Abstract
Traditional transfection agents including cationic lipids and polymers have high efficiency but cause cytotoxicity. While cell penetrating peptide based transfection agents exhibit improved cytotoxicity profiles, they do not have the efficiency of existing lipidic agents due to endosomal trapping. As a consequence, we propose an alternative method to efficient peptide based siRNA transfection by starting with melittin, a known pore-forming peptide. By incorporating modifications to decrease cytotoxicity and improve siRNA binding, we have developed p5RHH, which can complex siRNA to form nanoparticles of 190nm in diameter. p5RHH exhibits high efficiency with GFP knockdown at concentrations as low as 5nM, with negligible cytotoxicity. To date, p5RHH has shown the ability to transfect B16 cells, Human Umbilical Vein Endothelial Cells, and RAW264.7 cells with high efficiency. These in vitro models demonstrate that p5RHH mediated transfection can block cancer cell proliferation, angiogenesis, and foam cell formation. Moreover, p5RHH/siRNA nanoparticles maintain their size and transfection efficiency in the presence of serum proteins suggesting the potential for use of p5RHH in vivo. These data suggest that our strategy for development of siRNA transfecting peptides can provide an avenue to safe and effective siRNA therapeutics.
Keywords: Drug Delivery, Nanoparticle, Gene Therapy, Macrophage, Endothelial Cell
Introduction
RNA interference (RNAi) induced by small interfering RNA (siRNA) has been proposed as a highly effective therapy for myriad diseases including cancer and atherosclerosis.[1, 2] However, despite nearly two decades of intense research since ground breaking work by Tuschl et al. revealed the potential for siRNA in mammalian cells, siRNA therapeutics have demonstrated limited success in translation to clinical applications.[3, 4] The major barriers preventing successful siRNA based therapeutics comprise poor cellular uptake and instability of free siRNA in serum. Its large molecular weight (~14kDa) and high surface charge prevent siRNA from passing through the cellular membrane to reach the cytoplasmic compartment where siRNA is active, thus blocking successful induction of RNAi. These traits, combined with a serum half life of only ~10 minutes, necessitate the packaging of siRNA by transfection agents.[5] Such agents can protect siRNA from serum endonucleases, and promote siRNA uptake through endocytosis. Unfortunately, endocytotic pathways present another barrier, as siRNA must escape the endosomal/lysosomal compartment where it is degraded by an increasingly acidic environment.[5–9]
Despite these challenges, cationic lipids and polymers have been successfully employed for siRNA transfection.[2, 5, 6, 10–12] Unfortunately these types of transfection agents can exhibit unacceptable cytotoxicity.[13–16] The incorporation of cationic lipids into membrane bilayers within the cells promotes siRNA release into the cytoplasm, but also causes generation of reactive oxygen species (ROS) and Ca+2 leakage, a side effect shared by high molecular weight polyetheyleneimine cationic polymers.[15–17] Despite continued development of these siRNA carriers to reduce cytotoxicity, these agents have experienced difficulties when given systemically in vivo due to aggregation with serum proteins and complement activation.[18–20] If the problem of systemic siRNA delivery is to be solved, new classes of siRNA transfection agents need to be developed.
Cell penetrating peptide (CPP) based siRNA transfection agents have shown promise with respect to reducing cytotoxicity.[21–25] Although CPP based siRNA transfection appears nearly free of cytotoxicity, peptide based transfection agents have not achieved the high efficiency of traditional lipidic transfection agents. Some insight has been provided by the studies of Veldhoen et al., which suggest that peptide based transfection is limited by lysosomal trapping.[26] Despite early work showing that CPP mediates siRNA uptake in an energy independent manner[27, 28], it appears that nanoparticles produced by the assembly of CPP and siRNA are endocytosed and must escape the endosomal-lysosomal pathway to gain access to the cytosolic compartment.[21, 22, 29, 30] With this barrier in mind, existing CPP technology has achieved a new level of sophistication through the chemical conjugation of CPPs to membrane active lipids or endosomolytic agents, necessitating further peptide processing and purification.[24, 31–33]
In this work we propose an alternative strategy for efficient peptide based siRNA transfection based on modifications of the cytolytic peptide, melittin, which is the pore forming component of honey bee venom. Melittin’s ability to form pores in membrane bilayers suggests that it can serve as a basis for the development of simple peptides which can improve endosomal escape, thereby setting the stage for efficient siRNA delivery into the cytosolic compartment for improved RNAi. Previous work in our lab has shown that melittin can be modified to attenuate its cytotoxicity while maintaining its propensity for interacting with membrane bilayers.[34, 35] By incorporating these changes along with modifications to enhance peptide/siRNA interactions, we hypothesize that mellitin derived peptides can safely deliver siRNA to the cytoplasmic compartment owing to their inherent membrane active properties.
Materials and Methods
Preparation of peptide/siRNA nanoassemblies and analysis
Melittin derivatives were synthesized by Genscript (Piscataway, NJ), dissolved at 10mM in RNAse/DNAse free water (Sigma, St. Louis, MO) and stored in 4µl aliquots at -80°C before use. p5RHH/siRNA transfection complexes were prepared by diluting p5RHH 1:200 in phosphate buffered saline (PBS, Sigma), vortexed for 30 seconds followed by addition of the appropriate amount of siRNA (stock concentration of 10µM in 1x siRNA buffer (Thermo Scientific, Waltham, MA)) and incubated for 40 minutes at 37°C with shaking in an Eppendorf Thermomixer R. Resulting nanoparticles were analyzed for siRNA incorporation by resolution on a 12% polyacrylamide gel followed by ethidium bromide staining. Dynamic light scattering (DLS) and zeta potential measurements were performed on a Zeta Plus particle sizer (Brookhaven Instruments, Newton, MA). Serum stability analysis was performed by incubating freshly formed peptide/siRNA nanoparticles in 500µg/ml human serum albumin (HSA, Sigma) overnight followed by DLS and zeta potential measurements.
Cell culture
B16F10 and RAW264.7 (ATCC, Manassas, VA) cell lines were maintained under standard cell culture conditions (37°C and 5% CO2 in a humidified incubator) in DMEM (Gibco, Carlsbad, CA) supplemented with 10% fetal bovine serum (Gibco). B16F10 cells stably expressing GFP were produced as follows. B16F10 were transfected (Lipofectamine 2000, Invitrogen) with a fusion of EGFP (pEGFP-N1, Clontech) and the PEST sequence from mouse ornithine decarboxylase (S421-V461) in pEF6V5HisTOPO (Invitrogen). Cells were selected for four rounds with cell sorting by flow cytometry without antibiotic selection. An aliquot of cells was maintained in continuous culture for a month without a noticeable change in EGFP expression level. Human umbilical vein endothelial cells (HUVECs) were purchased from Lifeline Technologies (Frederick, MD) and cultured in VascuLife Basal Medium (Lifeline Technologies) supplemented with 5ng/mL EGF, 5ng/ml bFGF, 15ng/mL IGF-1, 50µg/mL ascorbic acid, 1µg/mL hydrocortisone hemisuccinate, 0.75U/mL Heparin Sulfate, 10mM L-glutamine, 2% fetal bovine serum in accordance with manufacturer instructions. For all experiments, HUVECs were used at passage 3.
siRNA transfection
Cells were plated in 6 well plates 12 hours before transfection and cultured under standard cell culture conditions. p5RHH/siRNA nanoparticles were prepared and incubated with cells for 4 hours in a final volume of 1mL OptimemI (Gibco) or appropriate media supplemented with 10% FBS. Transfections were scaled accordingly for cells plated in 12 well plates based on cell culture surface area. After transfection, cells were washed twice with PBS and incubated with standard cell culture medium for another 24–72 hours before analysis. Lipofectamine 2000 was used in accordance with the manufacturer’s protocol. Briefly, Lipofectamine 2000 was diluted in OptimemI to a final concentration of 8.4ug/ml and incubated at room temperature for 15 minutes. siRNA was then added to the diluted lipid and incubated for another 40 minutes before dilution to 1mL total volume with OptimemI for transfection. eGFP siRNA (Sense: 5’-GACGUAAACGGCCACAAGUUC-3’) was purchased from Sigma. siGENOME mouse MAPK9 siRNA1, siGENOME mouse STAT3 siRNA2, and siGENOME human STAT3 siRNA2 gene specific siRNAs were purchased from Dharmacon (Lafayette, CO). Scrambled siRNA was purchased from Qiagen (Valencia, CA)
Western blotting
24 or 48 hours after transfection, 100–200µl RIPA buffer (10 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1.0% IgepalCA-630, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 1 mM EDTA, 5% glycerol) with 1 mM PMSF and Complete Protease Inhibitor Cocktail (Roche) was added to each well of a 6 well plate and incubated on ice for 1 hour. Cell lysates were then centrifuged at 4°C for 5 minutes and supernatants stored at −20°C. Lysates were resolved on Nupage Bis-Tris gels (Life Technologies) and transferred to 0.22µm nitrocellulose before blocking in 5% bovine serum albumin (Sigma) in TBS-T. Primary antibodies used were: rabbit anti-GAPDH (1:1500, Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-βactin (1:1000, Sigma), mouse anti-STAT3 (1:1000, Cell Signaling, Danvers, MA), rabbit anti-JNK2 (1:1000, Cell Signaling). Secondary antibodies used were: anti-Rabbit HRP (1:5000, Santa Cruz Biotechnology) and anti-mouse HRP (1:5000, Santa Cruz Biotechnology). Blots were developed using ECL Western Blotting Substrate (Pierce, Rockford, IL). Knockdown was quantified using densitometry in ImageJ (NIH, Bethesda, MD) and normalized to untreated controls. All data are presented as an average of 3 separate experiments.
Real Time PCR
24 hours post transfection, cDNA was produced using the FastLane Cell cDNA kit (Qiagen). cDNA was stored at −20°C until use. mRNA levels were quantified using SYBR green detection on an Applied Biosystems 7300 System (Applied Biosystems, Carlsbad, CA) using iTaq SYBR green with ROX (Bio-Rad, Hercules, CA). Quantitect Primer Assay (Qiagen) provided gene specific primers for each gene. Genes of interested were normalized to species appropriate β-actin. Results are reported as the average “fold change” relative to untreated controls for 3 separate experiments.
Confocal microscopy
B16-F10 cells were cultured on glass coverslips and transfected with Cy3-labeled double stranded 21 base pair oligonucleotides (Sigma) according to standard transfection procedures. 12 hours post transfection, cells were washed 3x in PBS and fixed in 4% PFA before mounting on glass slides (Vectashield Mounting Medium with DAPI, Vector Labs, Burlingame, CA). Cells were imaged on a Zeiss Meta 510 (Thornwood, NY).
Flow cytometry
24 hours after B16-GFP cells were transfected with p5RHH/siRNA nanoparticles containing GFP specific or scrambled siRNA, cells were trypsinzed and resuspended in FACS buffer (0.2% FBS and 0.5mM EDTA) for analysis of GFP fluorescence.
Cell viability assays
Cell viability was determined 72 hours post transfection using Alamar Blue (Life technologies). Briefly, Alamar Blue was diluted 1:10 into phenol red free medium and incubated with cells for 2–4 hours. Fluorescence was measured on a fluorescent plate reader with excitation at 570nm and emission at 585nm (Varian Cary Eclipse, Agilent Technologies, Santa Clara, CA).
Tube formation assays
Matrigel (BD Biosciences, San Jose, CA) was thawed overnight at 4°C in an ice bath and subsequently allowed to gel in 24 well plates for 1 hour at 37°C. 24 hours after transfection with STAT3 specific or control siRNA, HUVECs were trypsinized and plated on matrigel at a cell density of 30,000 cells/well. Tube formation was allowed to proceed for 24 hours before visualization on an inverted microscope. A tube formation score was determined based on total tube length per field of view normalized to untreated controls as measured in ImageJ (NIH).
HUVEC migration assays
The bottoms of 12 well transwell inserts with 1.0µm pore size (Corning, Tewksbury, MA) were coated with 0.1% porcine gelatin (Sigma) at room temperature for 1 hour. HUVECs transfected with STAT3 specific or control siRNA 24 hours in advance were then trypsinized and resuspended in growth factor free media and added to the apical transwell chamber at a density of 30,000–50,000 cells/well. The bottom chamber contained growth factor free VascuLife basal media ± 5ng/ml bFGF. Cells were allowed to migrate through the polymer insert for 12 hours. Unmigrated cells were removed from the apical chamber with a sterile cotton swab, and migrated cell numbers were determined via Alamar Blue. Data are presented as the average normalized migration from 3 separate experiments. For visualization, inserts were cut out and mounted on glass slides. Cell nuclei were visualized with DAPI staining on an Olympus BX610 (Tokyo, Japan) and reported as average cell number per field of view.
Foam cell formation assay/Oil Red O Staining
48 hours after transfection with JNK2 specific or control siRNA, RAW264.7 cells were incubated ± 50ug/ml Ac-LDL (Intracel, Frederick, MD) for an additional 24 hours. Cells were then stained with Oil-Red O to visualize foam cell formation. Briefly, Oil-Red O was dissolved in neat methanol (0.5g/100mL) overnight before filtration through a 0.22µm filter. The Oil-Red O stock was then diluted 3:5 in distilled water to make up the Oil-Red O working solution and filtered a second time through a 0.22µm filter. Cells were fixed in 4% PFA for 10 minutes at room temperature and washed with 60% methanol before staining in the Oil-Red O working solution for 15 minutes. After staining, cells were washed once with 60% methanol and once with distilled water before mounting on glass slides.
Results
Screening for siRNA knockdown
Knockdown of B16 cells stably expressing GFP-PEST allowed quick screening for effective siRNA knockdown of GFP expression because the PEST sequence shortens GFP half life from 26 to 10 hours.[36] Melittin derivatives were chosen based on modifications designed to decrease cytotoxicity as well as improve interactions with oligonucleotides. We screened these peptides for their ability to deliver GFP siRNA to initiate GFP knockdown in B16 GFP cells (Supplemental Data Figure 1). p5RHH exhibited especially efficient siRNA transfection and was chosen for further characterization and optimization of formulation.
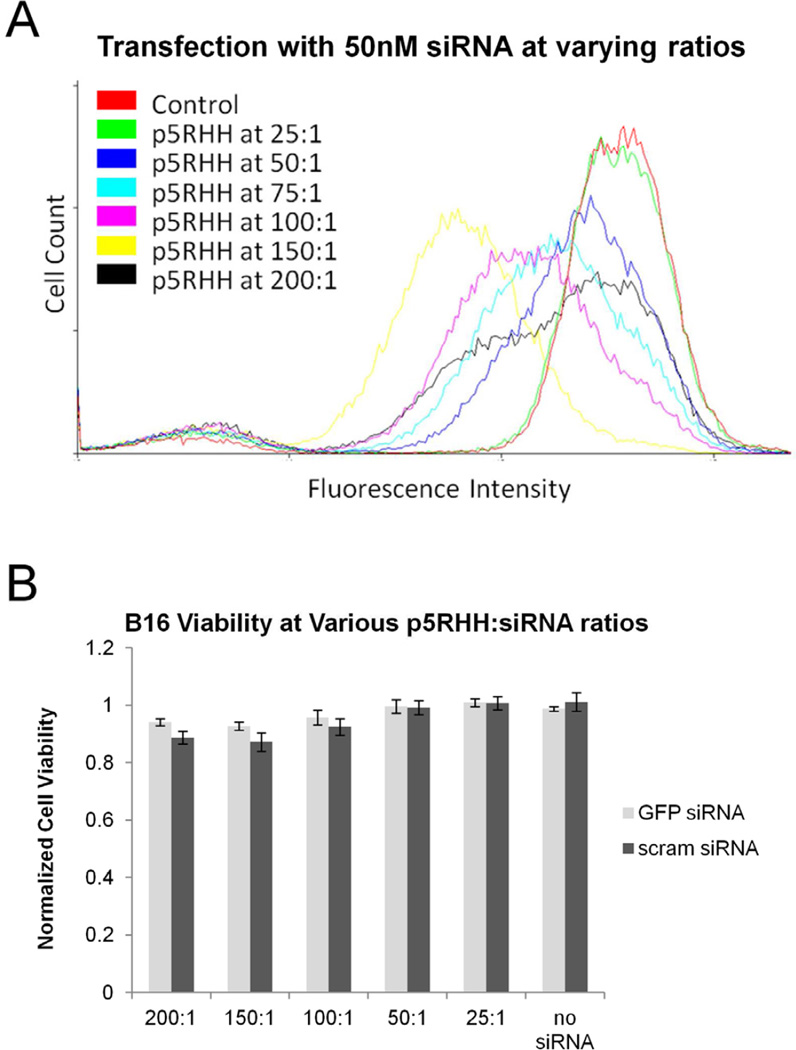
Flow cytometry was performed to determine the optimal p5RHH:siRNA ratio for transfection. Transfection efficiency improved with increasing p5RHH content until maximal GFP knockdown at a p5RHH:siRNA ratio of 150:1 (Figure 1a). In contrast, scrambled siRNA had no effect on GFP expression levels under the same conditions (unpublished observations). Although there was no sign of cytotoxicity associated with the peptide at ratios up to 200:1 (Figure 1b), we sought to minimize exposure to p5RHH and selected a p5RHH:siRNA ratio of 100:1 for the remaining experiments.
Figure 1.
A Optimization of p5RHH/siRNA ratios reveal an increasing transfection efficiency with increasing amounts of p5RHH until a maximum at 150:1 p5RHH:siRNA. B Alamar blue assays indicate no cytotoxicity at p5RHH:siRNA ratios up to 200:1 when transfecting 50nM siRNA.
Nanoparticle formation and characterization
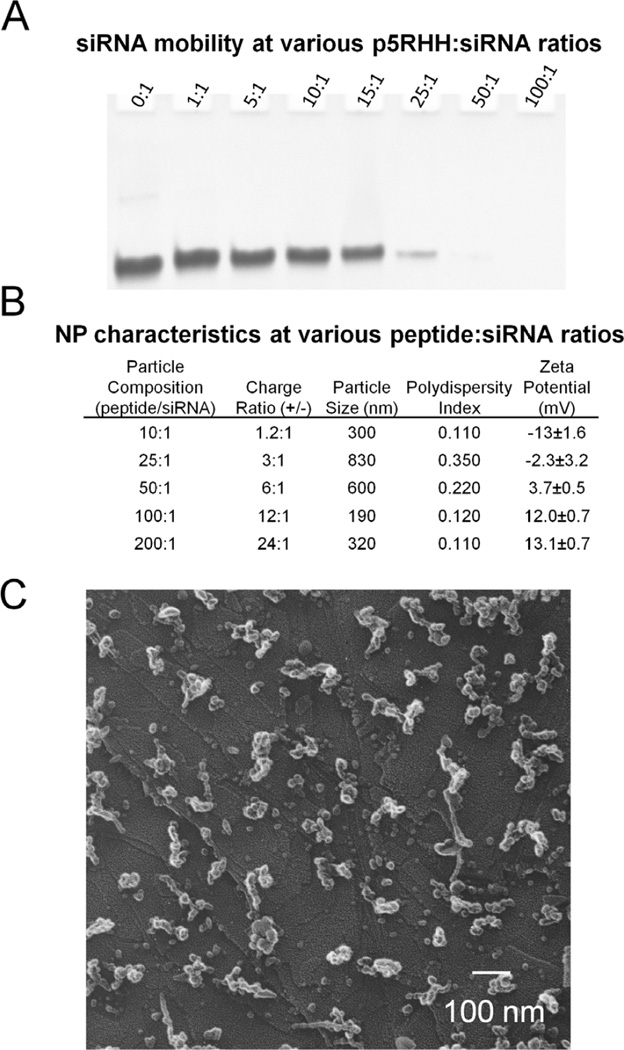
Based on the overall net positive charge of the melittin derivative, p5RHH, we anticipated that it would interact electrostatically with negatively charged siRNA. We monitored these interactions at varying peptide:siRNA ratios using gel retardation assays, in which only free unbound siRNA could migrate into the polyacrylamide gel under the presence of an electric field. In these assays, a set amount of siRNA was mixed with increasing amounts of p5RHH in PBS for 40 minutes before loading on the gel (Figure 2a). It is apparent that a peptide:siRNA ratio of at least 50:1 is required to completely compact the siRNA, which confirmed the lack of siRNA transfection noted by FACS at p5RHH:siRNA ratios below 50:1 (Figure 1a).
Figure 2.
A Gel retardation assays show that a p5RHH:siRNA ratio of 50:1 is required to completely complex siRNA. B Analysis of p5RHH:siRNA nanoparticles by dynamic light scattering and zeta potential analysis suggest that effective surface charge determines particle size. Nanoparticles with a surface charge of larger magnitude exhibit smaller diameters suggesting the importance of electrostatic interactions in stabilizing p5RHH:siRNA nanoparticles. A p5RHH:siRNA ratio of 100:1 generates the smallest particle size of 190nm. C SEM analysis of particle size confirms the dynamic light scattering data revealing small complexes of 100–150nm in diameter. (scale bar 100nm)
Dynamic light scattering and zeta potential measurements (Figure 2b) revealed that particle size is tied closely to the effective surface charge. Particles carrying a surface charge near 0mV exhibit the largest diameter, while particles with a zeta potential of greater magnitude have smaller diameters. The smallest particle size of 190nm was found to be produced with a peptide:siRNA molar ratio of 100:1 or a charge ratio around 12:1 (+/−). It is important to note that increasing p5RHH:siRNA ratio to 200:1 (doubling the +/− ratio to 24:1) does not increase the zeta potential, but does result in an increased particle size. This phenomenon has been previously reported with other peptide transfection agents, although the cause has not yet been established.[37] SEM imaging (Figure 2c) supports the DLS size measurements, showing the presence of distinct nanoparticles with an overall diameter near 150nm. We attribute the smaller particle size visualized by SEM to the drying process required during sample preparation.
Comparison with LF2000
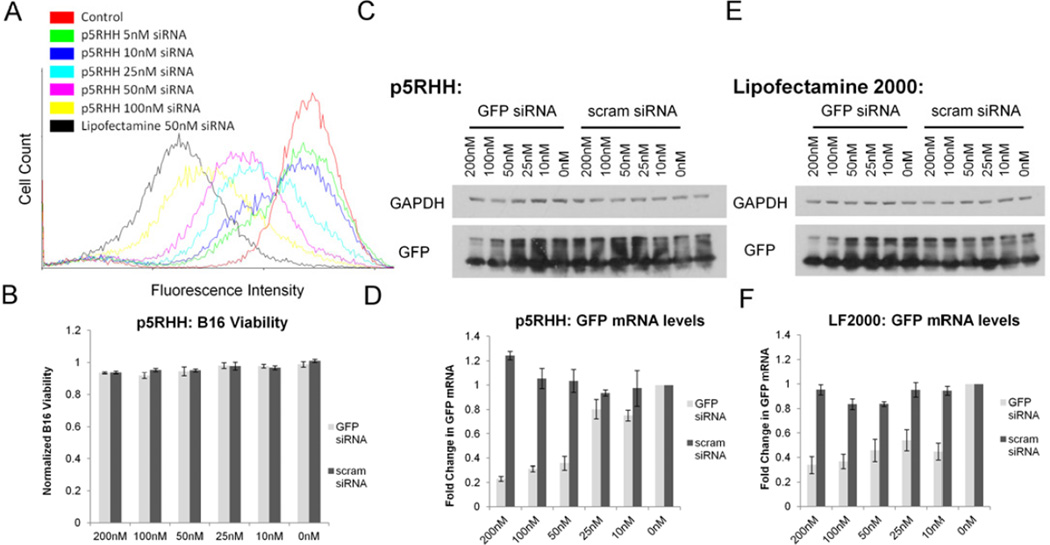
Dose response analysis using flow cytometry reveal that GFP knockdown by GFP siRNA transfected with p5RHH is highly efficient, with an ability to decrease GFP expression in ~15% of cells at concentrations as low as 5nM (Figure 3a). Western blotting (quantified in Supplemental Data Figure 2) reveals that scrambled siRNA had no effect on GFP protein levels when transfected under the same conditions (Figure 3c,d). However, in comparison to Lipofectamine 2000, p5RHH is less efficient at transfecting siRNA, with an IC50 at 50nM based on RT-PCR as compared to the IC50 of Lipofectamine 2000 at 10nM (Figure 3e,f). Additionally, the ability of p5RHH to transfect siRNA at low concentrations as noted by flow cytometry is not apparent via western blotting or RT-PCR, likely due to the low percentage of cells (~15%) showing knockdown by FACS. However, it is readily apparent that p5RHH dramatically improves the cytotoxicity profile over Lipofectamine 2000, exhibiting a minimal (~3%) decrease in cell viability, even at the highest concentrations tested (Figure 3b).
Figure 3.
A Dose response by flow cytometry shows that p5RHH mediated transfection is less efficient than Lipofectamine2000, but also shows high siRNA transfection efficiency with visible knockdown at concentrations as low as 5nM. B Alamar blue assays indicate that p5RHH exhibits minimal toxicity at siRNA concentrations up to 200nM. Western blotting (C) and RT-PCR (D) analysis of GFP mRNA confirms the ability of p5RHH to decrease mRNA levels in a sequence specific manner with an IC50 of ~50nM. Lipofectamine 2000 has a higher transfection efficiency with an IC50 of between 10–25nM as determined by western blotting (E) and RT-PCR (F).
Efficient siRNA release into the cytoplasm
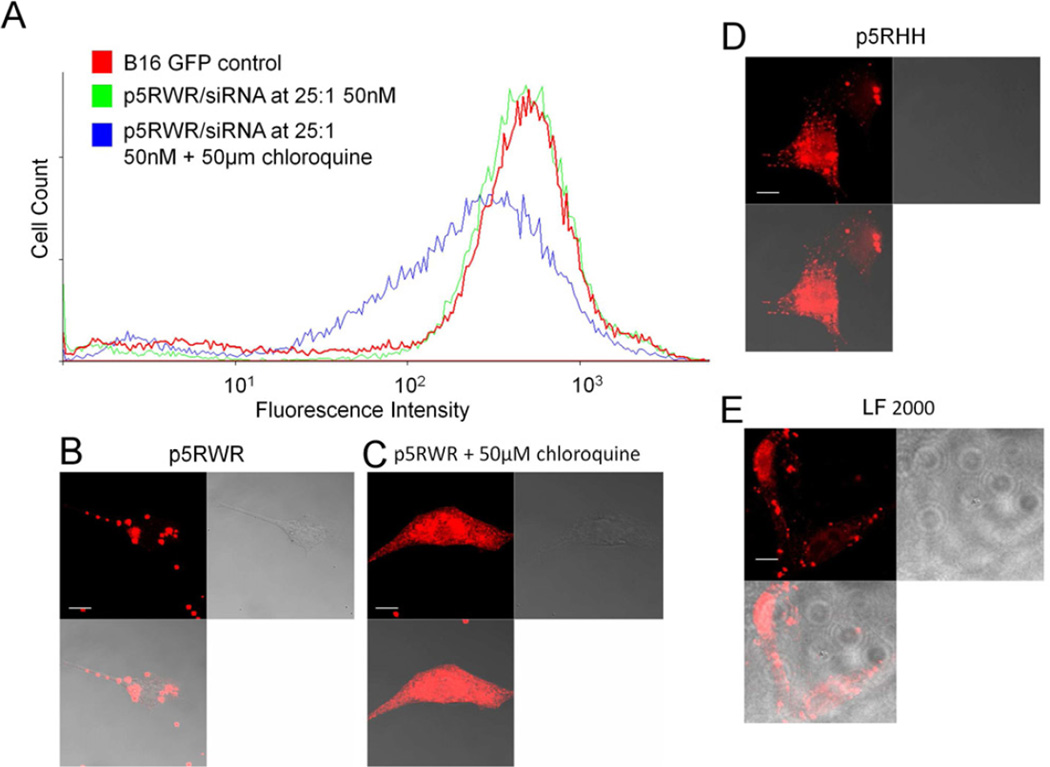
Analysis of a melittin derivative known to be inactive for siRNA transfection (p5RWR:VLTTGLPALISWIKRKRQQRWRRRR, Supplemental Data Figure 1) by confocal microscopy reveals that oligonucleotides packaged with p5RWR do not reach the cytoplasm without co-incubation in the presence of 50µM chloroquine, which is a known endosomolytic agent (Figure 4b,c). FACS analysis for GFP knockdown confirms that siRNA transfected by p5RWR is unable to initiate GFP knockdown without the aid of chloroquine (Figure 4a). These data suggest that p5RWR/siRNA nanoparticles remain trapped in the endosomal compartment and cannot initiate RNAi. In comparison, p5RHH alone is able to deliver oligonucleotides to the cytoplasm when analyzed 24 hours post transfection (Figure 4d), suggesting that p5RHH possesses innate endosomolytic capacity. Images of cells treated with Lipofectamine 2000 are shown for reference (Figure 4e).
Figure 4.
A The nonfunctioning melittin derivative, p5RWR, exhibits no siRNA transfection ability when screened for knockdown of GFP in B16GFP cells via flow cytometry. siRNA is being delivered, but does not reach cytoplasm until incubated with 50µM chloroquine. Confocal microscopy confirms the flow cytometry data, indicating that p5RWR(B) does not manifest appreciable oligonucleotide release into the cytoplasm unless incubated with 50µM chloroquine (C). D In comparison, p5RHH shows efficient oligo release into the cytoplasm similar to oligonucleotide delivery via Lipofectamine 2000.
siRNA delivery to slow cancer growth in vitro
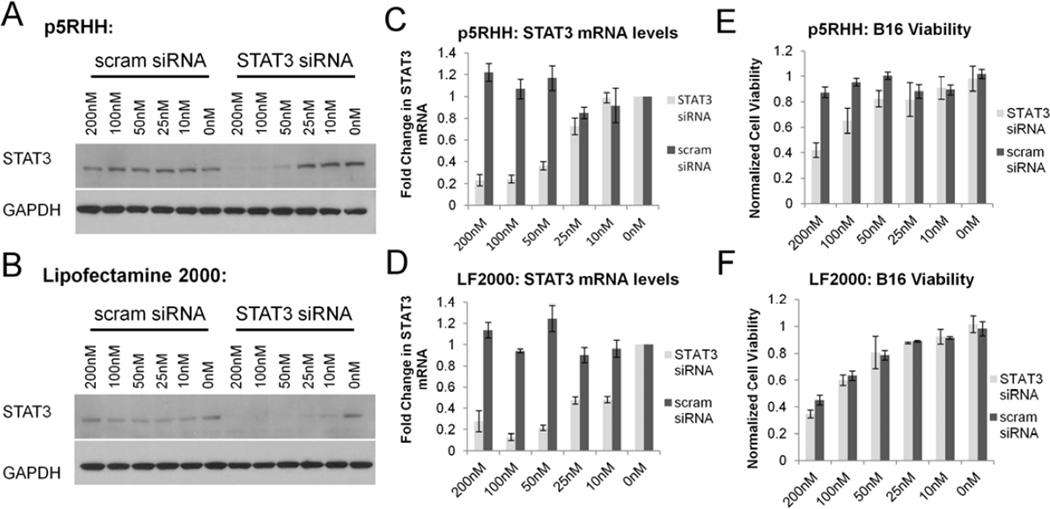
Signal Transducer and Activator of Transcription 3 (STAT3) is a well known oncogene believed to play a critical role in a wide variety of human malignancies.[38, 39] To test the ability of p5RHH to downregulate constitutively activated oncogenes, we targeted STAT3 expression in B16-F10 cells which are known to be STAT3 dependent.[40] Delivery of a STAT3 specific siRNA led to degradation of STAT3 mRNA with a subsequent decrease in STAT3 protein expression (p5RHH IC50: ~50nM, Lipofectamine 2000 IC50: ~10nM) (Figure 5a-d, Supplemental Data Figure 3). P5RHH mediated STAT3 siRNA transfection led to decreased B16-F10 viability (60% at 200nM) 72 hours following transfection as determined by Alamar Blue assays (Figure 5e). Importantly, scrambled siRNA showed no effect on B16 viability illustrating the safety of p5RHH in comparison to Lipofectamine 2000 (Figure 5f), which produced an equivalent decrease in cell viability (up to 60% at 200nM) when delivering either STAT3 specific or scrambled siRNAs, highlighting the need for non-cytotoxic siRNA transfection agents.
Figure 5.
Western blotting data indicate that p5RHH (A) is approximately 5-fold less efficient than Lipofectamine 2000 (B) at initiating a decrease in STAT3 protein levels in B16 cells. RT-PCR data show that p5RHH (C) loses activity at concentrations below 50nM while Lipofectamine 2000 (D) exhibits activity at doses as low as 10nM. B16 viability analysis via Alamar Blue demonstrates that p5RHH (E) transfection leads to a decrease in B16 viability by silencing oncogene expression in a sequence specific manner whereas Lipofectamine2000 (F) causes nonspecific dose dependent cytotoxicity.
siRNA delivery to prevent angiogenesis
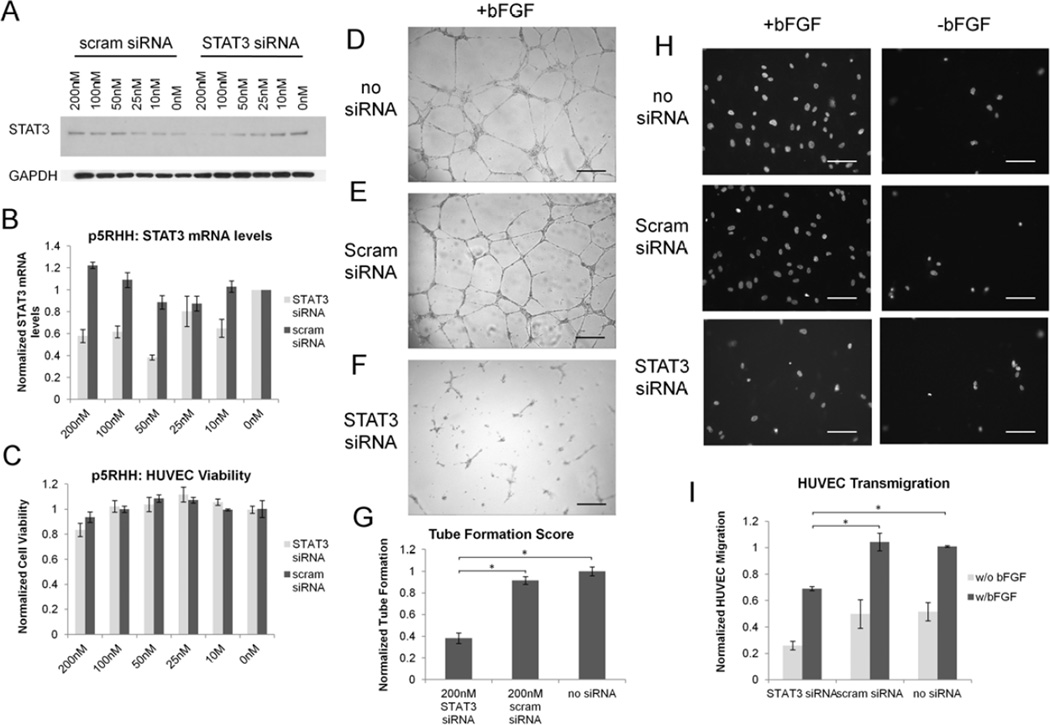
Pathological angiogenesis is a hallmark of many disease states, including cancer, atherosclerosis, and inflammation. STAT3 has previously been shown to be a key mediator in the migration and maturation of endothelial cells during angiogenesis.[41–44] Therefore, we delineated the ability of p5RHH to deliver STAT3 siRNA to HUVEC cells for the blockade of angiogenesis in vitro with the use of matrigel tube formation assays and transwell cell migration assays. HUVECs transfected with p5RHH/STAT3 siRNA nanoparticles exhibited a decrease in STAT3 mRNA and protein levels with an IC50 of ~50nM (Figure 6a,b, Supplemental Data Figure 4) without any accompanying decrease in HUVEC viability (Figure 6c). As with transfection of B16-F10 cells, Lipofectamine 2000 mediated transfection exhibits an IC50 of ~10nM, but strong cytotoxicity, with a 40% decrease in cell viability at siRNA doses as low as 25nM (Supplemental Data Figure 5).
Figure 6.
A Western blotting depicts a dose dependent decrease in STAT3 protein levels in HUVECs treated with STAT3 specific siRNA. B RT-PCR data illustrate a p5RHH-dependent 60% knockdown in STAT3 mRNA at concentrations as high as 200nM. C p5RHH has no cytotoxicity towards HUVEC cells when transfecting siRNA. D HUVECs treated with STAT3 siRNA show a 60% decrease in tube formation on matrigel when compared to controls (E, F) as quantified in G. A decrease in tube formation is accompanied by a 40% decrease in HUVEC migration in response to bFGF in transwell migration assays as determined by microscopy (H) and Alamar Blue assays (I)
Although p5RHH mediated STAT3 siRNA transfection did not impact cell viability, p5RHH/STAT3 siRNA nanoparticles used to treat HUVECs manifested a ~60% decrease in tube formation as compared to scrambled siRNA (Figure 6d-f). In addition, migration of HUVECs transfected by p5RHH was reduced by 50% as quantified by Alamar Blue (Figure 6i) and fluorescence microscopy (Figure 6h, Supplemental Data Figure 6). These data demonstrate the high efficiency with which p5RHH is able to safely transfect primary human endothelial cells for the prevention of pathological angiogenesis.
siRNA delivery to decrease foam cell formation
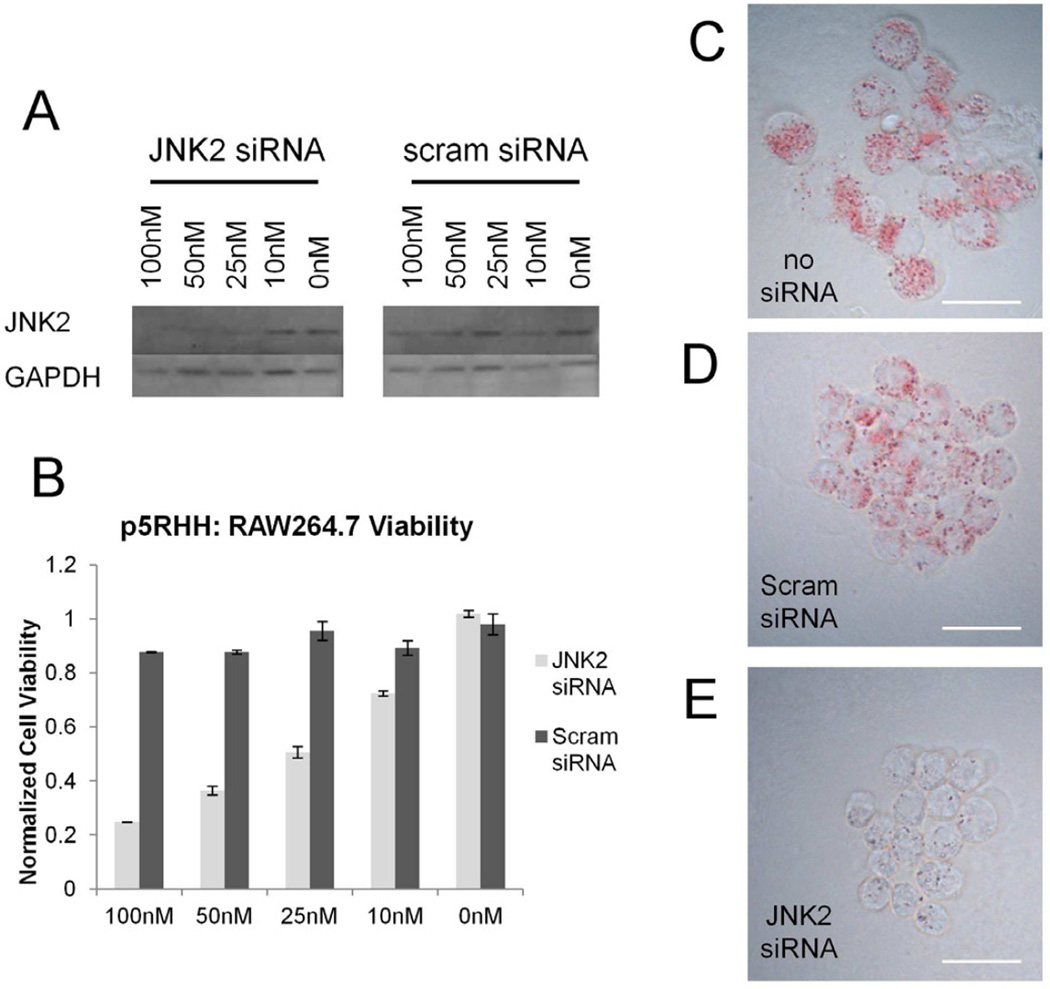
The disrupted endothelial barriers that characterize atherosclerotic plaques make atherosclerosis a prime target for nanoparticle-based therapies.[45] To ensure that we could block foam cell formation, the hallmark of atherosclerotic plaques, with p5RHH/siRNA nanoparticles, we delivered JNK2 siRNA to RAW264.7 (mouse monocyte/macrophage cell line) in vitro. JNK2 is a known mediator of foam cell formation and has been implicated in the uptake of both Ac-LDL by scavenger receptor A as well as oxLDL by CD36.[46, 47] p5RHH was able to deliver JNK2 siRNA to RAW264.6 cells without cytotoxicity (Figure 7a,b), leading to a strong decrease in JNK2 protein levels at concentrations as low as 25nM. Notably, JNK2 siRNA slowed RAW264.7 proliferation rates, but did not induce cell death (unpublished observations). In comparison, Lipofectamine 2000 has a similar IC50 as determined by western blotting, but also exhibits extensive cytotoxicity (Supplemental Data Figure 7). Decreased JNK2 protein levels suppressed foam cell formation in RAW264.7 cells that have been incubated in the presence of 50µg/ml Ac-LDL for 12 hours as determined by light microscopy following Oil-Red O staining (Figure 7c-e). These images show extensive lipid droplet accumulation in non-treated controls and scrambled siRNA treated cells, but no lipid droplet accumulation in RAW264.7 cells treated with a JNK2 specific siRNA.
Figure 7.
A Western blot analysis illustrates knockdown of JNK2 in RAW264.7 cells by p5RHH with IC50 <25nM. B Importantly, p5RHH causes only a ~5% decrease in cell viability when transfecting scrambled siRNA at 100nM. C–E Knockdown of JNK2 at 50nM siRNA shows a strong decrease in lipid droplet accumulation in RAW264.7 cells when incubated with 50µg/mL Ac-LDL overnight when compared to cells treated with Scrambled siRNA and untreated controls.
p5RHH Performance in the presence of serum
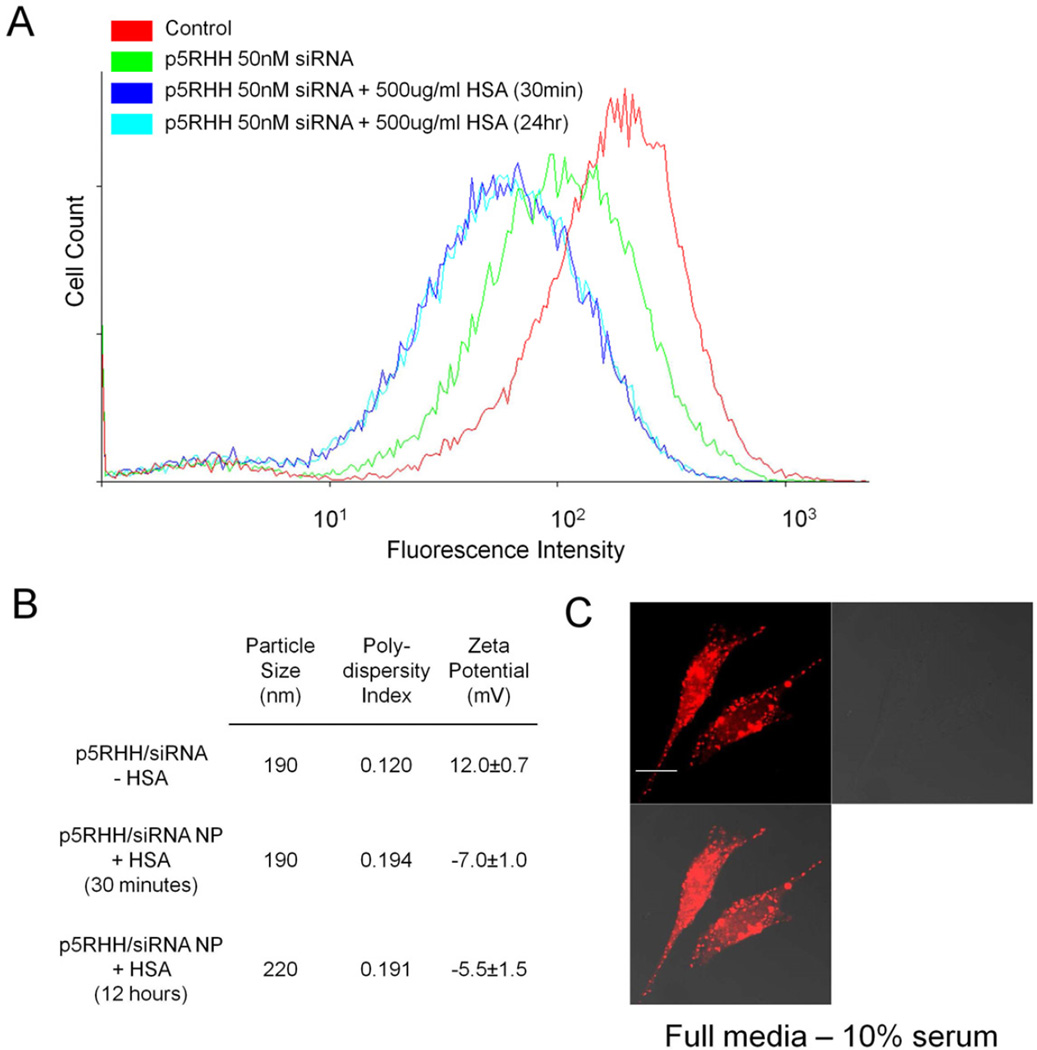
To ensure that our particles are stable in the presence of serum, we incubated p5RHH/siRNA nanoparticles in 500µg/ml human serum albumin (HSA) for 30 minutes or overnight. When we tested the activity of these particles, their ability to knockdown GFP expression was fortuitously enhanced as compared to fresh nanoparticles (Figure 8a). Confocal microscopy confirmed the ability of p5RHH to deliver oligonucleotides to the cytoplasm in the presence of serum proteins (Figure 8c). Dynamic light scattering revealed that the size of nanoparticles incubated with HSA increased marginally to ~220nm when compared with freshly prepared particles, but remained stable for up to 24 hours (Figure 8b). Moreover, the zeta potential of nanoparticles incubated with HSA became negative, which could be due to coating of the nanoparticles with negatively charged albumin (Figure 8b). These experiments demonstrate the serum stability of p5RHH/siRNA nanoparticles. Their maturation to even more potent transfection agents after incubation with serum proteins suggests potential applicability to in vivo settings.
Figure 8.
A Incubation of p5RHH:siRNA nanoparticles with 500µg/mL HSA for 30 minutes or overnight are characterized by improved GFP knockdown when compared to freshly prepared p5RHH:siRNA nanoparticles. B Particle size analysis of p5RHH:siRNA incubated in the presence of serum albumin overnight reveal a stable size. C Confocal microscopy of B16 cells transfected in normal cell culture media supplemented with 10% FBS shows efficient oligonucleotide release into the cytoplasm.
Discussion
Our lab has previously explored highly efficient siRNA delivery methods based on cationic lipids in a perfluorocarbon nanoemulsion formulation.[48] Despite the high efficiency achieved in vitro, difficulties with cytotoxicity at high nanoparticle concentrations reflected the challenges that accompany traditional cationic lipid transfection agents. In the present work, we have modified melittin peptides to realize high efficiency siRNA transfection based on the hypothesis that melittin’s membrane inserting and pore forming capacity would provide a means of endosomal escape, which is a key drawback to previously reported CPP based siRNA transfection agents.[26, 29, 49]
Indeed our experiments confirm that p5RHH is able to facilitate release of siRNA into the cytoplasm. Analysis of an inactive melittin derivative (p5RWR) suggests that, as with traditional CPP based siRNA delivery, p5RWR/siRNA particles are also taken up into endosomes and require endosomolytic agents to gain access to the cytoplasm. p5RHH/siRNA nanoparticles have a positive surface charge as determined by zeta potential measurements, which is a characteristic that has been shown to play an important role in nanoparticle association with the cell membranes, and subsequent endocytosis.[23, 50] Based on similarity of surface charge, it is likely that p5RHH/siRNA nanoparticles are handled via the same endocytotic machinery as inactive p5RWR/siRNA complexes, which suggests that p5RHH can promote the release of siRNA from the endosomal/lysosomal pathway in an efficient manner. Although the exact uptake pathway responsible for p5RHH/siRNA nanoparticle internalization remains to be determined, our analysis of the inactive p5RWR complexes provides insight into the potential mechanisms of p5RHH/siRNA nanoparticle processing by the cell.
While the exact properties responsible for efficient endosomal escape are not yet clarified, work on histidylated peptides used for oligonucleotide transfection provides some intuition regarding the function of p5RHH.[51, 52] Histidylated peptides and polymers were designed originally to aid plasmid release based on protonation of histidine’s imidazole group (pKa ~ 6.0) during lysosomal acidification (pH ~4.5). These polymers incorporate high histidine content (80–90% histidine) to drive endosomal lysis via the proton sponge effect. p5RHH possesses only two histidine residues and thus is unlikely able to buffer enough protons to lyse endosomes. However, protonation of histidine residues likely promotes p5RHH/siRNA nanoparticle disassembly and release of p5RHH to permeabilize the endosomal membrane for siRNA release. Detailed studies of p5RHH/siRNA disassembly and lytic capacity at low pH are currently underway.
The ability of p5RHH to deliver siRNA to the cytoplasm yields a quantifiable decrease in GFP expression at concentrations as low as 5nM. However, p5RHH is still unable to attain the level of transfection efficiency provided by Lipofectamine 2000 in B16-F10 cells or HUVECs. P5RHH seems to exhibit improved efficiency when transfecting RAW264.7 cells, with an IC50 that is approximately equal to that of Lipofectamine 2000. Not surprisingly, different cell types favor different endocytic mechanisms which could explain the differences between transfection efficiencies in different cell types. Nevertheless, p5RHH exhibits a substantial improvement over traditional cationic lipid based transfection in regards to cytotoxicity, exhibiting minimal decrease in cell viability against a variety of mouse and human cell lines at all tested concentrations. Moreover, it appears that the efficiency of p5RHH-mediated transfection can be further optimized as suggested by the observation of increased transfection efficiency when particles are first incubated with serum albumin in assays designed to determine serum stability. Zeta potential measurements suggest that albumin coats the p5RHH/siRNA nanoparticles, but it is unclear how this enhances transfection. Existing studies have shown that albumin can aid fusion of lipid bilayers at low pH and this activity may play a role in endosomal escape.[53, 54] The observed improvement in siRNA transfection efficiency should establish an interesting avenue for improved formulation methods to maximize the efficiency of p5RHH-mediated transfection.
The ability of p5RHH to transfect siRNA into cancer cells, endothelial cells, and even macrophages points to a broad spectrum of transfection activity while maintaining favorable cytotoxicity characteristics. Given the size of our nanoparticles (~190nm), we have targeted disease processes that do not require vascular extravasation through intact endothelial barriers. Cancer, angiogenesis, and atherosclerosis are all characterized by a discontinuous endothelial barrier with enhanced leakage of nanoparticles into the surrounding tissues. In cancer, this effect is widely known as “enhanced permeability and retention”, and similar effects have been previously reported for states of severe atherosclerosis.[45] Moreover, the size of our nanoparticles should provide favorable pharmacokinetics and delivery to intravascular targets by avoiding kidney filtration into the urine.[55] p5RHH/siRNA nanoparticles also exhibit size stability and improved siRNA transfection capacity when incubated in the presence of human serum albumin for 24 hours before transfection, an issue which has been acknowledged to diminish the activity of some CPP transfection agents.[56] Although detailed siRNA protection and long-term stability analysis remains to be performed, these data suggest that p5RHH/siRNA nanoparticles might provide therapeutic benefits when utilized for transfection in vivo.
Conclusions
The melittin derivative p5RHH exhibits the ability to interact with siRNA electrostatically and form nanoparticles which show efficient siRNA delivery into the cytoplasm with subsequent sequence specific degradation of mRNA and decreased protein expression in a variety of cell types. These studies validate our strategy of utilizing efficient pore-forming peptides as a basis for efficient siRNA transfection agents. Furthermore, our experiments did not reveal any signs of cytotoxicity in vitro, despite efficient siRNA transfection in a variety of cell types including primary human endothelial cells. The stability of these particles in the presence of serum proteins suggests that p5RHH/siRNA nanoparticles would be good test candidates for delivering siRNA in vivo to intravascular targets or to diseased tissue characterized by endothelial barrier dysfunction. Moreover, the increased efficiency of p5RHH/siRNA nanoparticles in the presence of serum proteins highlights the potential enhancement of p5RHH transfection capacity which can result from future studies of formulation conditions.
Supplementary Material
Acknowledgements
We thank Dr. Robyn Roth for help obtaining SEM images, Dr. Paul Schlesinger for insightful discussions, and Dr. Kent Boles for help producing the B16F10-GFP cell line used for siRNA screening. Research described here was primarily supported by the Sigma Aldrich Predoctoral Fellowship as well as grants from the National Institutes of Health (U01 CA141541 to Dr. Robert Schreiber and RO1 HL073646-08 to SAW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shen H, Sun T, Ferrari M. Nanovector delivery of siRNA for cancer therapy. Cancer Gene Ther. 2012;19:367–373. doi: 10.1038/cgt.2012.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miele E, Spinelli GP, Miele E, Fibrizio ED, Ferretti E, Tomao S, et al. Nanoparticle-based delivery of small interfering RNA: Challenges for cancer therapy. Int J Nanomedicine. 2012;7:3637–3657. doi: 10.2147/IJN.S23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 4.Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- 5.Wang J, Lu Z, Wientjes JG, Au JLS. Delivery of siRNA therapeutics: Barriers and carriers. AAPS J. 2010;12:492–503. doi: 10.1208/s12248-010-9210-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aigner A. Cellular delivery in vivo of siRNA-based therapeutics. Curr Pharm Des. 2008;14:3603–3619. doi: 10.2174/138161208786898815. [DOI] [PubMed] [Google Scholar]
- 7.Dominska M, Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. J Cell Sci. 2010;123:1183–1189. doi: 10.1242/jcs.066399. [DOI] [PubMed] [Google Scholar]
- 8.Varkouhi AK, Scholte M, Storm G, Haisma HJ. Endosomal escape pathways for delivery of biologicals. J Control Release. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 9.Kwon YJ. Before and after endosomal escape: Roles of stimuli-converting siRNA/polymer interactions in determining gene silencing efficiency. Acc Chem Res. 2012;45:1077–1088. doi: 10.1021/ar200241v. [DOI] [PubMed] [Google Scholar]
- 10.Gilmore IR, Fox SP, Hollins AJ, Akhta S. Delivery strategies for siRNA-mediated gene silencing. Curr Drug Del. 2006;3:147–155. doi: 10.2174/156720106776359159. [DOI] [PubMed] [Google Scholar]
- 11.Nimesh S, Gupta N, Chandra R. Strategies and advances in nanomdicine for targeted siRNA delivery. Nanomedicine. 2011;6:729–746. doi: 10.2217/nnm.11.15. [DOI] [PubMed] [Google Scholar]
- 12.Guzman-Villanueva D, El-Sherbiny IM, Herrera-Ruiz D, Vlassov AV, Smyth HDC. Formulation approaches to short interfering RNA and mircoRNA: Challenges and implications. J Pharm Sci. 2012;101:4046–4066. doi: 10.1002/jps.23300. [DOI] [PubMed] [Google Scholar]
- 13.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Filion MC, Phillips NC. Toxicity and immunomodulatory activity of liposomal vectors formulated with cationic lipids toward immune effector cells. Biochim Biophys Acta. 1997;1329:345–356. doi: 10.1016/s0005-2736(97)00126-0. [DOI] [PubMed] [Google Scholar]
- 15.Soenen SJH, Brisson AR, De Cuyper M. Addressing the problem of cationic lipid-mediated toxicity: The magnetoliposome model. Biomaterials. 2009;30:3691–3701. doi: 10.1016/j.biomaterials.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 16.Park K, Lee M-Y, Kim KS, Hahn SK. Target specific tumor treatment by VEGF siRNA complexed with reducible polyethyleneimine-hyaluronic acid conjugate. Biomaterials. 2012;31:5258–5265. doi: 10.1016/j.biomaterials.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Maruyama K, Iwasaki F, Takizawa T, Yanagie H, Nidome T, Etsuko Y, et al. Novel receptormediated gene delivery system comprising plasmid/protamine/sugar-containing polyanion ternary complex. Biomaterials. 2004;25:3267–3273. doi: 10.1016/j.biomaterials.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder A, Levins CG, Cortez C, Langer R, Anderson DG. Lipid-based nanotherapeutics for siRNA delivery. J Intern Med. 2009;267:9–21. doi: 10.1111/j.1365-2796.2009.02189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen D, Love KT, Chen Y, Eltoukhy AA, Kastrup C, Sahay SK, et al. Rapid discovery of potent siRNA-containing lipid nanoparticles enabled by controlled microfluidic formulation. J Am Chem Soc. 2012;134:6948–6951. doi: 10.1021/ja301621z. [DOI] [PubMed] [Google Scholar]
- 20.Plank C, Mechtler K, Szoka FC, Jr, Wagner E. Activation of the complement system by synthetic DNA complexes: A potential barrier for intravenous gene delivery. Hum Gene Ther. 1996;7:1437–1446. doi: 10.1089/hum.1996.7.12-1437. [DOI] [PubMed] [Google Scholar]
- 21.Abes R, Arzumanov AA, Moulton HM, Abes S, Ivanova GD GDPII, et al. Cell-penetrating-peptidebased delivery of oligonucleotides: An overview. Biochem Soc Trans. 2007;35:775–779. doi: 10.1042/BST0350775. [DOI] [PubMed] [Google Scholar]
- 22.Morris MC, Deshayes S, Heitz F, Divita G. Cell-penetration peptides: From molecular mechanisms to therapeutics. Biol Cell. 2008;100:201–217. doi: 10.1042/BC20070116. [DOI] [PubMed] [Google Scholar]
- 23.Meade BR, Dowdy SF. Enhancing the cellular uptake of siRNA duplexes following noncovalent packaging with protein transduction domain peptides. Adv Drug Del Rev. 2008;60:530–536. doi: 10.1016/j.addr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Endoh T, Ohtsuki T. Cellular siRNA delivery using cell-penetrating peptides modified for endosomal escape. Adv Drug Del Rev. 2009;61:704–709. doi: 10.1016/j.addr.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Laufer SD, Detzer A, Sczakiel G, Restle T. Selected strategies for the delivery of siRNA in vitro and in vivo. In: Erdmann VA, Barciszewski J, editors. RNA technologies and their applications. Berlin: Springer-Verlag; 2010. pp. 29–58. [Google Scholar]
- 26.Veldhoen S, Laufer SD, Trampe A, Restle T. Cellular delivery of small interfering RNA by a noncovalently attached cell-penetrating peptide: Quantitative analysis of uptake and biological effect. Nucleic Acids Res. 2006;34:6561–6573. doi: 10.1093/nar/gkl941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crombez L, Morris MC, Deshayes S, Heitz F, Divita G. Peptide-based nanoparticle for ex vivo and in vivo drug delivery. Curr Pharm Des. 2008;14:3656–3665. doi: 10.2174/138161208786898842. [DOI] [PubMed] [Google Scholar]
- 28.Deshayes S, Gerbal-Chaloin S, Morris MC, Aldrian-Herrada G, Charnet P, Divita G, et al. On the mechanism of non-endosomal peptide-mediated cellular delivery of nucleic acids. Biochim Biophys Acta. 2004;1667:141–147. doi: 10.1016/j.bbamem.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 29.Kim SW, Kim NY, Choi YB, Park SH, Yang JM, Shin S. RNA interference in vitro and in vivo using an arginine peptide/siRNA complex system. J Control Release. 2010;143:335–343. doi: 10.1016/j.jconrel.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Lee SH, Choe J, Park TG. Intracellular small interfering RNA delivery using genetically engineered double-stranded RNA binding protein domain. J Gene Med. 2009;11:804–812. doi: 10.1002/jgm.1365. [DOI] [PubMed] [Google Scholar]
- 31.Ezzat K, Andaloussi SEL, Zaghloul EM, Lehto T, Lindberg S, Moreno PMD, et al. Pepfect 14, a novel cell-penetrating peptide for oligonucleotide delivery in solution and as solid formulation. Nucleic Acids Res. 2011;39:5284–5298. doi: 10.1093/nar/gkr072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arthanari Y, Pluen A, Rajendran R, Aojla H, Demonarcos C. Delivery of therapeutic shRNA and siRNA by tat fusion peptide targeting bcr-abl fusion gene in chronic myeloid leukemia cells. J Control Release. 2010;145:272–280. doi: 10.1016/j.jconrel.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Mae M, Andaloussi SE, Lehto T, Langel U. Chemcially modified cell-penetrating peptides for the delivery of nucleic acids. Expert Opin Drug Deliv. 2009;6:1195–1205. doi: 10.1517/17425240903213688. [DOI] [PubMed] [Google Scholar]
- 34.Pan H, Ivashyna O, Sinha B, Lanza GM, Ratner L, Schlesinger PH, et al. Post-formulation peptide drug loading of nanostructures for metered control of NF-kB signaling. Biomaterials. 2011;32:231–238. doi: 10.1016/j.biomaterials.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan H, Marsh JN, Christenson ET, Soman NR, Ivashyna O, Lanza GM, et al. Chapter two - postformulation peptide drug loading of nanostructures. Methods Enzymol. 2012;508:17–39. doi: 10.1016/B978-0-12-391860-4.00002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corish P, Tyler-Smith C. Attenuation of green fluorescent protein half-life in mammalian cells. Protein Eng Des Sel. 1999;12:1035–1040. doi: 10.1093/protein/12.12.1035. [DOI] [PubMed] [Google Scholar]
- 37.Heitz F, Morris MC, Divita G. Twenty years of cell-penetrating peptides: From molecular mechanisms to therapeutics. Br J Pharmacol. 2009;157:195–206. doi: 10.1111/j.1476-5381.2009.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mankan AK, Greten FR. Inhibiting signal transducer and activator of transcription 3: Rationality and rationale design of inhibitors. Expert Opin Invest Drugs. 2011;20:1263–1275. doi: 10.1517/13543784.2011.601739. [DOI] [PubMed] [Google Scholar]
- 39.Zhao M, Jiang B, Gao F-H. Small molecule inhibitors of STAT3 for cancer therapy. Curr Med Chem. 2011;18:4012–4018. doi: 10.2174/092986711796957284. [DOI] [PubMed] [Google Scholar]
- 40.Yang CH, Fan M, Slominski AT, Yue J, Pfeffer LM. The role of consitutively activated STAT3 in B16 melanoma cells. Int J Infereron Cytokine Mediator Res. 2010;2:1–7. doi: 10.2147/IJICMR.S6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartoli M, Platt D, Lemtalsi T, Gu X, Brooks SE, Marrero MD, et al. VEGF differentially activates STAT3 in microvascular endothelial cells. FASEB J. 2003;17:1562–1564. doi: 10.1096/fj.02-1084fje. [DOI] [PubMed] [Google Scholar]
- 42.Yahata Y, Shirakata Y, Tokumaru S, Yamasaki K, Sayama K, Hanakawa Y, et al. Nuclear translocation of phosphorylated STAT3 is essential for vascular endothelial growth factor-induced human dermal microvascular enothelial migration and tube formation. J Biol Chem. 2003;278:40026–40031. doi: 10.1074/jbc.M301866200. [DOI] [PubMed] [Google Scholar]
- 43.Deo DD, Axelrad W, Robert EG, Marcheselli V, Bazan NG, D HJ. Phosphorylation of STAT-3 in response to basic fibroblast growth factor occurs through a mechanism involving platelet-activation factor, JAK-2, and src in human umbilical vein endothelial cells. J Biol Chem. 2002;277:21237–21245. doi: 10.1074/jbc.M110955200. [DOI] [PubMed] [Google Scholar]
- 44.Bid HK, Oswald D, Li C, London CA, Lin J, Houghton PJ. Anti-angiogenic activity of a small molecule STAT3 inhibitor LLL12. PLoS ONE. 2012;7:e35513. doi: 10.1371/journal.pone.0035513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Zhang L, Myerson J, Bibee K, Scott M, Allen J, et al. Quantifying the evolution of vascular barrier disruption in advanced atherosclerosis with semipermeant nanoparticle contrast agents. PLoS ONE. 2011;6:e26385. doi: 10.1371/journal.pone.0026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ricci R, Sumara G, Sumara I, Rozenberg I, Kurrer M, Akhmedov A, et al. Requirement of JNK2 for scavenger receptor A-mediated foam cell formation. Science. 2004;306:1558–1561. doi: 10.1126/science.1101909. [DOI] [PubMed] [Google Scholar]
- 47.Rahaman SO, Lennon DJ, Febbraio M, Podrez EA, Hazen SL, Silverstein RL. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metabolism. 2006;4:211–221. doi: 10.1016/j.cmet.2006.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaneda MM, Sasaki Y, Lanza GM, Milbrandt J, Wickline SA. Mechanisms of nucleotide trafficking during siRNA delivery to endothelial cells using perfluorocarbon nanoemulsions. Biomaterials. 2010;31:3079–3086. doi: 10.1016/j.biomaterials.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lundberg P, Andaloussi SE, Sutlu THJ, Langel U. Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. FASEB J. 2007;21:2664–2671. doi: 10.1096/fj.06-6502com. [DOI] [PubMed] [Google Scholar]
- 50.Li Y, Wang J, Gao Y, Zhu J, Wientjes JG, Au JLS. Relationships between liposome properties, cell membrane binding, intracellular processing, and intracellular bioavailability. AAPS J. 2011;13:585–597. doi: 10.1208/s12248-011-9298-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chou S-T, Leng Q, Scaria P, Woodle M, Mixson AJ. Selective modification of HK peptides enhances siRNA silencing of tumor targets in vivo. Cancer Gene Ther. 2011;18:707–716. doi: 10.1038/cgt.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pichon C, Roufai MB, Monsigny m, Midoux P. Histidylated oligolysines increase the transmembrane passage and the biological activity of antisense oligonucleotides. Nucleic Acids Res. 2000;28:504–512. doi: 10.1093/nar/28.2.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schenkman S, Araujo PS, Dijkman R, Quina FH, Chaimovich H. Effects of temperature and lipid composition on the serum albumin-induced aggregation and fusion of small unilamellar vesicles. Biochim Biophys Acta. 1981;649:633–641. doi: 10.1016/0005-2736(81)90168-1. [DOI] [PubMed] [Google Scholar]
- 54.Simoes S, Slepushkin V, Pires P, Gaspar R, Pedroso MC, Duzgunes N. Human serum albumin enhances DNA transfection by lipoplexes and confers resistance to inhibition by serum. Biochim Biophys Acta. 2000;1463:459–469. doi: 10.1016/s0005-2736(99)00238-2. [DOI] [PubMed] [Google Scholar]
- 55.Kaneda MM, Caruthers S, Lanza GM, Wickline SA. Perfluorocarbon nanoemulsions for quantitative molecular imaging and targeted therapeutics. Ann Biomed Eng. 2009;37:1922–1933. doi: 10.1007/s10439-009-9643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crombez L, Divita G. A non-covalent peptide-based strategy for siRNA delivery. In: Langel U, editor. Cell-penetrating peptides: Methods and protocols. New York, NY: Humana Press; 2011. pp. 349–360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.