Abstract
Objective
PAD is characterized by impaired blood flow to the lower extremities, causing claudication and exercise intolerance. The mechanism(s) by which exercise training improves functional capacity is not understood. This study tested the hypothesis that in peripheral artery disease (PAD) patients who undergo supervised exercise training, increases in capillary density (CD) in calf muscle take place before improvements in peak oxygen uptake (VO2).
Methods and Results
35 PAD patients were randomized to 12 weeks of directly-supervised or home–based exercise training. Peak VO2 testing and gastrocnemius muscle biopsies were performed at baseline and after training. Capillary density (endothelial cells/mm2) was measured using immunofluorescence staining. After 3 weeks of directly-supervised training, patients had an increase in CD (216±66 vs 284±77, p<0.01) but no increase in peak VO2. However after 12 weeks, peak VO2 increased (15.3±2.8 vs 16.8±3.8, p<0.01), while in muscle CD remained increased over baseline but there were no changes in markers of oxidative capacity. Within subjects, CD was related to peak VO2 before and after directly-supervised training.
Conclusions
Changes in capillary density in ischemic muscle with training may modulate the response to training and those changes precede the increase in VO2.
Keywords: Angiogenesis, capillaries, peripheral vascular disease, exercise
Peripheral artery disease (PAD) is caused by atherosclerotic stenoses in the peripheral arterial tree that impairs blood flow to the lower extremity. Recent statistics reported by the American Heart Association state that approximately eight million patients in the United States are affected with PAD, with an even larger number remaining undiagnosed.1,2 Approximately one-third of patients with PAD have intermittent claudication, defined as pain in one or both legs during exercise that is relieved with rest. Claudication causes severe exercise intolerance manifested by impaired walking ability and peak oxygen uptake values (VO2) that are 50% lower compared with matched controls (similar to class II–III heart failure), which both diminishes quality of life and is associated with an increase in mortality.3 To date, the best non-invasive treatment to improve functional capacity and peak VO2 in those who suffer from PAD remains supervised exercise training.4
The pathophysiology of intermittent claudication and the mechanism(s) by which exercise training improves functional capacity in patients with PAD remains poorly understood. Large conduit artery blood flow is certainly reduced relative to metabolic demand during exercise in PAD patients with claudication, but changes in calf blood flow that follow exercise training do not explain the increased functional capacity in PAD patients.5 However, less is known about the involvement of the microvasculature, which may have great impact on the metabolic potential of working muscle.6 A decreased capillary supply to skeletal muscle has been shown in PAD by others although correlation with functional outcomes is not well established.7,8 The role of angiogenesis, which is the growth and proliferation of small blood vessels from existing vascular structures, in improving exercise capacity in PAD is not known. However since exercise is a potent stimulus for angiogenic pathways and increased capillary density (CD),9,10,11,12,13,14,15 understanding whether exercise-induced angiogenesis occurs in patients with PAD is a key question.
Therefore, the rationale for this study was that exercise intolerance in PAD patients may be related to skeletal muscle microvascular perfusion, as measured by capillary density, and potentially improved by exercise training. This study tested the hypothesis that in PAD patients who undergo supervised exercise training, increases in CD in calf muscle occur and that these improvements take place before improvements in peak oxygen uptake (VO2). We also proposed in this study that there is a relationship between CD and peak VO2 in patients with PAD that to our knowledge has not been shown previously.
METHODS
Subjects
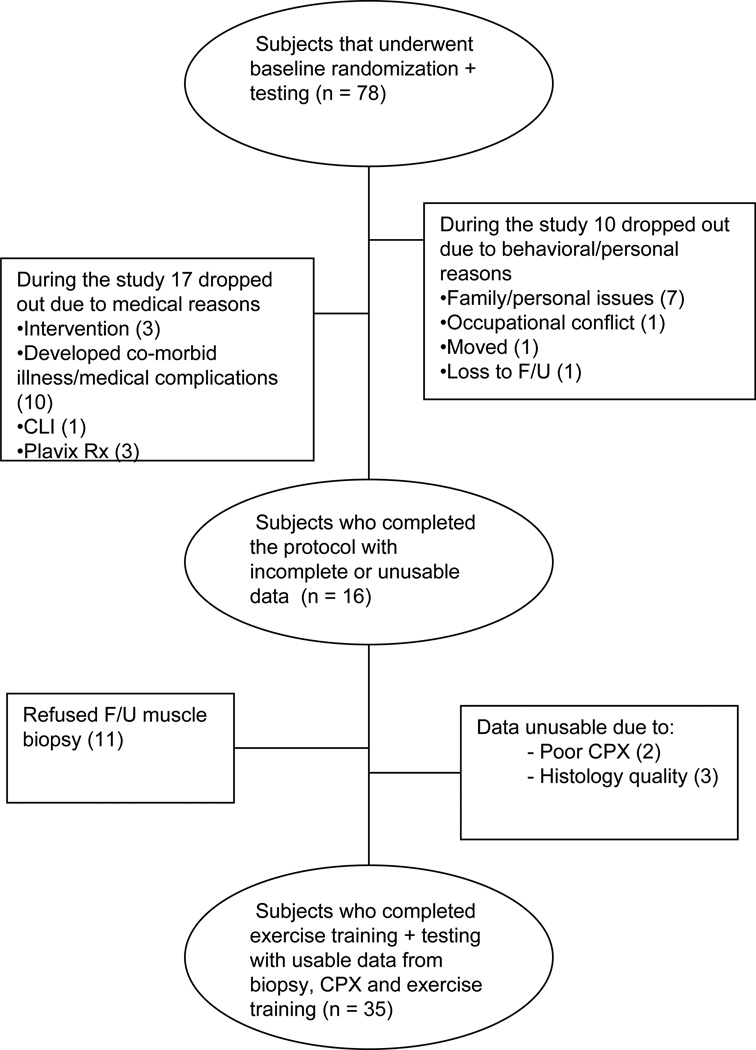
Subjects were selected from a larger randomized clinical trial of home vs. supervised-exercise in PAD subjects as shown in Figure 1. Seventy-eight subjects with PAD were randomized and completed baseline testing. During the course of the study, 27 subjects did not complete the protocol. Figure 1 categorized these reasons as due to personal (e.g. family, job) or medical reasons (e.g. intervention, illness). Fifty-one subjects completed the protocol; however, 16 of these did not have complete data sets. Figure 1 also lists the reasons for unusable data from those who did complete the protocol. Thirty-five subjects completed the protocol with complete usable data sets.
Figure 1.
Flow Chart of Enrollment and Completion of Subjects.
Subjects were recruited from the clinics and community at Duke University Medical Center and the University of Colorado School of Medicine. PAD subjects were selected who had symptom-limiting intermittent claudication and an ankle brachial index (ABI) <0.90 at rest or a 20% decrease in ABI post exercise or angiographic evidence of PAD. Subjects were required to be on a stable medical regimen including statin, anti-platelet, and anti-hypertensive medications, as indicated. Exclusion criteria included critical limb ischemia, severe peripheral neuropathy, diabetes mellitus, revascularization for PAD within the prior three months, unstable angina or severe coronary artery disease or other conditions that would prohibit cardiopulmonary exercise (CPX) testing or training. All subjects were sedentary prior to enrollment. All subjects were informed of testing protocols and the potential risks and benefits of participation. Each subject provided written informed consent before enrollment in the study. Group break-down was; supervised exercise (n=15) and home exercise (n=20) program. The Institutional Review Boards at Duke University and University of Colorado approved the research protocols.
Baseline Testing/Skeletal Muscle Biopsy
All subjects underwent ABI and cardiopulmonary exercise testing prior to skeletal muscle biopsy. ABI testing was performed in both legs in the supine position after 10 minutes of rest. The lowest ABI value was used for analysis. All subjects underwent a maximal CPX with a 12-lead electrocardiogram and expired gas analysis on a treadmill. Expired gases were analyzed continuously using a ParvoMedics (Sandy UT, USA) or a MedGraphics (St. Paul, MN, USA) unit and averaged in 15-second intervals. The Gardner graded treadmill protocol (performed at a standard speed (2 miles/hour) with a 2% grade increase every 2 minutes) was used for the cardiopulmonary test. Peak VO2 (ml/kg/min) was measured in all patients. Oxygen pulse was acquired by the following equation: Peak VO2 (L/min)/Peak heart rate.
Skeletal muscle biopsies were taken from the medial aspect of the gastrocnemius muscle at rest at least 24 hours after CPX testing. Biopsies were delayed by 24 hours in order to ensure that the skeletal muscle was in a resting metabolic state. A modified Bergstrom needle technique was utilized to obtain multiple 20–40 milligrams samples of skeletal muscle following local anesthesia with 2% lidocaine and a 1 cm skin incision.16 Two samples were snap frozen for citrate and myoglobin analysis. A separate sample was embedded in cross-section using optical cutting temperature (OCT) tissue freezing medium (Tissue-Tek®, Sakura Finetek USA, Inc., Torrance, CA), snap frozen in liquid nitrogen, and stored at −80°C for histologcal analyses. Muscle biopsies were performed at 0, 3 and 12 weeks in the supervised exercise group and 0 and 12 weeks in the home exercise group.
Exercise Training
PAD subjects randomized to supervised exercise came to medically supervised sessions three times a week until 36 sessions were completed. No subject exceeded 16 weeks to complete the 36 sessions. All subjects exercised on a treadmill at the workload that claudication onset was documented from the baseline CPX. Subjects were asked to exercise to near maximal pain utilizing a standardized claudication scale, at which time the subject stepped off the treadmill and rested until claudication pain subsided. Exercise and rest cycles were repeated during each exercise training session until the accumulation of 30–40 minutes of exercise was completed, referring to the actual time walked not including rest breaks. After a subject was able to walk for 8–10 minutes at their initial workload, speed and elevation was increased to elicit claudication again. To provide optimal medical care for subjects not randomized to supervised exercise, subjects in the home exercise group were given identical exercise instructions as the supervised group, however were asked to perform the exercise training on their own at home with an exercise prescription following the ACC-AHA guideline recommendation for PAD exercise training (Class1 level evidence A)17,18 at the time of the study according to current recommendations. Of note, these are also the current recommendations. All exercise training for both supervised and home groups were recorded for number of sessions per week and minutes per sessions. For supervised subjects this was recorded by an exercise physiologist conducting the training protocol. For the home subjects, patients were asked to complete an exercise diary which was collected at the end of the 12 weeks.
Histological Analysis/Indirect Immunofluorescence
Frozen muscle sections (7 micrometer thick) were cut using a Leica CM-1950 cryostat, and placed on positively charged slides. The slides were stored at −80°C until needed. Sections were removed from the freezer and allowed to reach room temperature (RT). Sections were fixed by immersion into 100% ice-cold acetone for 10 minutes, air dried for 10 min (RT), and then rehydrated in PBS for 5 minutes. Sections were blocked for 30 minutes with 10% normal goat serum in PBS containing 0.5% cold-water fish skin gelatin (Sigma). Endothelial cells were detected using a mouse anti-human CD31 (clone 9G11, 20 µg/ml; R&D, Inc.) followed by goat anti-mouse Alexa-Fluor-488 (40 µg/ml; Invitrogen) as previously described.19 Hybridoma lines BA-D5 and SC-71 were obtained from the ATCC (Manassas, VA).20 Hybridomas were cultured and purified by the Lymphocyte Culture Center at the University of Virginia. BA-D5 (8.9 µg/ml) and SC-71 (12.3 µg/ml) were co-incubated overnight at 4°C in blocking solution plus 5% normal mouse serum. Slides were washed twice in PBS and coverslips were applied using Prolong-Gold (Invitrogen).
Images were captured using a Zeiss LSM 510-UV confocal microscope at 100× magnification (final). A blinded observer with no knowledge of the group assignment, or time point with the group, analyzed the images using Image-Pro Plus 4.5.1. Fibers that were un-stained by either BA-D5 or SC-71 were counted as Type IId/x. Capillary density for each sample was calculated by dividing the total number of CD31-positive capillaries by the muscle fiber area (mm2 of tissue, which was measured using Image Pro Plus) per section. Examination of the capillary:fiber ratio were performed on individual fibers that a) did not touch 2 pre-selected adjacent boundaries of the image and b) where more than 75% of the circumference of the fiber was seen.
Hyperemic Limb Blood Flow
In a sub-set of (9 supervised-exercise and 15 home-exercise) subjects, calf blood flow was measured in the supine position by venous occlusion strain-gauge plethysmography (DE Hokanson, Issaquah, WA) at rest and during reactive hyperemia (RH) immediately after release of 5 minute cuff occlusion, as previously described.21,22,23 The involved leg (lowest ABI) was supported just above the level of the heart, and a mercury-in-Silastic strain gauge was placed around the widest part of the calf. Prior to all assessment an ankle cuff was inflated to 50 mmHg above systolic pressure for the 60 seconds to eliminate foot circulation from the measurement. A pneumatic cuff was placed on the thigh and inflated to 30 mmHg to achieve venous occlusion. The cuff occlusion was maintained for several cardiac cycles (4–6 cycles) to obtain resting blood flow measurements. Blood flow was expressed as ml/100 ml tissue/min. Resting blood flow was calculated as the average of five separate measurements in each limb. Peak RH blood flow was determined after limb ischemia induced by the proximal thigh cuff that was inflated 50 mmHg above systolic blood pressure for 5 min. Post-occlusion RH blood flow measurements were made every few seconds, and the highest value achieved was taken as the peak value. Analysis of peak blood flow was taken from nine supervised and 15 home exercise subjects.
Measures of Markers of Oxidative Metabolism
In addition to the assessment of fiber type composition by histology, a 25–30 mg sample that was immediately snap frozen into liquid nitrogen at the time of biopsy and stored at − 80° C and homogenized at the time of analaysis. Citrate synthase activity was measured using an enzymic colorimetric reaction between acetyl coenzyme A, oxaloacetic acid and 5,5’-Dithiobis-(2-nitrobenzoic acid)(DTNB) (Sigma, St. Louis, Missouri). The reaction was completed at room temperature where10 µg of protein lysate was combined with 178µl 1X reaction buffer, 2µl 30mM CoenzymeA and 2µl 10mM DTNB were pipette into a 96-well flat bottom plate (Costar, Corning, NY). Baseline activity was measured as repeated reads at 405nm using the Organon Teknika plate reader. Absorbency was measured in 10 second increments for 1.5 minutes. To each well, 10µl of 10mM oxaloacetic acid was added and enzyme activity was immediately re-measured with the plate reader. The absorbency over time was plotted for both baseline activity, and activity after the addition of oxaloacetic acid. The citrate synthase activity was calculated by the equation:
where Vol (ml) was the reaction volume, Venz(ml) was the volume of sample, and εmM was the extinction coefficient of TNB, which is 13.6 at 405nm. L(cm) is the pathlength for absorbance measurement which was 0.552 cm for the plates used. Citrate synthase activity was analyzed in 11 supervised exercise subjects and in six home exercise subjects. In addition, myoglobin levels were measured by ELISA (Calbiotech, Spring Valley, CA). Lysates were diluted 1:8000 and 20 µl of lysate was dispensed in each well (approximately 2.5 to 25ng of protein) with the enzyme conjugate reagent, incubated at room temperature for 45 minutes and the wells were washed with washing buffer. In each well 100 µl of TMB was dispensed and the reaction incubated at room temperature for 20 minutes. The absorbency of samples was read at 450nm using the Organon Teknika plate reader. The absorbency readings were plotted on a standard curve giving the myoglobin concentration per sample as (ng myoglobin/ng of soluble protein). Myoglobin was analyzed in 13 supervised exercise subjects and in 16 home exercise.
Statistical Analysis
Differences in demographic and clinical characteristics between supervised and home exercise groups were determined using a one-way ANOVA. For categorical variables, differences were determined by chi square analysis. The primary hypothesis was that with supervised training, capillary density would increase prior to any changes in peak VO2. Subjects randomized to supervised training had three time points for assessment; baseline, week 3 and week 12. Therefore, a repeated measures, mixed model ANOVA with post hoc testing using the Bonferroni adjustment for multiple comparisons was performed for this analysis. The same analysis was performed between supervised and home groups where the common time points were baseline and 12 weeks for capillary density and peak VO2. First, change scores after training between groups were analyzed. Second, post-training measures were tested for differences after controlling for baseline measures. Bivariate correlations were performed to determine the relationships between capillary density and the functional variables peak VO2. Tabular data are presented as means ± SD. P values of < 0.05 were considered significant for all tests.
RESULTS
Patient Demographics and Exercise Adherence
Table 1 shows subject demographics and clinical characteristics which represents a typical clinical population with intermittent claudication across all variables. Subjects were well matched for age, race, gender, body habitus, ABI, medication use and smoking history between supervised and home exercise groups. ABI did not change throughout the study for either the supervised (pre 0.62±0.23 and post 0.63±0.25) or the home exercise group (pre 0.68± 0.19 and post 0.69±0.21). Weight did not change throughout the study for either the supervised (pre 78.3±15.7 kg and post78.7±15.6 kg) or the home exercise group (pre 77.9 ±11.9kg and 78.4± 12.7kg post). The home-exercise group trended toward exercising more days per week versus the supervised exercise group (3.0±1.3 vs. 2.3±0.3) and more minutes per week (104±59 vs. 81±21), but neither difference reached statistical significance.
Table 1.
Demographic and Clinical Characteristics of PAD Subjects.
| Supervised Exercise | Home Exercise | |
|---|---|---|
| Number | 15 | 20 |
| Age, years | 69±10 | 67±10.1 |
| Race, % Caucasian | 80% | 75% |
| Sex (men/women) | 7/8 | 11/9 |
| BMI, kg/m2 | 27.3±5.2 | 26.1±3.9 |
| ABI | 0.63±0.2 | 0.65±0.1 |
| Current Smoking,% | 20% | 10% |
| Past Smoker, % | 80% | 90% |
| Statin Use, % | 53% | 60% |
| ACE/ARB Use, % | 40% | 30% |
| Beta Blocker Use, % | 40% | 20% |
| Antiplatelet Use, % | 33% | 30% |
| Aspirin Use, % | 73% | 80% |
Values are mean ± SD or percentages.
Changes in Capillary Density and Peak VO2 by Type of Exercise
Supervised Exercise Training
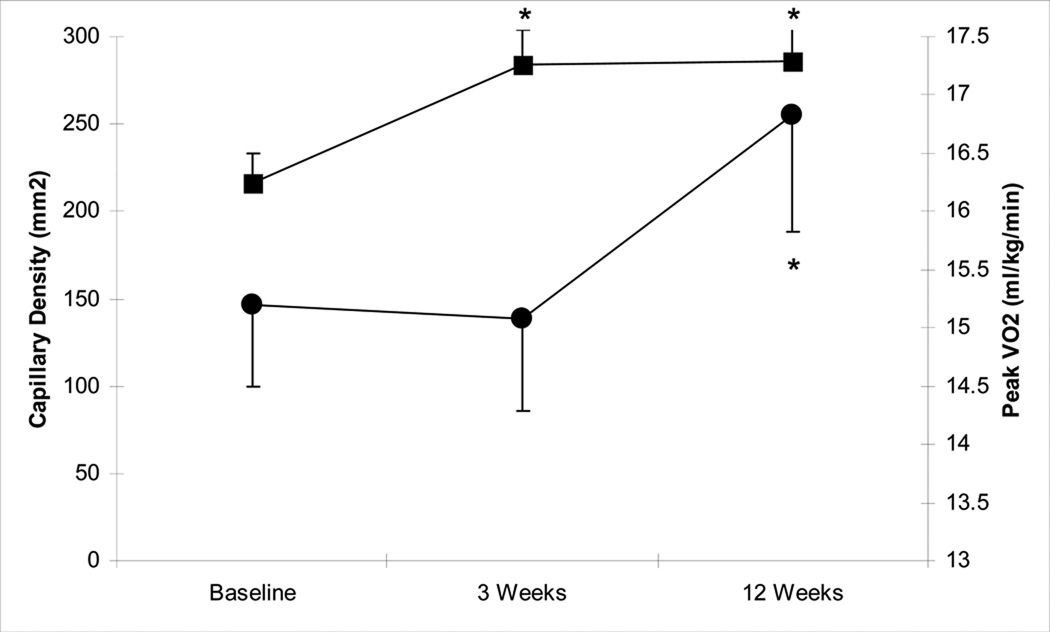
Figure 2 shows the changes during supervised exercise training for both capillary density and peak VO2. Subjects randomized to supervised training had three time points for assessment, baseline, Week 3 and Week 12. For the overall ANOVA model the p-value was significant at 0.0198. Post hoc testing revealed capillary density per area had a 31% increase from baseline (0 weeks) to three weeks post exercise training (216±66 vs. 284±77, p=0.049), while peak VO2 was unchanged. After 12 weeks of exercise training, capillary density (286±77) was virtually identical to the value obtained at the three week time point and remained greater than baseline (p=0.04). There was no statistically significant increase in capillary per fiber from baseline to three weeks post exercise training (1.72±0.5 vs 1.87±0.7, p=0.44), but approached significance at 12 weeks post exercise training (1.72±0.5 vs 2.04±0.7, p=0.08). Cross sectional fiber area was not different between time points in the supervised exercise group in either type I or type II fibers, nor did the cross sectional area change relate to changes in capillary density. Although peak VO2 did not show an increase from 0 to three weeks (p=0.68), it did increase significantly at 12 weeks compared to 0 weeks (15.3±2.8 vs. 16.8±3.8, p<0.01). From these results it appears that capillary density changes occur prior to changes in peak VO2 during supervised exercise training.
Figure 2.
Supervised exercise training concomitant time course of change for capillary density and peak VO2 (• peak VO2, ■ capillary density). * indicates significantly different versus 0 week, p<0.05.
Home Exercise Training
Capillary density per area remained unchanged from baseline to 12 weeks in the home exercise group (238±78 vs 235±91, p=0.91). Capillaries per fiber did not significantly increase (1.79±0.l5 vs 1.89±0.7, p=0.48). Similarly, peak VO2 remained numerically and statistically unchanged from baseline to 12 weeks (15.9±4.6 vs 15.9±4.7, p=0.99).
Between Group Changes in Capillary Density and Peak VO2
Our primary analysis was that the change in peak VO2 for the supervised group was 10.2 ± 13.1% versus 0.0 ± 15.7% for the home group approached significance (p = 0.09). Additional post hoc testing adjusting for baseline peak VO2, the 12 week peak VO2 was significantly different between groups (p=0.03). Capillary density per area approached significance at a p value of 0.09 as the supervised group showed a robust increase and the home group slightly decreased (Table 2).
Table 2.
Pre and Post Peak VO2, Skeletal Muscle and Oxygen Pulse Measures
| Supervised Exercise | Home Exercise | ||||
|---|---|---|---|---|---|
| 0 Week | 3 Week | 12 Week | 0 Week | 12 Week | |
| Peak VO2 | 15.3±2.8 | 15.1±3.1 | 16.8±3.7*§ | 15.9±4.6 | 15.9±4.7 |
| Capillarization (capillaries per mm2) | 216±66 | 284±77* | 286±76* | 238±78 | 235±91 |
| Capillary:Fiber | 1.72±0.5 | 1.87±0.7 | 2.04±0.7 | 1.79±0.5 | 1.89±0.7 |
| Fiber Type I (%) | 59±13 | 57±20 | 57±20 | 56±15 | 58±17 |
| Fiber Type IIa (%) | 21±9 | 22±10 | 25±11 | 23±10 | 22±12 |
| Fiber Type IId/x (%) | 20±13 | 20±16 | 17±15 | 21±11 | 20±13 |
| Oxygen Pulse (L/min) | 10.4±3.2 | 10.4±3.1 | 11.1±3.9† | 11.5±3.2 | 10.7±3.1 |
| Peak Blood Flow (ml/100 ml tissue/min) | 7.1±5.2 | 10.3±5.0 | 12.1±9.1† | 10.2±6.0 | 9.4±5.3 |
Values are mean ± SD .
Indicates difference between 0 Week in supervised group, p < 0.05
Indicates 12 week different between groups after controlling for baseline value, p <0.05
Indicates change from 0 to 12 weeks is different between groups, p < 05
Changes in Muscle Measures of Fiber Type, Peak Blood Flow, Oxygen Pulse and Oxidative Metabolism by Type of Exercise
Fiber-type composition (type I, IIa, and IId/x), peak blood flow and oxygen pulse (as shown in Table 2) did not change in the supervised or home-exercise group with training. In addition, analyses were performed on a sub-set of subjects to look for fold-changes in markers of oxidative metabolism specifically, citrate synthase activity and myoglobin from baseline to 12 weeks. In the supervised exercise group, citrate synthase was 1.10±0.89 fold increased at 12 weeks compared with baseline and in the home exercise group citrate synthase was 1.27 ± 0.51 fold increased at 12 weeks compared with baseline. In the supervised exercise group, myoglobin was 1.09 ± 0.74 fold increased at 12 weeks compared with baseline and in the home exercise group myoglobin was 0.73 ± 0.47 fold increased at 12 weeks compared with baseline. Thus, there was no significant difference between groups in terms of citrate synthase or myoglobin from baseline to 12 weeks.
Hemodynamic Changes Between Supervised and Home-Exercise Groups after 12 Weeks
Two measures, oxygen pulse and peak leg blood flow, changed significantly between groups at 12 weeks (Table 2). In both cases the supervised group showed a modest increase, while the home exercise group had a decrease.
Relationship Between Capillary Density and Peak VO2
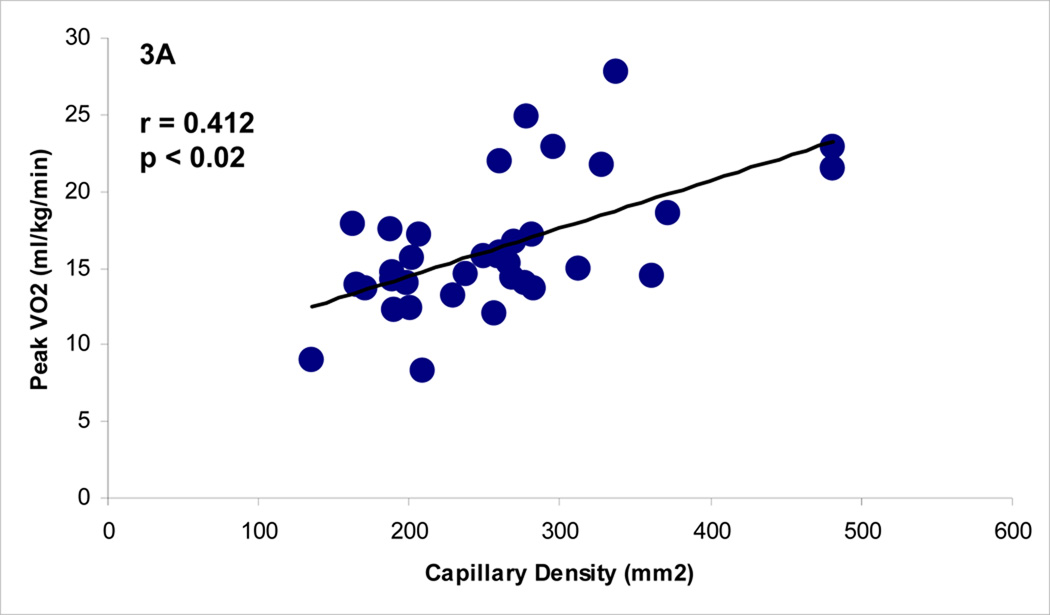
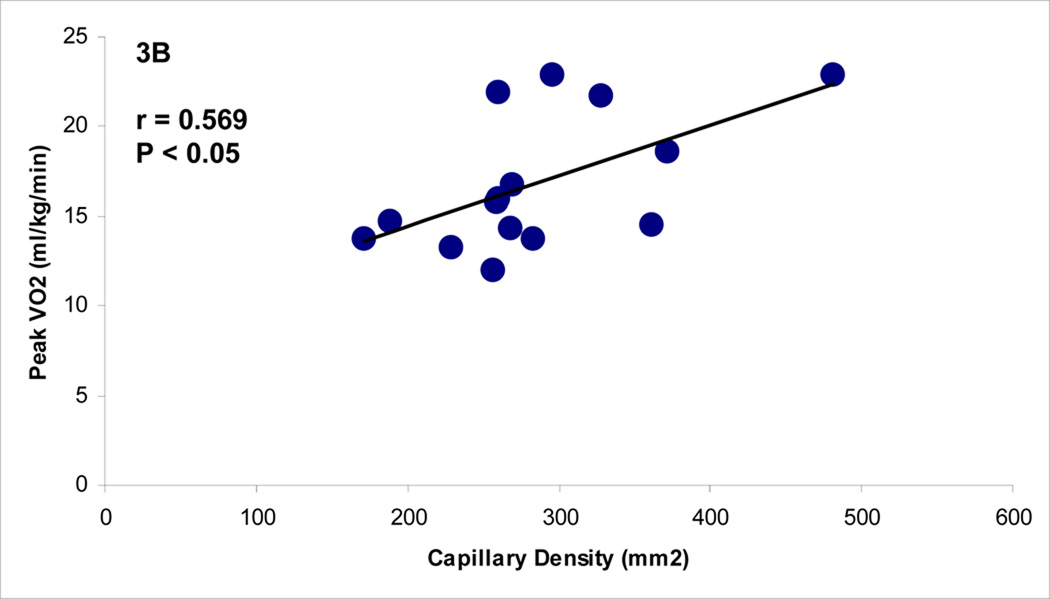
Before the start of exercise-training, there was a significant relationship between capillary density per area and peak VO2 (p < 0.02, r = 0.412) in all subjects, as shown in Figure 3. Following supervised exercise training, there also was a strong relationship between capillary density per area and peak VO2 (p < 0.03, r = 0.569). This relationship did not exist in the home exercise group at the end of the 12 weeks.
Figure 3.
A and 3B.The Relationship Between Capillary Density (mm2) and Peak VO2 at Baseline (all subjects) and Post-Exercise Training (Supervised Group).
DISCUSSION
This study was designed to test the hypothesis that increases in capillary density would occur in calf skeletal muscle prior to improvements in peak VO2 following exercise training in PAD. Indeed, after three weeks of supervised exercise training, PAD patients showed a significant increase in capillary density without a concomitant increase 15 in peak VO2 (Figure 2). However, after 12 weeks of exercise training, peak VO2 was also shown to increase without a significant additional increase in capillary density. These results were observed in the absence of changes in muscle oxidative markers such as fiber type, citrate synthase or myoglobin. The finding that citrate synthase, which is directly related to mitochondria content, did not increase after exercise training confirms our previous findings6 and may reflect a more global muscle myopathy associated with PAD. We are left to infer that the improved peak VO2 in subjects with PAD following supervised exercise is likely, at least in part, due to the increase in capillary density that might precede the collateral remodeling that is needed to increase perfusion; or that a period of increased perfusion may be necessary for an improvement in mitochondrial function, which may be disturbed in PAD. However we cannot exclude changes in other unidentified oxidative muscle measures.
The results of this study are worth comparing to what is known to occur in healthy subjects where two to five weeks of training induces an increase in peak VO2.24,25,26,27,28,29 Healthy individuals show an increased peak VO2 in the early phases of exercise training likely due predominantly to improved hemodynamics (e.g. stroke volume) and to a lesser extent to skeletal muscle adaptations. Furthermore, normal subjects also demonstrate improvements in oxygen pulse following 12 weeks or less of supervised exercise training that was not observed in the our present PAD study.30,31 The design of this study allowed for an intermediate time point, three weeks, measuring both capillary density (along with other oxidative markers) and peak VO2 in the supervised exercise group. A failure to improve peak VO2 following three weeks exercise training, as witnessed in this study, should be viewed as an abnormal maladaptive response to exercise training. Oxygen pulse, a surrogate for stroke volume, also failed to increase at this intermediate three week time point. It is possible that because claudication is the limiting factor for exercise tolerance in this population that exercise intensities or duration needed to achieve central hemodynamic improvements were not met. Despite limitations inherent in human studies, our data are consistent with the hypothesis that angiogenesis, manifested by an increase in capillary density in ischemic leg muscle, may be necessary before peak VO2 can be increased. To our knowledge, this is the first randomized, controlled human trial in subjects with PAD to demonstrate that finding.
In addition to the temporal association of changes in capillary density and peak VO2 in subjects with PAD following the onset of exercise, a second important finding was the relationship between capillary density and peak VO2 both at baseline in all subjects and following exercise training in the supervised group (Figure 3). Although this relationship exists in cross-sectional data with a wide range of peak VO2 in “healthy subjects”, it has not been demonstrated in a group of subjects with similar peak VO2 before or after exercise training. However we did not show a relationship between the change in capillary density and the change in peak VO2. Thus our data are not meant to suggest that angiogenesis in calf skeletal muscle is the sole mechanism that could account for the increases in peak VO2 seen in PAD patients following exercise training.
The third major finding was that despite being given similar exercise prescriptions, only patients in the supervised exercise training group, and not the home exercise group, improved peak VO2 and capillary density. It was hypothesized, based on previous investigations showing the superior improvements in exercise tolerance following supervised versus home training, that the home group would show little, if any, improvements. This hypothesis was correct and again shows the clinical efficacy of a supervised exercise intervention to improve functional capacity in PAD patients. Although when peak VO2 and capillary density (per area) were analyzed by change scores from 0 to 12 weeks, the differences between groups were close (p =0.07 and 0.09), they did not reach statistical significance. However, a second analysis comparing the 12 week peak VO2 between groups after controlling for baseline peak VO2 did show a difference (p <0.05). This observation, along with the acknowledgement that the pre-post peak VO2 in the supervised group improved (p <0.01) while the home group had no change demonstrates the superiority of a supervised exercise program in improving functional capacity.
The home exercise group was included as an optimal medical care group and interestingly the home exercise group tended to exercise more days per week than the supervised group (3.0 vs 2.3 days, NS) and did more minutes of exercise per week (104 vs 81 minutes, NS). However they did not improve their capillary density or peak VO2. Through training logs and empirical exit interviews (data not captured) we found that the home exercise subjects rarely exercised as recommended to the point of 3–4/5 pain severity of claudication, rested until it was relieved and then repeated to accumulate their minutes. Instead, despite instruction to the contrary, they exercised at an exercise level well below intolerable claudication thus failing to achieve an appropriate exercise intensity which have allowed the more substantial changes observed in the supervised group. Data from this study, as in previous studies, shows that supervised exercise is necessary for patients to achieve maximal benefit demonstrated by improved functional capacity. We now show that supervised exercise is also needed to gain increases in capillary density. If the home group had done the same intensity exercise (3–4/5 claudication) as the supervised group, we would have expected the results to be similar between groups. Although this was not the design or focus of the study, this is a key concept and supports the importance of supervised exercise versus home exercise for functional and skeletal muscle improvements in PAD. There are likely many reasons that home exercise has less clinical utility than supervised exercise; such as a patient not having access to a treadmill or gym membership, weather limiting outdoor walking, nowhere to rest while walking outside and a lack of motivation or natural avoidance to exercising in pain. Unfortunately, this study was not designed to determine why home exercise does not yield the beneficial results of supervised exercise, we can only speculate, at best, as to why this occurred in the present study and in previous studies. Future studies, capturing the minutes of exercise in 3–4/5 claudication versus < 3/5 pain, would be clinically helpful in understanding the mechanism of improvement.
Historically, measurements of hemodynamics by ABI and plethysmography have not proven to be strong predictors of exercise tolerance in PAD.32,33 In our study the ABI did not change in either group. Our data confirms previous investigations of leg blood flow in that despite a difference in peak blood flow between the supervised and home exercise, the increase in flow in the supervised group did not positively relate to increases in peak VO2. The relevance of microvasculature as measured by capillary density is that it represents the interface between hemodynamics and skeletal muscle; and therefore maybe more important than ABI. Improved capillary density increases the diffusion potential and blood resident time of the skeletal muscle, thereby improving oxygen extraction, substrate utilization, and oxidative capacity.13,34,35
There are several limitations in this study. First, we used five minutes of cuff occlusion during the plethysmography evaluation for post-hyperemic blood flow. Though this has been used by others 21,22,23 this is not a perfect measure of femoral artery endothelial function or true max flow measured with absolute minimum vascular resistance. A second limitation is that although we did not detect changes in CS or myoglobin with supervised exercise, we did not measure all proteins of oxidative metabolism and we cannot exclude that possibility that other proteins might be regulated in a different manner. Though not a true limitation, it is interesting to note that changes in capillary density (endothelial cells/mm2) were statistically significantly increased with supervised exercise. The two components of that measure (endothelial cells/ fiber and the mean area per fiber) changed but not statistically significantly in the expected direction to yield an overall change in capillaries/mm2. Specifically, following supervised training endothelial cells per fiber were numerically but not statistically greater and fiber area was numerically but not significantly lower. We did not power the study to detect these differences and additional studies would be needed to determine if one of these processes occurred first or was dominant in the angiogenic response we observed in subjects with PAD. A third limitation was that we did not prospectively collect detailed data in the home exercise training logs. Though, we collected total number of days and total number of minutes exercised in the home-exercise group but, unlike the supervised group, we do not have accurate data on whether that exercise induced claudication at all or for how long. Future studies are needed to test the hypothesis that the reason that supervised exercise resulted in angiogenesis in ischemic muscle and increases in peak VO2 in patients was due to presence and duration of exercise induced claudication.
In summary, the results of this study show a temporal dissociation between the timing of increased capillary density and the increased peak VO2 in patients with PAD treated with supervised exercise such that capillary density increased before a significant increase in peak VO2 occurred. The results also show a relationship between capillary density and peak VO2 both before and after supervised exercise training; such findings have not been reported in healthy normal subjects. These findings may suggest that it is necessary to increase microvasculature in order to improve exercise capacity in PAD patients. This is further supported by the lack of improvement in other skeletal muscle markers of oxidative capacity ; and the maladaptive response to peak VO2 and oxygen pulse compared to healthy subjects. Therefore, based on these results it is concluded that angiogenesis by supervised exercise training is an important mediator for improving peak VO2 in PAD patients.
Acknowledgments
Sources of Funding
This project was supported by R01 HL755752 from the National Institute of Health, National Heart, Lung, and Blood Institute and the Office of Research on Women’s Health, Office of the Director to BHA. Supported in part by Colorado CTSA grant 1 UL1 RR 025780 from NCRR/NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None of the authors have any conflicts of interest relative to the intervention.
REFERENCES
- 1.American Heart Association. Heart Disease and Stroke Statistics-2010 Update. http://americanheart.org.
- 2.Hirsch AT, Criqui MH, Treat-Jacobson D, Regensteiner JG, Creager MA, Olin JW, Krook SH, Hunninghake DB, Comerota AJ, Walsh ME, McDermott MM, Hiatt WR. Peripheral arterial disease detection, awareness, and treatment in primary care. JAMA. 2001;286:1317–1324. doi: 10.1001/jama.286.11.1317. [DOI] [PubMed] [Google Scholar]
- 3.Hiatt WR, Regesteiner JG, Hargarten ME, Wolfel EE, Brass EP. Benefits of exercise training for patients with peripheral arterial disease. Ciculation. 1990;81:602–609. doi: 10.1161/01.cir.81.2.602. [DOI] [PubMed] [Google Scholar]
- 4.Stewart KJ, Hiatt WR, Regensteiner JG, Hirsch AT. Exercise training for claudication. N Eng J Med. 2002;347:1941–1951. doi: 10.1056/NEJMra021135. [DOI] [PubMed] [Google Scholar]
- 5.Parmenter BJ, Raymond J, Fiatarone SMA. The effect of exercise on haemodynamics in intermittent claudication: a systematic review of randomized controlled trials. Sports Med. 2010;40:433–447. doi: 10.2165/11531330-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Hiatt WR, Regensteiner JG, Wolfel EE, Carry MR, Brass EP. Effect of exercise training on skeletal muscle histology and metabolism in peripheral arterial disease. J Appl Physiol. 1996;81:780–788. doi: 10.1152/jappl.1996.81.2.780. [DOI] [PubMed] [Google Scholar]
- 7.Clyne CA, Mears H, Weller RO, O'Donnell TF. Calf muscle adaptation to peripheral vascular disease. Cardiovasc Res. 1985;19:507–512. doi: 10.1093/cvr/19.8.507. [DOI] [PubMed] [Google Scholar]
- 8.Askew CD, Green S, Walker PJ, Kerr GK, Green AA, Williams AD, Febbraio MA. Skeletal muscle phenotype is associated with exercise tolerance in patients with peripheral arterial disease. J Vasc Surg. 2005;41:802–807. doi: 10.1016/j.jvs.2005.01.037. [DOI] [PubMed] [Google Scholar]
- 9.Annex BH, Torgan CE, Lin P, Taylor DA, Thompson MA, Peters KG, Kraus WE. Induction and maintenance of increased VEGF protein by nerve stimulation in rabbit skeletal muscle. Amer J Physiol. 1998;43:H860–H867. doi: 10.1152/ajpheart.1998.274.3.H860. [DOI] [PubMed] [Google Scholar]
- 10.Hudlicka O, Dodd C, Renkin EM, Gray SD. Early changes in fiber profiles and capillary density in long term stimulated muscle. Am J Phsyiol. 1982;243:H528–H535. doi: 10.1152/ajpheart.1982.243.4.H528. [DOI] [PubMed] [Google Scholar]
- 11.Ingjer F. Effects of endurance training on muscle fiber ATP-ase activity, capillary supply and mitochondrial content in man. J. Physiol. 1979;294:419–432. doi: 10.1113/jphysiol.1979.sp012938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hepple RT. Skeletal muscle: microcirculatory adaptation to metabolic demand. Medicine and Science in Sport and Exercise. 2000;32:117–123. doi: 10.1097/00005768-200001000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Andersen P, Henriksson J. Capillary supply of the quadriceps femoris muscle of man: adaptive response to exercise. J Physiol. 1977;270:677–690. doi: 10.1113/jphysiol.1977.sp011975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klausen K, Andersen LB, Pelle I. Adaptive changes in work capacity, skeletal muscle capillarization and enzyme levels during training and detraining. Acta Physiol Scand. 1981;113:9–16. doi: 10.1111/j.1748-1716.1981.tb06854.x. [DOI] [PubMed] [Google Scholar]
- 15.Robbins JL, Duscha BD, Bensimhon DR, Wasserman K, Hansen JE, Houmard JA, Annex BH, Kraus WE. A sex-specific relationship between capillary density and anaerobic threshold. J Appl Physiol. 2009;106:1181–1186. doi: 10.1152/japplphysiol.90947.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- 17.Hirsch AT, Haskal ZJ, Hertzer NR, et al. ACC/AHA 2005 guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): executive summary a collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease) endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. J Am Coll Cardiol. 2006;47:1239–1312. doi: 10.1016/j.jacc.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Gardner AW, Poehlman ET. Exercise rehabilitation programs for the treatment of claudication pain. A meta-analysis. JAMA. 1995;274:975–980. [PubMed] [Google Scholar]
- 19.Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary density of skeletal muscle: A contributing mechanism of exercise intolerance in class II–III chronic heart failure independent of other peripheral alterations. J Amer Coll Cardiol. 1999;33:1956–1963. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 20.Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lømo TJ. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. Mus. Res. Cell Mot. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- 21.Hellige G, Ensink FB, Baller D, Prennschutz-Schutzenau H, Sigmund-Duchanova H, Zipfel J. Measurement of arterial and venous reactivity by an advanced strain gauge plethysmograph. Angiology. 1979;30:539–548. doi: 10.1177/000331977903000804. [DOI] [PubMed] [Google Scholar]
- 22.Bartoli V, Dorigo B. Comparison between reactive and exercise hyperemia in normal subjects and patients with peripheral arterial disease. Angiology. 1979;30:40–47. doi: 10.1177/000331977903000105. [DOI] [PubMed] [Google Scholar]
- 23.Sanada H, Higashi Y, Goto C, Chayama K, Yoshizumi M, Sueda T. Vascular function in patients with lower extremity peripheral arterial disease: a comparison of functions in upper and lower extremities. Atherosclerosis. 2005;178:179–185. doi: 10.1016/j.atherosclerosis.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 24.Hickson RC, Hagberg JM, Ehsani AA, Holloszy JO. Time course of the adaptive responses of aerobic power and heart rate to training. Med Sci Sport Exer. 1981;13:17–20. [PubMed] [Google Scholar]
- 25.Gaessner GA, Poole DC, Gardner BP. Dissociation between VO2 max and ventilatory threshold responses to endurance training. Eur J Appl Physiol Occup Physiol. 1984;53:242–247. doi: 10.1007/BF00776597. [DOI] [PubMed] [Google Scholar]
- 26.Govindasamy D, Paterson DH, Poulin MJ, Cunnigham DA. Cardiorespiratory adaptation with short term training in older men. 1992;65:203–208. doi: 10.1007/BF00705082. [DOI] [PubMed] [Google Scholar]
- 27.Oberach A, Tonjes A, Kloting N, Fasshauer M, Kratzsch J, Busse MW, Paschke R, Stumvoll M, Bluther M. Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. 2006;154:577–585. doi: 10.1530/eje.1.02127. [DOI] [PubMed] [Google Scholar]
- 28.Aciero PJ, Vukovich MD, Holloszy JO, Racette SB, Kohrt WM. Comparison of short-term diet and exercise on insulin action in individuals with abnormal glucose tolerance. J Appl Physiol. 1999;86:1930–1935. doi: 10.1152/jappl.1999.86.6.1930. [DOI] [PubMed] [Google Scholar]
- 29.Farrell PA, Barboriak J. The time course of alterations in plasma lipid and lipoprotein concentrations during eight weeks of endurance training. Atherosclerosis. 1980;37:231–238. doi: 10.1016/0021-9150(80)90008-8. [DOI] [PubMed] [Google Scholar]
- 30.Wilmore JH, Costill DL. Physiology of Sport and Exercise. Champaign, IL: Human Kinetics; 1994. [Google Scholar]
- 31.Hartley LH, Grimby G, Kilbom A, Nilsson NJ, Astrand I, Bjure J, Ekblom B, Saltin B. Physical training in sedentary middle-aged and older men. Cardiac output and gas exchange at submaximal and maximal exercise. Scand J Clin Lab Invest. 1969;24:335–344. doi: 10.3109/00365516909080170. [DOI] [PubMed] [Google Scholar]
- 32.Brass EP, Hiatt WR. Acquired skeletal muscle metabolic myopathy in atherosclerotic peripheral arterial disease. Vasc Med. 2000;5:55–59. doi: 10.1177/1358836X0000500109. [DOI] [PubMed] [Google Scholar]
- 33.Gardner AW, Skinner JS, Cantwell BW, Smith LK. Prediction of claudication pain from clinical measurements obtained at rest. Med Sci Sports Exerc. 1992;24:163–170. [PubMed] [Google Scholar]
- 34.Richardson RS, Grassi B, Gavin TP, Haseler LJ, Tagore K, Roca J, Wagner PD. Evidence of O2 supply-dependent VO2 max in the exercise-trained human quadriceps. J Appl Physiol. 1999;86:1048–1053. doi: 10.1152/jappl.1999.86.3.1048. [DOI] [PubMed] [Google Scholar]
- 35.Egginton S. Invited review: activity-induced angiogenesis. Pflugers Arch. 2009;457:963–977. doi: 10.1007/s00424-008-0563-9. [DOI] [PubMed] [Google Scholar]