Abstract
Background
EUS-guided fiducial placement facilitates image-guided radiation therapy (IGRT).
Objective
To compare 2 types of commercially available fiducials for technical success, complications, visibility, and migration.
Design
Retrospective, single-center, comparative study.
Setting
Tertiary-care medical center.
Interventions
Traditional fiducials (TFs) (5-mm length, 0.8-mm diameter) and Visicoil fiducials (VFs) (10-mm length, 0.35-mm diameter) were compared. Fiducials were placed using linear 19-gauge (for TFs) or 22-gauge (for VFs) needles. A subjective visualization scoring system (0-2; 0 = not visible, 1 = barely visible, 2 = clearly visible) was used to assess visibility on CT. Fiducial migration was calculated as a change in interfiducial distance.
Main Outcome Measurements
Technical success, complications, visibility, and migration of 2 types of fiducials.
Results
Thirty-nine patients with locally advanced pancreatic cancer underwent EUS-guided placement of 103 fiducials (77 TFs, 26 VFs). The mean number of fiducials placed per patient was 2.66 (standard deviation 0.67) for the 19-gauge needle and 2.60 (standard deviation 0.70) for the 22-gauge needle (P = .83). No intra- or postprocedural complications were encountered. The median visibility score for TFs was significantly better than that for VFs, both when scores of 0 were and were not included (2.00, interquartile range [IQR] 2.00-2.00 vs 1.75, IQR 1.50-2.00, P = .009 and 2.00, IQR 2.00-2.00 vs 2.00, IQR 1.50-2.00, P < .0001, respectively). The mean migration was not significantly different between the 2 types of fiducials (0.8 mm [IQR 0.4-1.6 mm] for TFs vs 1.3 mm [IQR 0.6-1.5 mm] for VFs; P = .72).
Limitations
Retrospective, nonrandomized design.
Conclusions
Visibility was significantly better for TFs compared with VFs. The degree of fiducial migration was not significantly different for TFs and VFs. There was no significant difference in the mean number of fiducials placed, indicating a similar degree of technical difficulty for TF and VF deployment.
Pancreatic cancer is the second most common GI malignancy and the fourth leading cause of cancer deaths in the United States.1 Pancreatic cancer has a poor prognosis with postoperative 5-year survival rates of 3% to 25%.2,3 In two thirds of patients with resectable tumors, local recurrence develops within 2 years of surgery.4 The disease is often advanced at presentation, and nearly 90% of patients have inoperable disease at the time of diagnosis, with a median survival of approximately 4 months without treatment.3 Patients with locally advanced pancreatic adenocarcinoma most often have tumor involvement of celiac axis or superior mesenteric artery. In these patients, chemotherapy, conventional radiation therapy (RT), or a combination of both may positively influence overall survival and quality of life.5-8 The goal is to attempt to downstage the tumor, improve local control, and offer palliation.9 However, these treatment modalities have had a modest impact on the overall prognosis.5-8 In recent years, improvements in RT, namely, stereotactic body RT (SBRT), were possible because of advances in CT, magnetic resonance imaging, and positron emission tomography. SBRT delivers multiple beams of radiation with extreme accuracy, allowing the safe and effective delivery of RT to target sites.10-11 However, treatment of extracranial lesions with SBRT requires placement of intratumoral radiographic markers (fiducials) to allow image-guided RT (IGRT). With IGRT, it is possible to deliver high doses of RT with submillimeter accuracy, sparing surrounding organs at risk.9
Percutaneous radiographic marker placement is an established technique for the deployment of fiducials in pancreatic tumors.12 However, this approach is invasive and carries a nontrivial morbidity risk with a relatively high rate of fiducial migration.12 EUS-guided fiducial placement has been reported in recent years to be a less invasive and effective means for fiducial placement in patients with inoperable pancreatic cancer.13-19 EUS-guided fiducial placement is traditionally performed by using 19-gauge FNA needles because of the wide diameter (0.8 mm) of traditional fiducials.13,14,17-19 This has created technical difficulties in fiducial placement because of the stiffness of 19-gauge needles, especially in cases of cancers of the head of the pancreas. Recently, new, smaller fiducial markers, which can fit into 22-gauge FNA needles, were introduced.15,16 These may circumvent intricacies with fiducial placement because of the flexibility of the smaller needles.
Poor fiducial visualization and/or fiducial migration during IGRT can lead to insufficient dose coverage of the targeted tumor volume, excessive irradiation of adjacent normal structures, and compromised clinical outcomes.20 The aims of the current study were to compare 2 types of commercially available fiducials for technical success, complications, visibility, and migration.
METHODS
Patients
A prospectively collected radiation oncology database at the Johns Hopkins Hospital was searched for patients who underwent EUS-guided fiducial placement followed by IGRT between June 2010 and September 2011. The study was approved by the Institutional Review Board for Human Research and complied with Health Insurance Portability and Accountability Act regulations. Only patients with malignant pancreatic tumors were included. All tumors were locally unresectable because of vascular invasion. A retrospective analysis of institutional medical records was done to collect relevant data: demographic (age, sex), clinical (tumor location, tumor size), procedural (type of FNA needles used, type of fiducials used, number of fiducials placed, technical success, technical difficulty, technical failures and reasons for failure, use of fluoroscopy, complications).
Materials
Two kinds of commercially available fiducials were compared: traditional fiducials (TFs) (5-mm length, 0.8-mm diameter) and Visicoil fiducials (Core Oncology, Santa Barbara, Calif) (VFs) (10-mm length, 0.35-mm diameter). Unlike TFs, the VFs are flexible and have a coiled design, which theoretically reduces the incidence of fiducial migration. In addition, VFs are preloaded on a needle carrier delivery device that allows direct insertion of the fiducial into the EUS needle. The system used by our radiation oncologists for IGRT is the Synergy-S or Infinity platform (Elekta AB, Stockholm, Sweden). Both systems incorporate kilovoltage conebeam CT (CBCT) and planar x-ray imaging for initial positioning and monitoring during therapy.
EUS-guided fiducial placement and periprocedural care
If a tissue diagnosis had not been previously established, FNA with a 22- or 25-gauge Echotip needle (Cook Endoscopy, Winston-Salem, NC) was performed by using intraprocedural evaluation by an experienced cytopathologist to confirm malignancy. After FNA, fiducials were placed by 6 endosonographers using linear echoendoscopes (FG36-UA; Pentax Medical Corp, Montvale, NJ, or GF-UC140P-AL5; Olympus America, Center Valley, Pa) and 19-gauge (for TFs) or 22-gauge (for VFs) needles by using standard techniques under propofol-based sedation with monitored anesthesia care. An FNA needle (Echotip; Cook Endoscopy) was backloaded with 1 fiducial marker. The stylet of the EUS needle was withdrawn approximately 2 to 3 em and the fiducial was backloaded into the needle tip by using sterile techniques. The needle tip of the EUS needle was sealed with sterile bone wax to prevent unintended loss of the fiducial while advancing the needle through the therapeutic channel of the echoendoscope. The needle was then inserted into the target lesion under EUS guidance. The stylet of the EUS needle was advanced to maximal insertion, thus pushing the fiducial out of the needle and into the lesion (Fig. 1). The EUS needle was then withdrawn from the echoendoscope and reloaded with a new fiducial, and the technique was repeated until the desired number of fiducials were placed. The number of fiducials placed was left to the discretion of each individual endoscopist, but the goal was to place 2 to 4 fiducials to provide ample distance and angulation for IGRT. Fiducials were placed at the periphery of the tumor when possible. Fluoroscopy was used for initial cases, but this practice was subsequently abandoned as it was deemed unnecessary because the fiducials were readily visualized during EUS placement.
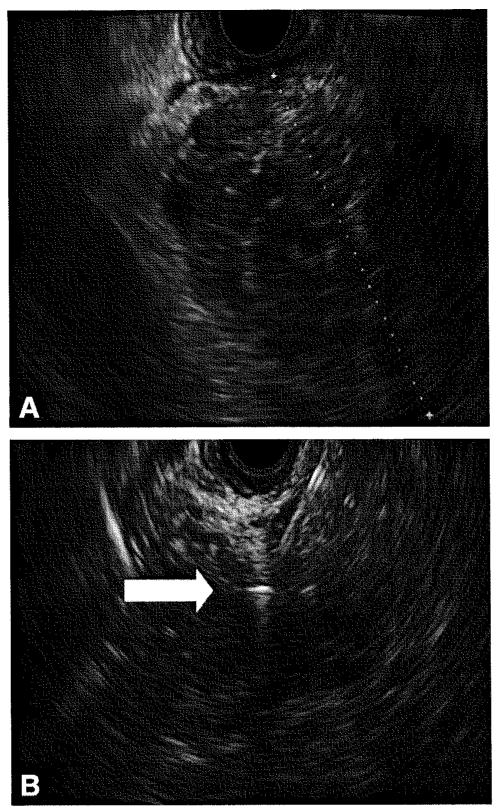
Figure 1.
A, EUS image of a mass in the head of the pancreas. EUS-guided FNA confirmed pancreatic ductal adenocarcinoma. B, EUS image showing FNA needle and a deployed hyperechoic fiducial (arrow).
Intraprocedural intravenous antibiotics (cefotetan 1 g, cefazolin 1 g, or clindamycin 600 mg) were administered prophylactically in all patients, followed by a 5-day course of oral ciprofloxacin (500 mg twice daily). All patients were monitored for 1 to 2 hours in the recovery area for symptoms of fever, abdominal pain, nausea, vomiting, dyspnea, hypoxia, and hypotension.
Outcomes
Technical difficulty was defined as the inability to place 1 or more fiducials in the desired location because of angulation and/or intervening vascular structures. Technical success was defined as the ability to place fiducials in the desired location. On discharge, patients were instructed to notify the nurse and/or physician of any delayed symptoms or procedure-related complications. A chart review for procedure-related complications was performed up to 30 days after discharge.
Assessment of fiducial visibility and migration
A subjective visualization scoring system (0-2; 0 = not visible, 1 = barely visible, and 2 = clearly visible) was used to assess visibility on the pretreatment planning CT scan and the last image associated with RT.21 Examples of the visibility scores are shown in Figure 2. The visibility of the fiducials was scored by 1 of the authors (K.J.K.) with experience in analyzing RT images and without previous knowledge of the type of fiducial(s) placed. To address the uncertainty of whether a score of 0 meant not visible or complete migration, a fiducial not seen in either the pretreatment planning study or the image from the last day of treatment was defined as complete migration.
Figure 2.
Examples of visibility scores (0, 1, and 2) on the primary treatment plan and the secondary treatment plan of the same patient (and example of blurriness of conebeam CT (CBCT) compared with treatment plan, as mentioned in the discussion section). A, Primary treatment plan (free breathing) showing 3 fiducials that were scored as a visibility score of 2 (patient also had a biliary stent in place). B, Secondary CBCT treatment plan showing 1 fiducial (visualization score of 1) and 2 fiducials with a score of 0. (The biliary stent is still clearly visible. This is the best contrast given the artifact from stent.)
Fiducial migration (calculated as a change in interfiducial distance) was assessed in Pinnacle (Philips Oncology Systems Amsterdam, the Netherlands). Three-dimensional fiducial locations were studied on the pretreatment planning study (either on a breath-held CT or on a reference phase from a 4-dimensional CT) versus on the free-breathing CT obtained during the last SBRT session (Fig. 3).22 The fiducials were then contoured on each image. Their average position was automatically placed at the geometric centroid of the segmented structures, allowing for computation of fiducial distances in 3-dimensional space in 3 principal directions (superoinferior, anteroposterior, and mediolateral) (Fig. 4).
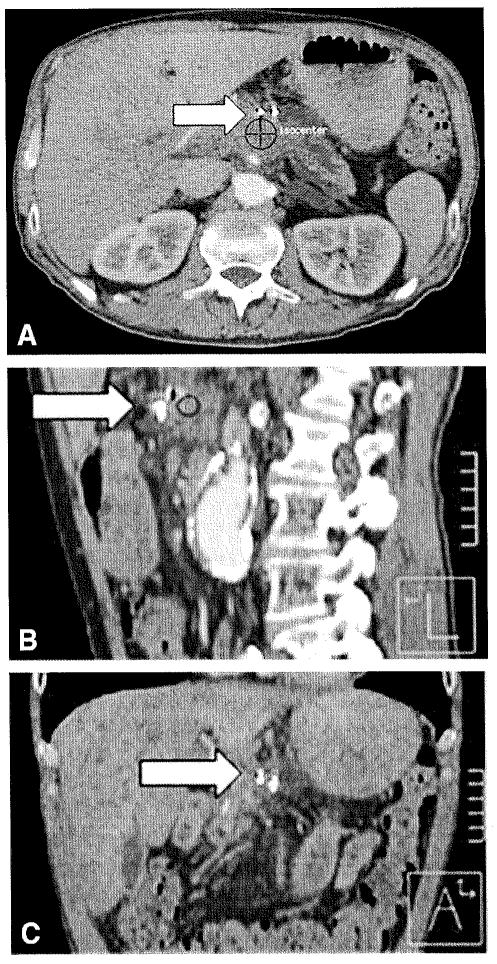
Figure 3.
Example of an unmarked breath-held CT treatment plan; 3 views (axial, sagittal, coronal); isocenter of pancreas tumor shown at target and 2 fiducials (arrow).
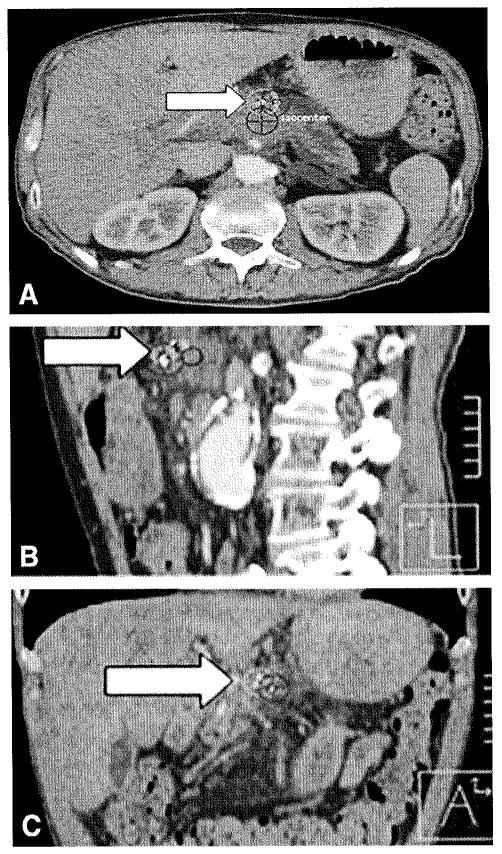
Figure 4.
Previously shown treatment plan with fiducials contoured and centroid (arrow) automatically placed on Pinnacle; 3 views (axial, sagittal, coronal).
Statistical analysis
Statistical analyses were performed with SPSS Statistics software, version 19 (International Business Machines Corporation, Armonk, NY). Patient characteristics consisting of continuous and dichotomous variables were summarized by using descriptive statistics. Although some of the patients had more than 1 fiducial placed, for the purposes of statistical analysis, the data for different fiducials were assumed to constitute statistically independent observations. Comparison of proportions between the TF and VF groups was performed by using the Pearson χ2 test by using the Yates correction for continuity where appropriate. To account for possible non-normality as well as possible unequal variance between datasets, comparison of quantitative data between the TF and VF groups was performed by using the non parametric Mann-Whitney U test. In all cases in which the Mann-Whitney U test was applied, the median and interquartile range (IQR) are given. In the few instances in which it was deemed preferable to compare means between the TF and VF groups, a 2-tailed unpaired Student t test was used, and the mean and standard deviation (SD) are given. For all tests, a 2-sided α level of ≤.05 was considered significant. Although there was multiple testing of outcome data arising from individual fiducials, correction by the Bonferroni method would not have removed significance from any findings; therefore, all P values are presented without correction for multiple comparisons. Because of the retrospective, observational nature of the study design, it is possible that some sampling bias may have occurred (especially with respect to tumor location because head/neck tumor location occurred with much greater relative frequency in the VF group than the TF group), so we recommend that the statistical findings be taken as descriptive only.
RESULTS
A total of 39 patients (25 male, 14 female) underwent EUS-guided fiducial placement. The median age of patients was 66.5 years (IQR 57.8-73.0 years). Patient and procedural characteristics are detailed in Tables 1 and 2. Targeted pancreatic masses measured a median of 3.1 em (IQR 2.7-3.5 em) and were located in the head/uncinate (66.7%), body (23.1%), or tail (5.1%). The cytological diagnosis of pancreatic adenocarcinoma was previously established in 28 patients. EUS-FNA was performed just before fiducial placement in the other 11 patients, and preliminary cytopathological interpretation was suspicious for adenocarcinoma in all of these patients. A total of 103 fiducials were placed: 77 TFs by using 19-gauge needles and 26 VFs by using 22-gauge needles. The mean number of fiducials placed per patient was 2.64 (SD 0.69, range 1.0-3.0). Of the 103 fiducials deployed, 37 were placed by using a trans gastric approach (34 TFs, 3 VFs) and 66 (43 TFs, 23 VFs) were placed by using a transduodenal approach. Fiducials could not be placed in 1 patient because the echoendoscope was not able to traverse a preexisting duodenal stent. This patient was excluded from analysis. Four patients who underwent placement of TFs had existing biliary stents compared with 1 patient who underwent VF placement. Technical difficulty was encountered during 18% of procedures (14% TF placement and 30% VF placement, P = .25). Technical difficulty was only encountered during transduodenal puncture of masses in the head/uncinate of pancreas. Existing biliary stents did not adversely affect ease or success of fiducial placement. No intraprocedural or delayed complications were encountered. Postprocedural fluoroscopy was performed in 33% of cases, and all clearly demonstrated implanted fiducials.
TABLE 1. Comparison of patient demographic and procedures.
| All fiducials | TFs | VFs | P value | |
|---|---|---|---|---|
| No. of patients | 39 | 29 | 10 | — |
| Total no. of fiducials placed | 103 | 77 | 26 | — |
| Median age (IQR), y | 66.5 (57.8-73.0) | 66.0 (57.0-72.3) | 70.5 (60.0-74.8) | .47 |
| Sex, no. (%female) | 14 (35.9) | 12 (41.4) | 2 (20) | .22 |
| Location of tumor, no. (%) | — | |||
| Uncinate | 1 (2.6) | 1/29 (3.4) | 0/10 (0) | .55 |
| Head/neck | 26 (66.7) | 17/29 (58.6) | 9/10 (90) | .07 |
| Body | 9 (23.1) | 8/29 (27.6) | 1/10 (10) | .26 |
| Tail | 2 (5.1) | 2/29 (6.9) | 0/10 (0) | .39 |
| Median (IQR) size of tumor, cm | 3.1 (2.7-3.5) | 3.0 (2.7-3.4) | 3.3 (3.0-3.9) | .12 |
| EUS-FNA approach, no. (%) | — | |||
| Transgastric | 13 (33.3) | 12/29 (41.4) | 1/10 (10) | .07 |
| Transduodenal | 26 (66.7) | 17/29 (58.6) | 9/10 (90) | .07 |
| Fluoroscopy used, no.(%) | 13 (33.3) | 9/29(31) | 4/10 (40) | .60 |
| Technical difficulty reported, no.(%) | 7 (17.9) | 4/29 (13.8) | 3/10 (30) | .25 |
| No. of immediate complications | 0 | — | ||
| No. of delayed complications | 0 | — | ||
| Median time from EUS to primary image, d (IQR)* | 6.0 (3.5-18.5) | 7.0 (4.0-16.0) | 4.5 (2.3-25.3) | .68 |
| Median time from EUS to secondary image, d (IQR)† | 27.0 (22.0-43.5) | 29.0 (22.0-44.0) | 24.0 (21.5-31.5) | .38 |
TFs, Traditional fiducials; VFs, Visicoil fiducials; IQR, interquartile range.
Primary image refers to the preradiotherapy imaging study (either a breath-held CT scan or a reference phase from a 4-dimensional CT scan) performed for the purposes of SBRT planning.
Secondary image refers to the free-breathing CT scan obtained during the last SBRT session.
TABLE 2. Patient and procedural characteristics for EUS-guided fiducial placement in 39 patients.
| Patient | Age,y | Sex | Location of tumor by EUS |
Largest diameter, mm |
Needle used, gauge |
Type of fiducials |
No. of fiducials |
Fluoroscopy used? |
Technical difficulty reported? |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 85 | Male | Head | 18 | 19 | TF | 1 | Yes | Yes |
| 2 | 52 | Male | Head | 27 | 19 | TF | 2 | Yes | No |
| 3 | 67 | Male | Body | 27.9 | 19 | TF | 3 | No | No |
| 4 | 61 | Female | Head | 30 | 19 | TF | 1 | Yes | Yes |
| 5 | 71 | Female | Head | 30 | 22 | VF | 1 | Yes | Yes |
| 6 | 66 | Male | Uncinate | 36.2 | 19 | TF | 2 | No | No |
| 7 | 56 | Female | Head | 38 | 19 | TF | 2 | Yes | No |
| 8 | 58 | Male | Body | 25.8 | 22 | VF | 3 | No | No |
| 9 | 66 | Male | Body | 36 | 19 | TF | 3 | No | No |
| 10 | 67 | Female | Head | 34.2 | 19 | TF | 2 | No | Yes |
| 11 | 56 | Male | Head | 25 | 19 | TF | 2 | Yes | No |
| 12 | 57 | Female | Head | 30.6 | 19 | TF | 3 | No | No |
| 13 | 73 | Female | Head | 42.9 | 19 | TF | 3 | No | No |
| 14 | 76 | Male | Head | 31.5 | 22 | VF | 3 | No | Yes |
| 15 | 71 | Male | Head | 31.4 | 22 | VF | 2 | Yes | Yes |
| 16 | 60 | Female | Head | 30 | 22 | VF | 3 | No | No |
| 17 | 58 | Male | Body | 25 | 19 | TF | 3 | Yes | No |
| 18 | 60 | Male | Head | 40 | 22 | VF | 3 | Yes | No |
| 19 | 78 | Female | Head | 30 | 19 | TF | 3 | No | No |
| 20 | 55 | Female | Head | 33 | 19 | TF | 3 | Yes | No |
| 21 | 83 | Female | Body | 32.5 | 19 | TF | 3 | Yes | No |
| 22 | 49 | Female | Body | 26 | 19 | TF | 2 | Yes | No |
| 23 | 69 | Male | Body | 34 | 19 | TF | 3 | Yes | No |
| 24 | 51 | Female | Head | 19 | 19 | TF | 3 | Yes | No |
| 25 | 76 | Male | Head | 33.8 | 22 | VF | 2 | Yes | No |
| 26 | 67 | Female | Head | 30 | 19 | TF | 4 | Yes | No |
| 27 | 55 | Male | Head | 35 | 22 | VF | 3 | No | No |
| 28 | 60 | Male | Head | 34 | 19 | TF | 3 | Yes | No |
| 29 | 81 | Male | Head | 40 | 22 | VF | 3 | No | No |
| 30 | 63 | Male | Head | 34.6 | 19 | TF | 2 | No | Yes |
| 31 | 70 | Male | Head | 50 | 22 | VF | 3 | No | No |
| 32 | 66 | Male | Head | 28 | 19 | TF | 3 | No | No |
| 33 | 77 | Male | Head | 25.3 | 19 | TF | 3 | No | No |
| 34 | 73 | Male | Body | 24.8 | 19 | TF | 3 | No | No |
| 35 | 82 | Female | Head | 20.7 | 19 | TF | 3 | No | No |
| 36 | 69 | Male | Body | 32 | 19 | TF | 3 | No | No |
| 37 | 57 | Male | Body | 33 | 19 | TF | 3 | No | No |
| 38 | 70 | Male | Neck | 36 | 19 | TF | 3 | No | No |
| 39 | 73 | Male | Body | 31 | 19 | TF | 3 | No | No |
TF, Traditional fiducial; VF, Visicoil fiducial.
Table 3 details differences between the fiducial (TF vs VF) groups. The mean number of fiducials placed per patient was 2.66 (SD 0.67, range 1.00-3.00) for the 19-gauge needle and 2.60 (SD 0.7, range 1.00-3.00) for the 22-gauge needle (P = .83). The median (IQR) distance between TFs was 1.09 (range 0.82-1.79) and that for VFs was 1.47 (range 1.21-2.34). In 6.5% (10/154) of TF images, fiducials were not visualized (score 0) compared with 7.7% (4/52) of VF images (P = .77). None (0/154) of the TF images had a visibility score of 1 compared with 19% (10/52) of VF images (P < .0001). Ninety-four percent (144/154) of TF images were clearly visible (score 2) compared with 73% (38/52) of VF images (P < .0001). The median visibility score of TFs was significantly better than that for VFs both when scores of 0 were and were not included (2.00 [IQR 2.00-2.00] vs 1.75 [IQR 1.50-2.00], P = .009; and 2.00 [IQR 2.00-2.00] vs 2.00 [IQR 1.50-2.00], P < .0001, respectively). Visibility scoring was also assessed on the pretreatment images, alone, and TFs remained more visible than VFs (P = .003, Mann-Whitney U test).
TABLE 3. Comparison of traditional fiducial (TF) and Visicoil fiducial (VF) group characteristics and outcomes.
| TF group (n = 29) |
VF group (n = 10) |
P value | |
|---|---|---|---|
| Total no. of fiducials placed | 77 | 26 | — |
| Mean no. of fiducials placed (SD) | 2.66 (0.67) | 2.60 (0.70) | .83 |
| Head/uncinate location, no.(%) | 19 (66) | 9 (90) | .14 |
| Technical difficulty, no.{%) | 4 (15) | 3 (30) | .25 |
| Median (IQR); mean (SD) visibility scores (with scores of 0 Included) | 2.00 (2.00-2.00); 1.87 (0.51) | 1.75 (1.50-2.00); 1.65 (0.46) | .009* |
| Median (IQR); mean (SD) visibility scores (without score of 0 included) |
2.00 (2.00-2.00); 2.00 (0.00) | 2.00 (1.50-2.00); 1.72 | (0.33) <.0001* |
| No. (%)with visibility score of O† | 10/154 (6.5) | 4/52 (7.7) | .77 |
| No.(%) with visibility score of 2† | 144/154 (94) | 38/52 (73) | <.0001 |
| Complete migration (defined as fiducial not seen in both images), no.(%) |
5/77 (6.5) | 1/26 (3.8) | .62 |
| Median migration (IQR), mm | 0.8 (0.4-1.6) | 1.3 (0.6-1.5) | .72 |
TF, traditional fiducial; VF, Visicoil fiducial; IQR, interquartile range; SD, standard deviation.
Although both medians and means are given, P values were computed by using the non parametric Mann-Whitney U test to account for possible non-normality and unequal variance between the 2 groups.
Two images per fiducial (from planning image and treatment image).
Because 9 of 10 patients (90%) who received VFs had tumor locations in the head/uncinate of the pancreas compared with only 19 of 29 patients (66%) who received TFs, we additionally compared visibility scores between only those fiducials located in the pancreatic head/uncinate to assess whether fiducial location within the pancreas could account for the difference in the visibility scores observed in TFs and VFs. For TFs located in the head/uncinate (n = 48), the median visibility score with scores of 0 included was 2.00 (IQR 2.00-2.00), whereas for VFs located in the head/neck (n = 22), the median visibility score with scores of 0 included was 1.75 (IQR 1.50-2.00) (P = .002). Similar results were obtained when scores of 0 were not included, with TFs demonstrating a superior median visibility score of 2.00 (IQR 2.00-2.00) compared with 2.00 (IQR 1.50-2.00) for VFs (P < .0001). Therefore, the difference in visibility between TFs and VFs persists when only fiducials implanted in the pancreatic head/uncinate are considered. These findings suggest that location in the pancreas alone cannot account for the difference in visibility between the 2 fiducial types.
The median time from EUS to primary CT image was not significantly different between the TF and VF groups (7.0 days [IQR 4.0-16.0 days] vs 4.5 days [IQR 2.3-25.3 days], respectively; P = .68). Migration was not significantly different between the 2 types of fiducials, with TFs exhibiting a median migration distance of 0.8 mm (IQR 0.4-1.6 mm) versus 1.3 mm (IQR 0.6-1.5 mm) for VFs (P = . 72). Complete migration, defined as nonvisualization of any fiducial in either primary or secondary CT images, was 6.5% (5/77) for TFs and 3.8% (1/26) for VFs (P = .62).
DISCUSSION
Strategies aimed at improving RO resection rates among patients with pancreatic cancer and minimal arterial involvement may provide a significant survival benefit in a group of patients with tumors that might otherwise be unresectable. SBRT is a novel technique that takes advantage of the technological advancements in image guidance and radiation dose delivery to direct potentially ablative doses of radiation to pancreatic tumors with acceptable toxicity that was not previously achievable with conventional techniques. SBRT of pancreatic cancer requires a high degree of confidence in tumor location, which can be provided by EUS-guided fiducial placement.
The current study compares 2 commercially available fiducials. The majority of pancreatic tumors were located in the head. There was 1 technical failure because the EUS endoscope was not able to traverse the duodenal stent. EUS-guided fiducial placement is a feasible technique and may be preferable to alternative approaches (radiology-guided placement or surgical placement) because of less invasiveness and high success rates. FNA is widely practiced by endosonographers. The technique of fine-needle injection of fiducials is similar to that for FNA. Hence, fine-needle injection can be performed by endosonographers who perform EUS-guided FNA. In addition, there were no complications encountered in the current study, suggesting that fiducial placement is safe and well tolerated. The use of Doppler US may minimize the risk of inadvertent placement of fiducials in a blood vessel.
The current study compares 2 commercially available fiducials in terms of technical success of placement, complications, visibility, and migration. Previous studies of EUS-guided fiducial placement evaluated either TFs with a length of 3 to 5 mm and a diameter of 0.8 mm or VFs with a length of 10 mm and a diameter of 0.35 mm. VFs have smaller diameters, which permits their insertion in 22-gauge FNA needles. The goal is to surmount technical difficulties encountered during insertion of the larger TFs into the stiffer 19-gauge FNA needles. In addition, VFs were also designed to have a coiled shape to prevent migration. Although technical difficulty was encountered more frequently during placement of TFs, the mean number of fiducials placed per patient was similar in both types of fiducials. This implies that placement of the larger fiducials may be more technically demanding, but failures are uncommon and placement of a similar number of fiducials compared with VF was still possible. However, selection bias may have occurred because the vast majority of VFs were placed in head lesions. Our data clearly show that visibility of TFs is significantly better than that of VFs. Our current preference is to place TFs (using 19-gauge needle) whenever possible. If this fails, changing to 22-gauge needle and VFs is advisable.
The visibility of TFs was significantly better than VFs, likely because of their larger diameter. It seems that fiducial diameter is more critical than fiducial length for optimal visibility during IGRT with conebeam and kilovolt imaging. Both types of fiducials demonstrated low migration rates, but the difference was not statistically significant. The coiled design of VFs did not result in less migration. It is worth mentioning that more TFs than VFs were not visible (score O) during IGRT, but the difference was not statistically significant. Whether the lack of visibility was attributable to fiducial migration, body mass index, image resolution, or other factors cannot be determined from this study. The optimal fiducial design would be a larger but compressed diameter fiducial that can be inserted in a 22-gauge needle but expands after deployment into the targeted tumor.
All patients received intra- and postprocedural antibiotics, and no infectious complications were seen. It is debatable whether antibiotics should be given for this indication, and some authors do not routinely prescribe antibiotics.16 However, infectious complications were previously reported in 2 patients, both of whom did not receive intra- or postprocedural antibiotics.16,17 In the first patient, cholangitis developed 25 days after the procedure, and the patient required treatment with antibiotics and percutaneous biliary drainage.17 In the second patient, there were fever and an increase in liver function test results 12 hours after the procedure, and the patient was treated with an outpatient course of antibiotics.16 The American Society for Gastrointestinal Endoscopy guidelines on antibody prophylaxis for GI procedures do not specifically discuss recommendations for EUS-guided fiducial placement or other EUS-guided fine-needle injection procedures.23 The current reported postprocedural infectious complication rate is 1.2% (2 of 165 patients reported to undergo EUS-guided fiducial placement). Because many of the studies are retrospective (with a tendency to underestimate late complications), most included patients were given antibiotics, and the fact that the 2 reported infectious complications were seen in patients who did not receive antibiotic prophylaxis, we believe that it is prudent to give antibiotics in these patients until further data are available.
Three patients underwent placement of 1 fiducial only. Although radiation oncologists preferentially ask for placement of multiple fiducials per tumor, this practice is not absolutely necessary. Planning images were 3-dimensional and, thus, even 1 fiducial was adequate in determining the 3-dimensional location of the tumor. Additionally, other tumor surrogates (such as biliary stents or local bony anatomy) were used to further assist in planning radiation treatment.
Our study has some limitations. This was a nonrandomized, retrospective study at a single tertiary referral center where all EUS procedures were performed by experienced endosonographers. A total of 6 fiducials (5.8%) were not visible during IGRT. It is not possible to determine whether this represents poor visibility of fiducials or complete migration outside the tumor field. However, TFs demonstrated improved visualization regardless of whether the nonvisualized fiducials were accounted for. The fact that the ontreatment CBCT images were always acquired during free breathing (whereas the planning study, being either a breath-held CT or reference phase of a 4-dimensional CT image, is ideally a motion-frozen representation) complicates our scoring of fiducial visibility on the CBCT. The specific issue is that the fiducials on CBCT appear blurred, where the degree of blurriness will depend on the amplitude of breathing motion. This may also influence our determination of migration, albeit to a lesser extent. With regard to visibility, to assess whether this complication biases our findings, we looked at our visibility scoring on the pretreatment images alone and found that the TFs remained more visible that VFs (P = .003).
In conclusion, visibility was significantly better for TFs compared with VFs. The degree of fiducial migration from the time of radiation planning simulation to the conclusion of IGRT was not significantly different for TFs and VFs. There was no significant difference in the mean number of fiducials placed, suggesting a similar success rate of fiducial placement for TF and VF deployment. Progress in fiducial design and custom-made FNA needles is needed to allow easy fiducial deployment, optimal visualization, limited migration, and multiple fiducial placement with 1 pass.
Take-home Message.
Progress in fiducial design and custom-made FNA needles is needed to allow easy fiducial deployment, optimal visualization, limited migration, and multiple fiducial placement with 1 pass.
Abbreviations
- CBCT
conebeam CT
- IGRT
image-guided radiation therapy
- IQR
interquartile range
- RT
radiation therapy
- SBRT
body radiation therapy
- SD
standard deviation
- TF
traditional fiducial
- VF
Visicoil fiducial
Footnotes
DISCLOSURE: This study was partially supported by NCI R01 CA 161613 (J. M. H.). Tbe following author disclosed a financial relationship relevant to this publication: Dr Khashab is a consultant to Boston Scientific. All other authors disclosed no financial relationship relevant to this publication.
REFERENCES
- 1.Greenlee RT, Hill-Harmon MB, Murray T, et al. Cancer statistics, 2001. CA Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Stephens J, Kuhn J, O’rien J, et al. Surgical morbidity, mortality, and long-term survival in patients with peripancreatic cancer following pancreaticoduodenectomy. Am J Surg. 1997;174:600–3. doi: 10.1016/s0002-9610(97)00204-3. discussion 603-4. [DOI] [PubMed] [Google Scholar]
- 3.Varadarajulu S, Eloubeidi MA. The role of endoscopic ultrasonography in the evaluation of pancreatico-biliary cancer. Surg Clin North Am. 2010;90:251–63. doi: 10.1016/j.suc.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 4.Buchler MW, Werner J, Weitz J. RO in pancreatic cancer surgery: surgery, pathology, biology, or definition matters? Ann Surg. 2010;251:1011–2. doi: 10.1097/SLA.0b013e3181e07dad. [DOI] [PubMed] [Google Scholar]
- 5.Moertel CG, Frytak 5, Hahn RG, et al. Therapy of locally unresectable pancreatic carcinoma: a randomized comparison of high dose (6000 rads) radiation alone, moderate dose radiation (4000 rads + 5-fluorouracil), and high dose radiation + 5-fluorouracil. The Gastrointestinal Tumor Study Group-Cancer. 1981;48:1705–10. doi: 10.1002/1097-0142(19811015)48:8<1705::aid-cncr2820480803>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 6.Huguet F, Andre T, Hammel P, et al. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol. 2007;25:326–31. doi: 10.1200/JCO.2006.07.5663. [DOI] [PubMed] [Google Scholar]
- 7.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 8.Burris HA, 3rd, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–13. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein SD, Ford EC, Duhon M, et al. Use of respiratory-correlated four-dimensional computed tomography to determine acceptable treatment margins for locally advanced pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2010;76:597–602. doi: 10.1016/j.ijrobp.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by a stereotactic radiosurgery boost in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2005;63:320–3. doi: 10.1016/j.ijrobp.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 11.Timmerman RD, Kavanagh BD, Cho LC, et al. Stereotactic body radiation therapy in multiple organ sites. J Clin Oncol. 2007;25:947–52. doi: 10.1200/JCO.2006.09.7469. [DOI] [PubMed] [Google Scholar]
- 12.Kothary N, Heit JJ, Louie JD, et al. Safety and efficacy of percutaneous fiducial marker implantation for image-guided radiation therapy. J Vase lnterv Radiol. 2009;20:235–9. doi: 10.1016/j.jvir.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Park WG, Yan BM, Schellenberg D, et al. EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest Endosc. 2010;71:513–8. doi: 10.1016/j.gie.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Sanders MK, Moser AJ, Khalid A, et al. EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc. doi: 10.1016/j.gie.2009.12.020. [DOI] [PubMed] [Google Scholar]
- 15.Ammar T, Cote GA, Creach KM, et al. Fiducial placement for stereotactic radiation by using EUS: feasibility when using a marker compatible with a standard 22-gauge needle. Gastrointest Endosc. 2010;71:630–3. doi: 10.1016/j.gie.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 16.DiMaio CJ, Nagula S, Goodman KA, et al. EUS-guided fiducial placement for image-guided radiation therapy in Gl malignancies by using a 22-gauge needle (with videos) Gastrointest Endosc. 2010;71:1204–10. doi: 10.1016/j.gie.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Pishvaian AC, Collins B, Gagnon G, et al. EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc. 2006;64:412–7. doi: 10.1016/j.gie.2006.01.048. [DOI] [PubMed] [Google Scholar]
- 18.Varadarajulu S, Trevino JM, Shen S, et al. The use of endoscopic ultrasound-guided gold markers in image-guided radiation therapy of pancreatic cancers: a case series. Endoscopy. 2010;42:423–5. doi: 10.1055/s-0029-1243989. [DOI] [PubMed] [Google Scholar]
- 19.Owens DJ, Savides TJ. EUS placement of metal fiducials by using a back-loaded technique with bone wax seal. Gastrointest Endosc. 2009;69:972–3. doi: 10.1016/j.gie.2008.05.052. [DOI] [PubMed] [Google Scholar]
- 20.Schiffner DC, Gottschalk AR, Lometti M, et al. Daily electronic portal imaging of implanted gold seed fiducials in patients undergoing radiotherapy after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2007;67:610–9. doi: 10.1016/j.ijrobp.2006.09.042. [DOI] [PubMed] [Google Scholar]
- 21.Henry AM, Stratford J, Davies J, et al. An assessment of clinically optimal gold marker length and diameter for pelvic radiotherapy verification using an amorphous silicon flat panel electronic portal imaging device. Br J Radiol. 2005;78:737–41. doi: 10.1259/bjr/97956788. [DOI] [PubMed] [Google Scholar]
- 22.Ford EC, Mageras GS, Yorke E, et al. Respiration-correlated spiral CT: a method of measuring respiratory-induced anatomic motion for radiation treatment planning. Med Phys. 2003;30:88–97. doi: 10.1118/1.1531177. [DOI] [PubMed] [Google Scholar]
- 23.Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for Gl endoscopy. Gastrointest Endosc. 2008;67:791–8. doi: 10.1016/j.gie.2008.02.068. [DOI] [PubMed] [Google Scholar]