Abstract
Purpose
To develop a model to assess the quality of an IMRT treatment plan using data of prior patients with pancreatic adenocarcinoma.
Methods
The dose to an organ at risk (OAR) depends in large part on its orientation and distance to the planning target volume (PTV). A database of 33 previously treated patients with pancreatic cancer was queried to find patients with less favorable PTV-OAR configuration than a new case. The minimal achieved dose among the selected patients should also be achievable for the OAR of the new case. This way the achievable doses to the OARs of 25 randomly selected pancreas cancer patients were predicted. The patients were replanned to verify if the predicted dose could be achieved. The new plans were compared to their original clinical plans.
Results
The predicted doses were achieved within 1 and 2 Gy for more than 82% and 94% of the patients, respectively, and were a good approximation of the minimal achievable doses. The improvement after replanning was 1.4 Gy (range 0–4.6 Gy) and 1.7 Gy (range 0–6.3 Gy) for the mean dose to the liver and the kidneys, respectively, without compromising target coverage or increasing radiation dose to the bowel. cord or stomach.
Conclusions
The model could accurately predict the achievable doses, leading to a considerable decrease in dose to the OARs and an increase in treatment planning efficiency.
Keywords: Pancreatic cancer, Intensity modulated radiotherapy (IMRT), Treatment plan prediction and optimization, Overlap volume histogram (OVH), Pancreas, Quality control
There has been a growing interest in radiotherapy, and particularly intensity modulated radiation therapy (IMRT), to deliver more conformal dose distributions to unresectable pancreatic tumors while limiting dose to organs at risk (OARs) [1,2]. In some cases IMRT in combination with chemotherapy can be used to down-stage tumors to increase the likelihood of subsequent surgical resection [3,4]. IMRT may also allow for dose escalation of pancreatic tumors while minimizing toxicity. Due to the low dose tolerance of the OARs in the abdomen, the tumor dose for these patients is restricted to 50–55 Gy in 1.8–2.0 Gy fractions when combined with chemotherapy [5], As a result local tumor control is poor, causing symptoms such as pain, bleeding and gastrointestinal ulceration [6].
With IMRT more conformal dose distributions can be generated than with conventional techniques, allowing less dose to the OARs without compromising target coverage [7]. However, due to the iterative nature of the IMRT planning process, dosimetrists manually optimize treatment objectives repeatedly to achieve a satisfactory dose distribution. Even for the most experienced treatment planner, it is very difficult to judge when a treatment plan cannot be further optimized in terms of OAR sparing. As a result, suboptimal plans may be inadvertently approved or dosimetrists’ may waste time trying to optimize a plan that is already maximally optimized. If the treatment planners were aware of the achievable degree of OAR sparing initially, plans could be generated in a more efficient fashion, potentially resulting in less toxicity and without delaying a patient’s treatment. Further, the treatment planner may be able to increase the dose to the tumor to more than the 50–55 Gy that is currently delivered once the achievable OARs doses have been achieved by up scaling the entire dose distribution to the maximum allowable OAR dose levels. This strategy therefore enhances the opportunity for both less normal tissue complications and a higher tumor control probability.
A number of methods have been proposed to give the dosimetrist insight into the lowest achievable OAR dose while maintaining adequate target coverage, such as lexicographic ordering [8] and other forms of multi-criteria optimization (MCO) [9,10]. Also anatomical data and treatment plans of previously treated patients can be used to predict what the achievable dose to an OAR can be for a new patient [11-13]. This is based on the observation that the minimal achievable dose to an OAR depends to a large extent on its distance and orientation to the planning target volume(s) (PTVs). Wu et al. have demonstrated this principle on head-and-neck cancer patients by lowering the parotid dose after implementing this strategy and replanning to achieve the predicted dose levels [12].
This method has led to excellent results in head-and-neck cancer patients, where all patients are treated with the same beam configuration. But this optimization is less likely to perform well for treatment sites in the abdomen such as pancreatic cancers. In contrast to head-and-neck cancer plans, in the abdomen, the OARs are larger compared to the tumor. Also parts of the OAR can be located superior and/or inferior to the treatment fields. As such, the assumption that the dose in a point depends mainly on the distance to the PTV is no longer valid. It is further unclear if this method performs well if the beam configurations vary among different patients.
The goal of the current study was to extend the method proposed by Wu et al. to predict the achievable dose to the OARs in the abdomen with IMRT without compromising PTV coverage. We focused on pancreatic cancer patients because this patient population may directly profit from improved treatment planning to allow dose escalation to the tumor while lowering or holding steady the dose to the OARs. A database of 33 previously treated pancreatic cancer patients was used. For 25 of these patients, selected at random, achievable OAR constraints were predicted using geometric and dosimetric data of the patients in the database. Then the patients were replanned to verify (1) if the predicted doses could have been achieved and (2) if the predicted dose was a good approximation of the minimal achievable dose. The new plans were compared to the original clinical plans to estimate the expected clinical gains of this technique.
Materials and methods
Patient population and treatment
A retrospective analysis was performed using the clinical IMRT treatment plans of 33 pancreatic cancer patients treated at Johns Hopkins University, Department of Radiation Oncology, between 2008 and 2010. Included were patients with tumors in the tail, head and body of the pancreas. The majority of the patients had tumors classified as resectable and were treated with upfront surgery followed by combined chemotherapy and radiation (45–54 Gy in 1.8 Gy fractions). Most patients received either 5-FU or gemcitabine-based chemoradiation. Following chemoradiation, patients were given an additional 4 months of gemcitabine. Patients with unresectable pancreatic cancer also received 5-FU or gemcitabine-based chemoradiation (45–54 Gy).
For resectable patients, the tumor bed was delineated from the pre-surgery diagnostic CT scans. The tumor bed, anastomoses, and adjacent lymph nodes (celiac, portocaval, peri-aortic) were added to the CTV, which was expanded another 1–2 cm to account for patient set-up error and breathing motion (PTV). After 45 Gy to the initial PTV, an additional 5.4–9.0 Gy was delivered to a cone down field (PTVCD) which included the tumor bed and adjacent anastomoses plus a 1–2 cm margin.
For unresectable patients, the GTV was defined as the gross tumor volume on the CT simulation scan. The diagnostic CT scan was used to assist in contouring the GTV. The GTV was expanded another 0.5–1.0 cm for microscopic extension (CTV) and another 0.5–1.5 cm for patient set-up error and breathing motion (PTV). For unresectable plans, the lymph node regions were not specifically targeted, but often the peripancreatic lymph nodes were covered in the PTV.
The original radiotherapy treatment plans were generated with Pinnacle v8.0 or v9.0 by experienced dosimetrists. At the time of treatment planning, the liver, kidneys and spinal cord were considered as the organs at risk (OAR). The following dose constraints were used: the mean liver dose must be below 30 Gy; the dose to at least 2/3 of 1 kidney must be below 18 Gy; and the maximum dose to the spinal cord must be below 45 Gy. The number of treatment beams varied from 5 to 9 and some patients also received a single non-coplanar beam. The energy of the photon beams was 15 megavolt (MV) and the total number of segments varied from 40 to 180. The dosimetrists first generated the initial treatment plan and then used the same beam configuration to generate the cone down plan. To generate the initial plan the original dose constraints were multiplied with the number of fractions of the initial plan divided by the total number of fractions. Because the PTVCD was always fully encompassed and smaller than the PTVinitial, this ensured that the original dose constraints were satisfied for the total dose distribution, i.e. initial plan plus cone down plan. This study focuses solely on improving the initial plan for both resectable and unresectable plans (45 Gy in 1.8 Gy fractions). Applying it afterward to the cone down plan is straightforward.
The overlap volume histogram (OVH)
An important parameter that influences the dose to OARs is the proximity to, or the overlap with, the PTV and the orientation of the OAR compared to the PTV. The overlap volume histogram (OVH) was introduced recently to describe this relationship [11,12]. The OVH(r) is a one dimensional function that describes the fraction of the OAR volume that is encompassed by a uniform expansion or contraction of the PTV by a distancer. This is schematically represented in Fig. 1. The shape of the OVH contains information about the ability to spare the OAR. If the expansion distance needed to reach a certain overlap is smaller for one patient than for another, the OAR of the first patient should be more difficult to spare than the OAR of the second. Stated otherwise, if the same expansion distance applied to two patients results in a greater overlap of the OAR for one of the patients, it will naturally be more difficult to spare the given OAR.
Fig. 1.
The overlap volume histogram (OVH) is constructed by calculating the overlap between the organ at risk (OAR) and the expanded/contracted PTV for each expansion distance as proposed by Kazhadan et al. [11]. In this study. the OVH was also calculated for the part of the OAR that lies within the treatment fields (OARfields). approximated by the 10% isodose line of a conformal plan with equal beam weights. The OVHs of both the entire OAR and of the OARfields, are shown as function of the expansion distance in arbitrary units.
It has been shown that the OVH is an effective tool to find achievable dose constraints for head-and-neck cancer patients [12]. but for treatment sites in the abdomen, the OVH has a potential weakness. Because the OARs are larger with respect to the PTV and because the length of the treatment fields is smaller in the cranial-caudal direction compared to head-and-neck, some organs, such as the liver and stomach, may be positioned to a large extent superior or inferior to the treatment fields. When this occurs, the usefulness of the OVH is limited because an organ may receive a low dose to a large part of its volume even though it can be very close to the PTV. If the same organ of a different patient is more distal to the PTV, but fully encompassed by the treatment fields, the predicted dose cannot be achieved. Even though the organ of the second patient is located further away from the PTV than that of the first, the organ of the second patient is more difficult to spare.
In the current study, we accounted for this effect by analyzing the part of the OAR that was within the treatment fields (OARfields) instead of the entire OAR as shown in Fig. 1. The OARfields was generated by setting the multi-leaf collimators (MLCs) of each beam to the beams-eye-view of the PTV expanded with a 1 cm margin. The weights of all beams were set to 1 and the OARfields was defined as the part of the OAR that was within the 10% isodose line of the resulting dose distribution. We chose the 10% to take into account scattered radiation and the penumbra of the beams. The 1 cm margin was used to account for small differences between the beam aperture of this forward plan and of the final IMRT plan. One centimeter corresponds to the width of a single leaf in the isocenter of most commercially available MLCs. The OVH (OVHfield) and DVH (DVHfields) of the OARfields, were then calculated for the original plan of each patient.
Predicting the achievable dose to OARs and replanning
Achievable dose-volume objectives (Le., points on the DVH curve) were predicted by searching in the database of all prior patients for patients where the OARfields, was more difficult to spare (i.e., which required a smaller PTV expansion distance to cover the same volume). The minimum achieved dose among the selected patients was determined and used as approximation of the lowest achievable dose to the OAR of the new patient. This procedure was performed for each of the 25 patients, selected randomly from the database. For each dose-volume objective of each OAR, an achievable dose was predicted. The volumes of the dose-volume objectives were defined with respect to the entire OAR and rescaled by the volume of the OARfields, to yield the corresponding relative volume of the OARfields. The achievability of the predicted dose-volume constraints was tested by generating a new treatment plan with the original beam configuration (i.e., number of beams, beam angles, number of segments and minimum number of monitoring units per segment) for each patient with the goal of achieving or surpassing the predicted dose-volume objectives.
OARs and treatment planning constraints
According to the latest RTOG pancreas protocol (RTOG 0848), the kidneys, liver, spinal cord, small intestine and the stomach are considered organs at risk. The mean dose to the bilateral kidneys must be <18 Gy, the mean dose to the liver must be <25 Gy and the maximum dose to the spinal cord is 45 Gy. The maximum dose to the stomach and bowel must be <54 Gy. Ninety percent of both organs must receive less than 50 Gy and 85% <45 Gy. Ninety percent of the PTV and 99% of the CIV are to receive 95% of the prescribed dose.
The 45 Gy dose limit to the spinal cord is on the flat region of the dose response curve and lowering the doses further will lead to an imperceptible decrease in the incidence of myelopathy [14]. Therefore, reducing the dose to the spinal cord to values lower than the dose constraint is not expected to substantially improve treatment. No dose constraints were applied to the stomach and bowel at the time the clinical treatment plans were generated. The database is thus not expected to contain optimized DVHs for both organs. Therefore the present study focuses on the kidneys and liver. The entire DVHs of the PTV, stomach, bowel and spinal cord were constrained to the DVHs of the original plan. A lower dose to the stomach, bowel and spinal cord was accepted; a higher dose was not.
Because not all treatment planning systems are capable of using constraints on the mean dose directly, we instead chose to constrain the mean organ dose by using multiple dose-volume objectives for the liver and kidneys. For the kidneys, the maximum doses to 25%, 50% and 75% of the volume were used. For the liver these volumes were 25%, 50% and 65%. The percentages refer to the entire OAR volume and not to the volume of the OARfields. The maximum value of the liver was smaller than for the kidneys because, for most patients, a large part of the liver was outside the treatment fields.
Results
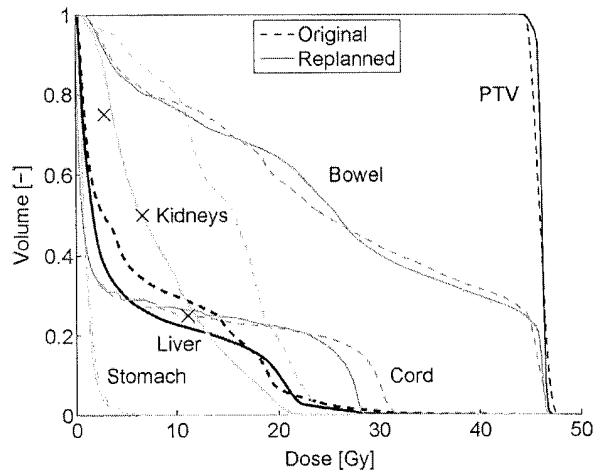
An overview of the patient and treatment characteristics is shown in Table 1. All of the initial clinical plans satisfied all of the dose constraints used at the time of planning. For 25 patients, the achievable doses at the volume constraints were predicted. New treatment plans were generated with the goal of achieving or surpassing these constraints. After replanning, the DVHs of the PTV, bowel, cord and stomach were similar to those from the original plans (Table 2). Fig. 2 shows for one of the patients the DVHs of the original and the replanned treatment plans and the predicted achievable doses. The predicted doses for the kidneys were more than 7.5 Gy lower than the original doses. After replanning, the achieved doses differed by less than 0.82 Gy from the predicted doses.
Table 1.
Patient and treatment characteristics.
| Patient | Resectable? | Location in pancreas | Planning target volume (cm3) | Number of beams | Replanned |
|---|---|---|---|---|---|
| 1 | Resectable | Head | 710 | 7 | Yes |
| 2 | Unresectable | Head | 459 | 7 | Yes |
| 3 | Resectable | Head | 754 | 7 | Yes |
| 4 | Resectable | Body and head | 735 | 7 | Yes |
| 5 | Resectable | Head | 691 | 5 | Yes |
| 6 | Resectable | Head | 896 | 5 | Yes |
| 7 | Resectable | Head | 463 | 5 | Yes |
| 8 | Resectable | Head | 831 | 5 | Yes |
| 9 | Resectable | Head | 841 | 6 | Yes |
| 10 | Resectable | Head | 669 | 6 | Yes |
| 11 | Resectable | Body and tail | 404 | 6 | Yes |
| 12 | Resectable | Ampulla | 960 | 5 | Yes |
| 13 | Resectable | Head | 486 | 6 | Yes |
| 14 | Resectable | Head | 944 | 7 | Yes |
| 15 | Resectable | Head | 496 | 7 | Yes |
| 16 | Unresectable | Head | 284 | 5 | Yes |
| 17 | Resectable | Tail | 1413 | 6 | Yes |
| 18 | Resectable | Head | 568 | 6 | Yes |
| 19 | Resectable | Tumor overlaps boundaries pancreas | 1035 | 6 | Yes |
| 20 | Resectable | Head | 522 | 6 | Yes |
| 21 | Resectable | Head | 843 | 7 | Yes |
| 22 | Unresectable | Body | 680 | 7 | Yes |
| 25 | Resectable | Body and tail | 1158 | 6 | Yes |
| 29 | Resectable | Head | 609 | 9 | No |
| 31 | Resectable | Head | 1637 | 6 | No |
| 23 | Resectable | Head | 315 | 6 | No |
| 24 | Resectable | Head | 349 | 6 | No |
| 26 | Unresectable | Body | 777 | 9 | No |
| 27 | Unresectable | Head | 1527 | 6 | No |
| 28 | Resectable | Head | 273 | 6 | No |
| 30 | Unresectable | Tail | 1609 | 6 | No |
| 32 | Unresectable | Body | 486 | 8 | No |
| 33 | Unresectable | Body | 330 | 6 | No |
Table 2.
Mean and range of the differences in dose at the dose-volume constraints between the replanned and original plan for the planning target volume (PTV), the stomach, bowel and spinal cord. Negative values indicate that the replanned dose was lower than the original dose.
| Volume constraint | Planing target volume (PTV) |
Stomach |
Bowel |
Cord | |||||
|---|---|---|---|---|---|---|---|---|---|
| 97% | Max | 15% | 10% | Max | 15% | 10% | Max | Max | |
| Mean | −0.16 | −0.18 | −0.63 | −0.48 | −0.47 | −0.39 | −0.24 | −0.33 | −0.74 |
| Range | −0.42, 0.39 | −1.01, 56 | −2.39, 0.93 | −1.78, 0.71 | −2.68, 0.32 | −2.73, 1.09 | −1.63, 0.45 | −1.27, 0.32 | −3.37, 0.03 |
Fig. 2.
The DVH of the original and replanned plans of one of the patients. After replanning the dose to the liver and kidneys is considerably decreased without increasing the dose to any of the other organs and without compromising PTV coverage. The crosses indicate the predicted dose values.
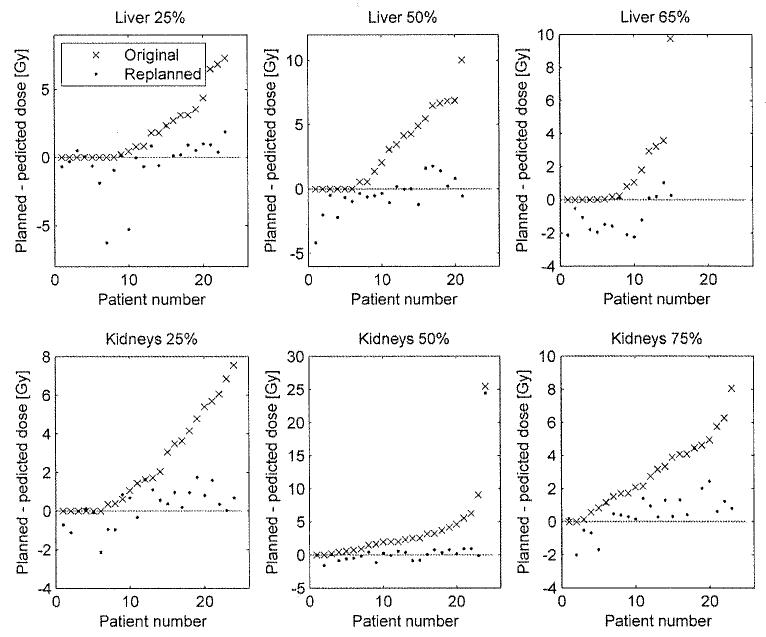
Fig. 3 shows, for all patients, the difference between the predicted dose and the original dose and between the predicted and replanned dose for the volume constraints of the liver and kidneys. For the liver the predicted dose was achievable within 1 Gy for 90% of the cases and for 98% of the cases within 2 Gy. For the kidneys the percentages were 82% within 1 Gy and 94% within 2 Gy. The predictions were thus achievable in most of the cases.
Fig. 3.
The difference between the planned and predicted dose for the original and the replanned plan at the different dose-volume constraints. The top and bottom rows show the three dose constraints on the liver and kidneys, respectively. A positive difference indicates that the planned dose was higher than the predicted dose. The distance between the bullets and dotted line represents the accuracy of the prediction. The distance between the bullets and crosses represents the relevance of the model. For some patients the difference between the original plan and the prediction was equal to zero. In these cases, the best prediction was based on the original plan of that patient. For clarity the results are sorted based on the results of the original plans.
For the liver, in 14% of the cases the replanned dose was more than 2 Gy lower than the predicted dose and for the kidneys only in 3% of the cases. This suggests that the prediction was a good approximation of the lowest achievable dose for the liver and kidneys for the majority of the patients.
Compared to the original plan, the mean liver dose decreased on average by 1.4 Gy (range 0–4.6 Gy) and the mean kidney dose decreased 1.7 Gy (range 0–6.3 Gy) after replanning. This demonstrates the relevance of this model for organ sparing.
The percentage of times a relevant prior patient was found in the database was 79% for the liver and 94% for the kidneys. The total number of prior patients that were selected at least once as the best candidate for any of the dose constraints was 21. For the individual constraints the numbers were 13 (25%, liver), 10 (50%, liver), 8 (65%, liver), 7 (25%, kidneys), 4 (50%, kidneys) and 4 (75%, kidneys).
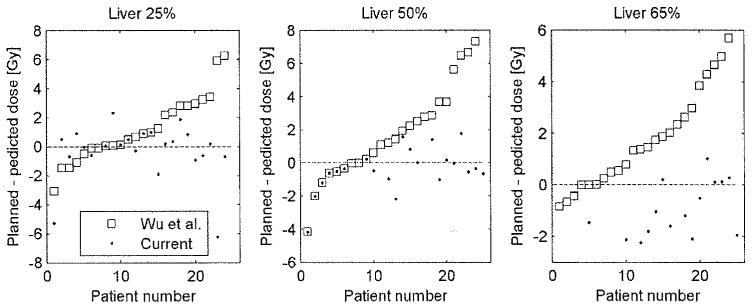
The average volume of the liver within the treatment fields was 67% (range 21%–94%). For the patients with less than 65% of the liver within the treatment fields, no prediction was generated for the 65% volume constraint, because the OVH comparison considers only the part of the OAR within the treatment fields. The combined kidney volume was almost entirely encompassed by the fields (mean 93%, range 53%–100%). The importance of considering the part of the OARs within the treatment fields is demonstrated in Fig. 4, where the current method is compared to the original method, proposed by Wu et al. The differences between the predicted and replanned doses of the liver are shown. Wu et al.’s method demonstrated that for 40% of the cases, the achieved dose was more than 2 Gy larger than the predicted dose compared to only 5% with the current method.
Fig. 4.
The results of the current method are compared with the method proposed by Wu et al., which does not take into account that large parts of the liver may not be encompassed by the treatment beams. The data is sorted based on the results of the Wu et al. method. The current method clearly results in more accurate predictions and especially less overestimations of the achievable dose.
The 10% isodose boundary of a 3D conformal plan exposing the PTV with equal beam weights was used to approximate the parts of the organs that were encompassed by the IMRT plans. The validity of this approach was verified by calculating the maximum dose to the part of the liver that was outside the treatment beams after replanning. The average maximum dose was 5.6 Gy (range 3.1–12.0), which corresponded to 12% of the prescribed dose. This indicates that the OARfields also corresponded to the part of the organ within the fields in the new treatment plans.
Discussion
A method was proposed to predict the achievable degree of organ sparing with IMRT for the liver and kidneys of pancreatic cancer patients. It was demonstrated that the predicted doses were achievable within 2 Gy for >94% of the patients and that they were a good approximation of the best achievable dose. This method can indicate if a treatment plan of a particular patient is maximally optimized as compared to the best plans of previously treated patients. Therefore it allows improved organ sparing and it can increase the efficiency of the treatment planning process.
For the patients included in the current study, the method would have resulted in a lower dose to the kidneys and liver without decreasing the dose to the PTV or increasing the dose to the spinal cord, bowel or stomach. As an alternative, this method allows the planner to increase the dose to the PTV without increasing the dose to the liver and kidneys. In fact, the decrease in liver and kidney dose was translatable to an increase in PTV dose by an average of 5 Gy (range 0–13 Gy) and 8.5 Gy (range 0–38 Gy) based on the liver and kidneys, respectively. Although in practice, these numbers are only achievable when the corresponding increase in dose to the cord, stomach and bowel is also acceptable.
Only 21 of 33 (63%) plans in the database were selected at least once as the lowest achievable dose. This indicates that the database does not need to contain hundreds of patients to find accurate predictions; rather, a few dozen patients with treatments plans of high quality can be sufficient. In 3–14% of the patients, depending on the organ, the current database overestimated the achievable dose (i.e., the achievable dose was lower than the predicted). Although this would not have posed a clinical problem (the predicted dose was achievable and lower than the original dose), it indicates that the database was not diverse enough to capture the space of variability.
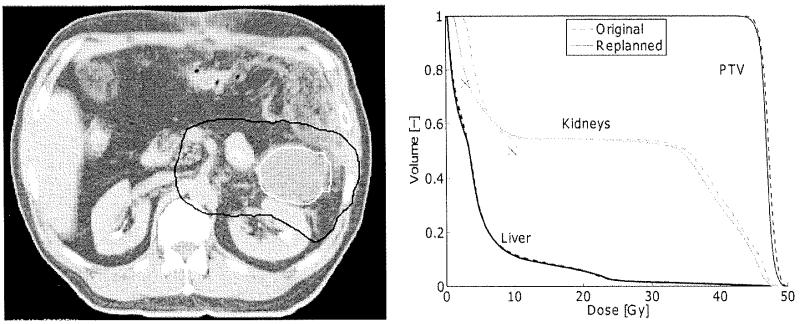
For only one patient, the predicted dose of any of the volume constraints was more than 3 Gy lower than the achievable dose. For all other patients, the PTV was located centrally at approximately the same distance from both kidneys. For this patient, however, the tumor bordered the left kidney and was located far away from the right kidney (Fig. 5). This is commonly seen in pancreatic tail lesions. This demonstrated that, as anatomical variation increases, accuracy is sacrificed, as is to be expected. If we had considered both kidneys separately, this discrepancy would not have occurred for this particular patient. As a matter of fact, no predictions would have been generated for the left kidney because none of the patients in the database had a kidney located closer to the PTV.
Fig. 5.
Left: A CT slice of the patient for which the difference between the replanned and predicted dose was more than 25 Gy. The GTV (white) and PTV (black) are indicated. Right: the DVH of the original and replanned plan. The predicted dose constraints for the kidneys are indicated by the crosses. The right kidney was positioned so close to the GTV that no patient existed in the database for which the dose at the 25% volume constraint was more difficult to achieve.
The predicted dose at the volume constraints depends on the quality of the treatment plans of the prior patients. One way to improve the database is to apply the method to new patients and add them to the database once the plans are complete and approved. To ensure that the database improves over time, it is advisable to aim for slightly lower doses at the volume constraints for the OARs than are predicted.
The numbers of beams and the beam angles varied considerably among the patients in the database. For each patient the beam configuration of the original plan was also used for replanning. Therefore it was expected that the predicted dose could not be achieved when the beam configuration of a patient was less favorable than the beam configuration of the patient that was used for the prediction. However, this effect proved to be small. A possible explanation is that the treatment planners had selected initially for each original plan a sufficiently good set of beams and beam parameters.
The achievable dose was predicted for each organ separately. Therefore it was expected that for some patients the tradeoff between sparing one organ vs. another would not allow achieving the predicted dose levels of both organs simultaneously. However this was not the case. This indicates that the treatment planners attempted to spare the liver and the kidneys simultaneously for the original plans of all patients without sacrificing either of the two.
Another approach that can give the treatment planner insight into the lowest achievable doses to the OARs was proposed by Halabi [15]. This multi-criteria optimization (MCO) approach calculates multiple treatment plans that are all optimal with respect to one dose constraint/objective. The user can navigate through the plans and learn the tradeoffs between sparing different OARs. This is not possible with the method proposed here. An advantage of the current method is that it is simple yet robust, it can be easily implemented in any clinic independent of the treatment planning system and the predictions reflect how different tradeoffs in treatment plan quality were dealt with in the past. The achievable dose values are predicted within seconds.
Because the current method is suitable for the pancreas, it is expected that it can also be used for other treatment sites in the abdomen and the pelvis (prostate) but this has to be confirmed with a follow-up study. Predicting achievable dose constraints can also allow the automation of the treatment planning process as was demonstrated successfully with a study at Johns Hopkins University for head-and-neck tumors [16]. Another application may be the comparison between treatment planning in different institutions or with different techniques, e.g., IMRT vs. arc therapy. Achievable treatment plans could be predicted for multiple techniques, allowing for an objective decision of which technique to use for a particular patient without actually going through the treatment planning process.
Conclusion
In this study we have proposed a method to predict achievable radiation doses to OARs of pancreatic cancer patients with IMRT. The predictions are based on data of prior patients. We have shown that the predicted doses indeed can be achieved for the vast majority of the patients and that they are a good approximation of the lowest achievable doses. This method can improve the logistics of the treatment planning process and yield treatment plans with less radiation exposure to the OARs. As an alternative this could be translated into an increase in tumor dose without increased normal tissue toxicity.
Acknowledgments
The authors would like to acknowledge financial support from the Pinnacle Student Award (S.P.), the Dutch Cancer Society (S.P.), Philips Radiation Oncology Systems (S.P.+B.W.+T.McN.) and Paul Maritz (B.W.), that made this research possible.
Footnotes
Conflict of interest
None.
References
- [1].Ben-Josef E, Shields AF, Vaishampayan U, et al. Intensity-modulated radiotherapy (IMRT) and concurrent capecitabine for pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;59:454–9. doi: 10.1016/j.ijrobp.2003.11.019. [DOI] [PubMed] [Google Scholar]
- [2].Ruano-Ravina A, Almazan Ortega R, Guedea F. Intraoperative radiotherapy in pancreatic cancer: a systematic review. Radiother Oncoi. 2008;87:318–25. doi: 10.1016/j.radonc.2007.12.002. [DOI] [PubMed] [Google Scholar]
- [3].Tempero MA, Arnoietti JP, Behrman S, et al. Pancreatic adenocarcinoma. J Natl Compr Canc Netw. 2010;8:972–1017. doi: 10.6004/jnccn.2010.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Valentini V, Calvo F, Reni M, et al. Intra-operative radiotherapy (IORT) in pancreatic cancer: joint analysis of the ISIORT-Europe experience. Radiother Oncol. 2009;91:54–9. doi: 10.1016/j.radonc.2008.07.020. [DOI] [PubMed] [Google Scholar]
- [5].Brunner TB, Scott-Brown M. The role of radiotherapy in multimodal treatment of pancreatic carcinoma. Radiat Oncol. 2010;5:64. doi: 10.1186/1748-717X-5-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ben-Josef E, Lawrence TS. Chemoradiotherapy for unresectable pancreatic cancer. Int J Clin Oncol. 2008;13:121–6. doi: 10.1007/s10147-007-0763-x. [DOI] [PubMed] [Google Scholar]
- [7].Zelefsky MJ, Fuks Z, Happersett L, et al. Clinical experience with intensity modulated radiation therapy (IMRT) in prostate cancer. Radiother Oncol. 2000;55:241–9. doi: 10.1016/s0167-8140(99)00100-0. [DOI] [PubMed] [Google Scholar]
- [8].Jee KW, McShan DL, Fraass BA. Lexicographic ordering: intuitive multicriteria optimization for IMRT. Phys Med Biol. 2007;52:1845–61. doi: 10.1088/0031-9155/52/7/006. [DOI] [PubMed] [Google Scholar]
- [9].Spalke T, Craft D, Bortfeld T. Analyzing the main trade-offs in multiobjective radiation therapy treatment planning databases. Phys Med Biol. 2009;54:3741–54. doi: 10.1088/0031-9155/54/12/009. [DOI] [PubMed] [Google Scholar]
- [10].Thieke C, Kufer KH, Monz M, et al. A new concept for interactive radiotherapy planning with multicriteria optimization: first clinical evaluation. Radiother Oncol. 2007;85:292–8. doi: 10.1016/j.radonc.2007.06.020. [DOI] [PubMed] [Google Scholar]
- [11].Kazhdan M, Simari P, McNutt T, et al. A shape relationship descriptor for radiation therapy planning. Med Image Comput Comput Assist Interv. 2009;12:100–8. doi: 10.1007/978-3-642-04271-3_13. [DOI] [PubMed] [Google Scholar]
- [12].Wu B, Ricchetti F, Sanguineti G, et al. Patient geometry-driven information retrieval for IMRT treatment plan quality control. Med Phys. 2009;36:5497–505. doi: 10.1118/1.3253464. [DOI] [PubMed] [Google Scholar]
- [13].Hunt MA, Jackson A, Narayana A, et al. Geometric factors influencing dosimetric sparing of the parotid glands using IMRT. Int J Radiat Oncol Biol Phys. 2006;66:296–304. doi: 10.1016/j.ijrobp.2006.05.028. [DOI] [PubMed] [Google Scholar]
- [14].Schultheiss TE, Kun LE, Ang KK, et al. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31:1093–112. doi: 10.1016/0360-3016(94)00655-5. [DOI] [PubMed] [Google Scholar]
- [15].Halabi T, Craft D, Bortfeld T. Dose-volume objectives in multi-criteria optimization. Phys Med Biol. 2006;51:3809–18. doi: 10.1088/0031-9155/51/15/014. [DOI] [PubMed] [Google Scholar]
- [16].Wu B, Ricchetti F, Sanguineti G, et al. Data-driven approach to generating achievable dose-volume histogram objectives in intensity-modulated radiotherapy planning. Int J Radiat Oncol Biol Phys. 2011;79:1241–7. doi: 10.1016/j.ijrobp.2010.05.026. [DOI] [PubMed] [Google Scholar]