29 (4%) | 165 (23%) |
Percentages, in parentheses, of overall families living in this particular public housing development.
Social Security.
Temporary Assistance for Needy Families program
Results
The sample collection started in late August 2010 and ended in early April 2011. The demographics of all families living in this particular low-income public housing development in Boston are shown in Table 2. A total of 720 families (1,880 household members) lived in this development as of January 1st 2012. The population of adult and children is 1,097 (58.3%) and 783 (41.7%) respectively, with the average household size of 2.6 persons and the average annual household income of $17,823. We enrolled 20 children, 10 males and 10 females, ages 3–11 and their families in this study (Table 3). In total, we collected 20 24-hr indoor air and 60 (3 per household) wipe samples. In addition, Table 3 also shows the results abstracted from the questionnaire in relation to household characteristics and hygienic practices that are relevant to either pest sighting or residential pesticide uses.
Table 3.
The demographics and household characteristics of children and their families participating in the study.
| Demography | No. (%) | |
|---|---|---|
| Ethnicity | Non-Hispanic Black | 7 (35) |
| Hispanic Black | 5 (25) | |
| Non-Hispanic White | 1 (5) | |
| Hispanic White | 7 (35) | |
| Gender | Female | 10 (50) |
| Male | 10 (50) | |
| Age | 3–5 | 6 (30) |
| 6–8 | 9 (45) | |
| 9–11 | 5 (25) | |
| Residence in current home | Less than 5 years | 13 (65) |
| Greater than 5 years | 7 (35) | |
| Pest sightings inside home – self reported | ||
| Any pest in last 3 months | 17 (85) | |
| Any pest in last month | 17 (85) | |
| Any pest in last week | 16 (80) | |
| Pesticide applications – self reported | ||
| On pets (at home) | 5 (25) | |
| Within last 3 months of first visit | 17 (85) | |
| Within last month | 8 (40) | |
| Within last week | 3 (15) | |
| Products used by professional pest control | ||
| Sprays | 5 (25) | |
| Traps or gels | 16 (80) | |
| Other | 2 (10) | |
| Other exposure characteristics | ||
| One or more family members contacting pesticides in workplace | 1 (5) | |
| Child washes hands before eating | No | 1 (5) |
| Yes | 19 (85) | |
| Child puts hands in mouth | No | 6 (30) |
| Yes | 14 (70) | |
| Child uses personal bug repellant | No | 17 (85) |
| Yes | 3 (15) | |
| Home characteristics observed by research staff during the first visit | ||
| Live pests or pest debris | 9 (45) | |
| Level of visible trash/clutter | ||
| Low | 11 (55) | |
| Medium | 5 (25) | |
| High | 4 (20) | |
| Overall condition/maintenance level of residence (housekeeping) | ||
| Good | 10 (50) | |
| Fair | 5 (25) | |
| Poor | 5 (25) | |
Among the pesticides targeted for analysis, 6 pyrethroids and 5 OP pesticides were found with quantifiable levels in the surface wipe samples (Table 4). The most commonly detected pyrethroids are permethrin (both cis- and trans-isomer) with 38% frequency of detection, followed by cypermethrin with 24% frequency of detection. Although cypermethrin was less frequently detected than permethrin, its average concentration in wipe samples was approximately 1.5 times higher than permethrin (3.87 vs. 2.47 µg/m2). Allethrin, fenvalerate, cyhalothrin, and fenpropath were only detected at 2–5% frequency of detection in those wipe samples. The most commonly detected OP was fenthion with 24% frequency of detection, followed by chlorpyrifos with 7% of samples detected. Diazinon, mevinphos, and phosmet were detected at 2–3% frequency of detection. The levels of OP pesticides found in the wipe samples (all below 0.01 µg/m2), however, are significantly lower than those of pyrethroids.
Table 4.
The descriptive statistics of dislodgeable pyrethroids and organophosphate (OP) pesticide residues (µg/m2) on wipe samples (n=60) collected from vinyl floor and kitchen countertop.
| LOD1 | FD2 (%) | Mean (St. Dev.) | p(50) 3 | p(75) 4 | p(95) 5 | Range6 | |
|---|---|---|---|---|---|---|---|
| Pyrethroids | |||||||
| Allethrin | 21.1 | 5 | 0.03 (0.12) | < LOD | < LOD | 0.04 | ND-0.65 |
| Cyhalothrin | 61.3 | 2 | n.a. 7 | < LOD | < LOD | < LOD | ND-2.46 |
| Cypermethrin | 62.4 | 24 | 3.87 (10.4) | < LOD | < LOD | 29.03 | ND-46.3 |
| Fenpropath | 3.4 | 2 | n.a. 7 | < LOD | < LOD | < LOD | ND-0.44 |
| Fenvalerate | 152.2 | 5 | 0.27 (1.24) | < LOD | < LOD | 0.40 | ND-6.89 |
| Permethrin | 8.1/6.9 | 38 | 2.47 (10.5) | < LOD | 0.84 | 5.79 | ND-74.5 |
| OP | |||||||
| Chlorpyrifos | 7.6 | 7 | 0.02 (0.1) | < LOD | < LOD | 0.09 | ND-0.73 |
| Diazinon | 7.0 | 3 | 0.01 (0.03) | < LOD | < LOD | < LOD | ND-0.22 |
| Fenthion | 6.8 | 24 | 0.06 (0.14) | < LOD | < LOD | 0.32 | ND-0.68 |
| Mevinphos | 5.2 | 2 | n.a. 7 | < LOD | < LOD | < LOD | ND-0.67 |
| Phosmet | 22.1 | 2 | n.a. 7 | < LOD | < LOD | < LOD | ND-0.84 |
limit of detection (ng/sample),
frequency of detection,
50th percentile,
75th percentile,
95 percentile,
from non-detected (ND) to the maximum value,
not applicable.
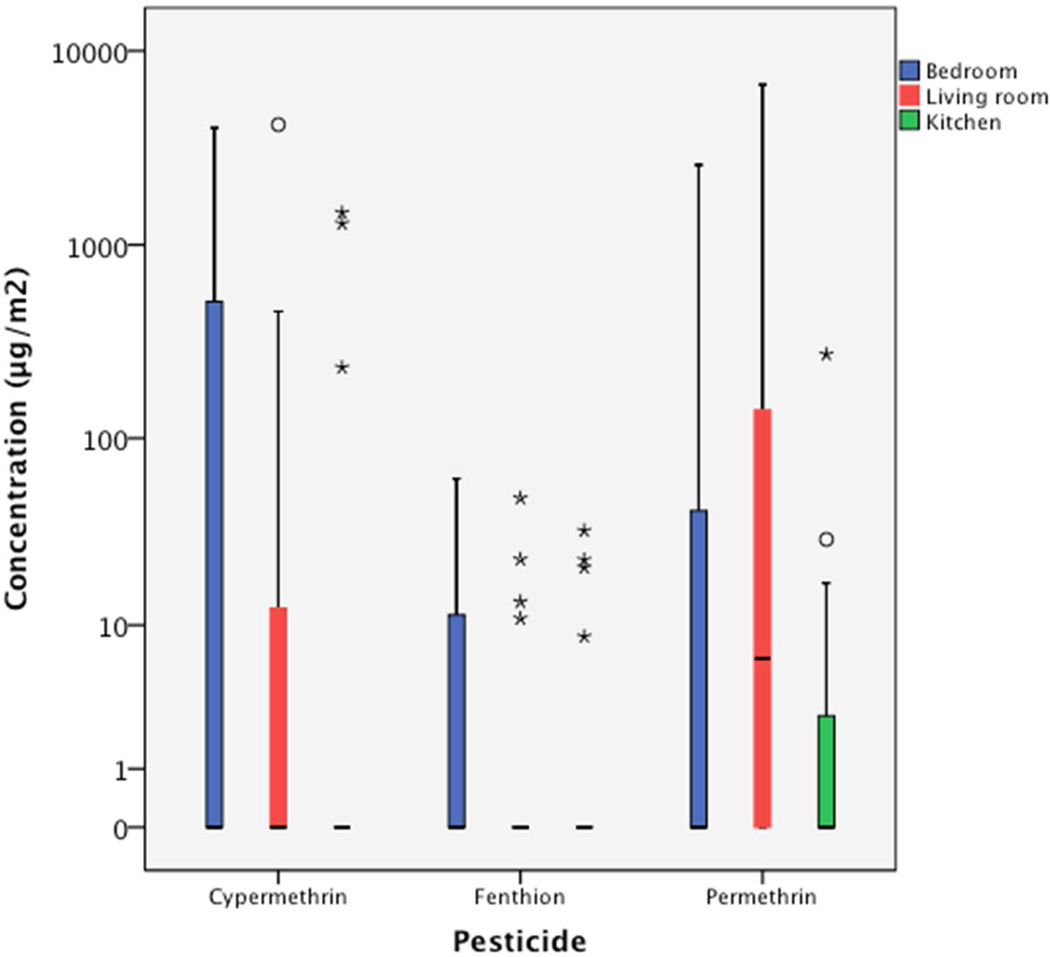
Among the 20 families, surface wipe samples collected from 3 families contained no detectable pyrethroids or OP residues. Samples collected from 3, 9, and 5 families contained 1, 2, and 3 different pyrethroids residues, respectively. We found that the living room was the most contaminated area in the homes with 15 of 20 wipe samples collected from living room floor containing detectable pyrethroids residues, and 3 of these 15 wipe samples containing multiple pyrethroids (Figure 1). The bedrooms where children slept were also commonly contaminated, as 14 of 20 wipe samples contained detectable pyrethroids residues. One wipe sample collected from bedroom floor contained multiple pyrethroids residues. The kitchen area was less contaminated by pyrethroids in which 8 of 20 wipe samples collected from the counter area contained detectable pyrethroids residues, and 2 of those 8 wipe samples contained multiple pyrethroids.
Figure 1.
The comparison of three most commonly detected pesticides, permethrin, cypermethrin and fenthion, in boxplots measured in wipe samples collected from bedroom floor, living room floor, and kitchen counter.
We found 5 pyrethroids and 3 OPs in the 20 indoor air samples that we collected from each household in the living room area over a 24-hour period (Table 5). Tefluthrin was the most commonly detected pyrethroids (40% detection frequency) with the average concentration of 0.06 ng/m3 in the indoor air samples. Cyhalothrin was the other pyrethroids that we detected (10%), however, its average concentration of 0.52 ng/m3 was 9 times higher than tefluthrin. Diazinon was detected in 14 samples (70%) with the average concentration of 0.59 ng/m3, followed by chlorpyrifos at 40% detection with the average concentration of 0.33 ng/m3. Indoor air samples collected from 10 families contained more than 1 pesticide, and among them, one indoor air sample contained 5 pesticides, allethrin, cyhalothrin, tefluthrin, chlorpyrifos, and diazinon at the levels exceeding the respective average concentrations of each pesticide.
Table 5.
The descriptive statistics of indoor air (n=20) pyrethroids and organophosphate (OP) pesticide concentrations (ng/m3) collected from living room area for 24 consecutive hours.
| LOD1 | FD2 (%) | Mean (St. Dev.) | p(50) 3 | p(75) 4 | p(95) 5 | Range6 | |
|---|---|---|---|---|---|---|---|
| Pyrethroids | |||||||
| Allethrin | 21.1 | 5 | n.a. 7 | < LOD | < LOD | < LOD | ND-3.76 |
| Cyhalothrin | 61.3 | 10 | 0.52 (2.1) | < LOD | < LOD | 1.59 | ND-9.18 |
| Cypermethrin | 62.4 | 5 | n.a. 7 | < LOD | < LOD | < LOD | ND-5.5 |
| Permethrin | 8.1/6.9 | 5 | n.a. 7 | < LOD | < LOD | < LOD | ND-3.03 |
| Tefluthrin | 7.1 | 40 | 0.06 (0.1) | < LOD | < LOD | 0.25 | ND-0.33 |
| OP | |||||||
| Chlorpyrifos | 7.6 | 40 | 0.33 (0.7) | < LOD | 0.24 | 1.68 | ND-2.61 |
| Diazinon | 7.0 | 70 | 0.59 (0.8) | 0.14 | 1.06 | 1.98 | ND-2.3 |
| Mevinphos | 5.2 | 5 | n.a. 7 | < LOD | < LOD | < LOD | ND-0.36 |
limit of detection (ng/sample),
frequency of detection,
50th percentile,
75th percentile,
95 percentile,
from non-detected (ND) to the maximum value,
not applicable.
During the initial home visits, we scored three homes that are high on the level of visible trash, household cluttering, and have poor overall housekeeping conditions by visual inspections (Table 3). These three families not only have pesticide residues above the median levels in the areas that we sampled but their households were also contaminated by multiple pesticides. The other two families have either a high level of visible trash or household cluttering, but the degree of household pesticide contamination was less than that of the three families.
Discussion
This study was conducted in response to the concern of many families living in Boston public housing developments in which they ranked the issue of pest infestation, pesticide uses, and pest allergens as their second concern, following by the fear of public safety, or crime. Similar concern of excessive pesticide use and the subsequent exposures have been reported as common risk factors that affect low-income housing residents' health (2). Therefore, we conducted this community-based participatory research (CBPR) study in collaboration among Boston Housing Authority, Committee for Boston Public Housing, and Harvard School of Public Health aiming to characterize the magnitude of residential pesticide exposures among young children living in Boston public housing developments prior to the implementation of a community-wide integrated pest management (IPM) program.
Since the phase out of several OP pesticides for residential uses in early 2000 by US EPA (18), pyrethroids as a group have become the most commonly used pesticides in residential settings, as reported by recent studies (3, 12, 16–18). Among the 63% of child care centers reporting pesticide applications in a recent study, pyrethroids were the most commonly used pesticides, and the frequency of pesticide applications in each center ranged from 1 to 107 times annually (16). In a study conducted by the Columbia Center for Children’s Environmental Health (3), researchers reported that among pesticides in various formulations, pyrethroid insecticides were the most common pesticide class as the spray formulation, and permethrin was the most common pyrethroids used by families living in inner communities in New York City. Regardless, all recent studies including ours continued to measure chlorpyrifos and diazinon (and few other OPs) in the indoor environment (via floor wipes or indoor air). The plausible explanation for this finding is that although OPs are considered non-persistent pesticides, the combined characteristics of semi-volatility of OPs and the repetitive applications over time that allow OP pesticides to accumulate in a reservoir in the indoor environment that later to be gradually released to the confined environment. The presumed lack of significant degradation pathways in the indoor environment also contributes to the persistent problem. The good news is that the levels of OP residues in indoor environment that were measured appear to be decreasing over the years.
The results from this study reinforced the evidence that many families living in public housing developments are constantly in battle with pest problems. More than 80% of families self-reported sighting pests inside their homes on a regular basis, and during the first home visits, we observed either live pests or pest debris in 9 out of 20 family’s homes (Table 3). The frequent sighting of pests would likely lead to additional pesticide applications, either by families themselves or via the contracted professional pest control applicators, and consequently pesticide contamination in their households. Even with repeated pest control applications within the public housing developments, we still observed either live pests or pest debris during our first home visit prior to the beginning of sample collections (Table 3).
The hygienic conditions of the household, as assessed by the maintenance level, visible trash, and the degree of cluttering, are correlated with the degree of household pesticide contamination, as measured in the floor wipes and indoor air samples. During our visual inspection at the initial home visits, we found several families not only have pesticide residues above the median levels in the areas that we sampled but their households were also contaminated by multiple pesticides. The correlation among household hygienic conditions, the sighting of live pests/pest debris, and the degree of indoor pesticide contamination indicated frequent pesticide uses, but also highlighted the failure of conventional chemical-based applications for pest controls. Regardless, none of the 20 families was free from pesticide contamination in their households. We found 17 of 20 families contain dislodgeable pesticide residues on the floor or kitchen counter surfaces that children may often come into contact, and 19 of 20 families contained detectable pesticide levels in the indoor air sample. Although we found that the living room and the child’s bedroom floor were more contaminated (Figure 1), compared to the counter surface in the kitchen, this finding however, is not inconsistent with a previous report in which similar pesticide contamination levels were found in living room and kitchen (12).
Unlike earlier studies in which efforts were made to quantify only OP pesticide residues in the homes of children (4, 5, 7), recent studies that are relevant for comparison to our present study focused on residential exposure to a broad range of pesticides. Quandt et al. (15) assessed farmworkers’ children’s exposures to a variety of pesticides used in agriculture, as well as in homes in North Carolina and Virginia. They reported fifteen different pesticides were measured (with average concentrations) in floor wipe samples including esfenvalerate (30 µg/m2), chlorpyrifos (9 µg/m2), diazinon (14 µg/m2), cis-permethrin (27 µg/m2) and trans-permethrin (38 µg/m2). Among all pesticides detected by Quandt et al. (15), permethrin and chlorpyrifos were the two most commonly detected in floor wipe samples with the frequencies of detection at 93% and 78%, respectively. The prevalence of permethrin and chlorpyrifos found in farmworker’s homes is consistent with studies conducted in urban public low-income housing units. A previous study aimed to investigate the magnitude of pesticide contamination was conducted in Boston public housing dwellings (12). Wipe samples taken from vinyl floor surfaces in both living room and kitchen area showed multiple pyrethroids and OP residues including chlorpyrifos, diazinon, cyfluthrin, cyhalothrin, cypermethrin, deltamethrin, esfenvalerate, permethrin, and tetramethrin. Overall, the median levels for all pesticides detected in the wipe samples ranged from 0.3 to 8.2 µg/m2, and chlorpyrifos and permethrin were again the two most commonly detected pesticides in the wipe samples with the detection frequency of 100% and 93% for both pesticides in kitchen and living room floor, respectively. The levels of pyrethroids and OPs reported by Julien et al. (12) were significantly lower than those by Quandt et al. (15) but consistent with those reported in the current study.
A collaborative study among the U.S. Department of Housing and Urban Development (HUD), the U.S. Consumer Product Safety Commission, and U.S. Environmental Protection Agency (EPA) was conducted to measure indoor pesticide contamination as the result of pest control applications in child care centers across the United States (16). Wipe samples collected from indoor surfaces (floors, tabletops, desks) contained chlorpyrifos (0.004−28 ng/cm2), diazinon (0.002−18 ng/cm2), cis-permethrin (0.004−3 ng/cm2), and trans-permethrin (0.004−7 ng/cm2) with the frequency of detection greater than 67%. Those pesticide levels measured in the wipe samples showed no regional difference in child care centers across the nation. Another similar collaborative study conducted by two federal government agencies, HUD and EPA was designed to measure lead, allergens, and insecticides in randomly selected nationally representative samples of 1,131 residential homes (17). Wipe samples from hard floor surface were collected from a subset of 500 randomly selected homes. The most commonly detected pesticides in those 500 homes were permethrin (89%), chlorpyrifos (78%), chlordane (64%), piperonyl butoxide (52%), cypermethrin (46%), and fipronil (40%). Similar to our study reported here in which the concentrations varied widely among pesticides, and the most commonly detected pyrethroids were trans-permethrin and cypermethrin. However, the reported mean concentrations for trans-permethrin (2.22 ng/cm2) and cypermethrin (2.9 ng/cm2) were significantly higher than the levels that we report here (2.47 and 3.87 µg/m2 for permethrin and cypermethrin, respectively).
The results from the current and previous studies have collectively shown that most non-carpeted floor surfaces in homes have detectable levels of common insecticides that are used mainly for pest control purpose. Those residues would pose significant risks of exposure because of the ease with which they are transferred to skin after contact and their close proximity to the residents. Studies have clearly demonstrated the continuum of available sources of exposures in the indoor environment, uptakes by the individuals, and the subsequent health outcomes (3, 5, 19–25). In addition to the concern for human exposure and health outcomes, these studies have suggested that the chemical-based pest control applications do not appear to be effective in eliminating the pest infestation problems in a sustainable manner. Despite the fact that repeated indoor pesticide applications are often required as reported here and in previous studies (16, 17), the sighting of pests in the dwellings is still common. Traditional pest control in low-income multi-family public housing usually consists of an initial ‘flush out’ (the intensive use of pesticides and other harsh chemicals) followed by periodic sprayings that only eliminates pests for the short term. As a result, it is often the toughest pests that survive, or bear the resistance to pesticides/chemicals. In desperation, residents often take pest control into their own hands and resort to the excessive use of over-the-counter products, as well as the use of restricted and illegal pesticides (e.g. Chinese chalk, a highly concentrated form of pyrethroid insecticide). A vicious cycle of frequent application with excessive amount of pyrethroids would only worsen the resistance issue in many common pests (26, 27). The results from studies conducted in low-income public housing, child care centers, and randomly selected homes in the U.S. should accentuate the need for alternative pest management programs that incorporate safer and more sustainable protocols for pest controls.
The most significant limitation of this study was the involvement of small numbers of families, which is mainly due to the lack of resources to support a large scale of study. In addition, the attrition of study participants during the first 6-month sampling period might affect the outcomes of IPM assessment. We were unable to stay in contact with two families during the 1st month, and one family after the 5th sampling month because they moved out of the public housing development. According to the study protocol, we replaced those lost families with three new families/children matched by gender, age, and race, to those that dropped out from the study. Since we had no prior knowledge of whether these 3 replaced families bear the identical household characteristics (such as indoor pest infestation issue) and hygienic practices (e.g. residential pesticide uses) to the families that we lost contact with, the inclusion of these new families may affect the outcomes of IPM assessment.
In conclusion, the data reported here indicate that children living in low-income public housing may have been exposed to higher pyrethroids in their households than those attending the child care centers. Our findings of the prevalence of OP and pyrethroids uses in these urban low-income public housing units are consistent with other studies (1, 2, 7, 9, 12). The presence of OP pesticides in the households years after the discontinuation of the availability for uses in residential environment highlights the risk of exposure to the occupants stemming from prior frequent indoor applications. This study is part of our ongoing CBPR efforts aiming to mitigate residential pesticide exposures in children living in low-income public housing dwellings by implementing an integrated pest management (IPM) program at the community-level. We will report the effectiveness of the IPM program in reducing residential pesticide exposures in children in future publications.
Acknowledgement
This publication was made possible by Grant Number 5R21ES017948 from the National Institute of Environmental Health Sciences (NIEHS) under its Partnerships for Environmental Public Health (PEHP) program. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIEHS. We thank Dr. Gail Livingston, John Kane, and Lori Luce at the Boston Housing Authority and Mae Fripp, Edna Carrassco, and Charity McNeil at the Committee for Boston Public Housing for their assistance in coordinating this study. We also thank Jose Vallarino at Harvard School of Public Health (HSPH) for making the indoor air sampling device, and Dr. Mei Chen and Erin Collins for their assistance in the Exposure Biology laboratory at HSPH. We thank all children and their families for their participation in this study.
References
- 1.Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, Wetmur JG, Matte TD, Gore AC, Godbold JH, Wolff MS. Pesticides and inner-city children: exposures, risks, and prevention. Environ. Health Perspect. 1999;107(Suppl 3):431–437. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang C, Abou El-Nour MM, Bennett GW. (2008) Survey of pest infestation, asthma, and allergy in low-income housing. J. Community Health. 2008;33(1):31–39. doi: 10.1007/s10900-007-9064-6. [DOI] [PubMed] [Google Scholar]
- 3.Horton MK, Jacobson JB, McKelvey W, Holmes D, Fincher B, Quantano A, Diaz BP, Shabbazz F, Shepard P, Rundle A, Whyatt RM. Characterization of residential pest control products used in inner city communities in New York City. J. Expo. Sci. Environ. Epidemiol. 2011;21(3):291–301. doi: 10.1038/jes.2010.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simcox NJ, Fenske RA, Wolz SA, Lee IC, Kalman DA. Pesticides in household dust and soil: exposure pathways for children of agricultural families. Environ. Health Perspect. 1995;103:1126–1134. doi: 10.1289/ehp.951031126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu C, Fenske RA, Simcox NJ, Kalman D. Pesticide exposures of children in an agricultural community: evidence of household proximity to farmland and take home exposure pathways. Environ. Res. 2000;84:290–302. doi: 10.1006/enrs.2000.4076. [DOI] [PubMed] [Google Scholar]
- 6.Fenske RA, Lu C, Barr D, Needham L. Children’s exposure to chlorpyrifos and parathion in an agricultural community in central Washington State. Environ. Health Perspect. 2002;110(5):549–553. doi: 10.1289/ehp.02110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whyatt RM, Camann DE, Kinney PL, Reyes A, Ramirez J, Dietrich J, Diaz D, Holmes D, Perera FP. Residential pesticide use during pregnancy among a cohort of urban minority women. Environ. Health Perspect. 2002;110(5):507–514. doi: 10.1289/ehp.02110507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bradman A, Whitaker D, Quirós L, Castorina R, Claus Henn B, Nishioka M, Morgan J, Barr DB, Harnly M, Brisbin JA, Sheldon LS, McKone TE, Eskenazi B. Pesticides and their metabolites in the homes and urine of farmworker children living in the Salinas Valley, CA. J. Expo. Sci. Environ. Epidemiol. 2007;17(4):331–349. doi: 10.1038/sj.jes.7500507. [DOI] [PubMed] [Google Scholar]
- 9.Williams MK, Barr DB, Camann DE, Cruz LA, Carlton EJ, Borjas M, Reyes A, Evans D, Kinney PL, Whitehead RD, Perera FP, Matsoanne S, Whyatt RM. An intervention to reduce residential insecticide exposure during pregnancy among an innercity cohort. Environ. Health Perspect. 2006;114(11):1684–1689. doi: 10.1289/ehp.9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner BL, Markowitz S, Rivera M, Romero H, Weeks M, Sanchez E, Deych E, Garg A, Godbold J, Wolff MS, Landrigan PJ, Berkowitz G. Integrated pest management in an urban community: a successful partnership for prevention. Environ. Health Perspect. 2003;111(13):1649–1653. doi: 10.1289/ehp.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Julien R, Levy JI, Adamkiewicz G, Hauser R, Spengler JD, Canales RA, Hynes HP. Pesticide in urban multiunit dwellings: hazard identification using classification and regression tree analysis. J. Air Waste Manag. Assoc. 2008;58(10):1297–1302. [PubMed] [Google Scholar]
- 12.Julien R, Adamkiewicz G, Levy JI, Bennett D, Nishioka M, Spengler JD. Pesticide loadings of select organophosphate and pyrethroid pesticides in urban public housing. J. Expo. Sci. Environ. Epidemiol. 2008;18(2):167–174. doi: 10.1038/sj.jes.7500576. [DOI] [PubMed] [Google Scholar]
- 13.Lu C, Barr DB, Pearson MA, Waller LA. Dietary intake and its contribution to the longitudinal organophosphorus pesticide exposure in urban and suburban children. Environ. Health Perspect. 2008;116(4):537–542. doi: 10.1289/ehp.10912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu C, Barr DB, Pearson MA, Walker LA, Bravo R. The attribution of urban and suburban children’s exposure to synthetic pyrethroid pesticides: a longitudinal assessment. J Exp Sci Environ Epidemiol. 2009;19(1):69–79. doi: 10.1038/jes.2008.49. [DOI] [PubMed] [Google Scholar]
- 15.Quandt SA, Arcury TA, Rao P, Snively BM, Camann DE, Doran AM, Yau AY, Hoppin JA, Jackson DS. Agricultural and residential pesticides in wipe samples from farmworker family residences in North Carolina and Virginia. Environ. Health Perspect. 2004;112(3):382–387. doi: 10.1289/ehp.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tulve NS, Jones PA, Nishioka MG, Fortmann RC, Croghan CW, Zhou JY, Fraser A, Cavel C, Friedman W. Pesticide measurements from the first national environmental health survey of child care centers using a multi-residue GC/MS analysis method. Environ. Sci. Technol. 2006;40:6269–6274. doi: 10.1021/es061021h. [DOI] [PubMed] [Google Scholar]
- 17.Stout DM, Bradham KD, Egeghy PP, Jones PA, Croghan CW, Ashley PA, Pinzer E, Friedman W, Brinkman MC, Nishioka MG, Cox DC. American healthy home survey: A national study of residential pesticides measured from floor wipes. Environ. Sci. Technol. 2009;43(12):4294–4300. doi: 10.1021/es8030243. [DOI] [PubMed] [Google Scholar]
- 18.Williams MK, Rundle A, Holmes D, Reyes M, Hoepner LA, Barr DB, Camann DE, Perera FP, Whyatt RM. Changes in pest infestation levels, self-reported pesticide use, permethrin exposure during pregnancy after the 2000–2001 U.S. Environmental Protection Agency restriction of organophosphates. Environ. Health Perspect. 2008;116(12):1681–1688. doi: 10.1289/ehp.11367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eskenazi B, Bradman A, Castorina R. Exposures of children to organophosphate pesticides and their potential adverse health effects. Environ. Health Perspect. 1999;107(Suppl 3):409–419. doi: 10.1289/ehp.99107s3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu C, Fisker-Anderson J, Knutson DE, Fenske RA. Biological monitoring survey of organophosphorus pesticide exposure among pre-school children in the Seattle metropolitan area. Environ. Health Perspect. 2001;109(3):299–303. doi: 10.1289/ehp.01109299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu C, Kedan G, Fisker-Andersen J, Kissel JC, Fenske RA. Multi-pathway organophosphorus pesticide exposures of pre-school children living in agricultural and non-agricultural communities. Environ. Res. 2004;96(3):283–289. doi: 10.1016/j.envres.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Whyatt RM, Rauh V, Barr DB, Camann DE, Andrews HF, Garfinkel R, Hoepner LA, Diaz D, Dietrich J, Reyes A, Tang D, Kinney PL, Perera FP. Prenatal insecticide exposures and birth weight and length among an urban minority cohort. Environ. Health Perspect. 2004;112(10):1125–1132. doi: 10.1289/ehp.6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, Barr DB, Furlong CE, Holland NT. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26(2):199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Eskenazi B, Huen K, Marks A, Harley KG, Bradman A, Barr DB, Holland N. PON1 and neurodevelopment in children from the CHAMACOS study exposed to organophosphate pesticides in utero. Environ. Health Perspect. 2010;118(12):1775–1781. doi: 10.1289/ehp.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, Whyatt R. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ. Health Perspect. 2011;119(8):1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davies TG, Field LM, Williamson MS. The re-emergence of the bed bug as a nuisance pest: implications of resistance to the pyrethroid insecticides. Med. Vet. Entomol. 2012;26(3):241–254. doi: 10.1111/j.1365-2915.2011.01006.x. [DOI] [PubMed] [Google Scholar]
- 27.Katsuda Y. Progress and future of pyrethroids. Top. Curr. Chem. 2012;314:1–30. doi: 10.1007/128_2011_252. [DOI] [PubMed] [Google Scholar]