Abstract
In the immediate management of patients with spinal cord injury (SCI), patients are typically observed for a period of time to determine whether voluntary control of bladder function returns. Therefore, bladder reinnervation surgeries are not likely to be performed immediately after the injury. We performed genitofemoral to pelvic nerve transfer (GF NT) surgery in canines at 1 and 3 months after bladder denervation (transection of S1 and S2 spinal roots) to determine whether this type of bladder reinnervation surgery has potential clinical feasibility. Nerve cuff electrodes were implanted on the genitofemoral nerves proximal to the pelvic nerve transfer site. Evidence for bladder reinnervation includes (1) increased bladder pressure and urethral fluid flow following electrical stimulation in four out of 20 nerve cuff electrodes implanted on the transferred GF nerves, (2) bilateral pelvic nerve stimulation induced bladder pressure and urethral fluid flow in three of four denervated animals with 1-month delay GF NT, and in five of six denervated animals with 3-month delay GF NT, and (3) abundant L1 and L2 spinal cord cell bodies (the origin of the GF nerve) retrogradely labeled with fluorogold injected into the bladder in all 10 of the GF NT animals, except one animal on one side. This study presents initial proof of concept that GF NT is a potentially viable clinical approach to reinnervation of the lower motor neuron–lesioned urinary bladder.
Keywords: animal studies, neural injury, recovery, regeneration, traumatic spinal cord injury
INTRODUCTION
One of the major medical and social problems of patients with spinal cord injury (SCI) is neurogenic bladder dysfunction. A recent survey of SCI patients indicates that restoration of bladder function is an important priority and in paraplegics is considered more important than recovery of walking (Anderson, 2004). Over the past several decades, functional electrical stimulation (FES) of sacral ventral roots has been developed and applied clinically to restore motor control after upper motor lesions above the T12-L1 vertebral level, which spare the S2-4 spinal cord segments where the parasympathetic nerve cell bodies innervating the urinary bladder are located (Brindley et al., 1986; Jezernik et al., 2002; Van Kerrebroeck et al., 1996). Injuries below the conus medullaris, including injuries to the conus itself and the cauda equina, result in a lower motoneuron (LMN) lesion, leading to a flaccid paralysis of the urinary bladder such that the bladder does not contract when filled (detrusor areflexia) and often results in a competent but non-relaxing smooth muscle sphincter and a striated sphincter that retains some fixed tone but is not under voluntary control. Of the patients we screened at the Philadelphia Shriners Hospital for possible inclusion in the FES program, 11 of 45 patients (24%) had flaccid bladders and 27% had flaccid lower extremities. FES of the sacral ventral roots in these patients with flaccid paralysis could not restore motor control of bladder function. Bladder reinnervation strategies needed to be developed for restoration of bladder motor control in this group of patients with LMN lesions.
Using a canine model, we have previously shown that the urinary bladder can become reinnervated following sacral nerve root transection and immediate end-on-end repair based on increased bladder pressure upon FES and neurotracing studies using fluorescent and lipophilic dyes (Ruggieri et al., 2006). Similar nerve transection and immediate end-to-end suturing studies in pigs has demonstrated return of the micturition reflex in two animals at 14 and 20 weeks postoperatively (Conzen and Sollmann, 1982). Using the canine model, we have recently reported that, after transection of the spinal roots innervating the urinary bladder, reinnervation can be accomplished by immediate nerve transfer using either intercostal nerves or coccygeal nerve roots in the lumbosacral spinal column, as well as peripheral genitofemoral (GF) nerves in the lower abdomen (Ruggieri et al., 2008). In the clinical management of patients following SCI, patients are typically evaluated for a period of time to determine whether they will recover motor control of bladder function. Thus, it is unlikely that nerve transfer surgeries would be applied immediately following human SCI. The aim of this study is to determine whether the bladder can be reinnervated if genitofemoral nerve transfer (GF NT) surgery is performed 1 and 3 months after the bladder denervation surgery to more closely mimic the possible clinical scenario. The GF nerve was chosen as the candidate for this transfer since its origin from L1 and L2 spinal cord segments is cranial to the sacral cord origin of parasympathetic nerves innervating the urinary bladder. Also, the GF nerve is a mixed nerve that carries sensory axons from the skin over the femoral triangle in the upper thigh and from the scrotum or labia majora, parasympathetic motor to blood vessels in the skin, and somatic motor input to the cremasteric muscle (Luria and Laufer, 2007; Zempoalteca et al., 2002).
METHODS
All studies were approved by the Temple University Institutional Animal Care and Use Committee in accordance with the laboratory animal care guidelines of both the U.S. Department of Agriculture and the Association for Assessment and Accreditation of Laboratory Animal Care. The study subjects were fully conditioned female mongrel hounds 6–12 months of age and 18–22 kg in body weight. A total of 13 dogs were used: four nerve transection with genitofemoral nerve transfer (GF NT) performed 1 month after nerve transection; six nerve transection with GF NT performed 3 months after nerve transection; and three sham-operated, nerve intact controls. These nerve intact sham-operated controls are the same animals as were included in a previous report (Ruggieri et al., 2008).
Surgical Preparation
Animals were fasted the day prior to surgery and covered with antibiotics (30 mg/kg trimethoprim and 6 mg/kg sulfadiazine P.O.). A fentanyl patch (75–100 mg/h for a 20-kg dog) was adhered to the shaved skin of the inner thigh and left in place for 3 days. Perioperative pain management included morphine (10 mg/L) in intravenous Ringers lactate delivered at 60–100 mL/h. Postoperative pain management also included 2 mg/kg ketoprofen IM for 2 days beginning on the second day post-surgery. Propofol (6 mg/kg iv) was administered to allow insertion of an endotracheal tube for isoflurane anesthesia (0.5–4% mean alveolar concentration) using oxygen as the carrier gas. For postoperative management of the neurogenic bladder, an abdominal vesicostomy was created as previously described (Ruggieri et al., 2006).
Bladder denervation was also performed as previously described (Ruggieri et al., 2006). Briefly, with the animal in the prone position, a 30-degree V-laminectomy of the L7 vertebral body and a partial laminectomy of the L6 and S1 vertebral bodies was done so that the S1 and S2 ventral roots innervating the bladder could be stimulated with a unipolar probe electrode. These two bilateral ventral roots that induced increased bladder pressure upon intraoperative electrical stimulation were transected, and 15-mm-long sections of the nerve roots were removed. Completeness of bladder denervation was confirmed by root transection–induced disappearance of bladder contractions upon stimulation of the entire conus medullaris with an epidural electrode placed in the mid-line under the L5 vertebral body. The incision was then closed in layers.
Genitofemoral Nerve Transfer
At 1 or 3 months after bladder denervation, the GF nerve was transferred to the pelvic nerve leading to the urinary bladder as previously described (Ruggieri et al., 2008). Briefly, the bladder vesicostomy was opened through a midline abdominal incision above and below the vesicostomy site. The GF nerves were mobilized bilaterally and attached to the distal severed end of the pelvic nerves leading to the bladder from the pelvic plexus by end-on-end anastomosis. In all nerve-transferred animals, a self-conforming, spiral, tripolar nerve cuff electrode (Axon Engineering, Cleveland, OH) was placed around the GF nerve approximately 3–4 cm proximal to the pelvic nerve anastomosis site. The electrode leads were coiled into a silicone pouch, which was inserted subcutaneously in the lower abdomen lateral to the incision.
Electrical Stimulation
At 2–3 months postoperatively, the silicone pouch containing the implanted electrode leads was retrieved under isoflurane anesthesia and stimulated (1.2 mA, 20 Hz, 0.5 msec quasi trapezoidal wave trains of 20-sec duration) while monitoring bladder pressure to determine return of bladder function. These stimulations were accomplished using a custom-made, current-regulated stimulator (Axon Engineering). Changes in pressure inside the urinary bladder were measured with an external pressure transducer interfaced with a data acquisition system (AD Instruments, Colorado Springs, CO). A Foley catheter was passed into the vesicostomy, and after inflating the balloon to 10 mL, gentle tension was placed on the catheter to prevent leakage. The catheter was connected via a three-way valve to the pressure recording system and a syringe infusion pump, which was used to fill the bladder with normal saline solution to approximately 30 mL. The pressure recording system was calibrated immediately before each use, and gentle manual pressure on the lower abdomen was used to ensure that the measurement system was functional and capable of detecting pressure changes inside the urinary bladder. During electrical stimulation, the abdomen was palpated to ensure that stimulation of the rectus abdominalis (and thus increasing the intra-abdominal pressure) was not being induced.
Immediately before the animals were euthanized, in addition to stimulation of the implanted electrodes, the pelvic nerves leading to the bladder were also evaluated for their ability to induce bladder pressure and urine flow by intraoperative electrical stimulation (20 Hz, 1 msec rectangular wave trains at 6, 9, 12, and 15 volts) using a unipolar probe electrode. The probe was touched to the surface of the nerve, and contact was continued until the induced bladder pressure reached a maximal response (3–5 sec). For these stimulations immediately prior to euthanasia, a Grass S88 stimulator (Astro-Med, West Warwick, RI) was used. Observation of increased bladder pressure following direct stimulation of the pelvic nerve (but not by stimulation of the implanted electrodes) was taken as evidence for successful bladder reinnervation, but failure of the implanted electrodes. For each FES session, bladder pressure was recorded for three to eight separate stimulations, and results are presented as means ± standard deviations. Differences between peak pressure during stimulation and the average baseline pressure for a 5–10-sec period immediately before stimulation was determined using Chart software (AD Instruments). For these stimulations, a catheter was not passed into the urethra so that electrical stimulation–induced flow of fluid from the bladder out the urethra could be observed.
Retrograde Neuronal Tracing and Collection of Spinal Cord Tissues
Fluorogold retrograde neuronal tracing from the urinary bladder to the spinal cord was performed on all animals as previously described (Ruggieri et al., 2006; Ruggieri et al., 2008). Briefly, at 3 weeks before animals were euthanized, injections of 4% (w/v) fluorogold were made lateral to each ureteral orifice. The dogs were euthanized with an intravenous injection of 360 mg/kg sodium pentobarbital. The spinal column with intact spinal cord, roots, and spinal nerves was removed and postfixed en bloc by immersion in freshly prepared 4% paraformaldehyde in 0.1M phosphate buffer (pH 7.4) for 3–5 days at 4°C. The spinal cord from the lumbar through the coccygeal regions was removed from the vertebral column, the dorsal roots on the right side marked with a permanent tissue marker for later identification purposes, cryoprotected in 30% sucrose in 0.1M phosphate buffer (pH 7.4) for 2 days, and frozen-sectioned into 18-μm coronal plane sections mounted immediately onto coated slides (Fisher Plus). The slides were coverslipped using 80% glycerol in 0.1M phosphate buffer, and used to measure mean number and diameter of fluorogold retrogradely labeled neuronal cell bodies. Cresyl violet staining of adjacent slides was used to further confirm segmental location.
Quantitative Analysis of Fluorogold Labeling
Spinal cord sections were analyzed quantitatively for the presence of fluorogold retrogradely labeled motor neuronal cell bodies using a Nikon fluorescence microscope interfaced with a bioquantitation system (Bioquant Osteo II, Bioquant, TN) and an X, Y motorized stage. Three sections per cord segment (lumbar, sacral, or coccygeal) were analyzed bilaterally for each animal using a independent-random sampling approach, as described in Mouton et al. (2002). To meet the needs of this sampling approach, every 10th serial section was collected at the sites of dorsal roots for the generation of at least six sets of slides per cord segment (with three sections of a cord segment per slide), for a total of at least 150 sections per cord. Then, within the gray matter of each section analyzed, four regions of the intermediate and ventral cord was examined at 20× magnification in order to determine the mean number of fluorogold labeled neurons per section, per side, and per segment for each surgical group. For the sham-operated animals, this was performed on the left side only because the right side of the cord was used for a different and separate experimental paradigm. Also, eight regions of the intermediate and ventral cord were examined at 40× magnification in order to quantify the mean cross-sectional diameter of the fluorogold labeled neurons per section, per side, and per segment for each surgical group. To avoid bias in estimating the number of neurons, only fluorogold-labeled cells in which the nucleus (unlabeled) was visible were measured. Also, the smallest cross-sectional diameter of these neurons was measured in order to assure that these measurements were made near the middle of cell bodies. The representative ventral horn micrographs shown in Figure 2 were generated using the Bioquant program topography, landmark, and region of interest tools (RO1) at 4× magnification, and then by topographically mapping the fluorogold labeled neurons at 20× using an object count array and the motorized stage within the larger ROI defined at the lower magnification in a manner similar to that used to count the labeled neurons.
FIG. 2.

Neuronal cell bodies in the spinal cord retrogradely labeled with fluorogold injected into the urinary bladder. Representative micrographs from dog 2 (Table 1). Arrows indicate cells that were counted as positive for retrograde fluorogold labeling. Increased labeled cell bodies as well as increased number of labeled axons in the lumbar cord (top), compared to the sacral cord of the same animal (bottom). Scale bar = 50 μm.
Statistical Analysis
Means and standard deviations (SD) are presented. Two-way analysis of variance (ANOVA) was used to compare the mean number of fluorogold labeled neurons per section using the factors cord region (lumbar, sacral, and coccygeal) and surgical group (GF NT at 1 month and GF NT at 3 months). Bonferroni post hoc analysis was then used to compare the differences between individual groups. A t-test was used to examine the differences in the mean diameter of fluorogold-labeled neurons in the lumbar cord region between the two different GF NT groups (there were no labeled neurons in the lumbar region in the sham group, thus eliminating that group from this particular analysis). Also, a one-way ANOVA was used to examine the differences in the mean diameter of fluorogold-labeled neurons in the sacral cord region among the three groups. A Tukey post hoc analysis was then used to compare the differences between individual groups. The p values of 0.05 or less were considered significant.
RESULTS
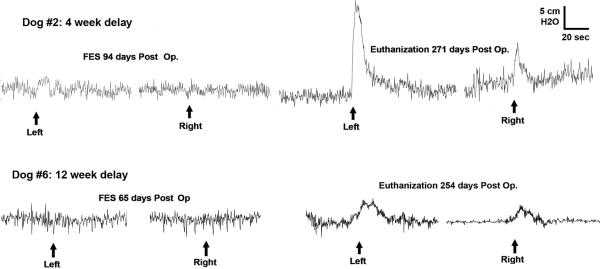
Figure 1 shows representative traces of the bladder pressure responses to functional electrical stimulation of the implanted electrodes on the left panel at 2–3 months postoperatively and stimulation of the pelvic nerves immediately prior to euthanasia on the right panel. All data for all animals is tabulated in Table 1, including the number and diameter of the cell bodies in the spinal cord that were retrogradely labeled with fluorogold injected into the bladder wall. For the FES results at euthanasia, maximal responses to electrical stimulations are shown that occurred in response a stimulus intensity of 12 volts with no further increased response at 15 volts. During stimulation of these implanted electrodes, no contraction of the abdominal musculature could be detected by direct palpation.
FIG. 1.
Functional electrical stimulation (FES): representative in vivo bladder pressure recordings during electrical stimulation. Stimulation of the implanted tripolar nerve cuff electrodes surrounding the genitofemoral nerve proximal to the nerve transfer site (left side) and intraoperative pelvic nerve stimulation with unipolar probe electrode immediately before the animals were euthanized (right side). Fluid flow out the urethra was noted during each electrical stimulation that induced increased bladder pressure. (Top) From dog 2 (Table 1) in which the genitofemoral nerve transfer (GF NT) surgery was performed 1 month after denervation. (Bottom) From dog 6 (Table 1) in which the GF NT surgery was performed 3 months after denervation.
Table 1.
Summary of Functional Electrical Stimulation and Neurotracing Results
| Implanted electrodes FES |
Pelvic nerve FES |
No. of fluorogold-labeled neurons |
Diameter of labeled neurons |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bladder pressure |
Bladder pressure |
Lumbar |
Sacral |
Coccygeal |
Lumbar |
Sacral |
Coccygeal |
||||||||||||||
| Dog | Surgery | Delay (months) | Postop. day | Left | Right | Both | Postop. day | Left | Right | L | R | L | R | L | R | L | R | L | R | L | R |
| 1 | GF NT | 1 | 91 | 2.9 ± 3.3 | 0.0 | 0.0 | 252 | 6.5 ± 2.2 | 3.0 ± 0.8 | 16.5 | 28.5 | 4.0 | 2.0 | 0.0 | 0.0 | 26 ± 7 | 23± 6 | 27 ± 6 | 15 ± 4 | 0 | 0 |
| 2 | GF NT | 1 | 94 | 0.0 | 0.0 | 5.6 | 254 | 23.5 ± 7.9 | 6.2 ± 1.3 | 13.5 | 12.0 | 0.5 | 0.0 | 0.0 | 0.0 | 21 ± 6 | 19 ± 8 | 24 | 0 | 0 | 0 |
| 3 | GF NT | 1 | 63 | 0.0 | 0.0 | 0.0 | 224 | 0.0 | 0.0 | 22.0 | 18.5 | 3.0 | 0.0 | 0.0 | 0.0 | 26 ± 1 | 21± 1 | 19 ± 2 | 0 | 0 | 0 |
| 4 | GF NT | 1 | 62 | 0.0 | 0.0 | 0.0 | 224 | 4.5 ± 0.9 | 2.4 ± 1.0 | 27.5 | 18.5 | 1.5 | 1.5 | 1.0 | 2.0 | 33 ± 2 | 32± 1 | 32 ± 5 | 32 ± 4 | 27± 9 | 22± 2 |
| 5 | GF NT | 3 | 57 | 1.2 ± 0.5 | 0.4 ± 0.3 | 0.0 | 335 | 6.0 ± 1.8 | 6.5 ± 1.2 | 13.5 | 25.0 | 6.5 | 2.0 | 0.0 | 0.5 | 30 ± 2 | 27± 2 | 25 ± 4 | 37 ± 4 | 0 | 22 |
| 6 | GF NT | 3 | 56 | 0.0 | 0.0 | 3.4 | 271 | 2.6 ± 1.3 | 2.7 ± 0.7 | 23.0 | 17.0 | 3.5 | 2.5 | 0.5 | 0.0 | 25 ± 8 | 27± 6 | 29 ± 8 | 25 ± 8 | 32 | 0 |
| 7 | GF NT | 3 | 48 | 0.0 | 4.9 ± 3.3 | 0.0 | 308 | 3.0 ± 1.5 | 8.5 ± 4.5 | 33.0 | 19.5 | 2.5 | 0.0 | 0.0 | 0.0 | 23 ± 7 | 22 ± 5 | 9 ± 3 | 0 | 0 | 0 |
| 8 | GF NT | 3 | 42 | 0.0 | 0.0 | 0.0 | 307 | 2.5 ± 0.5 | 4.5 ± 0.5 | 10.0 | 7.0 | 2.0 | 0.0 | 0.0 | 0.0 | 13 ± 5 | 23± 7 | 19 ± 4 | 0 | 0 | 0 |
| 9 | GF NT | 3 | 43 | 0.0 | 0.0 | 0.0 | 302 | 4.3 ± 1.2 | 3.6 ± 1.7 | 20.0 | 31.0 | 0.0 | 0.0 | 0.0 | 0.0 | 24 ± 5 | 23± 5 | 0 | 0 | 0 | 0 |
| 10 | GF NT | 3 | 39 | 0.0 | 0.0 | 0.0 | 306 | 0.0 | 0.0 | 16.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 15 ± 6 | 0 | 0 | 0 | 0 | 0 |
| 11 | SHAM | — | — | — | — | — | — | — | — | 0.0 | — | 16.0 | — | 0.0 | — | — | — | 18 ± 13 | — | — | — |
| 12 | SHAM | — | — | — | — | — | — | — | — | 0.0 | — | 10.0 | — | 0.0 | — | — | — | 14 ± 6 | — | — | — |
| 13 | SHAM | — | — | — | — | — | — | — | — | 0.0 | — | 11.5 | — | 0.0 | — | — | — | 20 ± 4 | — | — | — |
Pressure is expressed in units of cm H2O. FES, functional electrical stimulation; postop. day, number of days after the genitofemoral nerve transfer (GF NT) surgery.
Table 1 shows that, of the eight electrodes implanted in the four animals with GF NT performed 1 month after denervation, FES of the implanted electrodes induced an increase in bladder pressure in only one (dog 1, left side). Of the 12 electrodes implanted in the six dogs with GF NT performed 3 months after denervation, FES induced bladder pressure increases in three (bilaterally in dog 5 and right side of dog 7; Table 1). This resulted in an average increased pressure inside the bladder of 2.2 ± 2.7 cm H2O with 19 stimulations in four implanted electrodes. On the other hand, FES of the pelvic nerve immediately prior to euthanasia induced an increase in bladder pressure bilaterally in three of the four dogs with GF NT 1 month after denervation and in five of six dogs with GF NT 3 months after denervation. This resulted in an average increased pressure inside the bladder of 5.1 ± 4.7 cm H2O with 68 stimulations of 16 pelvic nerves. Stimulation of the implanted electrodes immediately prior to euthanasia did not induce an increase in bladder pressure in any of the animals. Considerable fluid flow out the urethra was observed during each stimulation, inducing increased pressure inside the bladder. Complete bladder denervation was confirmed in these animals by the sacral root transection–induced disappearance of bladder contraction upon conus medullaris stimulation at the time of bladder nerve transection.
In all experimental dogs that received surgical transection of ventral and dorsal spinal sacral (S1, S2) roots followed by GF NT to the bladder, fluorogold-labeled neuronal cell bodies were observed in the lateral ventral horns and in the zona intermedia of upper lumbar (L1, L2) spinal segments, indicating regrowth of motor axons from the lumbar cord to the bladder, and thus reinnervation of the bladder with the single exception of the right side of dog 10 in which no fluorogold-labeled lumbar cord cell bodies were observed. No fluorogold-labeled neurons were observed in the other lumbar cord segments (L3–L7). There were also a few fluorogold-labeled neuronal cell bodies in the sacral cord at S3, but considerably fewer in number than in the upper lumbar cord. Figure 2 shows representative micrographs of these fluorogold-labeled cells in the L1, L2, and S3 spinal cord segments. There were very few labeled cell bodies in the coccygeal cord bilaterally in one animal and unilaterally in two others (Table 1, dogs 4–6). ANOVA revealed significant differences in the number of fluorogold-labeled neurons between the sacral and lumbar cord regions (1.6 ± 1.4 and 20 ± 6, respectively, for 1-month delay versus 1.6 ± 2.0 and 18 ± 10, respectively, for the 3-month delay, p = 0.0059) and in the cord region by surgical group interaction (p < 0.0001). Post hoc analyses revealed statistically significant differences between each GF NT group compared to sham controls (p < 0.01 for each), but no differences between the 1-month and 3-month delay GF NT groups.
Since sacral cell bodies that innervate the bladder are parasympathetic motor, which are smaller in size than somatic motor cell bodes, and since the GF nerve contains both parasympathetic motor and somatic motor cell bodies to the cremasteric muscle, we wanted to examine whether the cell bodies innervating the bladder after GF NT were of similar diameters as in sham animals. We found that the mean diameters of fluorogold-labeled neurons in the upper lumbar spinal cord were not significantly different between the two GF NT groups (25 ± 2 μm for 1-month delay, 23 ± 5 for 3-month delay, p = 0.3154). We also found that there were also no statistically significant differences in the mean diameter of fluorogold-labeled neurons in the GF NT groups compared to sham control animals in the upper sacral region (25 ± 6, 24 ± 9, and 18 ± 10 μm for 1-month delay, 3-month delay, and sham-operated controls, respectively, p = 0.5101). This last results suggests that similar types of motor neurons reinnervate the bladder from the lumbar cord via the transferred GF nerves as in the sham animals.
DISCUSSION
While a cure for SCI-induced paralysis is certainly one of the ultimate goals of research in this area, an improvement in the quality of life by promoting functional recovery in the short term is a more realistic goal that may be achievable in a more immediate time frame. In a survey study of 681 SCI patients, regaining bladder and bowel function was of shared importance to both paraplegics and quadriplegics, and was rated as more important than regaining walking movement in paraplegics (Anderson, 2004). In patients with spastic bladders resulting from “upper motor lesions” which spare the cell bodies in the S2-4 spinal cord segments that innervate the bladder, bladder stimulation strategies with neuroprosthetic implants have been developed that can achieve bladder emptying (Brindley et al., 1986). However, bladder reinnervation strategies are required to achieve bladder emptying in patients with flaccid bladder paralysis resulting from “lower motor lesions” in which the sacral cord cell bodies innervating the bladder are damaged or their axons are severed.
Results of this study provide initial proof of concept that GF to pelvic nerve transfer is a potentially viable clinical approach to reinnervation of the LMN-lesioned urinary bladder. The 1-month time point was chosen as the shortest practical time point to allow the animal to completely recover from the first surgical procedure. The 3-month time point was chosen, since in human patients, following a traumatic SCI, this is the amount of time that some return of bladder function might be expected to occur if it is to occur at all (Little et al., 1999; Wein, 2002). The evidence presented in this canine model of bladder reinnervation by the transferred GF nerve includes the following: (1) increased bladder pressure and urethral fluid flow following electrical stimulation in four out of 20 nerve cuff electrodes implanted on the transferred GF nerves, (2) bilateral pelvic nerve stimulation induced bladder pressure and urethral fluid flow in three of four denervated animals with 1-month delay GF NT and in five of six denervated animals with 3-month delay GF NT, and (3) abundant lumbar spinal cord cell bodies retrogradely labeled with fluorogold injected into the bladder in all 10 of the GF NT animals except one animal on one side.
In a few of the animals, a small number of fluorogold-labeled cell bodies were observed in the S3 cord segment (bilaterally in two and unilaterally in two of the four 1-month delay GF NT animals; bilaterally in two and unilaterally in two of the six animals with 3-month delay GF NT). Complete functional denervation in all of these animals was achieved by transecting S1 and S2 spinal roots, and was confirmed at the time of nerve transection by the inability to induce bladder pressure increases during stimulation of the entire conus medullaris with an epidural electrode. The low number of sacral cord cell bodies that were retrogradely labeled from the bladder was primarily located in the S3 cord segment. Since we cut the S1 and S2 roots, any anomalous S3 innervation to the bladder would have been spared. In our pervious reports (Ruggieri et al., 2008; Ruggieri et al., 2006) and in this current report, only two nerve roots on each side of the spinal cord (S1 and S2) were found to induce bladder contractions during electrical stimulation in all dogs studied. However, it is possible that a very few number of motor fibers innervate the bladder in some animals through S3 roots; however, these were apparently below the level needed to induce bladder pressure increases during conus medullaris stimulation.
There were also a very few number of coccygeal cord cell bodies retrogradely labeled by fluorogold injected into the bladder. In our previous study with GF NT performed immediately following sacral bladder root transection, we found a similar few number of coccygeal cord cell bodies in one side of one of the four animals (Ruggieri et al., 2008). During the nerve transection procedure, no attempt was made to reinnervate the severed nerve roots in the lumbosacral spine and 15-mm sections of the transected roots that induced bladder contraction were removed in an attempt to prevent this possibility. Nevertheless, the possibility exists for that a coccygeal root was nicked during the laminectomy and transection procedure, and that these neural processes in these roots sprouted axons in that grew into the distal cut ends of the severed S1 and S2 roots to also reinnervate the bladder. However, because there were 2–18-fold more labeled cell bodies in the upper lumbar than the S3 cord, and 9–50-fold more labeled cell bodies in the upper lumbar than the coccygeal cord in these animals, bladder reinnervation by the genitofemoral nerve is the most likely explanation for the successful pelvic nerve stimulation induced bladder pressure increases in these animals.
In 16 out of the 20 implanted electrodes, quasi trapezoidal waveform electrical stimulation failed to induce increases in bladder pressure whereas in these same animals, direct stimulation of 16 out of 20 pelvic nerves with rectangular waveform electrical stimulation did induce increased bladder pressure. Explanations for this discrepancy include incomplete initial bladder denervation, incomplete bladder reinnervation at the time of stimulation of the implanted electrodes, the use of quasi trapezoidal waveform as opposed to rectangular waveform stimulation and disruption of electrical continuity between the ends of the electrode leads and the nerve contact surfaces. As described above, incomplete bladder denervation was mainly ruled out by stimulation of the entire conus medullaris after sacral root transection. At a nerve growth rate of 1 mm per day, sufficient time should have elapsed between the reinnervation surgery and the electrode stimulation for the nerve regrowth to reach the bladder. We chose to stimulate the implanted electrodes using quasi trapezoidal as opposed to rectangular waveform stimulation based on previous studies showing that this waveform selectively activates the small diameter axons that normally innervate the urinary bladder (Bhadra et al., 2001; Grunewald et al., 1998). It is possible that larger diameter axons reinnervated the bladder from the transferred genitofemoral nerve which were not activated by the quasi trapezoidal waveform stimulation and the difference in stimulation waveform as well as incomplete reinnervation remain possible explanations for the discrepancy between results of implanted electrode stimulation and direct pelvic nerve stimulation immediately prior to euthanasia. However, neither of these can provide an explanation for the discrepancy between the ability to increase bladder pressure by direct nerve stimulation in 16 of the 20 instances and the failure of the same, rectangular waveform stimulation to increase bladder pressure when applied to the implanted electrodes immediately prior to euthanasia. Based on our previous findings (Ruggieri et al., 2006) and the finding of the present study that some increase in bladder pressure could be produced by stimulation of four out of the 20 implanted electrodes using the quasi trapezoidal waveform, electrode failure in 16 of the 20 implants at the time of initial electrode stimulation and failure of all electrodes by the end of the study appears to be the most likely explanation.
The bladder pressure increases induced by FES stimulation was very low, an average of 2.2 cm H2O in the four of 20 implanted electrodes and 5.1 cm H2O in the 16 of 20 direct pelvic nerve stimulations. Bladder denervation by transection of the sacral roots likely results in denervation of urethral continence mechanisms as well as detrusor micturition mechanisms. Thus there was very little bladder outlet resistance to fluid flow in these animals so that these very low increases in bladder pressure allowed fluid to flow from the bladder out the urethra. These bladder pressure increases resulting from stimulation of the implanted electrodes as well as direct pelvic nerve stimulation are lower that what might be expected using a closed bladder outlet.
The findings of this study pave the path for clinical application of this nerve transfer surgery in human patients with LMN lesions resulting in flaccid bladder paralysis. Since the GF nerve is sensory to the skin over the femoral triangle in the upper thigh and from the scrotum or labia majora, and shares somatic motor input to the cremasteric muscle with the ilioinguinal nerve, it is unlikely that there is any clinical consequence to sacrificing the GF nerve for this purpose. Such a transfer would provide some degree of motor control of urinary bladder function for emptying but would likely not provide the voluntary motor control of the function of the bladder sphincter involved in controlling urinary continence. To achieve voluntary control of urinary continence as well as bladder emptying will require development of additional strategies for reinnervation of the pudendal nerve supplying innervation to the external urethral sphincter striated muscle.
ACKNOWLEDGMENTS
We would like to acknowledge the expert perioperative and post-operative veterinary care provided by Bernadette Simpkiss. Mamta Anim, Shreya Amin, and Phyllis Beaton are also acknowledged for their expert technical assistance in the histologic studies. This work was supported by a research grant from the Shriners Hospitals (to M.R.R.).
Footnotes
DISCLOSURE STATEMENT
No competing financial interests exist.
REFERENCES
- Anderson KD. Targeting recovery: priorities of the spinal cord–injured population. Journal of Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Bhadra N, Grunewald V, Creasey G, Mortimer JT. Urethral pressure profiles in the female canine implanted with sacral anterior nerve root electrodes. World J. Urol. 2001;19:272–277. doi: 10.1007/pl00007100. [DOI] [PubMed] [Google Scholar]
- Brindley GS, Polkey CE, Rushton DN, Cardozo L. Sacral anterior root stimulators for bladder control in paraplegia: the first 50 cases. J. Neurol. Neurosurg. Psychiatry. 1986;49:1104–1114. doi: 10.1136/jnnp.49.10.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzen MA, Sollmann H. Reinnervation of the urinary bladder after microsurgical reconstruction of transsected caudal fibres. An experimental study in pigs. Urol. Res. 1982;10:141–144. doi: 10.1007/BF00255957. [DOI] [PubMed] [Google Scholar]
- Grunewald V, Bhadra N, Creasey GH, Mortimer JT. Functional conditions of micturition induced by selective sacral anterior root stimulation: experimental results in a canine animal model. World J. Urol. 1998;16:329–336. doi: 10.1007/s003450050076. [DOI] [PubMed] [Google Scholar]
- Jezernik S, Craggs M, Grill WM, Creasey G, Rijkhoff NJM. Electrical stimulation for the treatment of bladder dysfunction: current status and future possibilities. Neurol. Res. 2002;24:413–430. doi: 10.1179/016164102101200294. [DOI] [PubMed] [Google Scholar]
- Little JW, Ditunno JF, Jr., Stiens SA, Harris RM. Incomplete spinal cord injury: neuronal mechanisms of motor recovery and hyperreflexia. Arch. Phys. Med. Rehabil. 1999;80:587–599. doi: 10.1016/s0003-9993(99)90204-6. [DOI] [PubMed] [Google Scholar]
- Luria V, Laufer E. Lateral motor column axons execute a ternary trajectory choice between limb and body tissues. Neural Dev. 2007;2:1–23. doi: 10.1186/1749-8104-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouton PR. Principals and Practices of Unbiased Sterology: An Introduction for Bioscienctists. The John Hopkins University Press; Baltimore: 2002. [Google Scholar]
- Ruggieri MR, Braverman AS, D'Andrea L, et al. Functional reinnervation of the canine bladder after spinal root transection and immediate end-on-end repair. J. Neurotrauma. 2006;23:1125–1136. doi: 10.1089/neu.2006.23.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri MR, Braverman AS, D'Andrea L, McCarthy J, Barbe MF. Functional reinnervation of the canine bladder after spinal root transection and immediate somatic nerve transfer. J. Neurotrauma. 2008;25:214–224. doi: 10.1089/neu.2007.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerrebroeck PE, Koldewijn EL, Rosier PF, Wijkstra H, Debruyne FM. Results of the treatment of neurogenic bladder dysfunction in spinal cord injury by sacral posterior root rhizotomy and anterior sacral root stimulation. J. Urol. 1996;155:1378–1381. doi: 10.1097/00005392-199604000-00069. [DOI] [PubMed] [Google Scholar]
- Wein AJ. Neuromuscular dysfunction of the lower urinary tract and its management. In: Walsh PC, Retic AB, Vaughan JED, Wein AJ, editors. Campbell's Urology. W.B. Saunders; Philadelphia: 2002. pp. 931–1026. [Google Scholar]
- Zempoalteca R, Martinez-Gomez M, Hudson R, Cruz Y, Lucio RA. An anatomical and electrophysiological study of the genitofemoral nerve and some of its targets in the male rat. J. Anat. 2002;201:493–505. doi: 10.1046/j.1469-7580.2002.00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]