Abstract
Purpose
Existing studies that examine the effect of neoadjuvant chemoradiation (CRT) for locally advanced rectal cancer on patient quality of life (QOL) are limited. Our goals were to prospectively explore acute changes in patient-reported QOL endpoints during and after treatment and to establish a distribution of scores that could be used for comparison as new treatment modalities emerge.
Methods and Materials
Fifty patients with locally advanced rectal cancer were prospectively enrolled at 2 institutions. Validated cancer-specific European Organization for Research and Treatment of Cancer (EORTC QLQ-CR30) and colorectal cancer-specific (EORTC QLQ-CR38 and EORTC QLQ-CR 29) QOL questionnaires were administered to patients 1 month before they began CRT, at week 4 of CRT, and 1 month after they had finished CRT. The questionnaires included multiple symptom scales, functional domains, and a composite global QOL score. Additionally, a toxicity scale was completed by providers 1 month before the beginning of CRT, weekly during treatment, and 1 month after the end of CRT.
Results
Global QOL showed a statistically significant and borderline clinically significant decrease during CRT (−9.50, P=.0024) but returned to baseline 1 month after the end of treatment (−0.33, P=.9205). Symptoms during treatment were mostly gastrointestinal (nausea/ vomiting +9.94, P<.0001; and diarrhea +16.67, P=.0022), urinary (dysuria +13.33, P<.0001; and frequency +11.82, P=.0006) or fatigue (+16.22, P<.0001). These symptoms returned to baseline after therapy. However, sexual enjoyment (P=.0236) and sexual function (P=.0047) remained persistently diminished after therapy.
Conclusions
Rectal cancer patients undergoing neoadjuvant CRT may experience a reduction in global QOL along with significant gastrointestinal and genitourinary symptoms during treatment. Moreover, provider-rated toxicity scales may not fully capture this decrease in patient-reported QOL. Although most symptoms are transient, impairment in sexual function may persist after the completion of therapy and merits further investigation.
Introduction
In 2012, approximately 40,300 patients will receive a diagnosis of rectal cancer in the United States (1). Although surgical resection remains the mainstay of curative therapy for these patients, it risks significant gastrointestinal and genitourinary morbidity. Although the introduction of newer surgical techniques such as total mesorectal excision has decreased surgical side effects through greater preservation of pelvic autonomic nerves, treatment regimens have also intensified through broader application of multimodality therapy, inasmuch as recent trials have shown benefit in local control and complete pathologic response rates when patients are treated with neoadjuvant radiation with or without concurrent chemotherapy (2–4). At our institutions, for example, neoadjuvant chemoradiation (CRT) is the standard of care for rectal cancer patients with locally advanced disease.
Given the significant adverse normal tissue effects from pelvic radiation therapy across multiple cancers (5), it is imperative that clinicians accurately characterize the morbidity of neoadjuvant chemoradiation so as to assess the risk-benefit profiles of treatment regimens and provide patients with more accurate expectations of likely complications. Although toxicity rates have been reported from major trials for locally advanced rectal cancer, patient-reported outcomes such as quality of life (QOL) are often underestimated or are not captured by physician-reported measures (6). Moreover, the current literature regarding health-related QOL for rectal cancer patients treated with pelvic radiation therapy is sparse and suffers from several limitations, including retrospective, cross-sectional study designs; the use of QOL instruments that have not been validated; and a lack of emphasis on the effects of nonsurgical treatment modalities on QOL (7).
We therefore sought to examine the QOL of rectal cancer patients undergoing neoadjuvant CRT for locally advanced disease using validated QOL questionnaires and a prospective study design. Our goals were to assess acute changes in QOL and symptoms during and after therapy and to establish a baseline of QOL scores in this population for future comparison as new treatment protocols emerge.
Methods and Materials
Study participants
The study was approved by the institutional review boards of the institutions at which the study was conducted. Patients with rectal cancer who were scheduled to receive concurrent neoadjuvant CRT and who did not have a history of pelvic radiation were eligible for the study. At our institutions, neoadjuvant CRT is generally administered for patients with locally advanced disease, defined as T3/T4 primary tumor stage, node-positive disease, or both. Patients were initially evaluated with ultrasonography, magnetic resonance imaging, or computed tomography to determine extent of disease. Chest imaging was obtained to exclude thoracic metastasis. Laboratory tests including full blood count, serum electrolytes, creatinine, urea, liver transaminases, alkaline phosphatase, total bilirubin, and carcinoembryonic antigen levels were obtained. Consecutive eligible patients consented to inclusion in the study and were prospectively evaluated by use of QOL instruments before, during, and after CRT as outlined below. Radiation therapy was administered in 1.8- to 2-Gy doses according to a standard 3-field technique (posterior-anterior, left lateral, and right lateral). The whole pelvis was treated to 45 Gy followed by a 5.4-Gy boost to the primary tumor and any grossly involved lymphadenopathy. In general, for anterior-posterior and posterior-anterior fields, the superior border was at the interspace between L5 and S1, the lateral border was 1.5 cm lateral to the widest bony margin of the true pelvic walls to include possible lateral extension and the iliac nodal chain, and the distal border was 3 to 5 cm distal to the tumor or flashing the anus if the tumor was less than 3 cm from the anal verge. The anterior border of the lateral fields was based on T stage, and the posterior border was set 1.5 cm behind the anterior bony sacral margin (8). Concurrent chemotherapy most commonly consisted of oral capecitabine given 7 days a week at a dosage of 825 mg/m2 twice daily.
General cancer-related QOL
We assessed general cancer-related QOL using the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30. The QLQ-C30 is a 30-item general cancer instrument that evaluates 5 domains of QOL (physical, role, cognitive, emotional, and social), 9 symptom scales (fatigue, nausea/vomiting, pain, dyspnea, sleep disturbance, appetite loss, constipation, diarrhea, and financial impact), and a global QOL score. Each domain is assessed by 2 to 5 questions, and responses are scored on a 4-point Likert scale, with higher scores representing better QOL. Higher scores on symptom items represent worse symptoms. The validity and reliability of the QLQ-C30 has been well documented (9).
Colorectal cancer-specific QOL
To collect data on colorectal cancer-specific QOL, we used the EORTC QLQ-CR38 and the EORTC QLQ-CR29. The 38-item QLQ-CR38 includes 4 functional domains (body image, sexual functioning, sexual enjoyment, and future perspective) and 8 symptom scales (micturition problems, chemotherapy side effects, symptoms associated with the gastrointestinal tract, male sexual problems, female sexual problems, defecation problems, stoma-related problems, and weight loss) (10). The 29-item QLQ-CR29, by contrast, represents an update to the QLQ-CR38 and consists of 6 scales and 11 single items (11). Questions regarding anorectal and urinary function were added in constructing the QLQ-CR29, and intrusive questions on sexual symptoms and enjoyment that were on the original 38-item measure were eliminated. Because international validation of the QLQ-CR29 had not occurred at the time of study design, patients completed both the QLQ-CR38 and the QLQ-CR29 (12). For both colorectal cancer-specific instruments, higher functional domain scores indicated increased function, and higher symptom scores signified more severe symptoms. All 3 questionnaires were administered at 3 time points: (1) within 3 weeks before the start of radiation therapy; (2) during the fourth week of radiation therapy; and (3) at a follow-up visit approximately 1 month after the end of radiation therapy.
Provider-rated toxicity scores
Additionally, patients were interviewed by a healthcare provider to determine the presence of treatment-related toxicities, including urinary frequency, urinary incontinence, bladder spasms, cystitis, diarrhea, stool incontinence, proctitis, nausea, vomiting, dehydration, vaginal mucositis, and dermatitis. Toxicities were graded in accordance with the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. The provider-rated toxicity scores were completed within 3 weeks before the start of radiation therapy, weekly during radiation therapy, and at a follow-up visit approximately 1 month after the end of radiation therapy.
Statistical analysis
The responses to the QOL questionnaires were linearly transformed to produce a semicontinuous 0 to 100 score based on EORTC scoring methodology (13). Missing responses were also treated according to EORTC procedures. Means and standard errors were calculated for all scale and single-item scores at each time point. Significant changes in all outcome variables between pretreatment and midtreatment scores, midtreatment and post-treatment scores, and pretreatment and posttreatment scores were calculated by repeated-measures analysis of variance techniques. To correct for multiple comparisons, P values <.01 were considered statistically significant. All statistical tests were conducted by use of the SAS system, version 9.2 (SAS Institute Inc, Cary, NC). Changes in global QOL greater than 10 points between time points were considered clinically significant according to prior convention (14). Given the level of variability in global QOL from prior studies, it was estimated that 50 patients would need to complete instruments at all 3 time points to provide sufficient power to enable detection of clinically significant differences in global QOL (15).
Results
From 2006 to 2010, 53 patients were enrolled, of whom 50 completed QOL questionnaires at all 3 time points and therefore served as our study population. The mean age was 59.2 years (range, 32.1–85.2 years), and 36 (72%) were men. Ninety-one percent of the sample (n=40) was Caucasian, 7% was African American (n=3), and 2% was Asian (n=1), with 6 participants missing racial data.
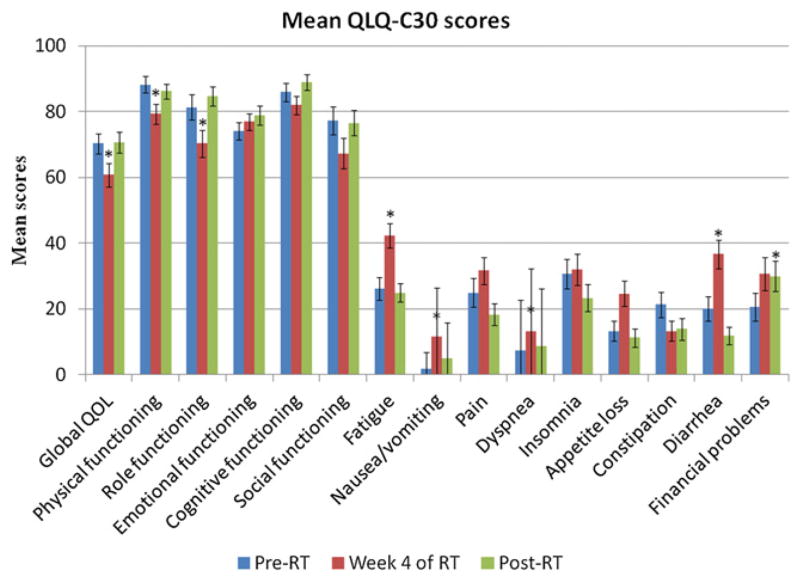
Figure 1 summarizes the scores from the QLQ-C30. Global QOL demonstrated a statistically significant and borderline clinically significant decrease during radiation therapy (−9.50, P=.0024) but returned to baseline 1 month after the end of treatment (−0.33, P=.9205). Physical functioning (−8.93, P=.0014) and role functioning (−11.00, P=.0010) also showed significant impairment during radiation therapy, both of which returned to baseline levels 1 month after treatment. Emotional, cognitive, and social functioning did not significantly change over the course of the study (P>.01). Of the symptoms captured by the QLQ-C30, fatigue (16.22, P<.0001), nausea/vomiting (9.94, P<.0001), diarrhea (16.67, P=.0022), and dyspnea (6.06, P=.0059) all significantly increased while patients were receiving treatment but returned to baseline after therapy. The only QLQ-C30 item that remained persistently elevated after the end of radiation therapy was financial problems (9.33, P=.0048).
Fig. 1.
Mean European Organization for Research and Treatment of Cancer QLQ-C30 scores by questionnaire item at each study time point. *Denotes a significant difference in item score compared with pretreatment levels. RT = radiation therapy.
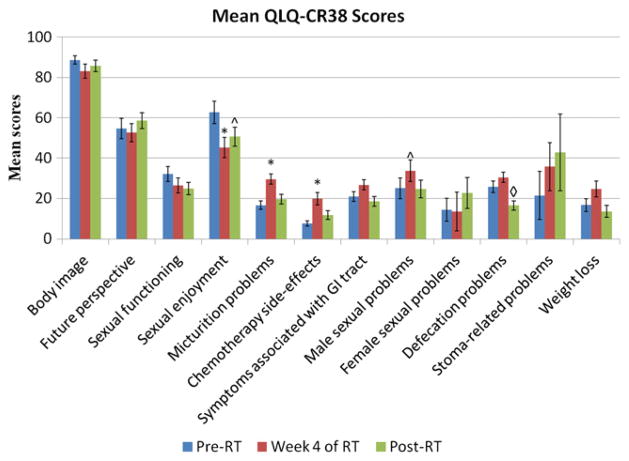
Figure 2 shows the scores from the QLQ-CR38. On the QLQ-CR38, patients experienced a significant decline in sexual enjoyment during therapy (−17.40, P=.0045) and a nonsignificant trend toward impaired sexual enjoyment at 1 month after treatment (−12.05, P=.0236). Although sexual function scores did not worsen during treatment, patients did experience significant impairment by 1 month after treatment (−7.24, P=.0047). Gender-specific items showed that male sexual problems marginally increased during radiation therapy (8.71, P=.0140), whereas female sexual problems could not be assessed because of poor response rates (<29% completion rate at each time point).
Fig. 2.
Mean European Organization for Research and Treatment of Cancer QLQ-CR38 scores by questionnaire item at each study time point. *Denotes a significant difference in item score compared with pretreatment levels. ^Denotes a borderline significant difference in item score compared with pretreatment levels. ◇ Denotes a significant difference in item score compared with levels at the fourth week of therapy. Note that only 2 patients had stomas. RT = radiation therapy.
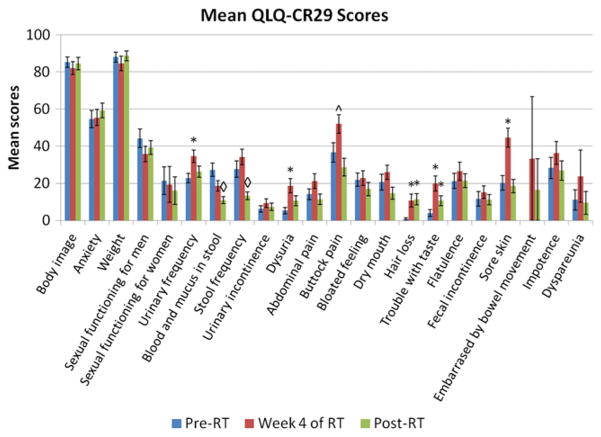
Figure 3 summarizes the scores from the QLQ-CR29. As part of the update to the QLQ-CR38, sexual enjoyment had been removed as an item in constructing the QLQ-CR29, and sexual function had been simplified into 1 question and separated by gender. Additionally, questions regarding ejaculation and vaginal dryness had been removed, given their perceived level of intrusiveness (11). None of the sexually related items on the QLQ-CR29 showed significant changes during or after treatment. The response rates to questions on sexual functioning decreased when separated by gender on the QLQ-CR29, primarily because of a low response rate among women (<64% completion rate at each time point). The response rate to the question on dyspareunia was particularly poor (<21% completion rate at each time point).
Fig. 3.
Mean European Organization for Research and Treatment of Cancer QLQ-CR29 scores by questionnaire item at each study time point. *Denotes a significant difference in item score compared with pretreatment levels. ^Denotes a borderline significant difference in item score compared with pretreatment levels. ◇ Denotes a significant difference in item score compared with levels at the fourth week of therapy. RT = radiation therapy.
On the QLQ-CR38, patients reported an increase in micturition problems during treatment (12.94, P<.0001), and the QLQ-CR29 also captured significant urinary symptoms, specifically frequency (11.82, P=.0006) and dysuria (13.33, P<.0001). Urinary symptoms returned to baseline after therapy on both questionnaires. None of the measures of anorectal function on either the QLQ-CR38 or the QLQ-CR29 declined during treatment, and defecation problems (−9.30, P=.0032), blood and mucus in the stool (−16.38, P<.0001), and stool frequency (−14.49, P=.0032) all were improved at 1 month after therapy when compared with pretreatment levels. Other symptoms that were exacerbated during treatment included chemotherapy-related side effects (12.33, P<.0001), sore skin (24.22, P<.0001), and buttock pain (15.34, P=.0179), all of which returned to baseline after therapy. By contrast, hair loss (10.00, P=.0029) and trouble with taste (15.99, P=.0005) remained below baseline after therapy.
Provider-rated toxicity scores indicated that the most common grade 3 toxicities were proctitis (10%) and diarrhea (8%). Other grade 3 toxicities included urinary frequency (4%), urinary incontinence (2%), stool incontinence (2%), vomiting (2%), dehydration (2%), and vaginal mucositis (2%). Grade 3 toxicities most commonly occurred at week 5 or 6. No patients experienced grade 3 cystitis, bladder spasms, nausea, or dermatitis.
Discussion
Despite the importance of patient-reported QOL endpoints for appreciating patient perspectives on treatment side effects, the current literature on the QOL effects of neoadjuvant CRT for rectal cancer is limited (7). We therefore conducted a prospective analysis examining QOL during neoadjuvant CRT in patients with locally advanced rectal cancer using validated QOL measures.
Notably, patients in our cohort experienced a clinically significant decline in global QOL during treatment as defined in the literature (14). This decrease in global QOL corroborates the results by Guren et al (15) and underscores the importance of treatment side effects on patient well-being. Adverse treatment effects that may have mediated global QOL impairment in our cohort primarily included gastrointestinal symptoms, genitourinary symptoms, and fatigue, inasmuch as the most common patient-reported symptoms included diarrhea, perianal soreness, urinary frequency, and dysuria. Although these are well-known side effects of pelvic CRT, a disconnect often existed between provider-rated toxicity scores and patient-reported QOL scores. Grade 3 urinary toxicity, for example, was infrequently reported by providers, but QOL scores indicated significant urinary symptoms. This discordance between physician-reported and patient-reported measures is also present in the literature, with trials reporting low levels of urinary toxicity but QOL studies suggesting the opposite (2–4, 15). The fact that urinary toxicity is a well-described side effect of pelvic radiation therapy for other cancers suggests that its occurrence in rectal cancer needs to be better appreciated by clinicians. Fortunately, it seems that many of these symptoms are transient, inasmuch as global QOL and almost all gastrointestinal, urinary, and fatigue symptom scores returned to baseline by 1 month after therapy.
A major finding of the current study is that in contrast with other domains of QOL and function, sexual problems increased during radiation therapy and persisted after therapy, with patients continuing to report diminished sexual function and enjoyment 1 month after receiving radiation. This finding adds to a growing body of evidence suggesting significant sexual dysfunction from radiation therapy in rectal cancer. Indeed, 2 recent randomized trials comparing preoperative radiation followed by surgery with surgery alone found higher levels of both male and female sexual dysfunction in the study arms receiving radiation than in the arms receiving surgery alone (16, 17). In the latter study, decreased rates of sexual activity, interest, pleasure, and satisfaction were reported in patients treated with radiation, along with impaired erectile and ejaculatory function in men and dyspareunia in women. Furthermore, our results confirm multiple prior studies showing that sexual problems are among the domains of QOL most likely to remain impaired after acute cancer treatment ends (18). Taken together, these findings suggest that CRT can contribute to sexual problems for rectal cancer and that these problems may persist after the completion of CRT. Although further study of the mechanism of chemoradiation-induced sexual dysfunction is needed, hypothesized mechanisms include fibrosis of normal tissue with secondary demyelination and vascular injury (19). Regardless, it is imperative that clinicians inform patients about the possible sexual side effects of CRT, particularly because research suggests that communication between cancer patients and providers about sexual issues related to their treatment is lacking (20).
Several limitations of our study must be acknowledged. First, although the rate of completed forms in our cohort was high, there was substantial variation in the response to individual items, which may have introduced selection bias. In particular, response rates to questions on sexual function items were low, especially for women. This may in part be due to the fact that female-specific questions on the QLQ-CR38 and QLQ-CR29 regarding vaginal dryness and dyspareunia require the respondent to be sexually active with a partner, whereas male-specific questions on erectile and ejaculatory function do not require a partner. Validated instruments that are specific to sexual function, such as the International Index of Erectile Function (IIEF) and the Female Sexual Function Inventory (FSFI), account for lack of sexual activity among respondents and have been used more recently in studies with rectal cancer patients (19). Interestingly, questions on the QLQ-CR38 regarding ejaculation and vaginal symptoms had been removed in the process of constructing the QLQ-CR29 because the perceived intimacy of these items was thought to have been compromising patient response rates. However, response to the QLQ-CR29 in our cohort failed to exceed response to the QLQ-CR38, and neither of the EORTC questionnaires in our study yielded a substantially improved rate of response when compared with response rates to the IIEF and FSFI from the aforementioned studies. Second, because the follow-up period in the current study was only 1 month, additional studies with a longer follow-up period are needed to establish the duration of sexual problems for patients undergoing radiation therapy for rectal cancer. Third, the current sample size was too small to enable meaningful analysis of the association of global QOL scores with other functional domain or symptom scores. Such analysis would be useful in characterizing the treatment effects that contribute most to changes in global QOL. In our cohort, for example, the parallel changes between global QOL and gastrointestinal and urinary symptoms are suggestive of a causal relationship. By contrast, whether sexual function significantly contributes to global QOL is uncertain, given that sexual function remained depressed after treatment but global QOL returned to baseline. Finally, analysis of radiation plans and dosages to organs of interest may have added important information on predictors of QOL outcomes.
Nevertheless, this study has several significant strengths, including a prospective design, the use of validated QOL questionnaires, and the incorporation of both patient-reported and provider-rated measures. Additionally, the majority of eligible patients consented to our study, and the vast majority of consenting patients completed questionnaires at all 3 time periods, supporting the application of our results to the population of patients with locally advanced rectal cancer undergoing neoadjuvant therapy. By prospectively examining patient-centered information in rectal cancer patients, the present study represents an important and rare contribution to the literature in this area. On the basis of on our findings, these patients may experience a temporary reduction in global QOL along with significant gastrointestinal and genitourinary symptoms. Although most symptoms are transient, impairment in sexual function may persist and merits further investigation.
Summary.
We prospectively analyzed acute changes in patient-reported quality of life (QOL) during and after neoadjuvant chemoradiation in patients with locally advanced rectal cancer. Our results indicate a reduction in global QOL along with significant gastrointestinal and genitourinary symptoms during treatment. Moreover, provider-rated toxicity scales may not capture the decrease in patient-reported QOL. Although most symptoms are transient, impairment in sexual function may persist after chemoradiation.
Acknowledgments
Supported in part by the National Cancer Institute core grant to the University of Michigan Comprehensive Cancer Center 5 P30 CA 46592.
Footnotes
Presented at the 2011 Gastrointestinal Cancers Symposium of the American Society of Clinical Oncology, January 20-22, 2011 in San Francisco, CA.
Conflict of interest: none.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:12–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246:693–701. doi: 10.1097/01.sla.0000257358.56863.ce. [DOI] [PubMed] [Google Scholar]
- 4.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 5.Pinkawa M, Gagel B, Piroth MD, et al. Erectile dysfunction after external beam radiotherapy for prostate cancer. Eur Urol. 2009;55:227–234. doi: 10.1016/j.eururo.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 6.Vistad I, Cvancarova M, Fosså SD, et al. Postradiotherapy morbidity in long-term survivors after locally advanced cervical cancer: how well do physicians’ assessments agree with those of their patients? Int J Radiat Oncol Biol Phys. 2008;71:1335–1342. doi: 10.1016/j.ijrobp.2007.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Grumann MM, Noach EM, Hoffman IA, et al. Comparison of quality of life of patients undergoing abdominoperineal extirpation or anterior resection for rectal cancer. Ann Surg. 2001;233:149–156. doi: 10.1097/00000658-200102000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gunderson LL, Tepper JE. Clinical Radiation Oncology. 3. Philadelphia: Churchill Livinstone; 2012. [Google Scholar]
- 9.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 10.Sprangers MA, te Velde A, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer. 1999;35:238–247. doi: 10.1016/s0959-8049(98)00357-8. [DOI] [PubMed] [Google Scholar]
- 11.Gujral S, Conroy T, Fleissner C, et al. Assessing quality of life in patients with colorectal cancer: an update of the EORTC quality of life questionnaire. Eur J Cancer. 2007;43:1564–1573. doi: 10.1016/j.ejca.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 12.Whistance RN, Conroy T, Chie W, et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer. 2009;45:3017–3026. doi: 10.1016/j.ejca.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Fayers P, Aaronson NK, Bjordal K, et al. EORTC QLQ-C30 Scoring Manual. 3. Brussels, Belgium: European Organization for Research and Treatment of Cancer; 2001. [Google Scholar]
- 14.Osoba D, Rodrigues G, Myles J, et al. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 15.Guren MG, Dueland S, Skovlund E, et al. Quality of life during radiotherapy for rectal cancer. Eur J Cancer. 2003;39:587–594. doi: 10.1016/s0959-8049(02)00741-4. [DOI] [PubMed] [Google Scholar]
- 16.Marijnen CA, van de Velde CJ, Putter H, et al. Impact of short-term preoperative radiotherapy on health-related quality of life and sexual functioning in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2005;23:1847–1858. doi: 10.1200/JCO.2005.05.256. [DOI] [PubMed] [Google Scholar]
- 17.Stephens RJ, Thompson LC, Quirke P, et al. Impact of short-course preoperative radiotherapy for rectal cancer on patients’ quality of life: data from the Medical Research Council CR07/National Cancer Institute of Canada Clinical Trials Group C016 randomized clinical trial. J Clin Oncol. 2010;28:4233–4239. doi: 10.1200/JCO.2009.26.5264. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt CE, Bestmann B, Kuchler T, et al. Ten-year historic cohort of quality of life and sexuality in patients with rectal cancer. Dis Colon Rectum. 2005;48:483–492. doi: 10.1007/s10350-004-0822-6. [DOI] [PubMed] [Google Scholar]
- 19.Brueheim K, Guren MG, Dahl AA, et al. Sexual function in males after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2010;76:1012–1017. doi: 10.1016/j.ijrobp.2009.03.075. [DOI] [PubMed] [Google Scholar]
- 20.Park ER, Norris RL, Bober SL. Sexual health communication during cancer care barriers and recommendations. Cancer J. 2009;15:74–77. doi: 10.1097/PPO.0b013e31819587dc. [DOI] [PubMed] [Google Scholar]