Abstract
Polysaccharides have been used in various biomedical applications due to availability and biocompatibility. In particular, polysaccharides have gained increasing interest in the development of functional nanomedicines as a component to provide a stealth function, improve interactions with target tissues or enable environment-responsive drug release. This review discusses recent advances in nanomedicine engineering based on polysaccharides with a specific emphasis on the rationale, applications and the remaining challenges.
In the treatment and diagnosis of cancer, nanoparticles (NPs) are considered promising carriers of drugs and imaging agents due to their ability to accumulate in solid tumors. A well-known explanation for this property is the so-called enhanced permeability and retention (EPR) effect [1], based on defective tumor vasculature and poor lymphatic drainage [2], which allows for the extravasation of NPs [3] and their accumulation in tumors [1]. For the efficient use of the EPR effect, NPs should be able to circulate in the body, avoiding clearance by the reticuloendothelial system [4]. In general, NP clearance from the circulation starts with the opsonization of NPs, followed by receptor-mediated phagocytosis [5]. Since hydrophobic and/or charged NPs are prone to opsonization, NPs are usually coated with electrically neutral hydrophilic polymers. This protective surface coating, also known as stealth coating, can extend the half-life of NPs significantly [6].
One of the most popular surface modification strategies is to conjugate PEG, a nonionic hydrophilic polymer, on the surface of NPs (PEGylation). Since the early use of PEG in extending the circulation half-life of a protein [7,8], PEGylation has been widely used to protect NPs such as liposomes [9], polymeric NPs [10] and micelles [11] from premature clearance during circulation. Here, the PEG that covers NPs forms a hydrated layer, which allows the NPs to evade opsonization and subsequently phagocytosis [12]. On the other hand, this protective surface also interferes with the desired interactions between NPs and targeted cells [13]. For example, PEGylated liposomal doxorubicin showed greater AUCplasma but lower tumor accumulation than those of the non-PEGylated counterpart, indicating the paradoxical effect of PEGylation [14]. A recent review article described that the PEGylated multifunctional envelope-type nano device was less effective in delivering genes into cells than the non-PEGylated device [15]. Furthermore, Lehtinen et al. demonstrated, using a computational model, that some targeting moieties could lose the functionality due to the steric hindrance effect of the PEG layer [16]. The increased stability by PEGylation can also hinder the endosomal escape of NPs, a critical step for effective intracellular delivery of nucleic acid therapeutics [17,18]. These challenges have prompted a search for new strategies to protect NP surface. Examples of recent efforts include the use of different polymers of synthetic or natural origin, biomimetic coating and conditional removal of PEG effect [19].
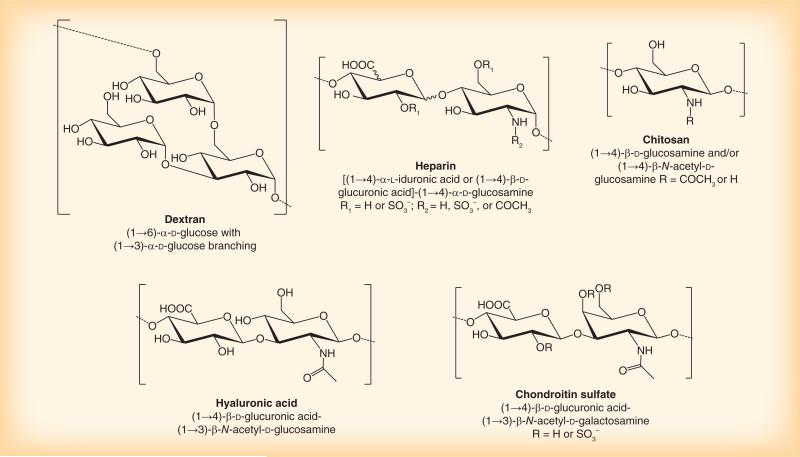
The discussion here focuses on the use of polysaccharides in nanomedicine. Polysaccharides have been widely used in the context of drug delivery and tissue engineering because of biocompatibility, availability and well-established modification schemes [20,21]. Some of the polysaccharides, such as dextran and heparin (Figure 1), have long been recognized as stealth-coating materials due to their ability to inhibit opsonization and complement activation [22,23]. Moreover, several studies demonstrate the ligand activities of polysaccharides such as chitosan, hyaluronic acid and chondroitin sulfate (Figure 1). NPs decorated with these polysaccharides showed more efficient cellular uptake than uncoated NPs, due to their specific interactions with various receptors on target cells [24–27]. For these reasons, polysaccharides have gained increasing interest in the field of nanomedicine as an alternative surface modification strategy. Examples of their applications in various types of nanomedicine follow.
Figure 1.
Polysaccharides frequently used for surface modifications of nanomedicines.
Production of polysaccharide-based NPs
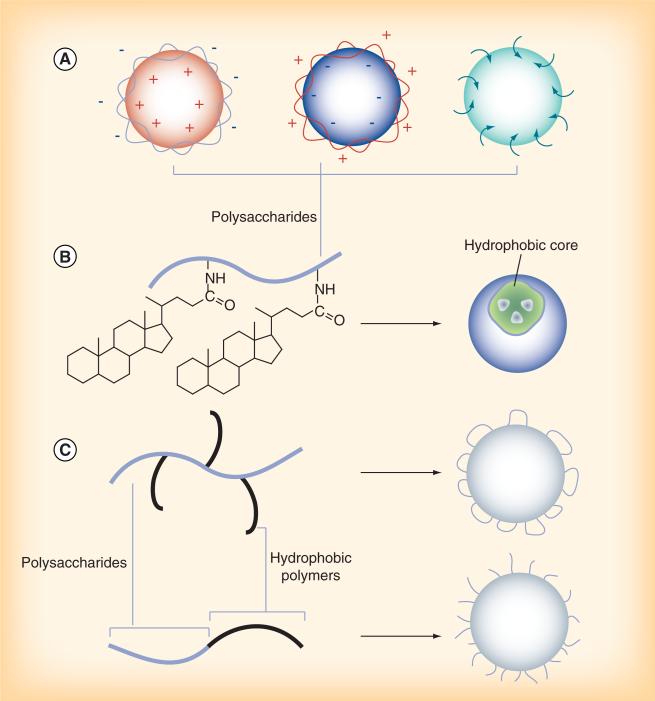
NPs incorporating polysaccharides are produced in various methods, as thoroughly reviewed elsewhere [28,29]. Polysaccharides can form surface layers on core NPs via electrostatic interactions or by anchoring on the NP surface during the formation of NPs (Figure 2a). Alternatively, hydrophilic polysaccharides are grafted with hydrophobic low-molecular-weight molecules such as cholesterol or bile acids to allow for the formation of self-assembled NPs, which carry hydrophobic drugs in the core (Figure 2B). NPs can be made of conjugates of polysaccharides and synthetic polymers, created by various conjugation methods. For example, linear hydrophobic polymers are grafted to a polysaccharide as side chains, creating a branched copolymer. Alternatively, a linear hydrophobic polymer can be conjugated to a polysaccharide terminus to create a linear diblock copolymer. Depending on the configuration of the copolymer, polysaccharides form a loop- (Figure 2c; top) or a brush-style (Figure 2c; bottom) surface layer.
Figure 2. Various types of polysaccharide-based nanoparticles.
(A) Polysaccharide-forming layers on the nanoparticle surfaces via electrostatic interactions or physical adherence. (B) Self-assembly of a hydrophilic polysaccharide with hydrophobic grafts. (C) Nanoparticles made of conjugates of polysaccharides and synthetic polymers.
Dextran
Dextran is a water-soluble polymer of (1→6)-α-d-glucose, with branches extending from the (1→3) position and occasionally from (1→4) or (1→2) positions [30]. Dextran is synthesized by bacteria, and the types and molecular weights of dextran vary with bacterial strains and culture conditions [30]. Dextran was first investigated as a plasma substitute in the early 1940s [31]. Due to the biocompatibility and degradability, dextran has long been a biomaterial of choice for various biomedical applications. Dextran has antifouling properties comparable to that of PEG [32] and, thus, been used to shield the surface of polymeric [33,34] or iron oxide NPs [28,35,36]. Dextran coating was shown to extend the circulation time of poly(methyl methacrylate) NPs [22,23]. However, another study reported that dextran-coated poly(caprolactone) NPs could induce complement activation [37]. The seemingly contrasting results are attributed to the difference of dextran conformation and density on the NP surface [38,39]. A dense brush-like dextran graft provides a steric barrier resistant to protein adsorption [23], whereas flexible dextran loops allow for binding of opsonic components [37]. The effect of dextran conformation on pharmacokinetics and biodistribution of poly(alkylacyanoacrylate) (PACA) NPs has been recently studied [40]. Here, PACA NPs with a dense dextran brush layer avoided liver uptake, whereas PACA NPs covered with dextran loops were rapidly accumulated in the liver [40]. It is noteworthy that the dextran-coated stealth PACA NPs accumulated in well-vascularized organs such as the heart and lungs in less than 48 h, a time typically needed for organ distribution of PEGylated NPs [40].
Heparin
Heparin is a linear polysaccharide with a repeating disaccharide unit of 1,4-linked uronic acid [d-glucuronicor l-iduronic acid] and d-glucosamine residues [41]. Heparin is one of the most negatively charged natural products [42] and implicated in various biological functions such as allergy, inflammation [43] and anticoagulation [42]. Its anticoagulant effect is initiated by the binding of heparin to antithrombin III and conformational change of the protein [42]. The activated antithrombin III functions as an inhibitor of thrombin and other serine proteases in the coagulation cascade [42]. Heparin is clinically used for extracorporeal procedures requiring anticoagulation and the treatment of thrombotic conditions [42]. In addition, several studies report antiangiogenic effects of heparin and its derivatives. For example, heparin and its fragments administered along with steroids show antiangiogenic effects [44,45]. 6-O-desulfated heparin was found to interfere with binding of FGF-2 to the receptor and, thus, inhibit FGF-2 induced angiogenesis [46]. Heparin and its modified version (periodate-oxidized and borohydride-reduced heparin) showed the potential to reduce lung metastasis by competitive inhibition of the cancer cell attachment to subendothelial matrix of lung capillaries [47]. To suppress tumor growth by site-specific inhibition of angiogenesis, heparin was conjugated with targeting ligands such as folate [48] and cyclic RGD peptide [49].
While the biological functions of heparin warrant the exploration as an active ingredient, the use of heparin as a component of NPs has been relatively limited due to the potential risk of hemorrhage. Nevertheless, heparin has been used with caution for surface-coating of NPs due to the ability to inhibit complement activation [22]. For example, heparin-coated poly(methyl methacrylate) NPs evaded reticuloendothelial system uptake and showed a longer circulation half-life than noncoated NPs [23]. Heparin-coated NPs were also used as a carrier of hemoglobin (Hb) [50,51]. Here heparin-poly(isobutylcyanoacrylate) copolymers were made into NPs, where heparin formed a hydrophilic stealth coating that spontaneously bound Hb with high efficiency [50]. The heparin-coated poly(isobutylcyanoacrylate) NPs coupled to Hb maintained the antithrombic activity of heparin and the ligand-binding capacity of Hb [50]. Heparin was also modified with a hydrophobic moiety such as deoxycholic acid (DOCA) to form self-assembled NPs [52] with the drug-loading capacity [53]. Doxorubicin loaded in the heparin–DOCA NPs induced tumor volume reduction to a greater extent than free doxorubicin, due to the extended circulation of the carrier and the antiproliferative effect of the heparin–DOCA conjugate itself [53].
The ability of heparin to control angiogenesis, tumor growth and metastasis in addition to the stealth function makes it an attractive biomaterial for the development of new nanomedicine. One of the ongoing efforts to enhance the utility of heparin in nanomedicine is to develop low-molecular-weight heparin derivatives with minimal anticoagulant effect yet high antiangiogenic activity [54].
Chitosan
Chitosan is a linear aminopolysaccharide composed of randomly distributed β-(1→4) linked d-glucosamine and N-acetyl-d-glucosamine units, obtained by the deacetylation of chitin from the exoskeleton of crustaceans [55]. Chitosan has been widely studied for the development of controlled drug/gene delivery systems, because of pH-sensitive water solubility, mucoadhesiveness and the ability to form a complex with nucleic acids [56]. The biological activity of chitosan varies with the physicochemical status determined by the molecular weight and degree of deacetylation [57]. The amine groups in chitosan make it uniquely suitable for covalent conjugation of anticancer drugs such as doxorubicin [58] and paclitaxel [59] for the treatment of cancer.
With a pKa value of 6.5, chitosan is typically insoluble in water at neutral pH. While this property is utilized to form self-assembled NPs [60], chitosan has also been modified in various ways to improve the water solubility in a broad range of pH, thereby enhancing its utility in physiological condition. For example, the amine groups of chitosan are partially quaternized [61] or conjugated with a sugar moiety [62]. Glycol chitosan (GC), a chitosan derivative with 2-hydroxyethylether groups in the 6-O position [63–67], low-molecular-weight chitosan (LMWC) [68] and zwitterionic chitosan [69] are also used when aqueous solubility of chitosan at neutral pH is desired.
For delivery of imaging or therapeutic agents, glycol chitosan is modified to hydrophobic moieties to form self-assembled structures, where hydrophilic GC forms a shell and hydrophobic moieties form a core that can encapsulate a therapeutic agent. These nanoparticulate assemblies based on hydrophobically modified GCs (HGC) showed enhanced accumulation in tumors via the EPR effect [70]. HGC made with 5β-cholanic acid has been used for the delivery of plasmid DNA (pDNA) [67], photosensitizers [71] and anticancer drugs such as paclitaxel [72], cisplatin [73] and doxorubicin [65]. HGC was also used as an imaging agent for tumor detection [70]. Here, a near-IR fluorescence dye, Cy5.5 was covalently conjugated to HGC to form approximately1 50 nm NPs. The Cy5.5-labeled HGC NPs showed excellent tumor accumulation, superior to polystyrene NPs of a comparable size [70]. In another example, GC was used as a delivery vehicle of polymerized siRNA (poly-siRNA) [74]. Here, the primary amine groups of GC were partially thiolated and crosslinked via disulfide bond to stabilize the electrostatic complex with poly-siRNA. The poly-siRNA-thiolated GC complex demonstrated quick cellular uptake and efficient in vivo gene silencing effects in solid tumors [74]. The excellent ability of GC-based NPs to accumulate in tumors is attributed to the stability during circulation and deformability that allows for efficient extravasation at the peritumoral vasculature [70]. Moreover, GC-based NPs are shown to enter cancer cells effectively [70] via several endocytic pathways [75].
Recently, chitosan has been explored as a material for coating the NP surface [68,76–78]. Our group used LMWC (<10 kDa) to decorate the surface of poly(lactic-co-glycolic acid) (PLGA) NP. Here, a conjugate of LMWC and PLGA was formed and made into NPs, where PLGA formed a drug-encapsulating core and LMWC, a surface coating. The rationale of using LMWC as a coating material was that it would develop cationic charge at pH below its pKa value (6.5), allowing the NPs to interact with cells located in acidic environment such as hypoxic tumors [68]. Owing to the low molecular weight, LMWC was more water soluble than high-molecular-weight chitosans and formed a hydrated surface layer that was resistant to protein adsorption and phagocytic uptake [68]. The authors also reported a new water-soluble chitosan derivative, created by partial amidation of amine groups [69]. The chitosan derivative shows negative charge at neutral pH, potentially useful for coating cationic NPs.
The cellular interaction of chitosan NPs is typically explained by the electrostatic interactions between positively charged chitosan and anionic substructures on the cell membrane [56]. In addition, chitosan (19 or 31 kDa) is reported to be taken up by the renal tubular cells via endocytosis mediated by megalin receptor [25]. In this study, chitosan uptake by the human renal tubular cells (HK-2) was decreased in the presence of gentamycin, another ligand of the megalin receptor. They also demonstrated that the accumulation of chitosan decreased in mice treated with disodium maleate, which caused depletion of the megalin receptor in the kidney [25].
Hyaluronic acid
Hyaluronan, or hyaluronic acid (HA), is a polysaccharide composed of alternating (1→4)-β-d-glucuronic acid and (1→3)-β-N-acetyl-d-glucosamine [79]. HA is a glycosaminoglycan abundant in the body, present in the skin, lung, synovial fluid and blood [80]. Biological roles of HA vary with the molecular weight [81]. For example, high-molecular-weight HA in the extracellular matrix was shown to have an antiangiogenic effect [82]; whereas later studies using HA fragments showed that it can be a stimulator of angiogenesis [83] and endothelial differentiation [84]. Several studies report receptors of HA. CD44 is a well-known receptor of HA [85]. HARE [86], a receptor for hyaluronate-mediated motility [87], and LYVE-1 [88] are also known as receptors of HA.
Because of the biological functions and known receptors, HA has been incorporated into a wide variety of nanomedicine constructs. HA has been used for the delivery of anticancer drugs such as paclitaxel [24,26,89,90], doxorubicin [91–93] and camptothecin [94]. In addition, HA is used along with cationic liposomes and polymers to reduce cytotoxicity and their interaction with serum proteins [95]. For example, HA conjugated with polyethyleneimine (HA–PEI) was used for the delivery of siRNA and has shown effective gene silencing effect in vitro [96] and in vivo [97,98]. The presence of HA prevented particle aggregation in blood and death related to pulmonary embolism [97]. The mechanism by which the siRNA/HA–PEI complex enters cells remains controversial [96,98], but there is a possibility of receptor-mediated endocytosis, given that the siRNA–HA–PEI complex, unlike siRNA–PEI, tends to accumulate in the tissues with HA receptors [27,98]. Here, LYVE-1 was identified as a receptor for HA-conjugated NPs [27]. More efficient gene silencing was observed in B16F1 murine melanoma cells overexpressing LYVE-1 than in HEK-293 human kidney cells without HA receptors [27]. In another study, HA was used for the targeting of sinusoidal endothelial cells (SECs) of the liver via HARE [99]. Here, HA was conjugated with poly-l-lysine and complexed with pDNA. Following the tail vein injection, the pDNA–HA-poly-l-lysine complex mainly accumulated in the liver SECs and expressed a reporter gene there via HARE-mediated endocytosis [99].
HA was also shown to contribute to gene transfection by increasing extracellular stability. Previously, a ternary gene complex consisting of pDNA, disulfide cross-linked PEI and HA coating (DPH complex) was created. The ternary complex was superior to a binary complex of pDNA and cationic polymers (i.e., crosslinked PEI) in the stability under the treatment with DNase and/or heparin and the transfection efficiency in the serum-containing medium [100]. A series of experiments suggest that HA not only protects the complex from unwanted interactions with serum proteins but also helps the complex dissociate in a timely manner once internalized by cells [100]. It is suggested that the intracellular decomplexation of the HA-containing complex facilitates the access of transcription factors to pDNA and subsequent gene expression processes [100,101].
Chondroitin sulfate
Chondroitin sulfate (CS) is a negatively charged glycosaminoglycan consisting of two alternating monosaccharides (N-acetylgalactosamine and glucuronic acid). CS is present on the cell surface and in the extracellular matrix [102], and covalently bound to proteins to form a proteoglycans [103]. Due to the good biocompatibility and hydrophilicity, several nanocarriers have been developed based on CS.
Since CS is highly hydrophilic, it is modified with additional moieties such as polylactide [103,104], acetyl groups [105] or methacrylate [106], which enable the formation of self-assembled nanocarriers and the loading of therapeutic agents. Alternatively, CS is used to coat a DNA–PEI complex to shield the cationic charge [107,108]. A ternary complex consisting of DNA, PEI and CS showed greater in vitro gene transfection efficiency and tumor accumulation than the DNA–PEI complex [108]. A ternary complex consisting of CS, PEI and DNA encoding mGM-CSF also inhibited the growth of intraperitoneal and subcutaneous tumors in a syngeneic mouse model to a greater extent than a binary complex of PEI and mGM-CSF DNA or another ternary complex using high-molecular-weight HA instead of CS [107].
The improvement of gene transfection and tumor-targeting effect by CS was attributed to the high expression of CD44, one of its receptors, on tumor cells [109]. Kurosaki et al. compared the effects of various polysaccharides as coating materials for pDNA–PEI complex and found that the CS-coated complex entered cells most effectively among the tested polysaccharide-based complexes [110]. Given that the uptake of the pDNA–PEI–CS complex was blocked by free CS added to the culture medium in a dose-dependent manner, the uptake of the CS-based ternary complex is likely to be through receptor-mediated endocytosis [110].
In addition, CS coating contributed to the safety of gene complexes. CS was added to a polymeric complex based on a polyaspartamide derivative to improve the safety and transfection efficiency [111]. As compared with a binary complex lacking CS, the ternary complex induced lower levels of lactate dehydrogenase, a marker of tissue damage, and pro-inflammatory cytokines such as TNF-α and COX-2 in lung tissues after gene delivery via intratracheal administration [111]. Serum creatine phosphokinase level, another indicator of tissue damage, measured after hydrodynamic gene introduction into skeletal muscle was also lower in the CS added group [111]. The protective effect of CS is attributable to neutralization of excessive positive charges, which cause cell-membrane damage when left unmasked [111].
Future perspective
Main advantages of polysaccharides are well-established biocompatibility, availability and abundant functional groups amenable to chemical modifications. Many research groups are exploring these advantages to improve the effectiveness of nanomedicines. However, several challenges remain to be overcome. First, most polysaccharides are of natural origin, and there is a high degree of variability with respect to the molecular weight and structure depending on the sources. Since these properties critically influence the biological activities of polysaccharides, alternative methods to produce polysaccharides with consistent properties need to be established for further advancement of polysaccharide-based nanomedicines. Second, depending on the origin, a polysaccharide may contain biologically active contaminants such as endotoxins and pathogens that may counteract the desired effect of the polysaccharide. Standardized procedures to purify polysaccharides are urgently needed. Third, the exact mechanisms of prominent biological actions of polysaccharides remain to be identified. It is not unusual to observe apparently contradicting activities of the ‘same’ polysaccharide. A subtle difference in molecular weight, degree of branching,or the arrangement of monomers can result in a significant differences in their biological effects. Moreover, the biological effects and fates of excess polysaccharides and degradation products are not entirely clear. An understanding of the mechanism of action is a prerequisite to successful utilization of a polysaccharide in nanomedicine development.
Executive summary.
Use of polysaccharides in nanomedicine
■ Naturally occurring polysaccharides, such as dextran, heparin, chitosan, hyaluronic acid and chondroitin sulfate are widely used for surface modification of nanomedicines.
■ These polysaccharides can reduce opsonization and complement activation due to nanomedicines in the blood and, thus, increase their circulation half-lives.
■ Some polysaccharides can function as a ligand and enhance the cellular uptake of nanomedicines via receptor-mediated endocytosis.
■ Advantages of polysaccharides include biocompatibility, cost–effectiveness and well-established modification schemes.
Remaining challenges
■ A high degree of variability, potential risk of bioactive contamination and insufficient mechanistic understanding of the biological actions remain as challenges.
Acknowledgments
This work was supported by NSF DMR-1056997, NIH R21 CA135130, a grant from the Lilly Endowment, Inc. to College of Pharmacy, Purdue University, and Intramural Research Program (Global RNAi Carrier Initiative) of Korea Institute of Science and Technology.
Key Terms
- Reticuloendothelial system
Special immune system primarily consisting of monocytes and macrophages located in reticular connective tissue such as lymph nodes and the spleen. The Kupffer cells of the liver and tissue histiocytes are also part of the reticuloendothelial system.
- Opsonization
Rendering of bacteria and other foreign substances subject to phagocytosis by opsonins such as antibodies and complements.
- Stealth coating
Covering of the nanoparticle surface with an electrically neutral hydrophilic polymer. The hydrated surface layer prevents protein adsorption and recognition by immune cells.
- Anticoagulation
Prevention of blood clotting. Heparin is a representative anticoagulant. Anticoagulants are used in medical conditions involving excessive blood clotting, such as deep vein thrombosis, pulmonary embolism, myocardial infarction and stroke.
- Angiogenesis
Physiological or pathological process involving the growth of new blood vessels. Although a normal process in growth, development and wound healing, it is also a crucial process in the growth of tumors.
- Self-assembled structures
Ordered arrangements of amphiphilic materials, which not only maintain molecular properties of individual building blocks, but also have novel properties that can perform specific functions.
- Endocytosis
Process by which cells take up molecules or nanoparticles. Examples of endocytosis pathways are clathrin-mediated endocytosis, caveolae-mediated endocytosis, macropinocytosis and phagocytosis.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
■ of interest
■■ of considerable interest
- 1.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46(12 Pt 1):6387–6392. [PubMed] [Google Scholar]
- 2.Hobbs SK, Monsky WL, Yuan F, et al. Regulation of transport pathways in tumor vessels: role of tumor type and microenvironment. Proc. Natl Acad. Sci. USA. 1998;95(8):4607–4612. doi: 10.1073/pnas.95.8.4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukumura D, Jain RK. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc. Res. 2007;74(2–3):72–84. doi: 10.1016/j.mvr.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao YJ, Juliano RL. Interactions of liposomes with the reticuloendothelial system. Effects of reticuloendothelial blockade on the clearance of large unilamellar vesicles. Biochim. Biophys. Acta. 1981;677(3–4):453–461. doi: 10.1016/0304-4165(81)90259-2. [DOI] [PubMed] [Google Scholar]
- 5.Patel HM, Moghimi SM. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system – the concept of tissue specificity. Adv. Drug Deliv. Rev. 1998;32(1–2):45–60. doi: 10.1016/s0169-409x(97)00131-2. [DOI] [PubMed] [Google Scholar]
- 6.Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol. Rev. 2001;53(2):283–318. [PubMed] [Google Scholar]
- 7.Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977;252(11):3582–3586. [PubMed] [Google Scholar]
- 8.Abuchowski A, van Es T, Palczuk NC, Davis FF. Alteration of immunological properties of bovine serum albumin by covalent attachment of polyethylene glycol. J. Biol. Chem. 1977;252(11):3578–3581. [PubMed] [Google Scholar]
- 9.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268(1):235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 10.Gref R, Minamitake Y, Peracchia MT, Trubetskoy V, Torchilin V, Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–1603. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 11.Lee SW, Yun MH, Jeong SW, et al. Development of docetaxel-loaded intravenous formulation, Nanoxel-PM™ using polymer-based delivery system. J. Control. Release. 2011;155(2):262–271. doi: 10.1016/j.jconrel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 12.Kenausis GL, Voros J, Elbert DL, et al. Poly(l-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: attachment mechanism and effects of polymer architecture on resistance to protein adsorption. J. Phys. Chem. B. 2000;104(14):3298–3309. [Google Scholar]
- 13.Du H, Chandaroy P, Hui SW. Grafted poly-(ethylene glycol) on lipid surfaces inhibits protein adsorption and cell adhesion. Biochim. Biophys. Acta. 1997;1326(2):236–248. doi: 10.1016/s0005-2736(97)00027-8. [DOI] [PubMed] [Google Scholar]
- 14.Hong RL, Huang CJ, Tseng YL, et al. Direct comparison of liposomal doxorubicin with or without polyethylene glycol coating in C-26 tumor-bearing mice: is surface coating with polyethylene glycol beneficial? Clin. Cancer Res. 1999;5(11):3645–3652. [PubMed] [Google Scholar]
- 15■■.Hatakeyama H, Akita H, Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv. Drug Deliv. Rev. 2011;63(3):152–160. doi: 10.1016/j.addr.2010.09.001. [Reviews the paradoxical role of PEG in nanomedicine delivery.] [DOI] [PubMed] [Google Scholar]
- 16■.Lehtinen J, Magarkar A, Stepniewski, et al. Analysis of cause of failure of new targeting peptide in PEGylated liposome: molecular modeling as rational design tool for nanomedicine. Eur. J. Pharm. Sci. 2012;46(3):121–130. doi: 10.1016/j.ejps.2012.02.009. [Demonstrates the cause of failure of targeting peptide in PEGylated liposome using computational modeling.] [DOI] [PubMed] [Google Scholar]
- 17.Remaut K, Lucas B, Braeckmans K, Demeester J, De Smedt SC. Pegylation of liposomes favours the endosomal degradation of the delivered phosphodiester oligonucleotides. J. Control. Release. 2007;117(2):256–266. doi: 10.1016/j.jconrel.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 18.Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur. J. Cell Biol. 2004;83(3):97–111. doi: 10.1078/0171-9335-00363. [DOI] [PubMed] [Google Scholar]
- 19■■.Amoozgar Z, Yeo Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2012;4(2):219–233. doi: 10.1002/wnan.1157. [Reviews alternative approaches for the stealth coating of nanoparticles.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008;60(15):1650–1662. doi: 10.1016/j.addr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Coviello T, Matricardi P, Alhaique F. Drug delivery strategies using polysaccharidic gels. Expert Opin. Drug Deliv. 2006;3(3):395–404. doi: 10.1517/17425247.3.3.395. [DOI] [PubMed] [Google Scholar]
- 22.Passirani C, Barratt G, Devissaguet JP, Labarre D. Interactions of nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate) with the complement system. Life Sci. 1998;62(8):775–785. doi: 10.1016/s0024-3205(97)01175-2. [DOI] [PubMed] [Google Scholar]
- 23.Passirani C, Barratt G, Devissaguet JP, Labarre D. Long-circulating nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate). Pharm. Res. 1998;15(7):1046–1050. doi: 10.1023/a:1011930127562. [DOI] [PubMed] [Google Scholar]
- 24.Li J, Huo M, Wang J, et al. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials. 2012;33(7):2310–2320. doi: 10.1016/j.biomaterials.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 25.Yuan ZX, Zhang ZR, Zhu D, et al. Specific renal uptake of randomly 50% N-acetylated low molecular weight chitosan. Mol. Pharm. 2009;6(1):305–314. doi: 10.1021/mp800078a. [DOI] [PubMed] [Google Scholar]
- 26.Rivkin I, Cohen K, Koffler J, Melikhov D, Peer D, Margalit R. Paclitaxel-clusters coated with hyaluronan as selective tumor-targeted nanovectors. Biomaterials. 2010;31(27):7106–7114. doi: 10.1016/j.biomaterials.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 27.Jiang G, Park K, Kim J, et al. Hyaluronic acid-polyethyleneimine conjugate for target specific intracellular delivery of siRNA. Biopolymers. 2008;89(7):635–642. doi: 10.1002/bip.20978. [DOI] [PubMed] [Google Scholar]
- 28■■.Lemarchand C, Gref R, Couvreur P. Polysaccharide-decorated nanoparticles. Eur. J. Pharm. Biopharm. 2004;58(2):327–341. doi: 10.1016/j.ejpb.2004.02.016. [Detailed review of preparation and characterization of polysaccharide-based nanoparticles.] [DOI] [PubMed] [Google Scholar]
- 29■■.Liu Z, Jiao Y, Wang Y, Zhou C, Zhang Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008;60(15):1650–1662. doi: 10.1016/j.addr.2008.09.001. [Detailed review of preparation, characterization and applications of polysaccharide-based nanoparticles.] [DOI] [PubMed] [Google Scholar]
- 30.Baldwin AD, Kiick KL. Polysaccharide-modified synthetic polymeric biomaterials. Biopolymers. 2010;94(1):128–140. doi: 10.1002/bip.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrison JH. Dextran as a plasma substitute with plasma volume and excretion studies on control patients. Ann. Surg. 1954;139(2):137–142. doi: 10.1097/00000658-195402000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Massia SP, Stark J, Letbetter DS. Surface-immobilized dextran limits cell adhesion and spreading. Biomaterials. 2000;21(22):2253–2261. doi: 10.1016/s0142-9612(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 33.Rodrigues JS, Santos-Magalhaes NS, Coelho LC, Couvreur P, Ponchel G, Gref R. Novel core(polyester)-shell(polysaccharide) nanoparticles: protein loading and surface modification with lectins. J. Control. Release. 2003;92(1–2):103–112. doi: 10.1016/s0168-3659(03)00296-7. [DOI] [PubMed] [Google Scholar]
- 34.Lemarchand C, Couvreur P, Besnard M, Costantini D, Gref R. Novel polyester-polysaccharide nanoparticles. Pharm. Res. 2003;20(8):1284–1292. doi: 10.1023/a:1025017502379. [DOI] [PubMed] [Google Scholar]
- 35.Bonnemain B. Superparamagnetic agents in magnetic resonance imaging: physicochemical characteristics and clinical applications. A review. J. Drug Target. 1998;6(3):167–174. doi: 10.3109/10611869808997890. [DOI] [PubMed] [Google Scholar]
- 36.Moore A, Marecos E, Bogdanov A, Jr, Weissleder R. Tumoral distribution of long-circulating dextran-coated iron oxide nanoparticles in a rodent model. Radiology. 2000;214(2):568–574. doi: 10.1148/radiology.214.2.r00fe19568. [DOI] [PubMed] [Google Scholar]
- 37.Lemarchand C, Gref R, Passirani C, et al. Influence of polysaccharide coating on the interactions of nanoparticles with biological systems. Biomaterials. 2006;27(1):108–118. doi: 10.1016/j.biomaterials.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 38.Labarre D, Vauthier C, Chauvierre C, Petri B, Müller R, Chehimi MM. Interactions of blood proteins with poly(isobutylcyanoacrylate) nanoparticles decorated with a polysaccharidic brush. Biomaterials. 2005;26(24):5075–5084. doi: 10.1016/j.biomaterials.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Vauthier C, Persson B, Lindner P, Cabane B. Protein adsorption and complement activation for di-block copolymer nanoparticles. Biomaterials. 2011;32(6):1646–1656. doi: 10.1016/j.biomaterials.2010.10.026. [DOI] [PubMed] [Google Scholar]
- 40.Alhareth K, Vauthier C, Bourasset F, Gueutin C, Ponchel G, Moussa F. Conformation of surface-decorating dextran chains affects the pharmacokinetics and biodistribution of doxorubicin-loaded nanoparticles. Eur. J. Pharm. Biopharm. 2012;81(2):453–457. doi: 10.1016/j.ejpb.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 41■.Kemp MM, Linhardt RJ. Heparin-based nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010;2(1):77–87. doi: 10.1002/wnan.68. [Detailed review of heparin as a material for nanomedicine.] [DOI] [PubMed] [Google Scholar]
- 42.Linhardt RJ. Claude S Hudson Award address in carbohydrate chemistry. heparin: structure and activity. J. Med. Chem. 2003;46(13):2551–2564. doi: 10.1021/jm030176m. 2003. [DOI] [PubMed] [Google Scholar]
- 43.Oschatz C, Maas C, Lecher B, et al. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity. 2011;34(2):258–268. doi: 10.1016/j.immuni.2011.02.008. [DOI] [PubMed] [Google Scholar]
- 44.Crum R, Szabo S, Folkman J. A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science. 1985;230(4732):1375–1378. doi: 10.1126/science.2416056. [DOI] [PubMed] [Google Scholar]
- 45.Folkman J, Langer R, Linhardt RJ, Haudenschild C, Taylor S. Angiogenesis inhibition and tumor regression caused by heparin or a heparin fragment in the presence of cortisone. Science. 1983;221(4612):719–725. doi: 10.1126/science.6192498. [DOI] [PubMed] [Google Scholar]
- 46.Lundin L, Larsson H, Kreuger J, et al. Selectively desulfated heparin inhibits fibroblast growth factor-induced mitogenicity and angiogenesis. J. Biol. Chem. 2000;275(32):24653–24660. doi: 10.1074/jbc.M908930199. [DOI] [PubMed] [Google Scholar]
- 47.Yoshitomi Y, Nakanishi H, Kusano Y, et al. Inhibition of experimental lung metastases of Lewis lung carcinoma cells by chemically modified heparin with reduced anticoagulant activity. Cancer Lett. 2004;207(2):165–174. doi: 10.1016/j.canlet.2003.11.037. [DOI] [PubMed] [Google Scholar]
- 48.Yu MK, Lee DY, Kim YS, et al. Antiang-iogenic and apoptotic properties of a novel amphiphilic folate-heparin-lithocholate derivative having cellular internality for cancer therapy. Pharm. Res. 2007;24(4):705–714. doi: 10.1007/s11095-006-9190-3. [DOI] [PubMed] [Google Scholar]
- 49.Park K, Kim YS, Lee GY, et al. Tumor endothelial cell targeted cyclic RGD-modified heparin derivative: inhibition of angiogenesis and tumor growth. Pharm. Res. 2008;25(12):2786–2798. doi: 10.1007/s11095-008-9643-y. [DOI] [PubMed] [Google Scholar]
- 50.Chauvierre C, Marden MC, Vauthier C, Labarre D, Couvreur P, Leclerc L. Heparin coated poly(alkylcyanoacrylate) nanoparticles coupled to hemoglobin: a new oxygen carrier. Biomaterials. 2004;25(15):3081–3086. doi: 10.1016/j.biomaterials.2003.09.097. [DOI] [PubMed] [Google Scholar]
- 51.Chauvierre C, Manchanda R, Labarre D, Vauthier C, Marden MC, Leclerc L. Artificial oxygen carrier based on polysaccharides-poly(alkylcyanoacrylates) nanoparticle templates. Biomaterials. 2010;31(23):6069–6074. doi: 10.1016/j.biomaterials.2010.04.039. [DOI] [PubMed] [Google Scholar]
- 52.Park K, Kim K, Kwon IC, et al. Preparation and characterization of self-assembled nanoparticles of heparin-deoxycholic acid conjugates. Langmuir. 2004;20(26):11726–11731. doi: 10.1021/la048646i. [DOI] [PubMed] [Google Scholar]
- 53.Park K, Lee GY, Kim YS, et al. Heparin-deoxycholic acid chemical conjugate as an anticancer drug carrier and its antitumor activity. J. Control. Release. 2006;114(3):300–306. doi: 10.1016/j.jconrel.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 54.Lee E, Kim YS, Bae SM, et al. Polyproline-type helical-structured low-molecular weight heparin (LMWH)-taurocholate conjugate as a new angiogenesis inhibitor. Int. J. Cancer. 2009;124(12):2755–2765. doi: 10.1002/ijc.24239. [DOI] [PubMed] [Google Scholar]
- 55.Kumar MN, Muzzarelli RA, Muzzarelli C, Sashiwa H, Domb AJ. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004;104(12):6017–6084. doi: 10.1021/cr030441b. [DOI] [PubMed] [Google Scholar]
- 56.Bernkop-Schnurch A, Dunnhaupt S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012;81(3):463–469. doi: 10.1016/j.ejpb.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 57.Park JH, Saravanakumar G, Kim K, Kwon IC. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010;62(1):28–41. doi: 10.1016/j.addr.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 58.Son YJ, Jang JS, Cho YW, et al. Biodistribution and anti-tumor efficacy of doxorubicin loaded glycol-chitosan nanoaggregates by EPR effect. J. Control. Release. 2003;91(1–2):135–145. doi: 10.1016/s0168-3659(03)00231-1. [DOI] [PubMed] [Google Scholar]
- 59.Lee E, Lee J, Lee IH, et al. Conjugated chitosan as a novel platform for oral delivery of paclitaxel. J. Med. Chem. 2008;51(20):6442–6449. doi: 10.1021/jm800767c. [DOI] [PubMed] [Google Scholar]
- 60.Bowman K, Leong KW. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomedicine. 2006;1(2):117–128. doi: 10.2147/nano.2006.1.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van der Merwe SM, Verhoef JC, Verheijden JHM, Kotzé AF, Junginger HE. Trimethylated chitosan as polymeric absorption enhancer for improved peroral delivery of peptide drugs. Eur. J. Pharm. Biopharm. 2004;58(2):225–235. doi: 10.1016/j.ejpb.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 62.Ono K, Saito Y, Yura H, et al. Photocrosslinkable chitosan as a biological adhesive. J. Biomed. Mater. Res. 2000;49:289–295. doi: 10.1002/(sici)1097-4636(200002)49:2<289::aid-jbm18>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 63.Amsden BG, Sukarto A, Knight DK, Shapka SN. Methacrylated glycol chitosan as a photopolymerizable biomaterial. Biomacromolecules. 2007;8(12):3758–3766. doi: 10.1021/bm700691e. [DOI] [PubMed] [Google Scholar]
- 64.Park JH, Kwon S, Nam J-O, et al. Self-assembled nanoparticles based on glycol chitosan bearing 5β-cholanic acid for RGD peptide delivery. J. Control. Release. 2004;95(3):579–588. doi: 10.1016/j.jconrel.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 65.Park K, Kim J-H, Nam YS, et al. Effect of polymer molecular weight on the tumor targeting characteristics of self-assembled glycol chitosan nanoparticles. J. Control. Release. 2007;122(3):305–314. doi: 10.1016/j.jconrel.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 66.Min KH, Park K, Kim Y-S, et al. Hydrophobically modified glycol chitosan nanoparticles-encapsulated camptothecin enhance the drug stability and tumor targeting in cancer therapy. J. Control. Release. 2008;127(3):208–218. doi: 10.1016/j.jconrel.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 67.Yoo HS, Lee JE, Chung H, Kwon IC, Jeong SY. Self-assembled nanoparticles containing hydrophobically modified glycol chitosan for gene delivery. J. Control. Release. 2005;103(1):235–243. doi: 10.1016/j.jconrel.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 68.Amoozgar Z, Park J, Lin Q, Yeo Y. Low molecular-weight chitosan as a pH-sensitive stealth coating for tumor-specific drug delivery. Mol. Pharm. 2012;9(5):1262–1270. doi: 10.1021/mp2005615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xu P, Bajaj G, Shugg T, Van Alstine WG, Yeo Y. Zwitterionic chitosan derivatives for pH-sensitive stealth coating. Biomacromolecules. 2010;11(9):2352–2358. doi: 10.1021/bm100481r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Na JH, Koo H, Lee S, et al. Real-time and non-invasive optical imaging of tumor-targeting glycol chitosan nanoparticles in various tumor models. Biomaterials. 2011;32(22):5252–5261. doi: 10.1016/j.biomaterials.2011.03.076. [DOI] [PubMed] [Google Scholar]
- 71.Lee SJ, Park K, Oh YK, et al. Tumor specificity and therapeutic efficacy of photosensitizer-encapsulated glycol chitosan-based nanoparticles in tumor-bearing mice. Biomaterials. 2009;30(15):2929–2939. doi: 10.1016/j.biomaterials.2009.01.058. [DOI] [PubMed] [Google Scholar]
- 72.Kim JH, Kim YS, Kim S, et al. Hydrophobically modified glycol chitosan nanoparticles as carriers for paclitaxel. J. Control. Release. 2006;111(1–2):228–234. doi: 10.1016/j.jconrel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 73.Kim JH, Kim YS, Park K, et al. Antitumor efficacy of cisplatin-loaded glycol chitosan nanoparticles in tumor-bearing mice. J. Control. Release. 2008;127(1):41–49. doi: 10.1016/j.jconrel.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 74■.Lee SJ, Huh MS, Lee SY, et al. Tumor-homing poly-siRNA/glycol chitosan self-cross-linked nanoparticles for systemic siRNA delivery in cancer treatment. Angew. Chem. Int. Ed. Engl. 2012;51(29):7203–7207. doi: 10.1002/anie.201201390. [Demonstrates delivery of poly-siRNA with glycol chitosan.] [DOI] [PubMed] [Google Scholar]
- 75.Nam HY, Kwon SM, Chung H, et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. J. Control. Release. 2009;135(3):259–267. doi: 10.1016/j.jconrel.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 76.Zhou J, Romero G, Rojas E, Ma L, Moya S, Gao C. Layer by layer chitosan/alginate coatings on poly(lactide-co-glycolide) nanoparticles for antifouling protection and folic acid binding to achieve selective cell targeting. J. Colloid Interface Sci. 2010;345(2):241–247. doi: 10.1016/j.jcis.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 77.Pastor E, Matveeva E, Valle-Gallego A, Goycoolea FM, Garcia-Fuentes M. Protein delivery based on uncoated and chitosan-coated mesoporous silicon microparticles. Colloids Surf. B Biointerfaces. 2011;88(2):601–609. doi: 10.1016/j.colsurfb.2011.07.049. [DOI] [PubMed] [Google Scholar]
- 78.Chung YI, Kim JC, Kim YH, et al. The effect of surface functionalization of PLGA nanoparticles by heparin- or chitosan-conjugated pluronic on tumor targeting. J. Control. Release. 2010;143(3):374–382. doi: 10.1016/j.jconrel.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 79.Chong BF, Blank LM, McLaughlin R, Nielsen LK. Microbial hyaluronic acid production. Appl. Microbiol. Biotechnol. 2005;66(4):341–351. doi: 10.1007/s00253-004-1774-4. [DOI] [PubMed] [Google Scholar]
- 80■.Oh EJ, Park K, Kim KS, et al. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release. 2010;141(1):2–12. doi: 10.1016/j.jconrel.2009.09.010. [Detailed review of hyaluronic acid as a material for nanomedicine.] [DOI] [PubMed] [Google Scholar]
- 81.Stern R, Asari AA, Sugahara KN. Hyaluronan fragments: an information-rich system. Eur. J. Cell Biol. 2006;85(8):699–715. doi: 10.1016/j.ejcb.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 82.Feinberg RN, Beebe DC. Hyaluronate in vasculogenesis. Science. 1983;220(4602):1177–1179. doi: 10.1126/science.6857242. [DOI] [PubMed] [Google Scholar]
- 83.West DC, Hampson IN, Arnold F, Kumar S. Angiogenesis induced by degradation products of hyaluronic acid. Science. 1985;228(4705):1324–1326. doi: 10.1126/science.2408340. [DOI] [PubMed] [Google Scholar]
- 84.Takahashi Y, Li L, Kamiryo M, et al. Hyaluronan fragments induce endothelial cell differentiation in a CD44- and CXCL1/GRO1-dependent manner. J. Biol. Chem. 2005;280(25):24195–24204. doi: 10.1074/jbc.M411913200. [DOI] [PubMed] [Google Scholar]
- 85.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 86.Weigel JA, Weigel PH. Characterization of the recombinant rat 175-kDa hyaluronan receptor for endocytosis (HARE). J. Biol. Chem. 2003;278(44):42802–42811. doi: 10.1074/jbc.M307201200. [DOI] [PubMed] [Google Scholar]
- 87.Hardwick C, Hoare K, Owens R, et al. Molecular cloning of a novel hyaluronan receptor that mediates tumor cell motility. J. Cell Biol. 1992;117(6):1343–1350. doi: 10.1083/jcb.117.6.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Banerji S, Ni J, Wang SX, et al. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J. Cell Biol. 1999;144(4):789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bajaj G, Kim MR, Mohammed SI, Yeo Y. Hyaluronic acid-based hydrogel for regional delivery of paclitaxel to intraperitoneal tumors. J. Control. Release. 2012;158(3):386–392. doi: 10.1016/j.jconrel.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Luo Y, Prestwich GD. Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjug. Chem. 1999;10(5):755–763. doi: 10.1021/bc9900338. [DOI] [PubMed] [Google Scholar]
- 91.Eliaz RE, Szoka FC., Jr Liposome-encapsulated doxorubicin targeted to CD44: a strategy to kill CD44-overexpressing tumor cells. Cancer Res. 2001;61(6):2592–2601. [PubMed] [Google Scholar]
- 92.Luo Y, Bernshaw NJ, Lu ZR, Kopecek J, Prestwich GD. Targeted delivery of doxorubicin by HPMA copolymer-hyaluronan bioconjugates. Pharm. Res. 2002;19(4):396–402. doi: 10.1023/a:1015170907274. [DOI] [PubMed] [Google Scholar]
- 93.Wu JL, Liu CG, Wang XL, Huang ZH. Preparation and characterization of nanoparticles based on histidine-hyaluronic acid conjugates as doxorubicin carriers. J. Mater. Sci. Mater. Med. 2012;23(8):1921–1929. doi: 10.1007/s10856-012-4665-8. [DOI] [PubMed] [Google Scholar]
- 94.Choi KY, Yoon HY, Kim JH, et al. Smart nanocarrier based on PEGylated hyaluronic acid for cancer therapy. ACS Nano. 2011;5(11):8591–8599. doi: 10.1021/nn202070n. [DOI] [PubMed] [Google Scholar]
- 95.Fischer D, Li Y, Ahlemeyer B, Krieglstein J, Kissel T. In vitro cytotoxicity testing of polycations: influence of polymer structure on cell viability and hemolysis. Biomaterials. 2003;24(7):1121–1131. doi: 10.1016/s0142-9612(02)00445-3. [DOI] [PubMed] [Google Scholar]
- 96.Han SE, Kang H, Shim GY, et al. Cationic derivatives of biocompatible hyaluronic acids for delivery of siRNA and antisense oligonucleotides. J. Drug Target. 2009;17(2):123–132. doi: 10.1080/10611860802472461. [DOI] [PubMed] [Google Scholar]
- 97.Park K, Hong SW, Hur W, et al. Target specific systemic delivery of TGF-beta siRNA/(PEI-SS)-g-HA complex for the treatment of liver cirrhosis. Biomaterials. 2011;32(21):4951–4958. doi: 10.1016/j.biomaterials.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 98.Jiang G, Park K, Kim J, Kim KS, Hahn SK. Target specific intracellular delivery of siRNA/PEI–HA complex by receptor mediated endocytosis. Mol. Pharm. 2009;6(3):727–737. doi: 10.1021/mp800176t. [DOI] [PubMed] [Google Scholar]
- 99.Takei Y, Maruyama A, Ferdous A, et al. Targeted gene delivery to sinusoidal endothelial cells: DNA nanoassociate bearing hyaluronan-glycocalyx. FASEB J. 2004;18(6):699–701. doi: 10.1096/fj.03-0494fje. [DOI] [PubMed] [Google Scholar]
- 100.Xu P, Quick GK, Yeo Y. Gene delivery through the use of a hyaluronate-associated intracellularly degradable crosslinked polyethyleneimine. Biomaterials. 2009;30(29):5834–5843. doi: 10.1016/j.biomaterials.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ito T, Iida-Tanaka N, Niidome T, et al. Hyaluronic acid and its derivative as a multi-functional gene expression enhancer: protection from non-specific interactions, adhesion to targeted cells, and transcriptional activation. J. Control. Release. 2006;112(3):382–388. doi: 10.1016/j.jconrel.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 102.Tomiyama T, Toita R, Kang JH, et al. Effect of introduction of chondroitin sulfate into polymer-peptide conjugate responding to intracellular signals. Nanoscale Res. Lett. 2011;6(1):532. doi: 10.1186/1556-276X-6-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee CT, Huang CP, Lee YD. Synthesis and characterizations of amphiphilic poly(l-lactide)-grafted chondroitin sulfate copolymer and its application as drug carrier. Biomol. Eng. 2007;24(1):131–139. doi: 10.1016/j.bioeng.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 104.Lee CT, Huang CP, Lee YD. Preparation of amphiphilic poly(l-lactide)-graft-chondroitin sulfate copolymer self-aggregates and its aggregation behavior. Biomacromolecules. 2006;7(4):1179–1186. doi: 10.1021/bm050995j. [DOI] [PubMed] [Google Scholar]
- 105.Park W, Park SJ, Na K. Potential of self-organizing nanogel with acetylated chondroitin sulfate as an anti-cancer drug carrier. Colloids Surf. B Biointerfaces. 2010;79(2):501–508. doi: 10.1016/j.colsurfb.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 106.Lim J, Hammoudi T, Bratt-Leal A, et al. Development of nano- and microscale chondroitin sulfate particles for controlled growth factor delivery. Acta Biomater. 2011;7(3):986–995. doi: 10.1016/j.actbio.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hamada K, Yoshihara C, Ito T, et al. Antitumor effect of chondroitin sulfate-coated ternary granulocyte macrophage-colony-stimulating factor plasmid complex for ovarian cancer. J. Gene Med. 2012;14(2):120–127. doi: 10.1002/jgm.1647. [DOI] [PubMed] [Google Scholar]
- 108.Pathak A, Kumar P, Chuttani K, et al. Gene expression, biodistribution, and pharmacoscintigraphic evaluation of chondroitin sulfate-PEI nanoconstructs mediated tumor gene therapy. ACS Nano. 2009;3(6):1493–1505. doi: 10.1021/nn900044f. [DOI] [PubMed] [Google Scholar]
- 109.Henke CA, Roongta U, Mickelson DJ, Knutson JR, McCarthy JB. CD44-related chondroitin sulfate proteoglycan, a cell surface receptor implicated with tumor cell invasion, mediates endothelial cell migration on fibrinogen and invasion into a fibrin matrix. J. Clin. Invest. 1996;97(11):2541–2552. doi: 10.1172/JCI118702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110■.Kurosaki T, Kitahara T, Kawakami S, et al. The development of a gene vector electrostatically assembled with a polysaccharide capsule. Biomaterials. 2009;30(26):4427–4434. doi: 10.1016/j.biomaterials.2009.04.041. [Compares various polysaccharides as a coating material of polyethyleneimine-based gene carriers.] [DOI] [PubMed] [Google Scholar]
- 111.Uchida S, Itaka K, Chen Q, et al. Combination of chondroitin sulfate and polyplex micelles from poly(ethylene glycol)-poly{N′-[N-(2-aminoethyl)-2-aminoethyl] aspartamide} block copolymer for prolonged in vivo gene transfection with reduced toxicity. J. Control. Release. 2011;155(2):296–302. doi: 10.1016/j.jconrel.2011.04.026. [DOI] [PubMed] [Google Scholar]