Abstract
Introduction
Assessing patient-specific risk factors for long-term mortality following resection of pancreatic adenocarcinoma can be difficult. Sarcopenia—the measurement of muscle wasting—may be a more objective and comprehensive patient-specific factor associated with long-term survival.
Methods
Total psoas area (TPA) was measured on preoperative cross-sectional imaging in 557 patients undergoing resection of pancreatic adenocarcinoma between 1996 and 2010. Sarcopenia was defined as the presence of a TPA in the lowest sex-specific quartile. The impact of sarcopenia on 90-day, 1-year, and 3-year mortality was assessed relative to other clinicopathological factors.
Results
Mean patient age was 65.7 years and 53.1 % was male. Mean TPA among men (611 mm2/m2) was greater than among women (454 mm2/m2). Surgery involved pancreaticoduodenectomy (86.0 %) or distal pancreatectomy (14.0 %). Mean tumor size was 3.4 cm; 49.9 % and 88.5 % of patients had vascular and perineural invasion, respectively. Margin status was R0 (59.0 %) and 77.7 % patients had lymph node metastasis. Overall 90-day mortality was 3.1 % and overall 1- and 3-year survival was 67.9 % and 35.7 %, respectively. Sarcopenia was associated with increased risk of 3-year mortality (HR=1.68; P<0.001). Tumor-specific factors such as poor differentiation on histology (HR=1.75), margin status (HR=1.66), and lymph node metastasis (HR=2.06) were associated with risk of death at 3-years (all P<0.001). After controlling for these factors, sarcopenia remained independently associated with an increased risk of death at 3 years (HR=1.63; P<0.001).
Conclusions
Sarcopenia was a predictor of survival following pancreatic surgery, with sarcopenic patients having a 63 % increased risk of death at 3 years. Sarcopenia was an objective measure of patient frailty that was strongly associated with long-term outcome independent of tumor-specific factors.
Keywords: Sarcopenia, Pancreas surgery, Morbidity, Mortality, Outcomes
Introduction
Pancreatic cancer is an aggressive malignancy and surgical resection is the only potentially curative treatment option with a 5-year survival ranging from 10 % to 20 %.1-5 Traditionally one of the most complex abdominal operations, improvements in operative technique, and perioperative care over the past several decades have resulted in improved morbidity and mortality. Operative mortality has decreased to less than 5 % at high volume centers and morbidity has improved, but still remains high at 40–60 %.6-8 In turn, high morbidity rates are associated with prolonged hospital stays, increased need for additional procedures or operations, and increased resource utilization.9-11 Quality improvement measures have focused on improving outcomes and decreasing morbidity and mortality following high-risk procedures. In addition, prognosis following surgery for pancreatic cancer remains poor. While previous studies have examined prognosis following surgery, these studies have largely focused on tumor-specific clinico-pathological factors such as lymph node metastasis, margin status, and tumor size, among others. 12–14
Prognosis following surgery is, however, undoubtedly multifactorial and is related not only to tumor-specific factors but also individual patient characteristics. The American Society of Anesthesiologist (ASA) classification, Eastern Cooperative Oncology Group (ECOG) performance status and body mass index (BMI)—among others—have been used with varying degrees of success, but often fail to identify those patients at highest risk for perioperative complications or mortality. Several groups have suggested the Estimation of Physiologic Ability and Surgical Stress Scoring (E-PASS) system as a more comprehensive physiologic estimation; however, results have been mixed.15-17 Frailty, a measure believed to estimate a patient’s physiologic reserves, has recently been proposed as a more robust predictor of outcomes following surgery.18,19 Initial assessments of frailty have been criticized for relying on scales dependent on subjective evaluations of weakness, exhaustion, and physical activity. Thus some investigators have proposed the use of sarcopenia—depletion of muscle mass as measured by cross-sectional imaging—as a more objective measure of frailty and, in turn, perhaps a better predictor of outcome. Previous data have suggested that sarcopenia may be associated with worse outcomes among patients being treated with chemotherapy for pancreatic, breast, prostate, and renal cell cancer.20-25 Only a few studies have examined the association between the presence of sarcopenia and outcomes following surgery. These studies have included patients undergoing surgery for melanoma, colorectal liver metastasis, and liver transplantation, but not pancreatic cancer.19,26,27 Given the importance of assessing patient-specific risk factors for resection of pancreatic adenocarcinoma, we sought to investigate the impact of sarcopenia among patients undergoing resection for pancreatic adenocarcinoma. Specifically, we hypothesize that preoperative sarcopenia would lead to worse short- and long-term outcomes among patients undergoing surgery for pancreatic adenocarcinoma.
Methods
Patients and data collection
Between 1999 and 2010, 1,593 patients who underwent curative intent surgery for pancreatic cancer were identified from the Johns Hopkins Hospital pancreas database. Perioperative abdominal CT images (i.e., within 30 days of surgery) were available for re-review for 557 patients, representing the study cohort. Clinical and pathological data were collected including information on demographics, tumor stage, tumor size, operative details, length of ICU, and hospital stay. Data on perioperative morbidity and mortality were also collected. Complications were scored by Clavien-Dindo classification with major complications being defined as Clavien grade ≥3.28 This study was approved by the Johns Hopkins Institutional Review Board.
Image Analysis
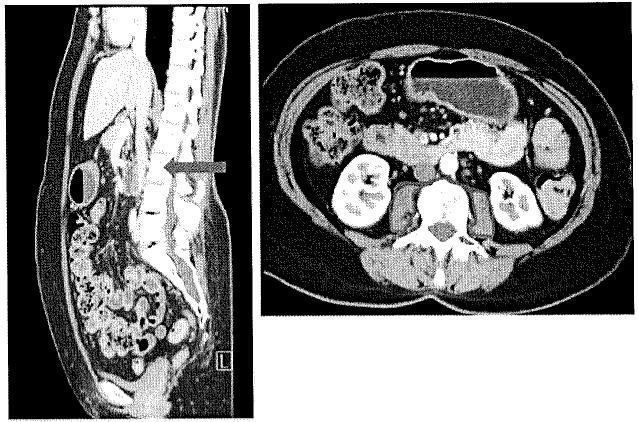
Sarcopenia was assessed by measuring the cross sectional area of the right and left psoas muscles (total psoas muscle area=TPA). TPA was measured at the level of L3 on the first image with both vertebral spines visible. Measurements were performed in a semi-automated fashion with manual outlining of psoas muscle borders and setting the density threshold between −30 and 110 Hounsfield Units (HU; Fig. 1). This allowed for automatic calculation of psoas muscle area by excluding vasculature and areas of fatty infiltration based on HU. The measured psoas area was then normalized for height, as per convention for body composition measurements (TPA mm2/m2).29-31
Fig. 1.
TPA was measured at the level of L3 on the first image with both vertebral spines visible. Measurements were performed in a semi-automated fashion with manual outlining of psoas muscle borders and setting the density threshold between −30 and 110 Hounsfield Units (HU)
Statistical analysis
Data are provided as mean, median, and standard deviation for continuous variables. The impact of sarcopenia was evaluated as both a continuous and categorical variable. Patients were stratified by quartiles according to TPA and sarcopenia was defined in the categorical analyses as the lowest quartile for men and women separately. The impact of sarcopenia on morbidity and mortality was examined using univariate and multivariate analyses. Overall survival was evaluated using the Kaplan–Meier method. A P value <0.05 was considered statistically significant. Statistical analyses were performed using Stata (Stata Corp, College Station, TX, USA).
Results
Demographic and Clinical Characteristics
The clinical and pathological characteristics of 557 patients included in the study are outlined in Table 1. The average age of the study population was 65.7 years. There were 296 men (53.1 %) and 261 women (46.9 %) in the study population. Overall, the majority of tumors (71.5 %) were stage III, with a mean tumor size of 3.4 cm. Margin status was R0 (59.0 %) and 77.7 % of patients had lymph node metastasis. There was an average of 3.3 lymph node metastases out of an average of 19.8 lymph nodes evaluated in the specimen. Vascular and perineural invasion were present in 49.9 % and 88.5 % of patients, respectively. Surgery consisted of a pancreaticoduodenectomy in the majority of patients (86.0 %) while 14.0 % of patients underwent a distal pancreatectomy.
Table 1.
Demographic and clinical characteristics of patients who underwent curative intent surgery for pancreatic adenocarcinoma
| All patients (n=557) |
Men (n=296) |
Women (n=261) |
P valuea | |
|---|---|---|---|---|
| Age at surgery (years); mean±SD | 65.7±10,6 | 65.2±10.8 | 66.3±10,3 | 0.21 |
| Total psoas area (cm2/m2); mean±SD | 537.7±168.1 | 611.0±167.9 | 454.3± 123.7 | <0.001 |
| Cutoff for lowest quartile (cm2/m2) | 413 | 492 | 362 | |
| Race | ||||
| White; n (%) | 475 (85.3) | 260 (87.8) | 215 (82.4) | 0.46 |
| Black; n (%) | 45 (8.1) | 20 (6.8) | 25 (9.6) | |
| Hispanic; n (%) | 12 (2.2) | 5(1.7) | 7 (2.7) | |
| Asian; n (%) | 9(1.6) | 3(1.0) | 6 (2.3) | |
| North American Native; n (%) | 1 (0.2) | 0 | 1 (0.4) | |
| Other; n (%) | 15 (2.7) | 8 (2.7) | 4 (2.7) | |
| Tumor characteristics | ||||
| Grade of tumor | ||||
| Well differentiated; n (%) | 27 (4.9) | 15 (5.0) | 12 (4.6) | 0.11 |
| Moderately differentiated; n (%) | 304 (54.6) | 150 (50.7) | 154 (59.0) | |
| Poorly differentiated; n (%) | 226 (40.6) | 131 (44.3) | 95 (36.4) | |
| Stage of tumor | ||||
| I; n (%) | 33 (5.9) | 13 (4.4) | 20 (7.7) | 0.36 |
| II; n (%) | 94 (16.9) | 51 (17.2) | 43 (16.5) | |
| III; n (%) | 398 (71.5) | 214 (72.3) | 184 (70.5) | |
| IV; n (%) | 22 (4.0) | 14 (4.7) | 8(3.1) | |
| is; n (%) | 1 (0.2) | 1 (0.3) | 0 | |
| Unknown; n (%) | 9(1.6) | 3(1.0) | 6 (2.3) | |
| Nodal metastasis; n (%) | 433 (77.7) | 237 (80.1) | 196 (75.1) | 0.16 |
| Distant metastasis; n (%) | 11 (2.0) | 5 (1.7) | 6 (2.3) | 0.73 |
| Tumor size (cm); mean±SD | 3.4± 1.5 | 3.5± 1.6 | 3.2±1.4 | 0.01 |
| Number of positive nodes; mean±SD | 3.3±3.5 | 3.5±3.5 | 3.0±3.5 | 0.13 |
| Number of resected nodes; mean±SD | 19.8±8.8 | 19.6±8.2 | 20.0±9.5 | 0.54 |
| Type of operation | ||||
| Pancreatoduodenectomy; n (%) | 479 (86.0) | 261 (87.8) | 219 (84.0) | 0.18 |
| Distal pancreatectomy; n (%) | 78 (14.0) | 36 (12.2) | 42 (16.1) |
P value for comparison between men and women
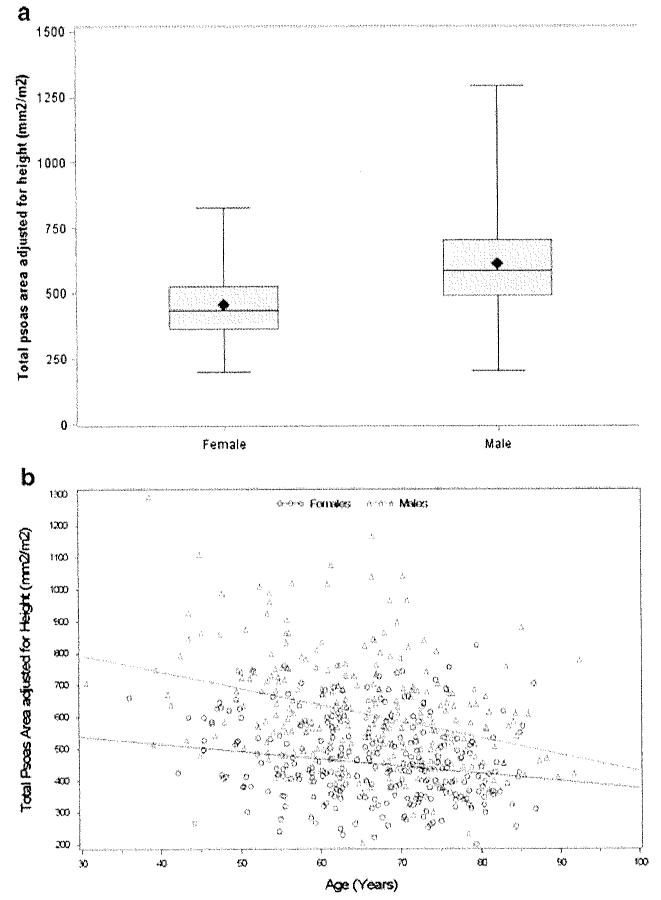
The average TPA was 537.7 mm2/m2 after normalizing for patient height. When stratified by gender, the mean adjusted TPA was statistically lower for women than for men (454.3 mm2/m2 vs. 611.0 mm2/m2, P<0.001; Fig. 2a). The lowest quartile TPA threshold for men was 492 mm2/m2 versus 362 mm2/m2 for women. Age also tended to be associated with the incidence of sarcopenia as there was a trend toward decreasing TPA with increasing age (Fig. 2b). Sarcopenia was noted across a wide range of BMIs. Sarcopenia was less frequently observed, however, among obese patients with a BMI≥30 kg/m2. Of the 112 patients who had a BMI≥ 30 kg/m2, 15 (13.3 %) also had sarcopenia and therefore were characterized as having sarcopenic obesity. In contrast, among patients with a BMI≤24.9 kg/m2 the incidence of sarcopenia was 36.2 %.
Fig. 2.
a When stratified by gender, the mean adjusted TPA was statistically lower for women than for men (454.3 mm2/m2 vs. 611.0 mm2/m2, P<0.001). b Age tended to be associated with the incidence of sarcopenia as there was a trend toward decreasing TPA with increasing age
Impact of Sarcopenia
Of the 557 patients who underwent pancreatic resection, 260 experienced a least one complication for an overall morbidity of 46.6 %. The most common postoperative complications included wound complications (15 %), delayed gastric emptying (15 %), and pancreatic fistula (8.5 %). Most complications were minor; 18.7 % of patients experienced a major (Clavien grade≥3) complication. The presence of sarcopenia was not associated with the risk of overall morbidity (OR=0.88, 95 % CI 0.60–1.29; P=0.51) or the risk of major complications (OR=0.72, 95 % CI 0.43–1.21; P=0.21; Table 2). Median ICU and overall length of hospital stay was similar among patients who had sarcopenia (ICU, 0.4 days; overall stay, 12.0 days) versus patients who did not have sarcopenia (ICU, 0.4 days; overall stay, 12.0 days) (P=0.92 for ICU, P=0.98 for length of stay).
Table 2.
Sarcopenia and hospital stay, morbidity, and mortality among men and women
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Sarcopenia (n=74) |
No Sarcopenia (n=222) |
P value | Sarcopenia (n = 65) |
No Sarcopenia (n=196) |
P value | |
| Hospital stay; mean±SD |
12,7±9.5 | 12.5±8,5 | 0.82 | 11,1 ±7.9 | 11,4±8.5 | 0.81 |
| ICU stay; mean±SD |
0.5±2.0 | 0.5±1.7 | 1.00 | 0.2±0.6 | 0.2±0.6 | 0.74 |
| Postoperative morbidity |
||||||
| Any complication; n (%) |
33 (44.6) | 115 (51.8) | 0.28 | 27 (41.5) | 85 (43.4) | 0.80 |
| Clavien grade ≥3 complication; n (%) |
14 (20.6) | 49 (24.8) | 0.49 | 7 (12.1) | 34 (20.5) | 0.15 |
| Postoperative mortality |
||||||
| 30 days; n (%) | 1 (1.4) | 1 (0.5) | 0.44 | 0(0) | 1 (0.5) | 1.00 |
| 90 days; n (%) | 7 (9.5) | 6 (2.7) | 0.02 | 1 (1.5) | 3 (1.5) | 1.00 |
| 1 year; n (%) | 36 (48.7) | 68 (30.6) | 0.01 | 24 (35.9) | 51 (26.0) | 0.09 |
| 3 years; n (%) | 59 (79.7) | 135 (60.8) | 0.003 | 48 (73.9) | 116(59.2) | 0.03 |
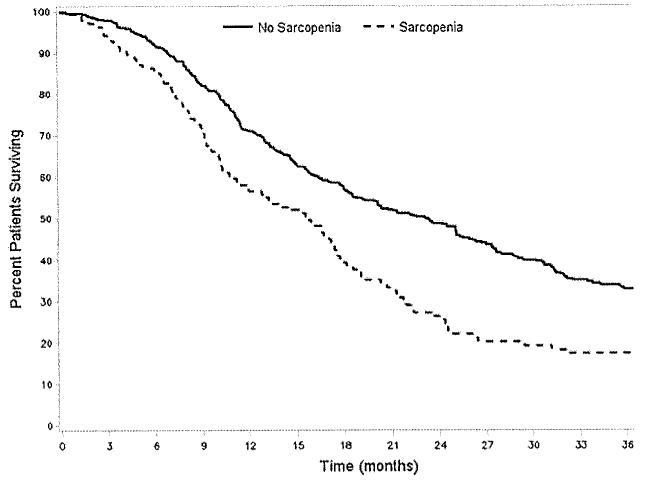
There were 17 deaths within 90 days for a mortality of 3.1 %. Sarcopenia was not associated with increased hazard of 90-day death (HR=2.31, 95 % CI 0.78–6.77; P=0.13). For the entire cohort, the median overall survival was 14 months. Overall 1- and 3-year survival was 67.9 % and 35.7 %, respectively. Several factors were associated with survival at 3 years on univariate analysis including tumor size (HR=1.13, 95 % CI 1.07–1.19), poor tumor differentiation (HR= 1.75, 95 % CI 1.40–2.12), vascular invasion (HR=1.57, 95 % CI 1.27–1.93), as well as lymph node metastasis (HR=2.06, 95 % CI 1.55–2.74; all P<0.001). The presence of sarcopenia was also associated with the risk of death at 3 years (HR=1.68, 95 % 1.34–2.11; P<0.001; Fig. 3). Deaths within the first 3 years of operation were found to be highest among patients in the lowest TPA quartile for both men (no sarcopenia, 60.8 % vs. sarcopenia, 79.7 %) and women (no sarcopenia, 59.2 % vs. sarcopenia, 73.9 %; both P<0.05). This translated into a worse 3-year survival for both men (no sarcopenia, 39.2 % vs. sarcopenia, 20.3 %) and women (no sarcopenia, 40.8 % vs. sarcopenia, 26.1 %; both P<0.05). On multivariate analysis, after controlling for tumor-specific factors, sarcopenia remained independently associated with an increased risk of death at 3 years (HR=1.63, 95 % CI 1.28–2.07; P<0.001; Table 3).
Fig. 3.
The presence of sarcopenia was also associated with the risk of death (no sarcopenia, 18.0 months; 40.0 % vs. sarcopenia, 13.7 months; 23.0 % median, 3-year survival, respectively; P=0.01)
Table 3.
Cox proportional hazard ratio estimates for the effect of sarcopenia on 3-year mortality after adjustment for other covariates
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Hazard ratio (95 % CI) |
P value | Hazard ratio (95 % CI) |
P value | |
| Sarcopenia | 1.68 (1.34–2.11) | <0.001 | 1.63 (1.28–2.07) | <0.001 |
| Age at surgery (years) | 1.01 (1.00–1.02) | 0.02 | 1.01 (1.00–1,03) | 0.01 |
| Weight (kg) | 0.99 (0.99–1.00) | 0.55 | – | – |
| Male gender | 1.16 (0.94–1.43) | 0.16 | – | – |
| Tumor size (cm) | 1.13 (1.07–1.19) | <0.001 | 1.13 (1.06–1.20) | <0.001 |
| Poorly differentiated tumor | 1.75 (1.40–2.12) | <0.001 | 1,76 (1.42–2.17) | <0.001 |
| Nodal metastasis | 2.06 (1.55–2.74) | <0.001 | 1.73 (1.29–2.32) | <0.001 |
| Vascular infiltration | 1.57 (1.27–1.93) | <0.001 | 1.25 (1.05–1.55) | <0.001 |
| Positive margins | 1.66 (1.33–2.06) | <0.001 | 1.46 (1.17–1.82) | <0.001 |
| Pancreatoduodenectomy (vs. distal pancreatectomy) |
1.07 (0.78–1.45) | 0.68 | – | – |
| Clavien–Dindo grade ≥3 complications |
1.70 (1.32–2.19) | <0.001 | 1.88 (1.44–2.44) | <0.001 |
| Race (reference: White) | ||||
| African American | 1.02 (0.70–1.47) | 0.93 | – | – |
| Other race | 0.58 (0.35–0.95) | 0.03 | 0.66 (0.40–1.09) | 0.11 |
Discussion
Advances in operative technique and perioperative care have improved outcomes following pancreatic surgery; however, surgical morbidity and mortality are still a concern. In addition, prognosis following surgery for pancreatic cancer remains poor. Studies examining prognosis following surgery have traditionally focused on tumor-specific clinico-pathological factors. For example, data from our group and others have noted that lymph node metastasis and margin status—among other factors—are associated with a worse prognosis following resection of pancreatic cancer.12-14 Prognosis following surgery is, however, undoubtedly multifactorial and is related not only to tumor-specific factors but also individual patient characteristics. While several scoring systems have been proposed, their prognostic accuracy in quantifying the risk associated with patient-level physiological/performance status has been questioned.15-17 Frailty has been proposed as a more global metric of patient physiological reserve and overall health status.32 Frailty can be assessed utilizing patient questionnaires or varied clinical measurements of weakness or physical activity.19,32 These measurements can be non-reproducible and subject to measurement or reporting bias. More recently, sarcopenia—as assessed by the measurement of muscle mass on cross-sectional imaging—has been reported to be an accurate and quantitative marker of frailty.19,27,32 The current study is important because we examined the prognostic impact of sarcopenia among a large cohort of patients who underwent pancreatic resection for pancreatic carcinoma. Specifically, we noted that sarcopenia was an independent predictor of mortality following pancreatic surgery, with sarcopenic patients having a 63 % increased risk of death at 3 years. As such, sarcopenia may be an important objective measure of patient frailty that can predict long-term outcome independent of tumor-specific factors. While it would be premature to advocate the use of sarcopenia as a factor to dictate postoperative therapy, similar to other important prognostic factors such as margin and node status, as well as T-stage, sarcopenia can assist in the risk stratification of patients in the postoperative period.
Sarcopenia is distinct from cancer related cachexia.33,34 Cancer patients often experience weight loss due to chemotherapy, symptoms from gastrointestinal malignancies, or cancer cachexia syndrome. Research in cancer cachexia has identified an association with endogenous transmitters and inflammatory markers which contribute to a negative nitrogen balance, fatigue, and weight loss.35,36 While weight remains an important measurement of nutritional status, various data have shown that there is differential loss of body fat and muscle in cancer cachexia.37 Recent studies have begun to focus on muscle mass measurements as a better predictor of outcome in cancer patients undergoing chemotherapy and operative resection. Sarcopenia refers specifically to muscle loss and is associated with normal aging and non-oncologic disease states.38-40 More recently, investigators have begun to examine the association between sarcopenia and outcomes among cancer patients receiving chemotherapy and have shown that sarcopenia is associated with worse outcomes among patients treated with chemotherapy for pancreatic, breast, prostate, and renal cell cancer.21-25 The prognostic association of sarcopenia with surgical outcomes is less defined. While a few studies have noted an association with sarcopenia and worse outcomes among patients undergoing surgery for melanoma, colorectal liver metastases, and hepatocellular carcinoma,19,26,27 the impact of sarcopenia on patients undergoing pancreatic surgery had not been examined prior to the current study.
The impact of sarcopenia on surgical morbidity remains ill defined. In our previous work, we had reported on the effect of sarcopenia among 259 patients who underwent liver resection for colorectal liver metastasis.19 In that study, we noted that patients with sarcopenia had longer hospital stays, a higher chance of extended ICU stay, and an increased risk of postoperative complications. In contrast, in the current study we did not find that sarcopenia impacted length of stay or morbidity among a much larger (n=557) cohort of patients undergoing pancreatic resection of carcinoma. In fact, the presence of sarcopenia was not associated with the risk of either overall morbidity or the risk of major complications (Table 2). This finding was somewhat surprising, as one might have expected sarcopenia to be associated with a worse short-term outcome. The reason for the discrepancy regarding the impact of sarcopenia on morbidity in our two studies is not clear. Given that the two study populations were divergent, our collective data may suggest that the impact of sarcopenia on morbidity may be a function of the underlying patient population and cancer being studied.
In the current study, we noted a strong association between sarcopenia and survival. Similar to previously reported data, we found that tumor-specific factors such as lesion size, tumor differentiation, presence of vascular invasion, and lymph node metastasis were associated with survival. More importantly, however, was the finding that sarcopenia remained an independent predictor of survival even after controlling for these risk factors. In fact, at 3 years patients with sarcopenia had a greater than 60 % increased risk of death compared with patients who did not have sarcopenia (Fig. 3). Englesbe et al. reported on the effect of sarcopenia on long-term mortality among patients undergoing liver transplantation.27 The authors similarly noted that 3-year survival following transplantation was only 26 % among patients with sarcopenia versus 77 % among patients without sarcopenia—a threefold increase in sarcopenia-associated mortality risk.27 In a separate study, Van Vledder et al. noted a near twofold decrease in 3-year survival among patients liver resection of colorectal liver metastasis who had sarcopenia (34.0 %) versus those patients who did not have sarcopenia (64.6 %).32 Taken together, our data corroborate and validate the importance of sarcopenia as a valuable preoperative tool to risk stratify patients with regard to prognosis and survival.
Several limitations must be bom in mind when considering our study. Similar to our previous work, we were not able to capture data on other frailty parameters such as grip strength, walking speed, or levels of exhaustion due to the study’s retrospective design.19 Despite having a large number of patients in the Johns Hopkins Hospital pancreatic database, many patients had cross-sectional imaging performed at an outside institution and therefore were not available for re-review. While these patients were not included in our analyses, it is likely that these data were missing/excluded at random and therefore did not pose a threat of selection bias. It was somewhat surprising that sarcopenia was not associated with short-term outcome. At very high volume centers such as Johns Flopkins, overall mortality following pancreatic surgery is a rare event (n= 17). Therefore, despite having a cohort size that was considerably larger than previous reports on the topic, the assessment of perioperative death and sarcopenia may have been susceptible to a type II error, especially if the effect was small. The interplay of frailty, sarcopenia and outcomes is undoubtedly complex and warrants future investigation. Finally, while we chose to utilize the psoas muscle-only approach to quantity sarcopenia in conjunction with BMI to quantify obesity, other approaches have been advocated.41
In conclusion, surgical resection of pancreatic adenocarcinoma remains the mainstay of treatment for those patients with potentially resectable disease. While much attention has been paid in the surgical literature to tumor-specific factors that may be associated with prognosis, other patient-centered factors have not received as much attention. In the current study, we report that measurement of muscle mass can be obtained from preoperative cross-sectional imaging and that loss of this muscle mass—defined as sarcopenia—was associated with a 60 % increased risk of mortality at 3 years. Similar to postoperative tumor-specific metrics of prognosis, pre-operative assessment of sarcopenia may be an important independent tool to inform clinical decision-making and assist in risk, stratification of patients with carcinoma undergoing pancreatic surgery.
Footnotes
Peter Peng and Omar Hyder contributed equally to the production of this manuscript.
Contributor Information
Peter Peng, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Omar Hyder, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Amin Firoozmand, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Peter Kneuertz, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Richard D. Schulick, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Donghang Huang, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Martin Makary, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Kenzo Hirose, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Barish Edil, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Michael A. Choti, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Joseph Herman, Department of Radiation Oncology, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
John L. Cameron, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Christopher L. Wolfgang, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA
Timothy M. Pawlik, Department of Surgery, Johns Hopkins University School of Medicine, Baltimore, MD, USA; Department of Surgery, Johns Hopkins University School of Medicine, Harvey 611 600 N. Wolfe Street, Baltimore, MD 21287, USA
References
- 1.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244(1):10–5. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4(6):567–79. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297(3):267–77. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 4.Ueno H, Kosuge T, Matsuyama Y, et al. A randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101(6):908–15. doi: 10.1038/sj.bjc.6605256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tact cancer cooperative group. Ann Surg. 1999;230(6):776–82. doi: 10.1097/00000658-199912000-00006. discussion 82-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seiler CA, Wagner M, Bachmann T, et al. Randomized clinical trial of pylorus-preserving duodenopancreatectomy versus classical Whipple resection-long term results. Br J Surg. 2005;92(5):547–56. doi: 10.1002/bjs.4881. [DOI] [PubMed] [Google Scholar]
- 7.Balzano G, Zerbi A, Capretti G, Rocchetti S, Capitanio V, Di Carlo V. Effect of hospital volume on outcome of pancreaticoduodenectomy in Italy. Br J Surg. 2008;95(3):357–62. doi: 10.1002/bjs.5982. [DOI] [PubMed] [Google Scholar]
- 8.Jilt Balcom, Rattner DW, Warshaw AL, Chang Y, Fernandez-del Castillo C. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136(4):391–8. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 9.Pratt W, Joseph S, Callery MP, Vollmer CM., Jr. POSSUM accurately predicts morbidity for pancreatic resection. Surgery. 2008;143(1):8–19. doi: 10.1016/j.surg.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 10.Emick DM, Riall TS, Cameron JL, et al. Hospital readmission after pancreaticoduodenectomy. J Gastrointest Surg. 2006;10(9):124352. doi: 10.1016/j.gassur.2006.08.016. discussion 52-3. [DOI] [PubMed] [Google Scholar]
- 11.Pratt WB, Maithel SK, Vanounou T, Huang ZS, Callery MP, Vollmer CM., Jr. Clinical and economic validation of the International Study Group of Pancreatic Fistula (ISGPF) classification scheme. Ann Surg. 2007;245(3):443–51. doi: 10.1097/01.sla.0000251708.70219.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141(5):610–8. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 13.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Annals of surgical oncology. 2008;15(1):165–74. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 14.Asiyanbola B, Gleisner A, Herman JM, et al. Determining pattern of recurrence following pancreaticoduodenectomy and adjuvant 5-flurouracil-based chemoradiation therapy: effect of number of metastatic lymph nodes and lymph node ratio. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2009;13(4):752–9. doi: 10.1007/s11605-008-0762-x. [DOI] [PubMed] [Google Scholar]
- 15.Deyle S, Banz VM, Wagner M, et al. Estimation of physiologic ability and surgical stress score does not predict immediate outcome after pancreatic surgery. Pancreas. 40(5):723–9. doi: 10.1097/MPA.0b013e318212c02c. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto D, Takamori H, Sakamoto Y, Tanaka H, Hirota M, Baba H. Can the physiologic ability and surgical stress (E-PASS) scoring system predict operative morbidity after distal pancreatectomy? Surg Today. 40(7):632–7. doi: 10.1007/s00595-009-4112-8. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto D, Takamori H, Sakamoto Y, et al. Is an estimation of physiologic ability and surgical stress able to predict operative morbidity after pancreaticoduodenectomy? J Hepatobiliary Pan-creat Sci. 17(2):132–8. doi: 10.1007/s00534-009-0116-4. [DOI] [PubMed] [Google Scholar]
- 18.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 210(6):901–8. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 19.Peng PD, van Vledder MG, Tsai S, et al. Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford) 13(7):439–46. doi: 10.1111/j.1477-2574.2011.00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3(4):269–75. doi: 10.1097/SPC.0b013e328331124a. [DOI] [PubMed] [Google Scholar]
- 21.Prado CM, Baracos VE, McCargar LJ, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15(8):2920–6. doi: 10.1158/1078-0432.CCR-08-2242. [DOI] [PubMed] [Google Scholar]
- 22.Galvao DA, Taaffe DR, Spry N, Joseph D, Newton RU. Combined resistance and aerobic exercise program reverses muscle loss in men undergoing androgen suppression therapy for prostate cancer without bone metastases: a randomized controlled trial. J Clin Oncol. 28(2):340–7. doi: 10.1200/JCO.2009.23.2488. [DOI] [PubMed] [Google Scholar]
- 23.Antoun S, Baracos VE, Birdsell L, Escudier B, Sawyer MB. Low body mass index and sarcopenia associated with dose-limiting toxicity of sorafenib in patients with renal cell carcinoma. Ann Oncol. 21(8):1594–8. doi: 10.1093/annonc/mdp605. [DOI] [PubMed] [Google Scholar]
- 24.Antoun S, Birdsell L, Sawyer MB, Venner P, Escudier B, Baracos VE. Association of skeletal muscle wasting with treatment with sorafenib in patients with advanced renal cell carcinoma: results from a placebo-controlled study. J Clin Oncol. 28(6):1054–60. doi: 10.1200/JCO.2009.24.9730. [DOI] [PubMed] [Google Scholar]
- 25.Tan BH, Birdsell LA, Martin L, Baracos VE, Fearon KC. Sarcopenia in an overweight or obese patient is an adverse prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15(22):6973–9. doi: 10.1158/1078-0432.CCR-09-1525. [DOI] [PubMed] [Google Scholar]
- 26.Sabel MS, Lee J, Cai S, Englesbe MJ, Holcombe S, Wang S. Sarcopenia as a Prognostic Factor among Patients with Stage III Melanoma. Ann Surg Oncol. 18(13):3579–85. doi: 10.1245/s10434-011-1976-9. [DOI] [PubMed] [Google Scholar]
- 27.Englesbe MJ, Patel SP, He K, et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 211(2):271–8. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 29.Baumgartner RN, Koehler KM, Gallagher D, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755–63. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 30.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol. 2004;97(6):2333–8. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 31.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33(5):997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 32.van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. The British journal of surgery. 2012 doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 33.Dodson S, Baracos VE, Jatoi A, et al. Muscle wasting in cancer cachexia: clinical implications, diagnosis, and emerging treatment strategies. Annual review of medicine. 2011;62:265–79. doi: 10.1146/annurev-med-061509-131248. [DOI] [PubMed] [Google Scholar]
- 34.Fearon KC, Voss AC, Hustead DS. Definition of cancer cachexia: effect of weight loss, reduced food intake, and systemic inflammation on functional status and prognosis. The American journal of clinical nutrition. 2006;83(6):1345–50. doi: 10.1093/ajcn/83.6.1345. [DOI] [PubMed] [Google Scholar]
- 35.Blum D, Omlin A, Fearon K, et al. Evolving classification systems for cancer cachexia: ready for clinical practice? Support Care Cancer. 18(3):273–9. doi: 10.1007/s00520-009-0800-6. [DOI] [PubMed] [Google Scholar]
- 36.Ockenga J, Valentini L. Review article: anorexia and cachexia in gastrointestinal cancer. Aliment Pharmacol Ther. 2005;22(7):58394. doi: 10.1111/j.1365-2036.2005.02628.x. [DOI] [PubMed] [Google Scholar]
- 37.Fouladiun M, Korner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care—correlations with food intake, metabolism, exercise capacity, and hormones. Cancer. 2005;103(10):2189–98. doi: 10.1002/cncr.21013. [DOI] [PubMed] [Google Scholar]
- 38.Goodpaster BH, Park SW, Hams TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61(10):1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 39.Park SW, Goodpaster BH, Lee JS, et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care. 2009;32(11):1993–7. doi: 10.2337/dc09-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park SW, Goodpaster BH, Strotmeyer ES, et al. Accelerated loss of skeletal muscle strength in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes Care. 2007;30(6):1507–12. doi: 10.2337/dc06-2537. [DOI] [PubMed] [Google Scholar]
- 41.Baracos VE, Reiman T, Mourtzakis M, Gioulbasanis I, Antoun S. Body composition in patients with non-small cell lung cancer: a contemporary view of cancer cachexia with the use of computed tomography image analysis. The American journal of clinical nutrition. 2010;91(4):1133S–37S. doi: 10.3945/ajcn.2010.28608C. [DOI] [PubMed] [Google Scholar]