Abstract
In this study, we assessed the effects of peripherally administered cannabinoids in an orofacial myositis model, and the role of sex hormones in cannabinoid receptor (CBR) expression in trigeminal ganglia (TG). Peripherally administered arachidonylcyclopropylamide (ACPA), a specific CB1R agonist, significantly attenuated complete Freund’s adjuvant (CFA)-induced mechanical hypersensitivity in the masseter muscle in male rats. The ACPA effect was blocked by a local administration of AM251, a specific CB1R antagonist, but not by AM630, a specific CB2R antagonist. In female rats, a 30-fold higher dose of ACPA was required to produce a moderate reduction in mechanical hypersensitivity. CFA injected in masseter muscle significantly upregulated CB1R mRNA expression in TG in male, but not in female, rats. There was a close correlation between the CB1R mRNA levels in TG and the antihyperalgesic effect of ACPA. Interleukin (IL)-1β and IL-6, which are elevated in the muscle tissue following CFA treatment, induced a significant upregulation of CB1R mRNA expression in TG from male rats. The upregulation of CB1R was prevented in TG cultures from orchidectomized male rats, which was restored by the application of testosterone. The cytokines did not alter the CB1R mRNA level in TG from intact as well as ovariectomized female rats. Neither estradiol supplement nor estrogen receptor blockade had any effects on CB1R expression. These data indicate that testosterone, but not estradiol, is required for the regulation of CB1Rs in TG under inflammatory conditions, which provide explanations for the sex differences in the antihyperalgesic effects of peripherally administered cannabinoids.
Keywords: Trigeminal ganglia, IL-6, IL-1β, Testosterone, Estrogen, Masseter muscle
1. Introduction
In recent years, cannabinoids have emerged as attractive alternatives or adjuncts for treatment of many types of diseases, including pain and inflammation [22]. However, psychotropic effects mediated by cannabinoid receptors (CBRs) in the central nervous system (CNS) limit the therapeutic use of cannabinoids as analgesics. Activation of peripheral CBRs, particularly CB1Rs, which are primarily expressed in primary sensory neurons, produces antinociceptive and antihyperalgesic effects in visceral, cutaneous, and muscle tissue [4,10,20,27]; and nociceptor-specific loss of CB1Rs in mice substantially reduces the analgesic effect produced by local and systemic delivery of cannabinoids [1]. These pertinent findings suggest targeting peripheral CB1R to provide effective pain relief without producing centrally mediated side effects.
It is well established that endocannabinoids produce sexually dimorphic cognitive and emotional responses [50]. Furthermore, several cannabinoids produce greater motoric effects in females [15], and more potent analgesia in females than in males in response to noxious mechanical and heat stimuli; the effects that are mediated by centrally located CB1Rs [55,56]. Sexually dimorphic responses in the cannabinoid system can be explained by sex differences in pharmacokinetics of cannabinoids [57], endocannabinoid levels [9], and CBR expression levels [11,35]. There is no information on whether selective activation of peripheral CBRs produces sex-dependent effects, and the potential mechanisms that might lead to sex differences in the cannabinoid effects are unknown.
While factors that modulate CBR expression are not well understood, sex steroids have been implicated as potential modulators of CBR expression in the periphery. Males exhibit higher levels of CB1R mRNA than females in the rat pituitary gland, and orchidectomy in males reduces CB1R transcripts, whereas chronic exposure to estradiol in ovariectomized females decreases CB1R transcripts [18]. Similarly, CB1R density decreases in parotid glands after castration in male rats, and is restored following testosterone replacement [12]. Inflammatory cytokines are also factors that modulate CBR expression. Cytokine-stimulated whole blood expresses greater CB1R and CB2R mRNA levels compared to nonstimulated blood [26]. CB2R expression in the spinal cord is upregulated along a time course consistent with the production of proinflammatory cytokines following the onset of multiple sclerosis [34]. These studies suggest that sex hormones and inflammation can influence the effect of endogenous cannabinoids as well as responses to cannabinoid treatments by modulating CBR expression.
In the present study, we used the rat model of orofacial myositis to evaluate the antihyperalgesic effects of peripheral CB1R activation in both sexes, and investigated the role of inflammatory cytokines, specifically IL-1β and IL-6 that are upregulated in the muscle under inflammatory conditions [38], on CB1R expression in primary sensory neurons in trigeminal ganglia (TG). We then examined whether cytokine-induced CB1R expression is modulated by sex hormones, estradiol, and testosterone.
2. Materials and methods
2.1. Animals
Age-matched adult male, female, orchidectomized (GDX) male, and ovariectomized (OVX) female Sprague-Dawley rats (8 weeks old; 250–300 g for males and 225–260 g for females; Harlan Laboratories Inc, Indianapolis, IN, USA) were used in the present study. The estrous cycle phase in female rats was not determined in this study. All animals were housed in a temperature-controlled room under a 12:12 light-dark cycle with access to food and water ad libitum. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and under a University of Maryland-approved Institutional Animal Care and Use Committee protocol.
2.2. Muscle inflammation
Inflammation was induced by injecting 50 μL of 50% complete Freund’s adjuvant (CFA) in isotonic saline (Sigma, St. Louis, MO, USA) into the mid-region of the masseter muscle via a 27-gauge needle. Rats were briefly anesthetized with 3% isoflurane for the injection procedure. The characteristics of inflammation and behavioral responses following CFA injections in the rat masseter have been described previously [5,25].
2.3. Drug preparation and administration
A CB1R agonist, ACPA in Tocrisolve 100 (Tocris, R&D Systems Inc, Minneapolis, MN, USA) was dissolved in phosphate-buffered saline (PBS; American Bioanalytical, Natick, MA, USA). A selective CB1R antagonist, AM251 (Tocris), and a CB2R antagonist, AM630 (Tocris), were dissolved in dimethyl sulfoxide (American Bioanalytical). Interleukin (IL)-1β (PeproTech, Rocky Hill, NJ, USA), IL-6 (PeproTech), soluble recombinant mouse IL-6 receptor (R&D Systems), recombinant human IL-1 receptor antagonist (IL-1RA; PeproTech), and recombinant human gp130 (R&D Systems) were dissolved in PBS. Cytokines and IL-6 receptor were prepared to a final concentration of 200 ng/20 μL. Cytokine antagonists were prepared to a final concentration of 2 μg/20 μL.
The above drugs were injected into the mid-region of the masseter muscle. In order to make sure that all drugs were injected in the same target region of the muscle, the injection site was determined by palpating the masseter between the zygomatic bone and the angle of the mandible. All drug injections were made via a 27-gauge needle. Injections were made for 5–10 seconds.
In culture studies, IL-1β, IL-6, tumor necrosis factor-alpha (TNF-α) (PeproTech), and soluble recombinant mouse IL-6 receptor were dissolved in PBS. Estradiol benzoate and testosterone (Sigma) were dissolved in dimethyl sulfoxide at a stock concentration of 2.66 mM. IL-1β, IL-6, TNF-α, and soluble recombinant mouse IL-6 receptor were applied to culture medium to a final concentration of 100 ng/mL. Estradiol benzoate and testosterone were applied to culture medium to a final concentration of 100 nM. Medium containing cytokine(s) and/or sex hormone were warmed to 37°C before being applied to cultured TG cells.
2.4. Behavioral studies
In this study, we utilized a behavioral model specifically developed for testing masseter sensitivity in rats [47,54]. In this model, a series of calibrated von Frey filaments were applied to the region over the masseter muscle. An active withdrawal of the head from the filament application was defined as a positive response. Each von Frey filament was applied 5 times and the response frequencies ([number of responses/number of stimuli] × 100%) to a range of filament forces were determined. After a nonlinear regression analysis, an EF50 value, the filament force (g) necessary to produce a 50% response frequency, was determined. The EF50 value was used as a measure of mechanical threshold. A reduction of EF50 after inflammation suggested the presence of mechanical hypersensitivity.
Mechanical sensitivity of the masseter muscle was determined before and 1, 2, 3, 5, 7, 10, and 14 days after the CFA injection. The effect of CB1R agonist on mechanical sensitivity was examined on the third day post CFA injection, a time point at which mechanical hypersensitivity was pronounced. Four doses of ACPA: 10 μg, 30 μg, 100 μg, and 300 μg/20 μL were administered into the masseter muscle to evaluate the dose-dependent effects on CFA-induced mechanical hypersensitivity. The proposed doses of ACPA were adapted from the literature [21]. Control groups received the vehicle injection in the same manner. To rule out the possibility that local administration of ACPA produced systemic effects by activating CB1Rs in the CNS, another group of rats received the highest doses of ACPA injections into the masseter muscle contralateral to the mechanical stimulation. To test the receptor specificity of the agonist, a selective antagonist for CB1Rs, AM251 (30 μg/10 μL), or for CB2Rs, AM630 (30 μg/10 μL), was administered 5 minutes before ACPA treatment in another group of animals. The selected doses of the antagonists have been shown to block the receptor-specific agonist effects [17,43]. In order to maintain the consistency in assessing behavioral responses, all behavioral experiments were conducted by an experimenter who was blinded to treatment conditions.
2.5. TG primary culture
All animals were euthanized by decapitation under pentobarbital anesthesia (100 mg/kg). Both TG from each animal were extracted and dissociated by sequential digestion with collagenase D (0.1%) in Dulbecco’s Modified Eagle Medium (DMEM-F12) (with l-glutamine) at 37°C for 30 minutes, followed by collagenase D (0.1%), trypsin (0.25%), DNase (50 μg), and EDTA (0.02%) in DMEM-F12 medium at 37°C for 15 minutes. After trituration, cells were plated on laminin precoated 12-well plates and kept in a 37°C incubator at 5% CO2 for 3 days.
2.6. Real-time RT-PCR
Total RNA was extracted from TG of naïve rats, 3 days after CFA-inflamed rats, 6 hours after the rats that received direct masseteric injections of cytokines, and 30 minutes after the cytokine and/or sex hormone application in cultured TG using Trizol (Sigma) and purified with an RNeasy kit (Qiagen Sciences, Germantown, MD, USA) that included a DNase treatment to remove genomic DNA. Reverse transcription was carried out using the Superscript First Stand synthesis kit (Invitrogen, Carlsbad, CA, USA). Superscript II (Invitrogen) was used to generate cDNA from 1 μg of RNA along with 2.5 ng of random primer per reaction. Real-time polymerase chain reaction (RT-PCR) analysis of cDNA equal to 25 ng RNA was performed on the Eppendorf Mastercycler ep realplex 2.0. The following primer pairs were used to detect CB1R mRNA: sense primer: 5′-CTACTGGTGCTGTGTGTCATC-3′, antisense primer: 5′- GCTGTCTTTACGGTGGAATAC-3′ [36]. The amount of a given CB1R mRNA was normalized to the glyceraldehyde 3-phosphate dehyrogenase (GAPDH) mRNA in the same sample. The primer pairs for detecting GAPDH mRNA were, sense primer: 5′-TCACCACCATGGAGAAGGC-3′, antisense primer: 5′- GCTAAGCAGTTGGTGGTGCA-3′. The cycling protocol used was 95°C for 10 minutes, followed by 45 cycles of 95°C for 10 seconds, 50°C for 15 seconds, and 72°C for 20 seconds. Relative quantification of the CB1R mRNA was calculated by the comparative CT method (ΔΔCT method) between control and experimental groups [53].
2.7. Statistics
The time-dependent changes in mechanical thresholds (EF50) before and after CFA treatment were analyzed with a 2-way analysis of variance (ANOVA) with repeated measures. In order to assess the effects of ACPA or vehicle, the post-CFA behavioral data were represented as a normalized EF50 value to the animals’ own baseline threshold. A 1-way ANOVA was then used to analyze mean percent changes in EF50 compared to baseline between experimental and control groups. A 2-way ANOVA was used to compare CB1R mRNA levels between control and experimental groups and between male and female intact TG. All other data obtained from RT-PCR experiments were analyzed with a 1-way ANOVA. All comparisons between multiple groups were followed by a post hoc test (Dunnett’s or Bonferroni’s). Data are presented as mean ± SE and differences were considered significant at P < 0.05.
3. Results
3.1. Sex differences in the effect of peripheral CB1R activation on masseter hypersensitivity
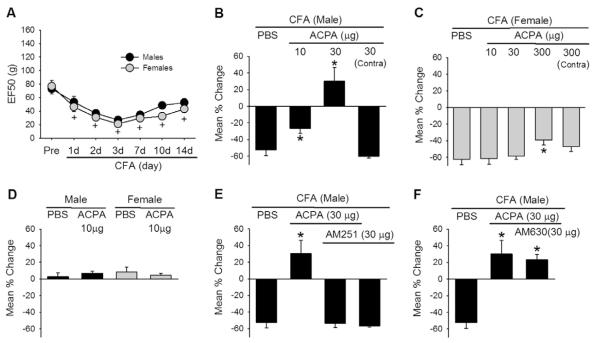
Masseteric injection of CFA in the rat induces a time-dependent and significant decrease in mechanical thresholds in as early as 30 minutes, that lasts over 12 days [5,54]. We have confirmed the development of mechanical hypersensitivity following CFA injection in the masseter, with a significant decrease in EF50 during the 14 days post CFA injection (Fig. 1A). There were no sex differences in the baseline thresholds, the time course, or the extent of mechanical hypersensitivity. In order to examine the effect of CB1R activation in CFA-induced masseter mechanical hypersensitivity, we injected the muscle with ACPA (20 μL) or vehicle 45 minutes prior to behavioral testing on the third day following CFA treatment. In males, 10 μg of ACPA significantly attenuated the CFA-induced mechanical hypersensitivity (Fig. 1B). ACPA at 30 μg not only reversed the hypersensitivity, but also produced a significant analgesic effect. To rule out possible systemic effects, the high dose of ACPA (30 μg) was injected into the masseter muscle contralateral to the testing site in a separate group of male rats. The contralateral ACPA treatment failed to attenuate the mechanical hypersensitivity, suggesting that ACPA at 30 μg was a systemically low dose and did not produce centrally mediated effects.
Fig. 1.
Effects of arachidonylcyclopropylamide (ACPA) on complete Freund’s adjuvant (CFA)-induced mechanical hypersensitivity. (A) CFA-induced mechanical hypersensitivity measured in the masseter muscle of male and normally cycling female rats. Mechanical force (g) that produced the head withdrawal responses 50% of the trials is plotted for pre- and 1, 2, 3, 7, 10, and 14 days post-CFA injection. +denotes significant time effects at P < 0.05 compared to the pre-CFA values. (B, C) Effects of intramuscular ACPA on mechanical sensitivity 3 days after CFA treatment in male and female rats. Bar graphs show mean % change in EF50 values in vehicle (phosphate-buffered saline [PBS])- and ACPA-treated rats. *denotes significant differences at P < 0.05 compared to the vehicle group. Contra-ACPA injected in the masseter muscle contralateral to the mechanical sensitivity testing. (D). Effects of ACPA on mechanical sensitivity of naïve male and female rats. (E, F). Effects of a CB1R antagonist (AM251) and a CB2R antagonist (AM630) on ACPA-mediated antihyperalgesic responses 3 days after CFA treatment in male rats. *denotes significant differences at P < 0.05 compared to the vehicle group. All data are shown as mean ± SE and each group consisted of 6–7 animals.
In contrast, the same doses of ACAP were ineffective in female rats. A 30-fold higher dose of ACPA (300 μg) was required to significantly attenuate the mechanical hypersensitivity in females (Fig. 1C). In a separate group of female rats, the highest dose of ACPA (300 μg) was also injected into the masseter muscle contralateral to the testing site. Similar to male rats, the contralateral ACPA treatment failed to attenuate the mechanical hypersensitivity. The dose of ACPA (10 μg) that significantly attenuated masseter mechanical hypersensitivity under the inflammatory condition in male rats did not affect the mechanical sensitivity in naïve male or female rats (Fig. 1D). These data demonstrated sex differences in the potency and efficacy of the local CB1R agonist in attenuating mechanical hypersensitivity under inflammatory conditions.
ACPA is a specific CB1R agonist with a 325-fold selectivity over CB2Rs (Tocris Bioscience). However, to further rule out the possible involvement of CB2Rs, additional behavioral experiments were conducted with specific antagonists for CB1Rs and CB2Rs. The effect of ACPA was completely blocked by the pretreatment of the muscle with a specific CB1R antagonist, AM251 (Fig. 1E). In contrast to AM251, pretreatment of the muscle with AM630, a specific CB2R antagonist, did not block the effect of ACPA (Fig. 1F). These data confirmed that the effect of ACPA was mediated by CB1Rs, and not by CB2Rs. AM251 alone, without ACPA, did not augment the masseter hypersensitivity, suggesting that endogenous cannabinoids do not play a major role in this model (Fig. 1E). Since AM630 was ineffective, we did not examine the effects of AM630 alone.
3.2. Sex differences in inflammation-induced CB1R expression in TG
In order to examine the impact of inflammation on CB1R expression, we measured the changes in CB1R mRNA in TG with a time course similar to the behavioral observations following CFA injection in the masseter. No sex differences in the level of CB1R mRNA in TG were observed under naïve conditions. CFA-induced masseter inflammation resulted in a time-dependent increase in the level of CB1R mRNA in TG of male, but not female, rats (Fig. 2A). In male rats, the level of CB1R mRNA was significantly greater in CFA-treated groups on day 3 and day 7, compared to the naïve group. These data suggested that inflammation-induced changes in CB1R mRNA levels in TG could underlie the sex differences observed in the behavioral studies.
Fig. 2.
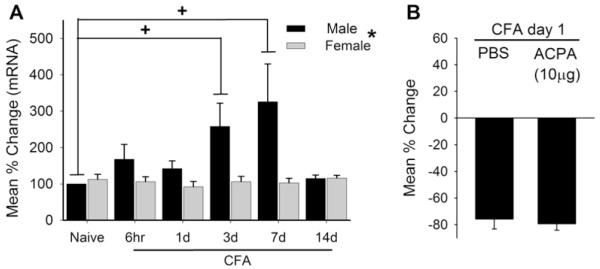
(A) Real-time polymerase chain reaction data showing complete Freund’s adjuvant (CFA)-induced changes in CB1R mRNA levels in intact trigeminal ganglia of male and female rats. All data are normalized to naïve male rats and presented as mean percent change. +denotes significant effects with respect to naïve condition at P < 0.05. (B) Effects of arachidonylcyclopropylamide (ACPA) on masseter hypersensitivity 1 day after CFA treatment in male rats. Bar graphs show mean % change in EF50 values in vehicle (phosphate-buffered saline [PBS])- and ACPA-treated rats. Data are shown as mean ± SE and each group consisted of 6 animals.
We showed that ACPA (10 μg) that was ineffective in attenuating masseter hypersensitivity under naïve conditions became efficacious on day 3 following CFA treatment, a time point when CB1R mRNA was significantly increased in male rats (Fig. 1B, D). To further correlate the effect of ACPA with the expression of CB1Rs in TG, we tested whether the same 10 μg dose attenuated masseter hypersensitivity 1 day after CFA-induced inflammation, when the level of CB1R mRNA in male TG was not significantly increased from the baseline. ACPA, which significantly attenuated the masseter hypersensitivity 3 days after inflammation, had no effect at day 1 (Fig. 2B). We observed that the same dose of ACPA was also ineffective at day 14 when the level of CB1R mRNA had returned to the baseline (data not shown). These data suggested that the antihyperalgesic effect of ACPA was largely due to an increased level of CB1R expression.
3.3. Inflammatory cytokines induce CB1R upregulation in TG cultures in a sex-dependent manner
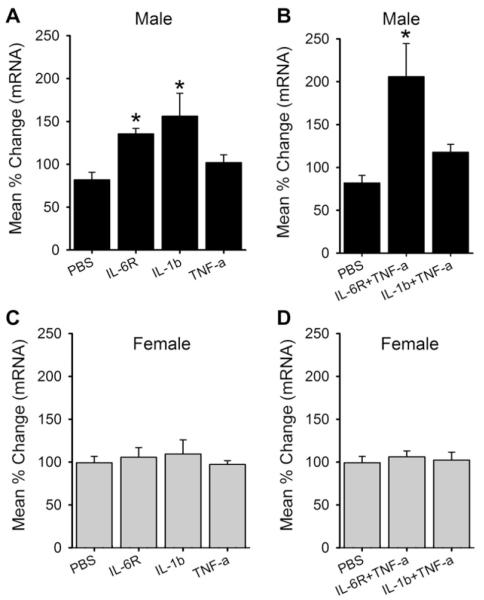
CFA-induced masseter inflammation leads to significant increases in the levels of IL-1β and IL-6, but not TNF-α, in the masseter muscle of both male and female rats [38]. Since the changes in IL-6 and IL-1β in the masseter muscle precede that of CB1R upregulation in TG, it is possible that local inflammatory cytokines could be involved in the regulation of CB1R expression. Interestingly, CFA increases the levels of IL-1β and IL-6 similarly between the 2 sexes [38], but modulates CB1R expression in TG in a sex-specific manner (Fig. 2A). These observations suggested that cellular mechanisms involved in transcriptional regulation of CB1Rs following inflammation could be different between males and females. In this experiment, we specifically investigated whether inflammatory cytokines induce differential upregulation of CB1R expression in male and female rats using primary cultures of TG.
In TG cultures from male rats, the level of CB1R mRNA increased significantly with 30 minutes application of IL-1β or IL-6 along with its soluble α-receptor subunit (IL-6R), but not of TNF-α (Fig. 3A). Since TNF-α has been shown to produce synergistic effects when combined with other cytokines [33,42], we also administered it in combination with IL-1β or IL-6R. The combination of IL-6R and TNF-α, but not the combination of IL-1β and TNF-α, also induced a significant upregulation of CB1R mRNA compared to controls (Fig. 3B). However, the same concentration of IL-1β, IL-6R, or TNF-α alone or as combinations of cytokines did not induce significant changes of CB1R mRNA in TG culture prepared from female rats (Fig. 3C, D).
Fig. 3.
Effects of inflammatory cytokines on CB1R mRNA expression in trigeminal ganglia primary cultures. Real-time polymerase chain reaction data following individual or combination of cytokines applications were compared to those of phosphate-buffered saline (PBS) treatment in cultures prepared from male (A,B) and female (C,D) rats. All data are normalized to untreated samples and presented as mean percent change in CB1R mRNA transcripts. Data are shown as mean ± SE and each group consisted of 5–6 replicates. *denotes significant differences compared to PBS group at P < 0.05. IL, interleukin; TNF, tumor necrosis factor.
3.4. IL-1β and IL-6 mediate CFA-induced CB1R upregulation and ACPA effects in vivo
We then investigated whether IL-1β and IL-6 are involved in inflammation-induced upregulation of CB1R mRNA in intact TG. IL-1RA or soluble gp130 (an inhibitor of IL-6 signaling complex) were directly administered into the masseter muscle 5 minutes before the induction of inflammation by CFA in male rats. TG from these rats were extracted 3 days later and the expression of CB1R mRNA was assessed. IL-1RA, at a dose that has been shown to effectively attenuate the IL-1β effect (2 μg) [23], completely prevented the upregulation of CB1R mRNA. Similarly, soluble gp130 at a dose that effectively antagonizes the IL-6 effect (2 μg) [41] also prevented the CFA-induced upregulation of CB1R mRNA (Fig. 4A).
Fig. 4.
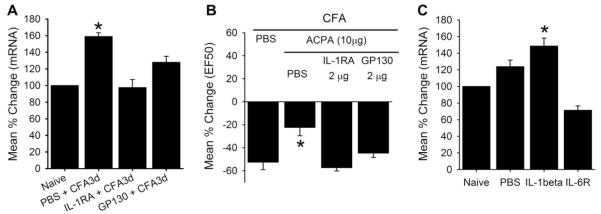
(A) Role of inflammatory cytokines in complete Freund’s adjuvant (CFA)-induced upregulation of CB1R mRNA in intact trigeminal ganglia (TG) of male rats. CFA-induced changes in CB1R mRNA levels were assessed in intact TG of male rats treated with interleukin (IL)-1 receptor antagonist (IL-1RA), IL-6 inhibitor, gp130, and vehicle. Data are normalized to that of TG obtained from naïve rats. Each group consisted of 6 rats. (B) Effects of arachidonylcyclopropylamide (ACPA) on CFA-induced masseter hypersensitivity were assessed under the same experimental conditions as in A. Each group consisted of 6–8 rats. (C) CB1R mRNA in intact TG from male rats treated with direct masseteric injections of IL-1β, IL-6 with its receptor (IL-6R), and vehicle control. Data are normalized to that of naïve male rats and each group consisted of 6 rats *denotes significant group effects at P < 0.05. PBS, phosphate-buffered saline.
In order to confirm that the blockade of CB1R upregulation by the cytokine antagonists is functionally relevant, we performed the same experiments and tested the effect of ACPA with the behavioral assay in additional groups of male rats. Consistent with the mRNA data, the dose of ACPA (10 μg) that significantly attenuated the CFA-induced masseter hypersensitivity was no longer effective in animals that were pretreated with either IL-1RA or gp130 (Fig. 4B).
Finally, we tested whether direct injections of these cytokines can induce CB1R mRNA upregulation in vivo. IL-1β (200 ng) or IL-6 (200 ng) and its soluble receptor (IL-6R) were injected into the mid-region of the masseter muscle of male rats, and CB1R mRNA was measured in intact TG 6 hours after the injection. IL-1β, but not its vehicle control, induced a significant increase in CB1R mRNA compared to that of TG from naïve rats (Fig. 4C). However, under the same experimental condition, IL-6R failed to induce a significant upregulation of CB1R mRNA.
3.5. Testosterone, but not estradiol, modulates cytokine-induced CB1R upregulation
Since cytokine-induced upregulation of CB1R mRNA was observed only in TG from male rats, we investigated whether sex hormones play a role in modulating cytokine-induced CB1R expression. First, since it is possible that circulating estradiol could have persistent inhibitory effects on cytokine-induced CB1R expression, we applied the same concentrations of inflammatory cytokines to TG cultures prepared from OVX female rats. None of the cytokines exerted a significant effect on CB1R expression (Fig. 5A). We then tested whether additional estradiol is required for cytokine-induced upregulation of CB1R mRNA. TG cultures from intact female rats were supplemented with estradiol benzoate (100 nM). The estradiol treatment alone or in combination with cytokines did not induce a significant upregulation of CB1R mRNA (Fig. 5B). Finally, we tested whether estradiol exerts residual inhibitory effects on TG cultures from intact female rats. The application of estrogen receptor antagonist (ICI 182, 780, 100 nM; Tocris) along with the cytokines in TG cultures from intact females also failed to increase the CB1R mRNA (Fig. 5C). Collectively, these data suggested that estradiol is not involved in the modulation of CB1R expression by inflammatory cytokines in TG.
Fig. 5.
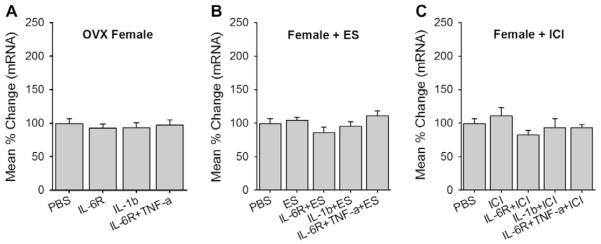
Role of estrogen in CB1R expression in trigeminal ganglia (TG) from female rats. (A) CB1R mRNA levels were assessed from TG of ovariectomized (OVX) rats treated with cytokines. (B) Estradiol benzoate (ES) alone or ES combined with cytokines were applied to TG cultures from intact female rats and CB1R mRNA levels assessed. (C) An estrogen receptor antagonist, ICI-182,780 (ICI) alone or ICI combined with cytokines were applied to TG cultures from intact female rats and CB1R mRNA assessed. All data are normalized to untreated samples and presented as mean percent change in CB1R mRNA transcripts. Data are shown as mean ± SE and each group consisted of 5–6 replicates. *denotes significant differences compared to PBS group at P < 0.05. PBS, phosphate-buffered saline; IL, interleukin; TNF, tumor necrosis factor.
If estradiol is not preventing the CB1R mRNA upregulation in females, it is possible that testosterone is playing a role in cytokine-induced modulation in males. Therefore, we applied the same cytokine treatments that induced CB1R mRNA upregulation in TG cultures from intact males to those prepared from GDX rats. Under this condition, neither IL-1β nor IL-6R was effective in upregulating CB1R mRNA (Fig. 6A). IL-6R applied in combination with TNF-α was also ineffective. Testosterone treatment in cultures from GDX rats significantly restored the cytokine-induced upregulation of CB1R mRNA (Fig. 6B). IL-6R in combination with TNF-α also induced a significant upregulation of CB1R mRNA in testosterone-treated cultures of GDX rats.
Fig. 6.
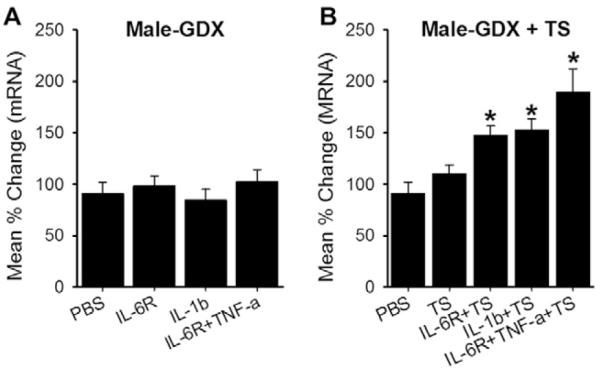
Role of testosterone in CB1R mRNA expression in trigeminal ganglia (TG) from male rats. (A) Real-time polymerase chain reaction data following individual or combination of cytokines applications were compared to those of PBS treatment in orchidectomized (GDX) rats. (B) Testosterone (TS) alone or TS combined with cytokines were applied to TG cultures from GDX rats and CB1R mRNA levels assessed. All data are normalized to untreated samples and presented as mean percent change in CB1R mRNA transcripts. Data are shown as mean ± SE and each group consisted of 5–6 replicates. *denotes significant differences compared to PBS group at P < 0.05. (C) Effects of arachidonylcyclopropylamide (ACPA) on masseter hypersensitivity 3 days after complete Freund’s adjuvant treatment in GDX rats. Bar graphs show mean % change in EF50 values in vehicle (phosphate-buffered saline [PBS])- and ACPA-treated rats. Data are shown as mean ± SE and each group consisted of 6 animals.
Since the cytokine treatment no longer induced CB1R mRNA upregulation in TG cultures prepared from GDX rats, ACPA should not be efficacious in attenuating CFA-induced mechanical hypersensitivity in GDX animals. Our behavioral data confirmed that the dose of ACPA (10 μg) that significantly attenuated the CFA-induced mechanical hypersensitivity in intact male rats was no longer effective in GDX rats (Fig. 6C). Taken together, these data suggested that testosterone is required for cytokine-induced CB1R upregulation in male rats, and further corroborate the correlation between the efficacy of peripheral CB1R and CB1R expression in TG.
Finally, we examined whether testosterone modulates cytokine-induced CB1R expression in female rats. IL-1β, IL-6R, or the combination of IL-6R and TNF-α was applied to cultures from female rats in the presence of testosterone. The testosterone treatment did not result in cytokine-induced CB1R upregulation in cultures prepared from female rats (Fig. 7).
Fig. 7.
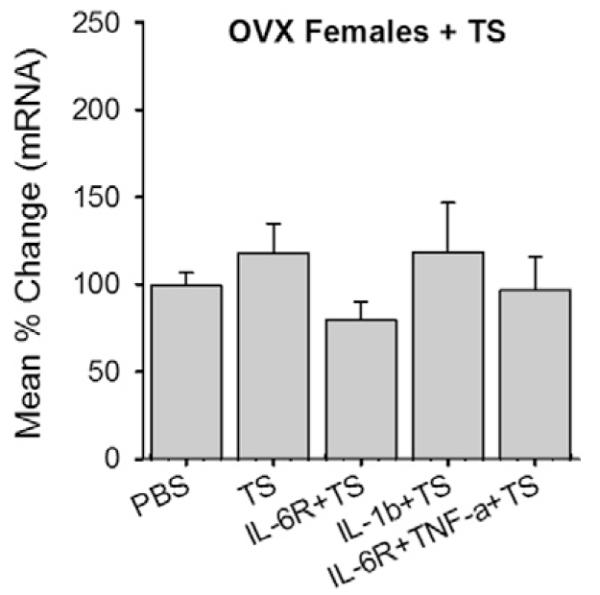
Role of testosterone (TS) in CB1R mRNA expression in trigeminal ganglia (TG) from female rats. TS alone or TS combined with cytokines were applied to TG cultures from intact female rats and CB1R mRNA levels assessed. All data are normalized to untreated samples and presented as mean percent change in CB1R mRNA transcripts. Data are shown as mean ± SE and each group consisted of 5–6 replicates. OVX, ovariectomized; PBS, phosphate-buffered saline; IL, interleukin; TNF, tumor necrosis factor.
4. Discussion
Recent studies continue to demonstrate peripherally localized CBRs as an attractive target to garner cannabinoids’ therapeutic effects in many types of pain conditions without producing unwanted side effects [19,46,52,59]. Selective knockout of CB1Rs in nociceptors in mice substantially reduces the analgesic effect produced by systemic delivery of cannabinoids, suggesting peripheral CB1Rs as the major site for cannabinoid-mediated analgesia [1]. Consistent with this finding, systemic delivery of peripherally restricted cannabinoid compounds produces robust and sufficient analgesic and antihyperalgesic effects in animal models of inflammatory and neuropathic pain [16,59]. Studies implicating the peripheral CB1R in the blockade of not only the development, but also the maintenance, of pathological pain conditions lend further support for therapeutic interventions targeting the CBR at the site of pathophysiology [20,30]. In clinical trials, Sativex, a cannabis extract, provides significant improvement on movement-induced pain in rheumatoid arthritis patients [6], and effectively relieves peripheral neuropathic pain without causing significant psychotropic effects, suggesting a peripheral action of the drug [40].
The results from the present study provide additional evidence that activation of CB1Rs in TG afferents effectively attenuates inflammation-induced mechanical hyperalgesia in the masseter muscle. Systemically inactive doses of ACPA reversed the mechanical hyperalgesia in inflamed muscle, but did not alter the mechanical sensitivity in noninflamed muscle. Although we did not perform complete dose-response studies to compare the efficacy of ACPA between normal and inflamed conditions, our data are consistent with previous studies that showed enhanced effects of cannabinoids at the peripheral CB1R under inflammatory conditions [4,29].
Cannabinoids produce greater antinociception in female rats compared to male rats, effects predominantly mediated by CB1Rs in the brain [55,56]. Based on this animal study, the analgesic activity of Cannador, a whole plant extract, was tested in female subjects in human experimental pain models [31], but no analgesic or antihyperalgesic activities were found with this cannabis extract. However, Nabilone, a synthetic analogue of tetrahydrocannabinol, significantly attenuates hyperalgesia only in women [45].
As far as we know, there is no study that showed sex differences in behavioral responses mediated by peripheral CB1Rs under either normal or pathological conditions. Many studies that showed a potent peripheral CB1R effect under inflammatory or neuropathic pain conditions used male subjects [1,20,37,48,61], and the few studies that utilized female subjects did not directly assess sex differences [19,59]. Thus, we provide a novel and interesting finding that locally administered CB1R agonist produces more potent antihyperalgesic effects in males compared to females in a rat myositis model.
The sex differences in ACPA effects can be explained by multiple factors such as differences in cannabinoid metabolism in local tissue, CB1R expression level, G-protein coupling efficiency, and endocannabinoid signaling between males and females. It is possible that inflammation or injury can have sex-dependent impacts on these factors. For example, CFA in the hind paw induced a significant upregulation of CB1R expression in dorsal root ganglia (DRG), which resulted in enhanced antihyperalgesic effects of a peripheral CB1 agonist in male rats, but female rats were not studied [4]. In the present study, the antihyperalgesic effects of ACPA were observable only in male rats in which CB1R mRNA was significantly increased. In female rats, masseter inflammation did not alter the CB1R expression level in TG, and ACPA was ineffective in attenuating mechanical sensitivity in either inflamed or noninflamed muscle. Therefore, our data suggest that sex differences in local ACPA effects are a result of a differential impact of inflammation on CB1R expression between male and female rats. Although we did not systematically investigate this in the present study, CB2R expressed in primary sensory neurons also mediate analgesic activity in chronic inflammatory and neuropathic pain conditions [24]. It is possible that the limited effect observed in females at a high dose of ACPA may be due to CB2R activation. Sex differences in peripheral CB2R require further studies.
Of the multitude of inflammatory mediators that are released in the local tissue, cytokines have been frequently implicated in the modulation of both ORs and CBRs. In the OR system, proinflammatory and antiinflammatory cytokines, IL-1β, IL-6, TNF-α, and IL-4 are powerful modulators of OR expression in neuronal and non-neuronal cells [7,8,32,51,58]. Direct injection of IL-1β into the hind paw mimics CFA-induced upregulation of kappa OR in DRG [44]. Similarly, in the cannabinoid system, CB1R mRNA and protein levels increase significantly in IL-1β-, TNF-α-, or IL-6-stimulated whole blood compared to nonstimulated blood [26].
In this study, we showed that inflammatory cytokines such as IL-1β and IL-6 induce CB1R expression in TG. Since we only measured CB1R mRNA from whole TG or dissociated TG, we cannot claim that the cytokines elevated neuronal CB1Rs. In our hands, several commercially available CB1R antibodies did not yield reliable and quantifiable immunohistochemical staining in TG neurons. However, CB1Rs are expressed primarily in neurons in DRG [4], and there is a high level of colocalization of CB1Rs and transient receptor potential V1 channel in small-diameter DRG neurons [2]. These studies strongly suggest that the cytokine-induced upregulation involves neuronal CB1Rs.
While both IL-1β and IL-6 produced moderate but significant upregulation of CB1R mRNA, IL-6 and TNF-α combined treatment resulted in a more pronounced increase in CB1R expression. This was not surprising because TNF-α has been shown to potentiate IL-6 effect in other systems [33,42]. An interesting observation was that the IL-1β effect is diminished when combined with TNF-α. Therefore, unlike the synergistic interactions between IL-6 and TNF-α, a high level of TNF-α could inhibit IL-1β in inducing CB1Rs. CFA treatment did not result in CB1R upregulation in intact animals when IL-1β and IL-6 were blocked, confirming the involvement of IL-6 and IL-1β in CB1R expression in TG. Furthermore, direct injection of IL-1β mimicked the CFA effect of CB1R expression in TG. Thus, the involvement of IL-1β in CB1R regulation is shown at 3 different levels.
Direct injection of IL-6, however, failed to mimic the CFA effect. We collected evidence that IL-1β treatment in the masseter muscle evokes a significant release of IL-6 (data not shown), suggesting that IL-1β treatment accompanies the IL-6 effect. In fact, IL-1β leads to IL-6 production in various systems such as human orbital fibroblasts, placental mesenchymal cells, and Caco-2 cells [13,14,28]. These observations suggest that under in vivo conditions, both IL-6 and IL-1β are required for CB1R induction. This interpretation is consistent with the data in Fig. 4 that showed a significant but only partial effect following the blockade of IL-6 compared to IL-1β. Taken together, our data provided compelling evidence that IL-1β and IL-6 are critically involved in the regulation of CB1Rs in TG under inflammatory conditions.
Currently, the effect of sex hormones on CBR expression is not well understood. 17β-Estradiol treatment induces CB1R expression in human colon cancer cells, and the coadministration of an estrogen receptor antagonist blocks this effect [39]. In the hippocampus and the hypothalamus, however, OVX females have higher amounts of cannabinoid receptor binding relative to both cycling females and estradiol-replaced OVX females [49]. Similarly, in the anterior pituitary gland, the CB1R mRNA transcript is at the lowest level during the estrous stage in normally cycling female rats, and estradiol-replaced OVX rats exhibit significantly lower CB1R mRNA compared to OVX animals that had not been replaced with estradiol [18]. Interestingly, the same study also showed that males maintain higher levels of CB1R mRNA than female rats, and that orchidectomy in males reduced CB1R mRNA levels. In the parotid gland, castration in male rats significantly reduces CB1R expression, which is restored after testosterone treatment [12]. Given that a higher density of CB1R levels is observed in male animals in many brain regions [50], it seems that CB1R expression is influenced by gonadal hormones and that testosterone plays a key role in the regulation of CB1R in the CNS as well as in the periphery.
Gonadal hormones do not seem to modulate basal CB1R level in TG, as there were no significant sex differences in the CB1R mRNA levels between the 2 sexes. A significant role of estradiol in CB1R expression is not supported in our data. We could not determine the estrous stage of female rats because CFA-induced inflammation disrupts estrous cycling [60]. Therefore, the impact of fluctuations in estrogen levels in normally cycling female rats on CB1R is yet to be determined. In our hands, the cytokine-induced CB1R upregulation was restored following a brief exposure to testosterone in GDX animals. These data have to be confirmed in GDX animals with testosterone replacement that more closely restore the serum testosterone level. Nevertheless, the present observations suggest that testosterone, but not estradiol, is required in the upregulation of peripheral CB1R under inflammatory conditions. A testosterone deficiency has been reported in chronic pain patients, and testosterone replacement therapy is necessary for satisfactory pain control [3]. Our data suggest that testosterone may be involved in maintaining endogenous antinociceptive systems such as CBR in chronic pain conditions and that effective treatment strategies targeting peripheral CB1R should consider the hormonal status of patients. Furthermore, our data offer important clues to further investigate cellular mechanisms that link cytokines and sex hormones in various inflammatory conditions.
Acknowledgement
The authors thank Gregory Haynes and Dr. Jongseok Lee for technical assistance. This study was supported by National Institutes of Health Grants RO1 DE019448 to J.Y.R. and F30 DE020988 to K.Y.N.
Footnotes
Conflict of interest statement There is no conflict of interest to declare.
References
- [1].Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nat Neurosci. 2007;10:870–9. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors are expressed in nociceptive primary sensory neurons. Neuroscience. 2000;100:685–8. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- [3].Aloisi AM, Ceccarelli I, Carlucci M, Suman A, Sindaco G, Mameli S, Paci V, Ravaioli L, Passavanti G, Bachiocco V, Pari G. Hormone replacement therapy in morphine-induced hypogonadic male chronic pain patients. Reprod Biol Endocrinol. 2011;9:26. doi: 10.1186/1477-7827-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Amaya F, Shimosato G, Kawasaki Y, Hashimoto S, Tanaka Y, Ji RR, Tanaka M. Induction of CB1 cannabinoid receptor by inflammation in primary afferent neurons facilitates antihyperalgesic effect of peripheral CB1 agonist. PAIN®. 2006;124:175–83. doi: 10.1016/j.pain.2006.04.001. [DOI] [PubMed] [Google Scholar]
- [5].Ambalavanar R, Moutanni A, Dessem D. Inflammation of craniofacial muscle induces widespread mechanical allodynia. Neurosci Lett. 2006;399:249–54. doi: 10.1016/j.neulet.2006.02.003. [DOI] [PubMed] [Google Scholar]
- [6].Blake DR, Robson P, Ho M, Jubb RW, McCabe CS. Preliminary assessment of the efficacy, tolerability and safety of a cannabis-based medicine (Sativex) in the treatment of pain caused by rheumatoid arthritis. Rheumatology (Oxford) 2006;45:50–2. doi: 10.1093/rheumatology/kei183. [DOI] [PubMed] [Google Scholar]
- [7].Börner C, Höllt V, Kraus J. Involvement of activator protein-1 in transcriptional regulation of the human mu-opioid receptor gene. Mol Pharmacol. 2002;61:800–5. doi: 10.1124/mol.61.4.800. [DOI] [PubMed] [Google Scholar]
- [8].Börner C, Kraus J, Schröder H, Ammer H, Höllt V. Transcriptional regulation of the human mu-opioid receptor gene by interleukin-6. Mol Pharmacol. 2004;66:1719–26. doi: 10.1124/mol.104.003806. [DOI] [PubMed] [Google Scholar]
- [9].Bradshaw HB, Rimmerman N, Krey JF, Walker JM. Sex and hormonal cycle differences in rat brain levels of pain-related cannabimimetic lipid mediators. Am J Physiol Regul Integr Comp Physiol. 2006;291:R349–58. doi: 10.1152/ajpregu.00933.2005. [DOI] [PubMed] [Google Scholar]
- [10].Brusberg M, Arvidsson S, Kang D, Larsson H, Lindström E, Martinez V. CB1 receptors mediate the analgesic effects of cannabinoids on colorectal distension-induced visceral pain in rodents. J Neurosci. 2009;29:1554–64. doi: 10.1523/JNEUROSCI.5166-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB1 receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br J Pharmacol. 2010;161:103–12. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Busch L, Sterin-Borda L, Borda E. Effects of castration on cannabinoid cb receptor expression and on the biological actions of cannabinoid in the parotid gland. Clin Exp Pharmacol Physiol. 2006;33:258–63. doi: 10.1111/j.1440-1681.2006.04355.x. [DOI] [PubMed] [Google Scholar]
- [13].Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem. 2008;283:25900–12. doi: 10.1074/jbc.M707692200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chen B, Tsui S, Smith TJ. IL-1 beta induces IL-6 expression in human orbital fibroblasts: identification of an anatomic-site specific phenotypic attribute relevant to thyroid-associated ophthalmopathy. J Immunol. 2005;175:1310–9. doi: 10.4049/jimmunol.175.2.1310. [DOI] [PubMed] [Google Scholar]
- [15].Cohn RA, Barnes PR, Barratt E, Pirch JH. Sex differences in response to marijuana in the cat. In: Singh JM, Miller LH, Lal H, editors. Drug addiction experimental pharmacology. Vol. 1. Futura; New York: 1972. pp. 227–34. [Google Scholar]
- [16].Dziadulewicz EK, Bevan SJ, Brain CT, Coote PR, Culshaw AJ, Davis AJ, Edwards LJ, Fisher AJ, Fox AJ, Gentry C, Groarke A, Hart TW, Huber W, James IF, Kesingland A, La Vecchia L, Loong Y, Lyothier I, McNair K, O’Farrell C, Peacock M, Portmann R, Schopfer U, Yaqoob M, Zadrobilek J. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone: a potent, orally bioavailable human CB1/CB2 dual agonist with antihyperalgesic properties and restricted central nervous system penetration. J Med Chem. 2007;50:3851–6. doi: 10.1021/jm070317a. [DOI] [PubMed] [Google Scholar]
- [17].Gibson HE, Edwards JG, Page RS, Van Hook MJ, Kauer JA. TRPV1 channels mediate long-term depression at synapses on hippocampal interneurons. Neuron. 2008;57:746–59. doi: 10.1016/j.neuron.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].González S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, Di Marzo V, Ramos JA, Fernández-Ruiz JJ. Sex steroid influence on cannabinoid CB(1) receptor mRNA and endocannabinoid levels in the anterior pituitary gland. Biochem Biophys Res Commun. 2000;270:260–6. doi: 10.1006/bbrc.2000.2406. [DOI] [PubMed] [Google Scholar]
- [19].Guerrero AV, Quang P, Dekker N, Jordan RC, Schmidt BL. Peripheral cannabinoids attenuate carcinoma-induced nociception in mice. Neurosci Lett. 2008;433:77–81. doi: 10.1016/j.neulet.2007.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gutierrez T, Farthing JN, Zvonok AM, Makriyannis A, Hohmann AG. Activation of peripheral cannabinoid CB1 and CB2 receptors suppresses the maintenance of inflammatory nociception: a comparative analysis. Br J Pharmacol. 2007;150:153–63. doi: 10.1038/sj.bjp.0706984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hayase T, Yamamoto Y, Yamamoto K. Protective effects of cannabinoid receptor ligands analogous to anandamide against cocaine toxicity. Nihon Arukoru Yakubutsu Igakkai Zasshi. 2001;36:596–608. [PubMed] [Google Scholar]
- [22].Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2005–2009. Cannabinoids. 2010;5:1–21. [Google Scholar]
- [23].Hook MA, Washburn SN, Moreno G, Woller SA, Puga D, Lee KH, Grau JW. An IL-1 receptor antagonist blocks a morphine-induced attenuation of locomotor recovery after spinal cord injury. Brain Behav Immun. 2011;25:349–59. doi: 10.1016/j.bbi.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hsieh GC, Pai M, Chandran P, Hooker BA, Zhu CZ, Salyers AK, Wensink EJ, Zhan C, Carroll WA, Dart MJ, Yao BB, Honore P, Meyer MD. Central and peripheral sites of action for CB2 receptor mediated analgesic activity in chronic inflammatory and neuropathic pain models in rats. Br J Pharmacol. 2011;162:428–40. doi: 10.1111/j.1476-5381.2010.01046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Imbe H, Dubner R, Ren K. Masseteric inflammation-induced Fos protein expression in the trigeminal interpolaris/caudalis transition zone: contribution of somatosensory-vagal-adrenal integration. Brain Res. 1999;845:165–75. doi: 10.1016/s0006-8993(99)01913-7. [DOI] [PubMed] [Google Scholar]
- [26].Jean-Gilles L, Gran B, Constantinescu CS. Interaction between cytokines, cannabinoids and the nervous system. Immunobiology. 2010;215:606–10. doi: 10.1016/j.imbio.2009.12.006. [DOI] [PubMed] [Google Scholar]
- [27].Johanek LM, Simone DA. Activation of peripheral cannabinoid receptors attenuates cutaneous hyperalgesia produced by a heat injury. PAIN®. 2004;109:432–42. doi: 10.1016/j.pain.2004.02.020. [DOI] [PubMed] [Google Scholar]
- [28].Kauma SW, Turner TT, Harty JR. Interleukin-1 beta stimulates interleukin-6 production in placental villous core mesenchymal cells. Endocrinology. 1994;134:457–60. doi: 10.1210/endo.134.1.8275959. [DOI] [PubMed] [Google Scholar]
- [29].Kelly S, Jhaveri MD, Sagar DR, Kendall DA, Chapman V. Activation of peripheral cannabinoid CB1 receptors inhibits mechanically evoked responses of spinal neurons in noninflamed rats and rats with hindpaw inflammation. Eur J Neurosci. 2003;18:2239–43. doi: 10.1046/j.1460-9568.2003.02957.x. [DOI] [PubMed] [Google Scholar]
- [30].Khasabova IA, Khasabov SG, Harding-Rose C, Coicou LG, Seybold BA, Lindberg AE, Steevens CD, Simone DA, Seybold VS. A decrease in anandamide signaling contributes to the maintenance of cutaneous mechanical hyperalgesia in a model of bone cancer pain. J Neurosci. 2008;28:11141–52. doi: 10.1523/JNEUROSCI.2847-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kraft B, Frickey NA, Kaufmann RM, Reif M, Frey R, Gustorff B, Kress HG. Lack of analgesia by oral standardized cannabis extract on acute inflammatory pain and hyperalgesia in volunteers. Anesthesiology. 2008;109:101–10. doi: 10.1097/ALN.0b013e31817881e1. [DOI] [PubMed] [Google Scholar]
- [32].Kraus J, Börner C, Giannini E, Höllt V. The role of nuclear factor kappaB in tumor necrosis factor-regulated transcription of the human mu-opioid receptor gene. Mol Pharmacol. 2003;64:876–84. doi: 10.1124/mol.64.4.876. [DOI] [PubMed] [Google Scholar]
- [33].Kuhweide R, Van Damme J, Ceuppens JL. Tumor necrosis factor-alpha and interleukin 6 synergistically induce T cell growth. Eur J Immunol. 1990;20:1019–25. doi: 10.1002/eji.1830200511. [DOI] [PubMed] [Google Scholar]
- [34].Loría F, Petrosino S, Mestre L, Spagnolo A, Correa F, Hernangómez M, Guaza C, Di Marzo V, Docagne F. Study of the regulation of the endocannabinoid system in a virus model of multiple sclerosis reveals a therapeutic effect of palmitoylethanolamide. Eur J Neurosci. 2008;28:633–41. doi: 10.1111/j.1460-9568.2008.06377.x. [DOI] [PubMed] [Google Scholar]
- [35].Mateos B, Borcel E, Loriga R, Luesu W, Bini V, Llorente R, Castelli MP, Viveros MP. Adolescent exposure to nicotine and/or the cannabinoid agonist CP 55,940 induces gender-dependent long-lasting memory impairments and changes in brain nicotinic and CB(1) cannabinoid receptors. J Psychopharmacol. 2011;25:1676–90. doi: 10.1177/0269881110370503. [DOI] [PubMed] [Google Scholar]
- [36].Mitrirattanakul S, Ramakul N, Guerrero AV, Matsuka Y, Ono T, Iwase H, Mackie K, Faull KF, Spigelman I. Site-specific increases in peripheral cannabinoid receptors and their endogenous ligands in a model of neuropathic pain. PAIN®. 2006;126:102–14. doi: 10.1016/j.pain.2006.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Nackley AG, Suplita RL, 2nd, Hohmann AG. A peripheral cannabinoid mechanism suppresses spinal fos protein expression and pain behavior in a rat model of inflammation. Neuroscience. 2003;117:659–70. doi: 10.1016/s0306-4522(02)00870-9. [DOI] [PubMed] [Google Scholar]
- [38].Niu KY, Ro JY. Changes in intramuscular cytokine levels during masseter inflammation in male and female rats. Neurosci Lett. 2011;487:223–7. doi: 10.1016/j.neulet.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Notarnicola M, Messa C, Orlando A, Bifulco M, Laezza C, Gazzerro P, Caruso MG. Estrogenic induction of cannabinoid CB1 receptor in human colon cancer cell lines. Scand J Gastroenterol. 2008;43:66–72. doi: 10.1080/00365520701559011. [DOI] [PubMed] [Google Scholar]
- [40].Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: a randomised, double-blind, placebo-controlled clinical trial. PAIN®. 2007;133:210–20. doi: 10.1016/j.pain.2007.08.028. [DOI] [PubMed] [Google Scholar]
- [41].Obreja O, Schmelz M, Poole S, Kress M. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. PAIN®. 2002;96:57–62. doi: 10.1016/s0304-3959(01)00420-1. [DOI] [PubMed] [Google Scholar]
- [42].Poli G, Bressler P, Kinter A, Duh E, Timmer WC, Rabson A, Justement JS, Stanley S, Fauci AS. Interleukin 6 induces human immunodeficiency virus expression in infected monocytic cells alone and in synergy with tumor necrosis factor alpha by transcriptional and post-transcriptional mechanisms. J Exp Med. 1990;172:151–8. doi: 10.1084/jem.172.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Potenzieri C, Harding-Rose C, Simone DA. The cannabinoid receptor agonist, WIN 55, 212–2, attenuates tumor-evoked hyperalgesia through peripheral mechanisms. Brain Res. 2008;1215:69–75. doi: 10.1016/j.brainres.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Puehler W, Rittner HL, Mousa SA, Brack A, Krause H, Stein C, Schäfer M. Interleukin-1 beta contributes to the upregulation of kappa opioid receptor mrna in dorsal root ganglia in response to peripheral inflammation. Neuroscience. 2006;141:989–98. doi: 10.1016/j.neuroscience.2006.03.078. [DOI] [PubMed] [Google Scholar]
- [45].Redmond WJ, Goffaux P, Potvin S, Marchand S. Analgesic and antihyperalgesic effects of nabilone on experimental heat pain. Curr Med Res Opin. 2008;24:1017–24. doi: 10.1185/030079908x280635. [DOI] [PubMed] [Google Scholar]
- [46].Reis GM, Ramos MA, Pacheco Dda F, Klein A, Perez AC, Duarte ID. Endogenous cannabinoid receptor agonist anandamide induces peripheral antinociception by activation of ATP-sensitive K+ channels. Life Sci. 2011;88:653–7. doi: 10.1016/j.lfs.2011.01.017. [DOI] [PubMed] [Google Scholar]
- [47].Ren K. An improved method for assessing mechanical allodynia in the rat. Physiol Behav. 1999;67:711–6. doi: 10.1016/s0031-9384(99)00136-5. [DOI] [PubMed] [Google Scholar]
- [48].Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. PAIN®. 1998;75:111–9. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- [49].Riebe CJ, Hill MN, Lee TT, Hillard CJ, Gorzalka BB. Estrogenic regulation of limbic cannabinoid receptor binding. Psychoneuroendocrinology. 2010;35:1265–9. doi: 10.1016/j.psyneuen.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Rubino T, Parolaro D. Sexually dimorphic effects of cannabinoid compounds on emotion and cognition. Front Behav Neurosci. 2011;5:64. doi: 10.3389/fnbeh.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Ruzicka BB, Thompson RC, Watson SJ, Akil H. Interleukin-1 beta-mediated regulation of mu-opioid receptor mRNA in primary astrocyte-enriched cultures. J Neurochem. 1996;66:425–8. doi: 10.1046/j.1471-4159.1996.66010425.x. [DOI] [PubMed] [Google Scholar]
- [52].Sánchez E, Bagües A, Martin M. Cannabinoids and muscular pain. Effectiveness of the local administration in rat. Eur J Pain. 2012 doi: 10.1002/j.1532-2149.2012.00115.x. http://dx.doi.org/10.1002/j.1532-2149.2012.00115.x [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- [53].Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–8. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- [54].Shimizu K, Guo W, Wang H, Zou S, LaGraize SC, Iwata K, Wei F, Dubner R, Ren K. Differential involvement of trigeminal transition zone and laminated subnucleus caudalis in orofacial deep and cutaneous hyperalgesia: the effects of interleukin-10 and glial inhibitors. Mol Pain. 2009;21:75. doi: 10.1186/1744-8069-5-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabinoids. Eur J Pharmacol. 2001;430:41–7. doi: 10.1016/s0014-2999(01)01267-5. [DOI] [PubMed] [Google Scholar]
- [56].Tseng AH, Craft RM. CB(1) receptor mediation of cannabinoid behavioral effects in male and female rats. Psychopharmacology (Berl) 2004;172:25–30. doi: 10.1007/s00213-003-1620-x. [DOI] [PubMed] [Google Scholar]
- [57].Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in Delta 9-tetrahydrocannabinol-induced behavioral effects in rats. Behav Brain Res. 2004;154:77–83. doi: 10.1016/j.bbr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- [58].Vidal EL, Patel NA, Wu G, Fiala M, Chang SL. Interleukin-1 induces the expression of mu opioid receptors in endothelial cells. Immunopharmacology. 1998;38:261–6. doi: 10.1016/s0162-3109(97)00085-4. [DOI] [PubMed] [Google Scholar]
- [59].Walczak JS, Cervero F. Local activation of cannabinoid CB1 receptors in the urinary bladder reduces the inflammation-induced sensitization of bladder afferents. Mol Pain. 2011;7:31. doi: 10.1186/1744-8069-7-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang X, Traub RJ, Murphy AZ. Persistent pain model reveals sex difference in morphine potency. Am J Physiol Regul Integr Comp Physiol. 2006;291:R300–6. doi: 10.1152/ajpregu.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yu XH, Cao CQ, Martino G, Puma C, Morinville A, St-Onge S, Lessard E, Perkins MN, Laird JM. A peripherally restricted cannabinoid receptor agonist produces robust anti-nociceptive effects in rodent models of inflammatory and neuropathic pain. PAIN®. 2010;151:337–44. doi: 10.1016/j.pain.2010.07.019. [DOI] [PubMed] [Google Scholar]