Abstract
Radiation treatment or chemotherapy has been linked with a higher risk of secondary cancers such as therapy related Acute Myeloid Leukemia (tAML). Several of these cancers have been shown to be correlated to the introduction of double stranded breaks (DSB) and rearrangements within the Mixed Lineage Leukemia (MLL) gene. We used Zinc Finger Nucleases (ZFNs) to introduce precise cuts within MLL to examine how a single DNA DSB might lead to chromosomal rearrangements. A ZFN targeting exon 13 within the breakpoint cluster region of MLL was transiently expressed in a human lymphoblast cell line originating from a CML patient. Although FISH analysis showed ZFN DSB at this region increased the rate of MLL fragmentation, we were unable to detect leukemogenic rearrangements or translocations via inverse PCR. Interestingly, gene fragmentation as well as small interstitial deletions, insertions and base substitutions increased with the inhibition of DNA-PK, suggesting repair of this particular DSB is linked to non-homologous end joining (NHEJ). Although mis-repair of DSBs may be necessary for the initiation of leukemogenic translocations, a MLL targeted DNA break alone is insufficient.
Introduction
Under normal conditions, the MLL gene on Chromosome 11q23 (also called ALL-1, HRX and HTRX1) (1, 2, 3,4) encodes a transcription regulator of the HOX gene family and plays an important role in hematopoiesis and embryogenesis (5). Breakpoints in MLL are commonly found in both Infant Acute Leukemia (IAL), tAML and tALL (6, 7, 8). These breakpoints exhibit a bias towards the telomeric portion of the 8.3kb Breakpoint Cluster Region (BCR) of MLL (9, 10, 11). The unfaithful repair of breaks within this promiscuous gene can lead to translocations and subsequently the production of fusion proteins, such as MLL-AF4 and MLL-AF9 (12, 13). Typical oncogenic fusion proteins combine the N terminal portion of MLL containing a region with three AT hook DNA-binding motifs and the DNA methyltransferase homology region, and the C terminal portion of a fusion partner (5). These fusions often lead to a gain of function of MLL that play a role in either nuclear factors involved in transcription processes or signaling molecules localized to cytoplasm/cell junctions (14,15).
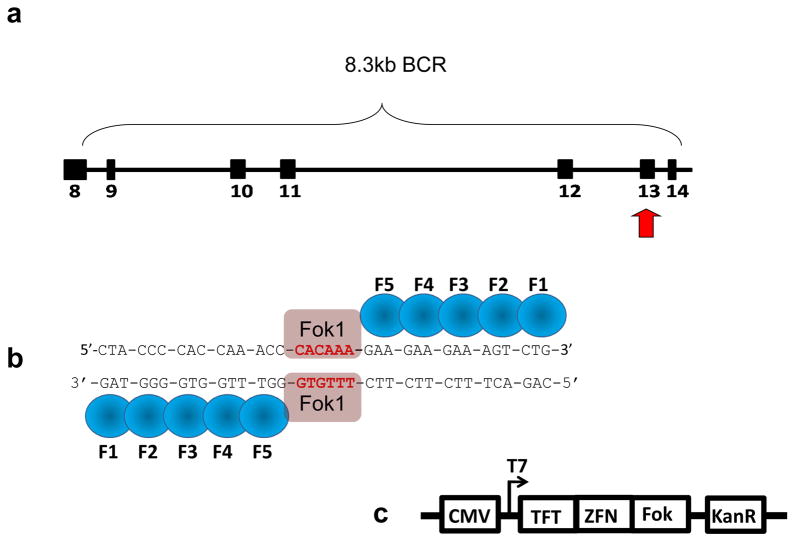
Clinical analysis has linked leukemogenic translocations to exposure to irradiation and Topoisomerase II poisons such as Etoposide. However, these systems deliver genome wide DSB in a random, non-targeted manner (16, 17, 18), making it difficult to correlate breaks at specific regions to common rearrangements that may lead to leukemogenesis. Therefore, we utilized custom designed Zinc Finger Nuclease (ZFN) technology to deliver a site-specific DSB to the telomeric portion of the MLL Breakpoint Cluster Region (BCR) (Fig. 1a). Our goal was to analyze chromosomal translocations linked to MLL leukemogenesis via DSB mis-repair, using the human lymphoblast cell line K562.
Figure 1.
ZFN were custom designed to target chromosome 11’s MLL BCR (exons 8–14) and induce DSB. (a) Translocations driving some AML and ALL may be triggered by DSBs within the 11q23 region of MLL, specifically the telomeric portion of the BCR. (b) ZFNs designed to specifically cut within exon 13 of the MLL BCR region consist of fingers that recognize triplet base pairs. Together the “left and right hands” work as dimers through the attached FokI endonuclease and induce a site specific DSB. (c) ZFN pUC vector. CMV promoter was used to express Kanomycin resistance gene (KanR) and the ZFN protein fused to the FokI endonuclease (Fok) and a Triple Flag tag (TFT) (obtained through Sigma-Aldrich).
The application of ZFN technology has been used to modify genetic information to answer many basic biological questions, or as a means of therapy for inherited disorders through the use of site-directed recombination (19, 20, 21). Each C2H2-zinc finger motif recognizes three bases at the major groove of DNA. Assembled in a specific order, our five fingered ZFN recognizes a specific 15 bp binding site within MLL exon 13 (Fig. 1b) and induces a DSB through heterodimerization of the FokI endonuclease. ZFN’s have been used previously to induce chromosomal translocations (22). In this case, DSBs targeting loci on chromosome 19 and the X chromosome in human embryonic stem and precursor cells that are uninvolved in clinically relevant translocations. However, in this study, we asked if gross chromosomal rearrangements could be linked to the mis-repair of a single DSB within the telomeric portion of the MLL BCR. FISH analysis showed an increased rate of MLL signal separation indicative of gross chromosomal rearrangements or unrepaired gene fragmentation. However IPCR of the sequences flanking the site specific DSB, were unable to detect either gross chromosomal rearrangements or leukemogenic translocations. We found that a single DNA DSB at exon 13 commonly gave insertions, deletions and single base pair substitutions. More infrequent were two base pair substitutions and a t(4;11)(q31.1,q23) chromosomal translocation was only detected with Nu7026 treatment prior to induction of DSB by our ZFN. An increase in the rates of single strand annealing (SSA), elevation of Rad51 levels and subsequent increase in rearrangements with the addition of the DNA-PK inhibitor, suggests the DSB produced are coupled to NHEJ repair.
Materials and Methods
Cell Culture, ZFN Transfection and Nu 7026 Treatment
The K562 cell line was obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured in Iscove’s Modified Dulbecco’s Medium, 10% fetal bovine serum with 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a 5% CO2 humidified incubator.
ZFNs targeting MLL BCR exon 13 (Sigma, St. Louis, MO) were transfected using the Amaxa Nucleofector (Walkersville, MD). 2×106 K562 cells were washed twice in 1mL DPBS (Gibco, Carlsbad, California) and centrifuged at 200×g for 10 minutes at room temperature. The outline of their construction is shown as Fig. 1. Cells were resuspended in 100 μL room temperature Nucleofector solution with 5 μg of each ZFN DNA (total of 10 μg) and electroporated using Nucleofector T-016 setting per manufacturer’s recommendations. Cells were maintained in 6 well Nunc plates (Fisher Scientific, Pittsburgh, PA) after transfection for 6, 12, 24, 48 and 72 hours.
ZFN “left hand” and “right hand” amino acid sequences respectively:
5′ AAMAERPFQCRICMRKFARSDYLTKHTKIHTGEKPFQCRICMRNFSRSDHLSQHIRTHTGEKPFACDICGKKFARSDVRKNHTKIHTGSQKPFQCRICMRNFSRSDSLSAHIRTHTGEKPFACDICGRKFATSGHLSRHTKIHLR 3′
5′ AAMAERPFQCRICMRNFSRSDHLSTHIRTHTGEKPFACDICGRKFADSSSRKKHTKIHTGEKPFQCRICMRKFAQSNHRKTHTKIHTGGGGSQKPFQCRICMRNFSQSGNLARHIRTHTGEKPFACDICGRKFAQSGNLARHTKIHLR 3′
ZFN “left hand” and “right hand” MLL binding sites respectively:
5′ CTACCCCACCAAACC 3′
5′ GAAGAAGAAAGTCTGG 3′
ZFN cleavage site: 5′ CACAAA 3′
Nu7026 (Sigma, St. Louis, MO) pretreatment: cells were washed 3x in DPBS and centrifuged at 200xg for 10 minutes at room temperature and resuspended into a 75cm2 flask with fresh media containing 10mM Nu7026 16 hours prior to ZFN electroporation.
Flow cytometry and Western analysis
24 and 48 hours after ZFN electroporation, cells were collected, washed 2x in TBS, fixed with methanol:acetone (1:1) for 1 min, washed 4x in TBS. Pellets were then resuspended and incubated in 10μg/mL of anti-FlagM2-FITC monoclonal antibody (Sigma, St. Louis, MO) at room temperature for 1 hour. Cells were then washed 2x with TBS and examined using FACS Calibur (BD, Franklin Lakes, NJ).
6, 12, 24, 48 and 72 hours after ZFN electroporation, cells were collected for protein isolation, washed 3x in DPBS, and resuspended in lysis buffer (100mM Tris-HCl, 150mM NaCl, 5mM EDTA, 1% Triton X-100 and protease inhibitor), incubated on ice for 20 minutes and centrifuged at 14,000xg for 20min @ 4°C. Total protein concentration was measured using a BCA protein assay (Thermo Scientific, Rockford, IL) per manufacturer’s recommendations. 30μg of total protein was used for all western blot analysis. To determine ZFN protein expression levels, blots blocked with 5% milk-TBST were incubated overnight at 4°C in 1:1000 dilution of mouse monoclonal [M2] DDDDK-tag-HRP antibody (abcam, Cambridge, MA). To determine Rad51 protein expression, blots were incubated in 1μg/mL anti-Rad51 mouse monoclonal antibody (Calbiochem, Darmstadt, Germany) overnight at 4°C, washed 3x with TBST and incubated with 1:5000 dilution of goat anti-mouse-HRP secondary antibody (Calbiochem, Darmstadt, Germany) for 3 hours at room temperature. Subsequently all blots were co-incubated with 1:2000 dilution anti-α-tubulin (Cell Signaling, Danvers, MA) primary antibody overnight at 4°C, washed and incubated with 1:5000 dilution of goat anti-rabbit-HRP secondary antibody (Calbiochem, Darmstadt, Germany). To assess upregulation of Rad51, Image J software (NIH, Bethesda, MD) program was used to quantify Rad51 protein levels and normalized to α-tubulin.
Mutation detection and Fluorescence In Situ Hybridization
Genomic DNA from the 48 hour samples treated with 400ng, 2μg, 5μg and 10μg ZFN (i.e. 400ng ZFN1 and 400ng ZFN2 for a total DNA of 800ng) were isolated per Qiagen DNeasy Blood and Tissue kit’s protocol (Qiagen, Valencia, CA). PCR amplification of reference and test samples were performed with 100ng of genomic DNA and the following cycles: denaturing for 5 min at 95°C, 30 cycles of 94°C (45 sec), 57°C (30 sec), and 72°C (1 min), and final extension at 72°C for 10 min. Forward and reverse primer sequences respectively:
5′ GTCTTTAGCATTTAATTGGGTGTAATCAGTTGCCTA 3′ and
5′ CATTACTAGGAAATCATCTCAGCAGAGAAA 3′.
Amplification, mismatch nuclease digestion and hybridization of G/C positive controls and homoduplex/heteroduplex samples were carried out per Surveyor Mutation Detection kit’s recommendations (Transgenomics, Omaha, NE).
Whole cell populations of 48 hour ZFN (10μg total) treated with and without Nu7026 pretreatment were also prepared for FISH analysis. Cells were washed 2x in 10mL DPBS, centrifuged at 1200 rpm for 10 minutes, resuspended and incubated for 15 minutes with 10 mL of prewarmed (37°C) 0.075 M KCl. Cells were then fixed 3x with 10mL of fresh methanol: glacial acetic acid (3:1) and dropped onto slides. Denaturation and hybridization to an MLL Dual Color Break-Apart Rearrangement probe (Abbott Molecular, Des Plaines, IL) were carried out per the manufacturer’s protocol. Slides were subsequently counterstained with DAPI mounting medium (Santa Cruz Biotech, Santa Cruz, CA), a coverslip applied, and viewed under a Leica DMR fluorescent microscope. Photographs were taken with a Leica DC300F (Leica, Heerbrugg, Switzerland) camera and processed with Image-Pro Plus 5.1 software (Media Cybernetics, Bethesda, MD).
Ligation mediated PCR, Inverse PCR
LMPCR and IPCR sample preparation was performed as previously described (23, 24). A blunt end linker is ligated to all available double-strand DNA breaks and PCR is carried out using linker and sequence-specific primers adjacent to a pre-identified break-site. Here, MLL cleavage was introduced in K562 cells with ZFN. After a minimum of 6 hour treatment, genomic DNA was isolated per Qiagen DNeasy Blood and Tissue kit’s protocol (Qiagen, Valencia, CA) and ligated with linker. 1 μg BB9.0 positive control plasmid was digested with 10 units of PvuII (New England Biolabs, Ipswich, MA) for 3 hours at 37°C and purified using Quickspin kit (Qiagen, Valencia, CA) prior to linker ligation.
Linkers: Linker 11 (5′-GAATTCAGATC-3′) and Linker 25 (5′GCGGTGACCCGG GAGATCTGAATTC-3′). The detail on making of the linker and its ligation to genomic DNA has been previously described (24). For the first round of PCR, the reaction mixture uses linker-ligated DNA as the template (1 μL from the ligation reaction) with Linker 25 primer plus LM-PCR Forward primer. Prior to ligation-mediated PCR (LM-PCR), the reaction mixture was heated to 72°C for 3 minutes to dissociate the 11-mer oligomer, leaving a 50, 25-mer overhang. Taq DNA Polymerase (2.5 Units) was added and LM-PCR executed with the following settings: 1 × (72°C for 5 minutes), 1 × (95°C for 4 minutes), 30 × (95°C for 30 seconds, 57°C for 30 seconds, 72°C for 45 seconds), 1 × (72°C for 10 minutes) and left at 4°C. The expected PCR product generated for ZFN treated cells was 610 bp for round 1 LM-PCR and 380 bp for the second round nested LM-PCR. The expected PCR product generated for B99.0 positive control plasmid was 540 bp for round one and 310 bp for the second round nested PCR. LMPCR forward and reverse primer sequences: 5′ GTCTTTAGCATTTAATTGGGTGTAATCAGTTGCCTA 3′ and 5′-GCGGTGACCCGGGAGATCTGAATTC-3′ respectively.
IPCR primers for exon 13
Round 1: Forward 5′CCAAGGATGACTG TGCTTAGAG 3′ and reverse 5′ ATTTTGTCTAGGGACTTGGGC 3′.
Round 2: Forward 5′ TTGGGTGTA ATCAGTTGCCTA 3′ and reverse 5′ ATTTTGTCTAGGGACTTGGGC 3′.
IPCR primers for exon 12
Round 1: Forward 5′ CACTCTTAGGTCACTTAGCATGTT 3′ and 5′ CAAA CCAGACCTTACAACTGT 3′.
Round 2: Forward 5′ TACTCTGAATCTCCCGCAATGTC 3′ and reverse 5′ ATTTTGTCTAGGGACTTGGGC 3′. Gel excision, TOPO-TA vector pCR2.1 cloning and sequencing was also performed as previously described (22).
SSA luciferase construct
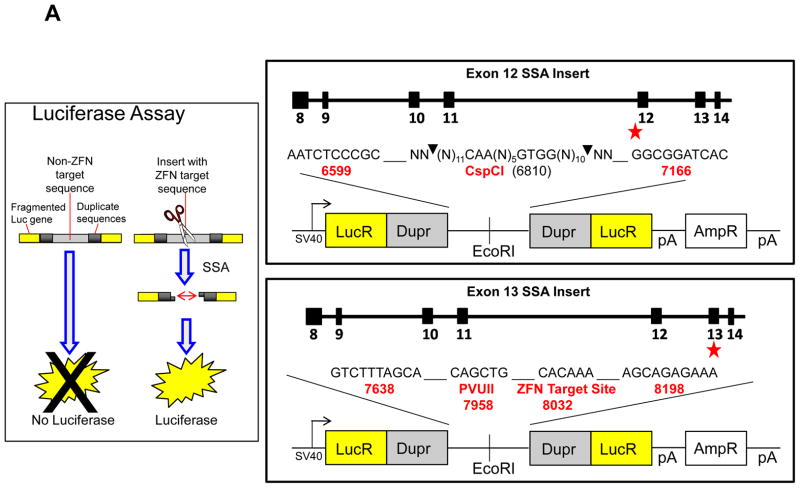
We generated luciferase reporter constructs containing sequences from exon 13 and exon 12 separately. 200ng pGL3 vectors (a kind gift from D. Segal, University of California Davis Genome Center, Davis, CA) digested with EcoRI (NEB, Ipswich, MA) were ligated to 500ng exon 13 or exon12 PCR amplified inserts using T4 ligase (Promega, Madison, WI) overnight at 4°C. Constructs were transformed into chemically competent One Shot Top10 (Invitrogen, Carlsbad, CA) and further propagated in 200mL LB broth (Sigma, St. Louis, MO) and purified with Qiagen Plasmid Maxi kit (Qiagen, Valencia, CA). 2μg of either exon 13 or exon 12 construct and 5μg of each ZFN plasmid (total of 10μg) were electroporated into 2×106 K562 cells, with and without Nu7026 pretreatment. 24, 48 and 72 hours post ZFN induction, luciferase levels were determined using Luciferase assay reagents per manufacturer’s protocol (Promega, Madison, WI).
Exon 13 insert sequence:
GTCTTTAGCATTTAATTGGGTGTAATCAGTTGCCTATTTTGTGTTTTAATTTTGGGACTATAGCAGAAAACATGATGTTGAATAAAATTCCAAAAATAAGTCAAATCTACCTAATATGAATACTCATCACTGAGTGCCTTTGGCCAGGAAATAAATCTATCTCAATGCTTTAATTGGGAGTAAATAATGTATGAGGAAATTTAAACTCATAATTGTGTGCTGTACTTACTTGCCAGTAAATGTGAAATGGGGTACTAAGTAATAGGTGTTGGGTGAAGGTAATATGATGCTTATCTTTTTGCCATTATATTTTCTTACAGCAGCTGCTGGAGTGTAATAAGTGCCGAAACAGCTATCACCCTGAGTGCCTGGGACCAAACTACCCCACCAAACCCACAAAGAAGAAGAAAGTCTGGGTGAGTTATACACATGATGCTCTTTTATAGAGAACCACCATGTGACTATTGGACTTATGTAACTTGTATTACAATATCTATGCTTGAGGATGTCAGTATGACAATCTTTTTGCCTCATTACTAGGAAATCATCTCAGCAGAGAAA
Exon 12 insert sequence:
AATCTCCCGCAATGTCCAATACTGTACTTTTTTACATAGTCATTGCTTAATGAATATGTATTGAATTAAATATATGCCAGTGGACTACTAAAACCCAAAGTATATAAGAAGGGTATGGTTGATTATGTTTTTCTACATATTATTTGACATACTTCTATCTTCCCATGTTCTTACTATAGTTTGTGTATTGCCAAGTCTGTTGTGAGCCCTTCCACAAGTTTTGTTTAGAGGAGAACGAGCGCCCTCTGGAGGACCAGCTGGAAAATTGGTGTTGTCGTCGTTGCAAATTCTGTCACGTTTGTGGAAGGCAACATCAGGCTACAAAGGTACAAAACTTGGTAATAGAACTACAGCTGGGCCTCTGTATCAGTGGGTTCTGTATCCCTGGACTCAACCAACCTTGGATTGAATGTATCTGGGAAAAAATGAGTAGTTGCCTCTGTACTCTATGTGAACAGACTTTTTCTTGTCATTATTTCCTAAACAATACAGTATAACAACTATTTACATTGTATTAGGTATGATAAGTAATCTAGAGATAATTTAAAGTATATGGTGGGCGGATCAC
Results
Generation of MLL exon 13 specific ZFN
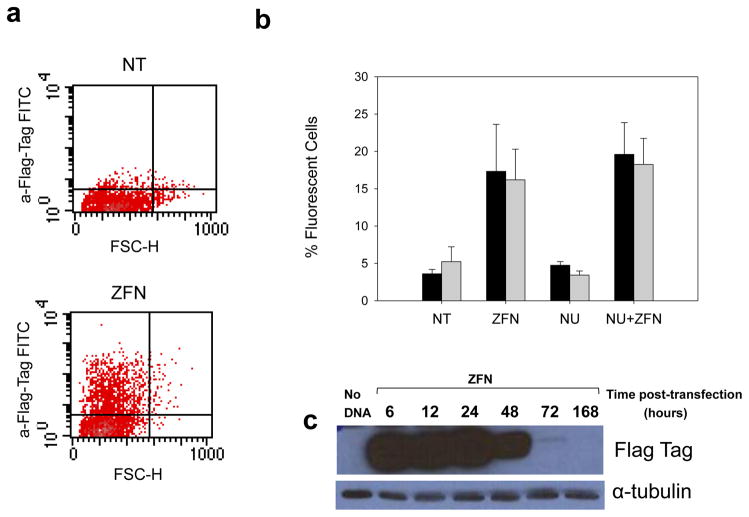
In order to generate site-specific DSB’s within the telomeric portion of the MLL BCR, we acquired a custom designed ZFN targeting exon 13. Two plasmids, each containing one ZFN “hand” and an added triple FLAG-tag protein, were electroporated into K562 cells. The ability to efficiently introduce both ZFN hands into the cells was integral to the requisite heterodimerization of the Fokl endonuclease leading to the site specific scission event. Because no antibody specific to our ZFN protein is currently commercially available, detection of our unique ZFN protein for both intracellular fluorescence flow cytometry staining and western blot analysis was carried out using an anti-Triple Flag Tag antibody (Fig 2c) directly conjugated to FITC. We determined that approximately 20% of the K562 cell population expressed ZFN protein 24 hours post transfection (Fig 2a). Pretreatment with Nu7026 had minimal effect on ZFN expression levels (Fig 2b). Western blot analysis showed the presence of ZFN protein as early as 6 hours post transfection. Transient protein expression diminished by 48 hours and was nearly undetectable at 72 hours (Fig 2c)
Figure 2.
Expression of ZFN and site-specific DNA DSB in K562 cell line. (a) Flow cytometry analysis of ZFN protein expression 24 hours post transfection was detected using anti-FlagTag-FITC antibody. Negative control K562 cells electroporated with no ZFN (NT) (top) compared to cells treated with ZFN (bottom). b) Relative expression of ZFN 24 hours (black) and 48 hours (gray) post transfection in cells without ZFN DNA (NT) compared to ZFN induction (ZFN), cells pretreated with Nu7026 (NU) and pretreatment with NU7026 followed by ZFN induction (NU+ZFN). Signal from NT and NU populations reflect background fluorescence. (c) Western blot time-course expression of 40.4 kD ZFN (10 μg) and 52 kD alpha-tubulin (lane 1: negative control cells electroporated with no DNA, 2: 6h post electroporation with ZFN, 3: 12h, 4: 24h, 5: 48h, 6: 72h, 7: 1 week).
Verification of target specificity of our ZFN
The schema used for these analyses, LM-PCR, the Surveyor assay and inverse PCR are shown schematically in Fig. 3.
Figure 3.
Schematic diagram of analytical methods used. a) The surveyor assay detects ZFN induced mis-repair leading to base mispairing that is specifically cleaved by a celery derived enzyme. b) Ligation mediated PCR detects DNA breaks at a site adjacent to the ZFN cleavage site, mediated via DNA ligase (green ovals). c) ZFN induced aberrations can be detected using inverse PCR with primers upstream of the cleavage site. Sau3AI restriction digest allows for ligation and circularization of the DNA sequence encompassing exon 13 and any mis-repair event due to ZFN cleavage. Any rearrangement, insertion or deletion within the MLL gene will be captured (blue line) generating the sequence MLL-Unknown-MLL upon sequencing. Though usually applied to the detection of translocations, it is capable of reporting a range of aberrations
LMPCR
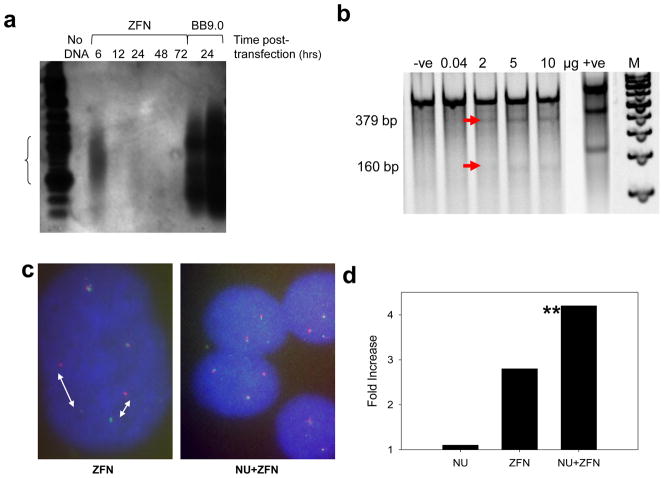
At various time points after ZFN transfection, genomic DNA was isolated and available blunt ended DNA breaks, likely generated by end-trimming of the staggered ZFN cleavage, ligated to a linker. Primer sets targeting a region upstream of the ZFN cut site and to the linker itself allowed for the PCR amplification of a 610 base pair product. Without a DSB, amplification of the linker would give no signal as seen with the negative control. Southern blot analysis showed breaks could be detected as early as 6 hours after ZFN introduction into the cells, the signal fading thereafter (Fig. 4a). The positive control for the LM-PCR reaction (BB9.0) plasmid containing the 8.3 kb BCR was digested with PVUll, linker-ligated and PCR amplified to give the expected 540 base pair product.
Figure 4.
Detection of gross chromosomal changes induced by site specific DNA DSB within MLL BCR exon 13. (a) Southern blot of K562 cells transfected with 10μg ZFN and analyzed at various times after transfection by ligation-mediated PCR Lane 1: Ladder, 2: no DNA, Lanes 3–7 analysis after the times shown, lanes 8 &9: BB9 positive control (0.5 μl and 1 μl loaded). Experiment run in triplicate with similar results. (b) Expected nested PCR heteroduplex cleavage products of samples with misrepaired DSB induced by ZFN are 160 and 379 bp (lane 1: negative control cells transfected with no DNA, lanes 2–6 (5 empty), ZFN DNA in the amount shown, 6: positive control heteroduplex sample gives two cleavage products of 217 and 416 bp, 8 (empty) 7: 100bp ladder). (c) FISH analysis of K562 cells at 48 hours post transfection with ZFN (left) and cells pretreated with Nu7026 followed by ZFN induction (right). Intact K562 cells contain three normal copies of chromosome 11. (d) Quantitative evaluation of gross chromosomal changes induced by ZNF and ZFN and Nu7026 pretreatment (NU+ZFN) compared to Nu7026 treatment alone (NU) via FISH. Fold induction is percent changes in treated/control. ** Statistically significant increase compared to controls (one tailed Fishers Exact test used as fragmenting activity was predicted to increase number of alterations P = 0.03).
Surveyor analysis
To determine the frequency of ZFN treated cells that accumulate single base mutations subsequent to ZFN cleavage, we designed primers flanking the site of putative ZFN-induced breaks. With the use of the mutation detection Surveyor assay, that uses a celery derived enzyme that cleaves specifically at sites of mis-matched bases, we expected that any mismatched base pairs would yield 379 and 160 base pair products. Using varying concentrations of ZFN (Fig 4b), 2μg of each ZFN hand (4 μg total) yielded 12% heteroduplex/homoduplex mismatch when compared to the homoduplex intact sample, 5 μg of each ZFN yielded 19% and 10 μg of each ZFN yielded 16%. In comparison, the hetero/homoduplex G/C positive control provided with the kit showed a 42% yield of the hetero/homoduplex versus the homoduplex alone.
ZFN induced gross chromosomal changes observed by FISH
Assessment of MLL rearrangements can be achieved using separate green and red fluorescent probes targeting the 5′ and 3′ location of the MLL BCR. Using the Vysis Breakapart probes, cells with intact MLL BCR emit a single yellow signal from the co-localized probes, cells that exhibited rearrangements, or which were fragmented and remain separated, generate distinct green and red signals (Fig 4c, d). FISH analysis resulted in a 2.8 fold increase in signal separation in cells treated with ZFN (7/106 events) in comparison to control samples (2/84 events). To determine if suppression of the error prone non-homologous end joining (NHEJ) repair pathway would lead to any change in gross chromosomal rearrangements, cells pretreated with the DNA-PK inhibitor, Nu7026 were also stained with the Vysis probe. Surprisingly, pretreatment with Nu7026 prior to ZFN induction exhibited a higher incidence (7/68 events, 4.3 fold over control cells) of signal separation than cells treated with ZFN alone (Fig 4d). This may imply that what is observed are free DNA ends that remain widely separated. However it cannot be excluded that some of these widely separated signals reflect specific rearrangements. In comparison, control cells treated with Nu7026 alone showed only a 1.1 fold increase over control levels (events = 6/189) (Fig. 4d). Statistical analysis showed that only the ZFN + Nu7026 treated group was significantly different from either control samples (P=0.04) or Nu7026 alone (P=0.03) one tailed Fishers exact test.
Chromosomal rearrangements induced by ZFN targeting
To assess the presence of any chromosomal rearrangements associated with a single DSB at the telomeric portion of the MLL BCR, we screened separate populations of cells using IPCR. Sequencing revealed that K562 cell samples treated with ZFN (6 hours n=8, 24 hours n= 6, 48 hours n=9) at various times showed small interstitial deletions, insertions and base substitutions. In comparison, all control samples (transfected without any ZFN DNA and no Nu7026 pretreatment, n= 9) had an intact DNA sequence (Fig. 5a). Interestingly, K562 samples pretreated with NU7026 resulted in an increase in mis-repair events after ZFN induction. The only translocation detected, was from a sample treated with Nu7026 prior to ZFN induction (6 hours n=10). We have previously shown that the intron 11/exon 12 border is unusually susceptible to both DNA breaks and rearrangements (10). To address whether this intrinsically unstable region would be affected by a single DSB, roughly 1200 base pairs upstream of the ZFN target site, we also screened the same samples using primers targeting intron 11/exon 12 (Fig. 5b). The results show that a single DSB downstream of this hotspot site had little effect on the distal hotspot region. However, the negative control samples showed considerably higher incidence of aberrations indicating that this region is unstable, regardless of ZFN induction or Nu7026 treatment (Fig. 5b).
Figure 5.

Aberrations induced by ZFN targeting MLL Fig 5(a) ZFN DSB induced sequence changes in MLL exon 13 with (lower) and without (top) Nu7026 pretreatment Fig. 5(b) MLL exon 12, a region of known instability, remains relatively unaffected by the downstream cleavage of ZFNs and/or pretreatment with Nu7026. Panel shows code for types and times of alterations observed which for substitutions, insertions and deletions are shown to scale.
Upregulation of SSA pathway and expression of DSB induced repair proteins induced by Nu7026
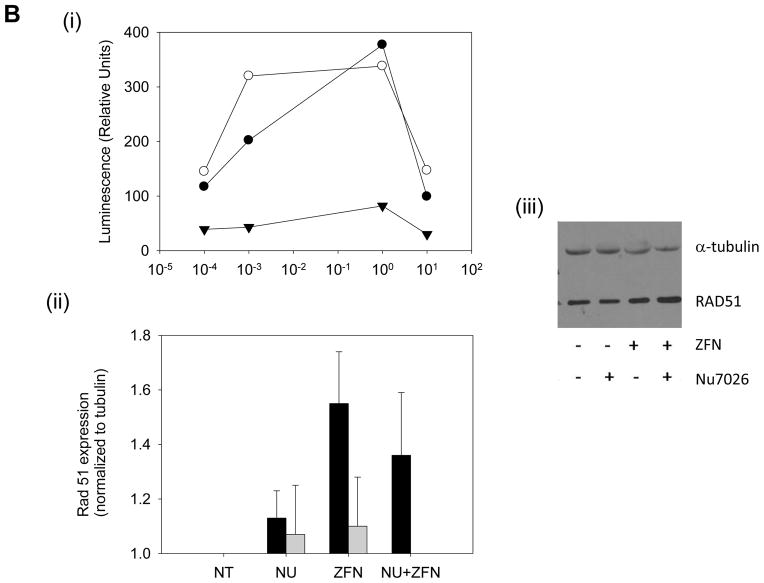
The SSA assay served dual purposes: to examine ZFN induced mis-repair and also to gain insight on the relative contribution of the various repair pathways (Fig 6a). By inserting the ZFN target site within two repeat sequences flanked by a fragmented luciferase gene, a DSB undergoing accurate SSA will allow for the expression of a functional luciferase gene. We expected that by inhibiting the error prone NHEJ pathway, the incidence of mis-repair post ZFN induction would decrease. However, the luciferase reporter system shows an increase in the error prone SSA pathway after DNA-pk inhibition (Fig. 6b)
Figure 6.
Upregulation of SSA repair pathway and expression of DSB induced repair protein Rad 51 induced by ZFN and Nu7026. Fig 6(a) Schematic of SSA luciferase reporter responsive to cleavage of MLL exon 13 insert; also shown structure of Luciferase SSA repair plasmids with recognition sites for the negative control (exon 12) and the ZFN target (exon 13). Flanking inserted targeted sites are duplicate sequences (Dupr). Faithful SSA repair of the luciferase fragmented gene (Lucr) after DSB induction will yield a functional luciferase gene. Fig. 6(b) (i) Concentration dependent Nu7026 pretreatment potentiates SSA repair pathway upregulation in K562 transfected with ZFN over a period of time: ● (24 hours post transfection), ○ (48 hours post transfection) and ▼ (72 hours post transfection). Luciferase reporter assay fold induction is treated/untreated. (ii) Quantitative analysis of Rad 51 at 24h (black) and 48h (gray) post ZFN transfection following Nu7026 pretreatment or ZFN treatment alone from Western blots. iii) Representative western after either no ZFN DNA (NT) compared to ZFN induction (ZFN), cells pretreated with Nu7026 (NU) and pretreatment with NU7026 followed by ZFN induction (NU+ZFN). (n=3) (paired student T test * p ≤ 0.05 for ZFN alone compared to untreated samples).
Discussion
The ability to faithfully repair DNA DSB is integral to the stability of the genome. Specifically, DNA breaks within the telomeric portion of the MLL BCR have been linked to the generation of MLL fusions in both tAML and IAL patients (9, 10, 11). Using the K562 cell line derived from a patient with CML in terminal blast crisis, our aim was to analyze the fate of a single ZFN DSB targeting the telomeric portion of the MLL BCR. This would address the question of whether a DNA break alone was sufficient to destabilize this region of MLL, promoting rearrangements. In addition, the specificity of the break would permit an assessment of those factors impacting restitution or rearrangement at this sensitive target site. Parallel studies addressing the same question have used genetically engineered I-SceI cells. Although this rare cutting restriction enzyme system allows a site-specific single DSB to be inserted, the host genome has to be modified to incorporate the artificial target (25). ZFN technology has the advantage of recognizing unique, biologically significant genomic sequences in vivo, preserving context, without either the separate integration of a recognition sequence or application of a plasmid based model system (26). We report here the ability of a ZFN targeting MLL exon 13 to introduce DNA double strand breaks, where their repair was linked to the introduction of single base changes. Such base pair anomalies were then detected using the Surveyor assay and gross chromosomal alterations assessed using both FISH and IPCR analysis.
Introduction of a plasmid coding for a ZFN targeting MLL exon 13 into K562 cells was associated with robust expression of a linked Flag tag with approximately 20% of the treated population (Fig. 2). Three independent screens were employed to confirm ZFN function (Fig. 3). In the first, using the commercial “Surveyor” technology the presence of a ZFN-induced DNA break is inferred by the presence of mis-repair events at the ZFN target site. Such base mis-pairings lead to a local helix distortion that is recognized by a celery derived enzyme, cleaving the test substrate (27). As shown in Fig 4b, enhanced DNA cleavage is observed as the amount of transfected ZFN is increased. In the second, a dual fluorescent probe system was employed where each maps to contiguous tracts of MLL. Fragmentation by the ZFN will allow each probe target to separate, if not immediately repaired. As shown in Fig. 4c&d, introduction of the ZFN generated an increase in the incidence of probe separation. Such a finding may indicate gene rearrangement, however the elevation in MLL probe separation in the presence of the NHEJ inhibitor, NU7026, is consistent with ZFN cleavage and non-repair. Finally, a more direct approach was utilized where individual DNA breaks were measured using ligation mediated PCR. Here, a signal is only observed if the DNA is physically broken at the predicted ZFN target site. As seen in Fig. 4a, a robust LM-PCR signal is observed at 6h, fading at later times. Unexpectedly, the LM-PCR products covered a substantial range of sizes, depicted as a smear, suggesting the initial DNA breaks at this site are rapidly subject to repeat exonuclease attack producing mis-repair products of varying sizes.
Using IPCR to screen for sequence alterations at the ZFN target site, multiple small interstitial insertions, deletions and base pair substitutions were detected as a result of the ZFN treatment (Fig. 5a). Major deletions were clustered around the cut site however small insertions and substitutions were distributed throughout the ~1kbp+ region interrogated by IPCR. This suggests that repair of the targeted DNA double strand break facilitates errors at multiple locations as a part of the repair process. However IPCR showed only a single MLL translocation in cells treated with both ZFN and the DNA-PK inhibitor, NU7026. This particular translocation partner involved the human mineralocorticoid receptor, which regulates blood pressure and has not been clinically observed to be involved in leukemia (28). The limited chromosomal rearrangement detection through IPCR could be attributed to the necessary pre-selection of individual amplicons chosen for sequencing or that primers were either deleted or separated by large distances (insertions, translocations, duplications). To bypass this in future studies, a “shotgun” approach where all IPCR products identified may be processed for massively parallel sequencing so as to reduce selection bias.
We also wanted to determine the relative contribution of NHEJ, SSA and HR to the restitution of a ZFN-induced single DSB within MLL. Previous work has indicated that the majority of MLL fusion genes contain short regions of microhomology at the break point – an observation consistent with NHEJ being involved in their formation (10). NU7026 is a potent and specific inhibitor of DNA-PK and thus of the NHEJ repair pathway (29). Inhibition of the error prone NHEJ pathway via NU 7026 treatment increased the separation of the contiguous FISH probes, indicative of either DNA fragmentation and non-repair or gene rearrangement (30 and our results). It has previously been reported in CHO cells that a majority of endonuclease-induced DSB are repaired almost exclusively by HR (31). However, Liang et al. reported random endonuclease cleavage induced NHEJ and HR at similar rates (38). We therefore wanted to determine whether disruption of the conventional NHEJ pathway would engage error prone SSA, and subsequently increase or decrease repair errors. In addition to validating ZFN site specificity, the luciferase reporter construct revealed efficient ZFN DSB repair through the SSA pathway (32 and Fig. 6b). Interestingly, preference for this pathway was enhanced with the addition of NU7026, supporting the contention that at least some of the widely separated FISH signals observed after NU7026/ZFN treatment may be related to SSA pathway use. Non-conservative and error prone SSA requires homologous sequences to promote single strand pairing, with the sequences between repeats being deleted following recombination. This role of SSA may provide a partial explanation for the prevalence of IPCR samples with interstitial deletions. However, upon further analysis, the direct repeats required for SSA were not observed, suggesting access to other repair mechanisms, such as the error prone alternative end joining pathway, were possibly involved (33).
Previous studies have shown that the alternative end joining pathway is suppressed by Xrcc4-ligase IV and independent of DNA-PK, a protein competitively inhibited by NU7026 (33). This pathway is reportedly upregulated by DNA ligase III (34, 32). Upregulation of Rad 51 protein in cells has also been implicated in genomic instability (32). An increase in Rad 51 protein expression may also be an indication that repair of DSB via the more accurate HR pathway has also increased. However, this pathway is only accurate so long as the homologous sister chromosomes are intact. Other studies have shown that, although HR does not result in the small deletions often seen in NHEJ or SSA, it may lead to loss of heterozygosity or chromosomal translocations (30, 35). FISH data revealed that the targeted ZFN DSB induced gross chromosomal separation in more than one chromosome 11 copy, perhaps linked to the anueploid status of K562 cells for chromosome 11. Recent studies have shown that ZFN infrequently cleave off target, it is thus likely that more than one MLL allele was cleaved within a cell (36, 37, 30, 25).
Approximately 1200 base pairs upstream of the ZFN target site, at the intron 11/exon 12 border of MLL, is a region that is particularly sensitive to both DNA breaks and rearrangements (10, 23). We have previously proposed that this region contained a structurally unstable palindrome, linked to a Topoisomerase ll consensus sequence binding site (23). In addition, this location is also a site of nuclease hypersensitivity (11). We therefore also screened this region for any chromosomal aberrations triggered by the distal ZFN DSB to determine if cleavage at exon 13 would destabilize this location. We found no aberrations specifically associated with ZFN alone or with Nu7026 addition at the intron 11/exon 12 border (Fig. 5b). There was however an increase in aberration incidence within the control samples at this location in comparison to those studied at exon13, indicating that this region is unstable regardless of ZFN or Nu7026 treatment. Other studies have also attempted to induce a single DSB to determine if this would result in tumorigenic translocations or rearrangements (35, 30, 38). The use of I-SceI in these systems, targeting different genetic locations, also resulted in only interstitial deletions and insertions, and similarly did not detect gross chromosomal rearrangements through PCR analysis. A previous study has shown that although a single DSB is 50 fold more likely to induce interchromosomal recombinants over spontaneous events, the frequency of recombinants was 80 fold less than with two DSB (25). Consistent with these results, Brunet et al., reported translocation frequencies of 10−4–10−5 by inducing two targeted DSBs using ZFN’s as the mediators of DNA breaks (22).
The factors linked to the initiation of tAML are still incompletely understood. Though a key role of MLL fusions has been demonstrated, factors mediating the initial fragmentation events remain unclear. Recently, the possibility of co-transcribing genes has been proposed as a means whereby potential fusion partners are placed within the same time and space, and thus may promote their mutual interaction (39, 40). This process may facilitate inter-gene exchange particularly if the transcription process itself can enhance fragmentation –perhaps through recruitment of AID (41). In this context it has been noted that an internal gene promoter site has been identified adjacent to the MLL rearrangement hotspot in exon 12 (42).
We have shown here that a single DSB within MLL exon 13 is inefficient at generating leukemogenic translocations. Massively parallel sequencing paired with clonogenic studies may be key in determining the relative frequency of leukemogenic rearrangements and translocations induced by DSB at various regions of MLL.
Highlights.
A Zinc Finger Nuclease (ZFN) targeting a leukemogenic hot spot for rearrangement in MLL is created
The novel ZFN efficiently cleaves MLL exon 13
Despite MLL cleavage and evidence of mis-repair, no leukemogenic translocations were produced
MLL cleavage alone is insufficient to generate leukemogenic translocations
Acknowledgments
Support: This work was supported by P01CA105049 to AV
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tkachuk DC, Kohler S, Cleary ML. Involvement of a homolog of Drosophila trithorax by 11q23 chromosomal translocation in acute leukemias. Cell. 1992;71:691. doi: 10.1016/0092-8674(92)90602-9. [DOI] [PubMed] [Google Scholar]
- 2.Djabali M, Selleri L, Parry P, Bower M, Young BD, Evans GA. A trithorax-like gene is interrupted by chromosome 11q23 translocations in acute leukaemias. Nat Genet. 1992;2:113. doi: 10.1038/ng1092-113. [DOI] [PubMed] [Google Scholar]
- 3.Gu Y, Cimino G, Alder H, Nakamura T, Prasad R, Canaani O, Moir DT, Jones C, Nowell PC, Croce CM, Canaani E. The (4;11)(q21; q23) chromosome translocations in acute leukemias involve the VDJ recombinase. Proc Natl Acad Sci USA. 1992;89:10464. doi: 10.1073/pnas.89.21.10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ziemin-Van Der Poel S, McCabe NR, Gill HJ, Espinosa R, III, Patel Y, Harden A, Rubinelli P, Smith SD, LeBeau MM, Rowley JD, Diaz MO. Identification of a gene, MLL, that spans the breakpoint in 11q23 translocations associated with human leukemias. Proc Natl Acad Sci USA. 1991;88:10735. doi: 10.1073/pnas.88.23.10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ernst P, Wang J, Korsmeyer SJ. The role of MLL in hematopoiesis and leukemia. Curr Opin Hematol. 2002;9:282–287. doi: 10.1097/00062752-200207000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Mitelman F. Catalog of Chromosome Aberrations in Cancer. 5. New York, NY: Wiley-Liss; 1994. [Google Scholar]
- 7.Thirman MJ, Gill HJ, Burnett RC, Mbangkollo D, Mccabe NR, Kobayashi H, Ziemin-Van Der Poel S, Kaneko Y, Morgan R, Sandberg AA, Chaganti RSK, Larson RA, LeBeau MM, Diaz MO, Rowley JD. Rearrangement of the MLL gene in acute lymphoblastic and acute myeloid leukemias with 11q23 chromosomal translocations. N Engl J Med. 1993;329:909. doi: 10.1056/NEJM199309233291302. [DOI] [PubMed] [Google Scholar]
- 8.Felix CA, Hosler MR, Winick NJ, Masterson M, Wilson AE, Lange BJ. ALL-1 gene rearrangements in DNA topoisomerase II inhibitor-related leukemia in children. Blood. 1995;85:3250–3256. [PubMed] [Google Scholar]
- 9.Stanulla M, Wang J, Chervinsky DS, Thandla S, Aplan PD. DNA cleavage within the MLL breakpoint cluster region is a specific event which occurs as part of higher-order chromatin fragmentation during the initial stages of apoptosis. Mol Cell Biol. 1997;17:4070–4079. doi: 10.1128/mcb.17.7.4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betti CJ, Villalobos MJ, Diaz MO, Vaughan AT. Apoptotic triggers initiate translocations within the MLL gene involving the nonhomologous end joining repair system. Cancer Res. 2001;61:4550–4555. [PubMed] [Google Scholar]
- 11.Strissel PL, Strick R, Rowley JD, Zeleznik L. An in vivo topoisomerase II cleavage site and a DNase I hypersensitive site colocalize near exon 9 in the MLL breakpoint cluster region. Blood. 1998;92:3793–3803. [PubMed] [Google Scholar]
- 12.Cimino G, Moir DT, Cana ani O, et al. Cloning of ALL-1, the locus involved in leukemias with the t(4;11)(q21;q23), t(9;11)(p22;q23), and t(11;19)(q23;p13) chromosome translocations. Cancer Res. 1991;51:6712–4. [PubMed] [Google Scholar]
- 13.Ayton PM, Cleary ML. Molecular mechanisms of leukemogenesis mediated by MLL fusion proteins. Oncogene. 2001;20:5695–5707. doi: 10.1038/sj.onc.1204639. [DOI] [PubMed] [Google Scholar]
- 14.So CW, Cleary ML. Dimerization: a versatile switch for oncogenesis. Blood. 2004;104:919–921. doi: 10.1182/blood-2004-03-0992. [DOI] [PubMed] [Google Scholar]
- 15.Collins EC, Rabbitts TH. The promiscuous MLL gene links chromosomal translocations to cellular differentiation and tumour tropism. Trends Mol Med. 2002;8:436–442. doi: 10.1016/s1471-4914(02)02397-3. [DOI] [PubMed] [Google Scholar]
- 16.Sak A, Stuschke M. Repair of ionizing radiation induced DNA double-strand breaks (dsb) at the c-myc locus in comparison to the overall genome. Int J Radiat Biol. 1998;73(1):35–43. doi: 10.1080/095530098142680. [DOI] [PubMed] [Google Scholar]
- 17.Sak A, Stuschke M, Stapper N, Streffer C. Induction of DNA double-strand breaks by ionizing radiation at the c-myc locus compared with the whole genome: a study using pulsed-field gel electrophoresis and gene probing. Int J Radiat Biol. 1996 Jun;69(6):679–85. doi: 10.1080/095530096145418. [DOI] [PubMed] [Google Scholar]
- 18.Aplan PD, Chervinsky DS, Stanulla M, Burhans WC. Site-specific DNA cleavage within the MLL breakpoint cluster region induced by topoisomerase II inhibitors. Blood. 1996;87:2649–2658. [PubMed] [Google Scholar]
- 19.Bibikova M, Beumer K, Trautman JK, Carroll D. Enhancing gene targeting with designed zinc finger nucleases. Science. 2003;300:764. doi: 10.1126/science.1079512. [DOI] [PubMed] [Google Scholar]
- 20.Bibikova M, et al. Stimulation of homologous recombination through targeted cleavage by chimeric nucleases. Mol Cell Biol. 2001;21:289–297. doi: 10.1128/MCB.21.1.289-297.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porteus MH, Baltimore D. Chimeric nucleases stimulate gene targeting in human cells. Science. 2003;300:763. doi: 10.1126/science.1078395. [DOI] [PubMed] [Google Scholar]
- 22.Brunet E, Simsek D, Tomishima M, DeKelver R, Choi VM, Gregory P, Urnov F, Weinstock DM, Jasin M. Chromosomal translocations induced at specified loci in human stem cells. Proc Natl Acad Sci U S A. 2009 Jun 30;106(26):10620–5. doi: 10.1073/pnas.0902076106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le H, Singh S, Shih SJ, Du N, Schnyder S, Loredo GA, Bien C, Michaelis L, Toor A, Diaz MO, Vaughan AT. Rearrangements of the MLL Gene Are Influenced by DNA Secondary Structure, Potentially Mediated by Topoisomerase II Binding. Genes Chromosomes Cancer. 2009;48(9):806–15. doi: 10.1002/gcc.20685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Betti CJ, Villalobos MJ, Jiang Q, Cline E, Diaz MO, Loredo G, Vaughan AT. Cleavage of the MLL gene by activators of apoptosis is independent of topoisomerase II activity. Leukemia. 2005;19:2289–2295. doi: 10.1038/sj.leu.2403966. [DOI] [PubMed] [Google Scholar]
- 25.Richardson C, Jasin M. Frequent chromosomal translocations induced by DNA double-strand breaks. Nature. 2000;405:697–700. doi: 10.1038/35015097. [DOI] [PubMed] [Google Scholar]
- 26.Szczepek M, Brondani V, Büchel J, Serrano L, Segal DJ, Cathomen T. Structure-based redesign of the dimerization interface reduces the toxicity of zinc-finger nucleases. Nature Biotechnology. 2007;25:786–793. doi: 10.1038/nbt1317. [DOI] [PubMed] [Google Scholar]
- 27.Qiu P, Shandilya H, D’Alessio JM, O’Connor K, Durocher J, Gerard GF. Mutation detection using Surveyor nuclease. Biotechniques. 2004 Apr;36(4):702–7. doi: 10.2144/04364PF01. [DOI] [PubMed] [Google Scholar]
- 28.Morrison N, Harrap SB, Arriza J, Boyd E, Michael Connor J. Regional chromosomal assignment of the human mineralocorticoid receptor gene to 4q31.1. Hum Genet. 1990;85:130–132. doi: 10.1007/BF00276340. [DOI] [PubMed] [Google Scholar]
- 29.Veuger SJ, Curtin NJ, Smith GC, Durkacz BW. Effects of novel inhibitors of poly(ADP-ribose) polymerase-1 and the DNA-dependent protein kinase on enzyme activities and DNA repair. Oncogene. 2004 Sep 23;23(44):7322–9. doi: 10.1038/sj.onc.1207984. [DOI] [PubMed] [Google Scholar]
- 30.Soutoglou E, Dorn JF, Sengupta K, Jasin M, Nussenzweig A, Ried T, Danuser G, Misteli T. Positional stability of single double-strand breaks in mammalian cells. Nat Cell Biol. 2007;9(6):675–682. doi: 10.1038/ncb1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taghian DG, Nickoloff JA. Chromosomal double-strand breaks induce gene conversion at high frequency in mammalian cells. Mol Cell Biol. 1997;17:6386–6393. doi: 10.1128/mcb.17.11.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richardson C, Stark JM, Ommundsen M, Jasin M. Rad51 overexpression promotes alternative double-strand break repair pathways and genome instability. Oncogene. 2004;23:546–553. doi: 10.1038/sj.onc.1207098. [DOI] [PubMed] [Google Scholar]
- 33.Simsek D, Jasin M. Alternative end-joining is suppressed by the canonical NHEJ component Xrcc4-ligase IV during chromosomal translocation formation. Nat Struct Mol Biol. 2010;17(4):410, 6. doi: 10.1038/nsmb.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simsek, et al. DNA Ligase III Promotes Alternative Nonhomologous End-Joining during Chromosomal Translocation Formation. PLoS Genet. 2011 Jun;7(6) doi: 10.1371/journal.pgen.1002080. and mediates translocation events in mammalian cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y, Zhang Z, Keenan B, Roschke AV, Nakahara K, Aplan PD. Efficient repair of DNA double-strand breaks in malignant cells with structural Instability. Mutation Research. 2010;683(1–2):115–22. doi: 10.1016/j.mrfmmm.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gabriel R, et al. An unbiased genome-wide analysis of zinc-finger nuclease specificity. Nature Biotechnology. 2011:1948. doi: 10.1038/nbt.1948. [DOI] [PubMed] [Google Scholar]
- 37.Pattanayak V, et al. Revealing off-target cleavage specificities of zinc-finger nucleases by in vitro selection. Nature Methods. 2011:1670. doi: 10.1038/nmeth.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang F, Han M, Romanienko PJ, Jasin M. Homology-directed repair is a major double-strand break repair pathway in mammalian cells. Proc Natl Acad Sci. 1998;95:5172–5177. doi: 10.1073/pnas.95.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitchell JA, Fraser P. Transcription factories are nuclear subcompartments that remain in the absence of transcription. Genes Dev. 2008;22:20–5. doi: 10.1101/gad.454008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osborne CS, Chakalova L, Mitchell JA, Horton A, Wood AL, Bolland DJ, Corcoran AE, Fraser P. Myc dynamically preferentially relocates to a transcription factory occupied by Igh. PLoS Biol. 2007;5:e192. doi: 10.1371/journal.pbio.0050192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robbiani DF, Bothmer A, Callen E, Reina-San-Martin B, Dorsett Y, Difilippantonio S, Bolland DJ, Chen HT, Corcoran AE, Nussenzweig A, Nussenzweig MC. AID is required for the chromosomal breaks in c-myc that lead to c-myc/IgH translocations. Cell. 2008 Dec 12;135(6):1028–38. doi: 10.1016/j.cell.2008.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scharf S, Zech J, Bursen A, Schraets D, Oliver PL, Kliem S, Pfitzner E, Gillert E, Dingermann T, Marschalek R. Transcription linked to recombination: A gene-internal promoter coincides with the recombination hot spot II of the human MLL gene. Oncogene. 2007;26:1361–1371. doi: 10.1038/sj.onc.1209948. [DOI] [PubMed] [Google Scholar]