Abstract
Background and Objectives
Following resection of pancreatic adenocarcinoma, tumor size has been considered a key prognostic feature; however, this remains controversial. We sought to examine the association of size with outcomes following resection of pancreatic adenocarcinoma.
Methods
Between 1970 and 2010, 1,697 patients with pancreatic adenocarcinoma at the Johns Hopkins Hospital underwent curative intent pancreaticoduodenectomy. Prognostic factors were identified by univariate and multivariate analyses.
Results
Of 1,697 patients, tumor size was ≤2 cm in 418 (24.6%) patients, 2–5 cm in 1,070 (63.1%) patients, and ≥5 cm in 209 (12.3%) patients. On univariate analyses, 5-year survival was inversely proportional to tumor size (≤2 cm: 28.8% vs. 2–5 cm: 19.4% vs. ≥5 cm: 14.2%: P < 0.001). Size correlated with the risk of other adverse factors, with larger tumors being more likely to be associated with nodal disease and poor differentiation (both P < 0.05). On multivariate analysis, the 2 cm cut-off was not associated with survival, while nodal disease (HR = 1.59; P = 0.006) and poor differentiation (HR = 1.59; P = 0.04) remained predictive of outcome, regardless of size.
Conclusion
The cut-off value of 2 cm is not independently associated with outcome, however, tumor size was strongly associated with the risk of other adverse prognostic factors. The effect of size on prognosis was largely attributable to these other biologic factors rather than tumor size itself.
Keywords: pancreas adenocarcinoma, tumor size, prognosis
INTRODUCTION
With over 40,000 patients diagnosed and approximately 35,000 attributable deaths on an annual basis, pancreatic cancer is the fourth most common cause of cancer-related death in the United States [1,2], Without surgery, few—if any—patient with pancreatic cancer will survive to 5 years and median survival is 4–6 months [3-5]. While surgical resection remains the only chance at cure, the prognosis following surgical resection of pancreatic adenocarcinoma remains poor, with median survival ranging from 11.9 to 22.0 months [6-11] and 5-year overall survival ranging from 12% to 21% [6,7,9,10].
Several factors have been noted to impact survival follow surgical resection of pancreatic adenocarcinoma. Specifically, lymph node status, peri-neural/vascular invasion, histological tumor grade, and margin status have all been reported to affect outcome [4,10,12-16], Tumor size has also been considered a key prognostic feature, as noted by its incorporation into the American Joint Committee on Cancer (AJCC) staging system for pancreatic cancer [17], In fact, in the AJCC staging system the difference between a T1 and T2 tumor is defined solely by the size of the tumor (≤2 cm vs. >2 cm). The impact of tumor size on outcome, however, remains controversial. Several studies have noted no association between tumor size and long-term survival [16,18-20], while other studies have noted that patients with smaller pancreatic lesions had abetter overall survival [8-10,21], Most previous studies, however, have analyzed a heterogeneous array of patient and tumor factors and few [22] have specifically examined the prognostic effect of tumor size. In the current study, we sought to examine the association of tumor size on peri-operative outcomes among patients with pancreatic head lesions, as well as the long-term survival following resection of pancreatic adenocarcinoma. We also examined how tumor size was associated with the presence of other pathologic findings, as well as performed stratified analyses to assess the impact of tumor size relative to these other tumor characteristics.
METHODS
Prospective data on patients who underwent pancreaticoduodenectomy for pancreatic adenocarcinoma from 1970 to 2010 were identified from the Johns Hopkins Hospital institutional database. Patients who were operated on with palliative intent (e.g., palliative bypass), as well as patients who had less than 6 months of follow-up, were excluded from consideration. The remaining 1,697 patients who underwent curative intent surgery for pancreatic adenocarcinoma are the focus of this study. The study was approved by the Johns Hopkins Hospital Institutional Review Board.
Prior to surgery, all patients were evaluated with a baseline history and physical exam, serum laboratory tests, and imaging studies (e.g., computed tomography or magnetic resonance imaging scan of the abdomen and pelvis) at the discretion of the treating surgeon. Following surgery, all patients were regularly followed and prospectively monitored.
Data Collection
Standard demographic characteristics, as well as intra-operative, pathologic, and outcome data, were collected. Operative details including type of operation and operative blood loss were recorded. Peri-operative morbidity and complications were also noted. Peri-operative mortality was defined as death within 30 days of surgery. Pathologic tumor characteristics were recorded including, tumor location, histological differentiation, presence of peri-neural or vascular invasion, margin status, lymph node status, and tumor size. Tumor size was determined based on measurements taken from the pathologic specimen. Tumor-node metastasis (TNM) staging was performed according to the 7th Edition AJCC Staging system [17]. Date of last follow-up and vital status were recorded.
Statistical Analyses
Summary statistics were obtained using established methods and presented as percentages or median values. Survival was estimated using Kaplan–Meier cumulative survival estimates [23], with survival calculated from the time of pancreatic surgery. Differences in survival were examined using the log-rank test. Factors associated with survival were examined using univariate and multivariate Cox regression analyses. The hazard ratio and the 95% confidence intervals (CI) were estimated and a P-value <0.05 was considered significant. For purposes of analyses, tumor size was analyzed both as a continuous and a categorical variable. Specifically, tumor size was categorized using the cut-offs, ≤2, 2–5, and ≥5 cm. The cut-off of 2 cm is designated by the AJCC T classification [17], All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 17.0 (Chicago, IL).
RESULTS
Overall Patient, Operative and Tumor Characteristics
Our selection criteria identified 1,697 patients who underwent pancreaticoduodenectomy for pancreatic adenocarcinoma (Table I). Overall, these patients had a median age of 67 years (range: 18–93). A majority of patients was male (n = 897; 52.9%). More patients underwent resection in recent years (1970–1989, 5.3%. vs. 1990–2007, 94.7%). At the time of surgery, most patients underwent a pylorus preserving pancreaticoduodenectomy (n = 1,138; 67.1 %), while fewer patients underwent a classic pancreaticoduodenectomy (n = 502; 29.6%); a small number of patients (n = 57; 3.3%) underwent a total pancreatectomy. The median duration of the operation was 380 min (range: 200–1,020). The median amount of blood loss during the operation was 800 ml (range: 50–18,000); with 486 (28.6%) patients receiving more than 2 U. Post-operatively, the overall median hospital stay was 10 days (range: 5–73); over the last decade (2000–2010), the median hospital stay had decreased (9 days; range: 3–65) (P < 0.001). The peri-operative complication rate was 47.3% (n = 803). Morbidity was mostly associated with surgical site infection (n = 112; 13.9%), pancreatic fistula (n = 108; 13.4%), or intra-abdominal abscess (n = 53; 6.6%). Twenty-seven patients died within 30 days of the operation for an overall peri-operative mortality rate of 1.6%.
TABLE I.
Patient and Operative Characteristics
| Patient characteristics (n = 1,697) |
|
|---|---|
| Variable | Number (%) |
| Age (median [range]) | 67 [18–93] |
| Gender, male | 897 (52.9) |
| Race, white | 1,498 (88.3) |
| Extent of surgery | |
| Classic pancreaticoduodenectomy | 502 (29.6) |
| Pylorus preserving pancreaticoduodenectomy | 1,138 (67.1) |
| Total pancreatectomy | 57 (3.4) |
| Adjuvant chemotherapy | 343 (24.5) |
| Adjuvant radiotherapy | 293 (20.9) |
Percentages exclude missing values.
On final pathologic assessment, overall median tumor size was 3.0 cm (range: 0.10–15.5). The majority of patients (n = 1,279; 75.4%) had a tumor >2 cm, while only 418 (24.6%) patients had a tumor ≤2 cm. Among those patients with a tumor ≤2 cm, 1,070 (83.7%) had a tumor measuring between 2 and 5 cm, while 209 (16.3%) had a tumor ≥5 cm (Table II). There was no change in tumor size over the time periods examined (median tumor size: 1970–1989, 3.0 cm vs. 1990–2007, 3.0 cm; P = 0.4). The majority (n = 985; 60.3%) of tumors were moderately to well-differentiated. With regard to pathologic staging, most patients had a T3 tumor (64.3%), while 35.7% had a T2 tumor. The median number of nodes evaluated was 17 (range: 1–94). Of the 1,697 patients, 1,280 (75.4%) had lymph node metastasis (Nl), while 410 (24.2%) did not (N0). The surgical margin was microscopically negative (R0) in 1,213 (71.8%) patients and microscopically or macroscopically positive in 476 (28.2%) patients.
TABLE II.
Patient, Tumor and Operative Characteristics, Stratified by Tumor Size
| Size of pancreatic lesion (n = 1,697) |
||||
|---|---|---|---|---|
| >2 cm (n = 1,279), number (%) |
||||
| Variable | ≤2 cm (n = 418), number (%) |
2–5 cm (n = 1,070) | ≥5 cm (n = 209) | p-value |
| Age (median [range]) | 66 [33–92] | 67 [20–93] | 67 [30–87] | 0.53 |
| Gender, male | 217 (51.9) | 542 (50.7) | 138 (66.0) | 0.66 |
| Race, white | 365 (87.3) | 938 (87.7) | 195 (93.3) | 0.49 |
| Extent of surgery | ||||
| Classic pancreaticoduodenectomy | 98 (23.4) | 327 (30.6) | 77 (36.8) | 0.002 |
| Pylorus preserving pancreaticoduodenectomy | 310 (74.2) | 716 (66.9) | 112 (53.6) | |
| Total pancreatectomy | 10 (2.4) | 27 (2.5) | 20 (9.6) | |
| Estimated blood loss, median [range] | 750 [15–11,500] | 750 [50–18,000] | 1,000 [200–15,000] | 0.04 |
| Receipt of >2 PRBCs during surgery | ||||
| Yes | 102 (50.7) | 260 (20.5) | 75 (37.7) | <0.001 |
| No | 99 (49.3) | 759 (74.5) | 124 (62.3) | |
| Operative time, minutes, median [range] | 370 [200–790] | 390 [230–1,020] | 425 [200–765] | <0.001 |
| AJCC T category | ||||
| T1/T2 | 226 (54.2) | 326 (30.7) | 56 (26.8) | <0.001 |
| T3/T4 | 191 (45.8) | 735 (69.3) | 153 (73.2) | |
| AJCC N category | ||||
| NO | 156 (37.4) | 208 (19.5) | 46 (22.0) | <0.001 |
| N1/N2 | 261 (62.6) | 856 (80.5) | 163 (88.0) | |
| Differentiation | ||||
| Poor | 129 (32.7) | 427 (40.9) | 93 (47.7) | 0.001 |
| Moderate/well | 265 (67.3) | 618 (59.1.) | 102 (52.3) | |
| Vascular invasion | ||||
| Yes | 124 (43.8) | 462 (51.1) | 100 (55.2) | 0.02 |
| No | 159 (56.2) | 442 (48.9) | 81 (44.8) | |
| Peri-neural invasion | ||||
| Yes | 218 (80.4) | 774 (91.5) | 134 (79.3) | 0.001 |
| No | 53 (19.6) | 72 (8.5) | 35 (20.7) | |
| Pre-operative CA 19-9 >100 mg/dl | ||||
| Yes | 56 (37.8) | 234 (63.2) | 39 (56.5) | <0.001 |
| No | 92 (62.2) | 136 (36.8) | 30 (43.5) | |
| Resection margin | ||||
| Positive | 83 (20.0) | 319 (29.9) | 74 (35.9) | <0.001 |
| Negative | 333 (80.0) | 748 (70.1) | 132 (64.1) | |
| Adjuvant chemotherapy | ||||
| Yes | 82 (22.5) | 231 (26.9) | 30 (17.1) | 0.50 |
| No | 282 (77.5) | 627 (73.1) | 145 (82.9) | |
| Adjuvant radiotherapy | ||||
| Yes | 93 (25.5) | 170 (19.9) | 30 (17.1) | 0.01 |
| No | 271 (74.5) | 686 (80.1) | 145 (82.9) | |
Percentages exclude missing values.
p-value reflect comparison between tumor size ≤2 cm versus >2 cm.
Impact of Tumor Size on Operative Details and Tumor Characteristics
Further stratified analyses focused on the impact of tumor size on operative outcomes and pathological tumor characteristics. Patients with tumors >2 cm were more likely to have undergone a classic pancreaticoduodenectomy versus a pylorus preserving procedure compared with patients who had tumors ≤2 cm (P = 0.002). In fact, of those patients with a tumor ≥5 cm, 36.8% (n = 77) underwent a classic pancreaticoduodenectomy compared with only 28.6% (n = 425) among patients with tumors <5 cm. The median amount of blood loss and the need for intra-operative blood transfusion was also higher among patients with larger tumors (Table II). Post-operatively, the incidence of peri-operative complications and post-operative deaths within 30 days of surgery were similar (morbidity: tumors ≤2 cm, 45.6% vs. tumors >2 cm, 50.9%; 30 mortality: tumors ≤2 cm, 1,4% vs. tumors >2 cm, 1.6%, respectively; all P > 0.05).
When stratified by size of the pancreatic lesion, pathological tumor characteristics were noted to vary considerably. Specifically, patients with a tumor >2 cm, had a higher incidence of vascular and peri-neural invasion (Table II). Tumor size was also directly correlated with histological differentiation, with larger tumors more likely to be poorly differentiated (Table II). With regard to lymph node status, of the 418 patients with tumor ≤2 cm 62.6% patients had lymph node metastasis compared with 80.5% and 88.0% of patients with tumors measuring 2–5 cm and ≥5 cm, respectively (P < 0.001). The median number [range] of nodes evaluated was 15 [1–94] among patients with tumors measuring ≤2 cm compared with 17 [1–74] among those patients with tumors >2 cm (P < 0.001). Furthermore, the status of the surgical margin was also associated with tumor size. Specifically, the likelihood of achieving a microscopically negative (R0) margin decreased as the tumor size increased (≤2 cm 80.0%, 2–5 cm 70.1%, ≥5 cm 64.1%; P < 0.001).
Survival: Does Tumor Size Matter?
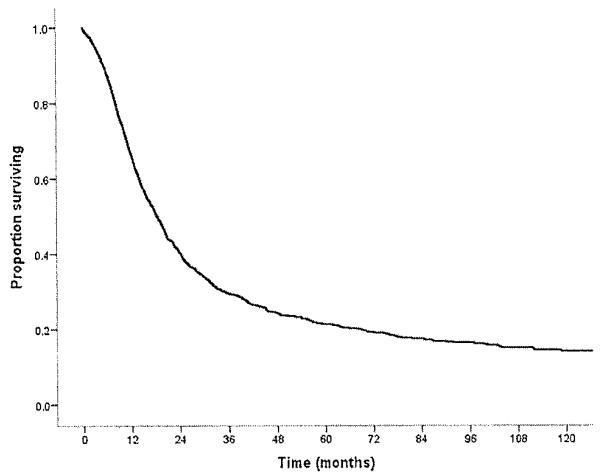
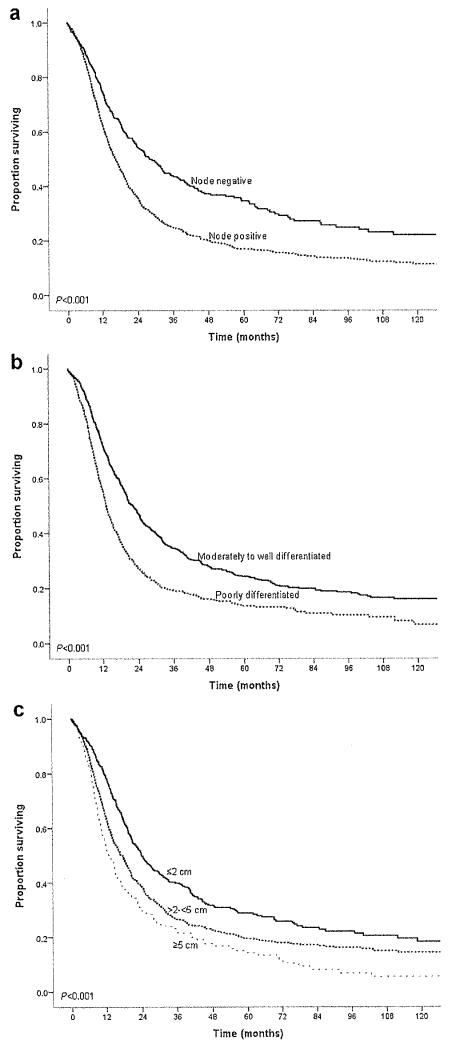
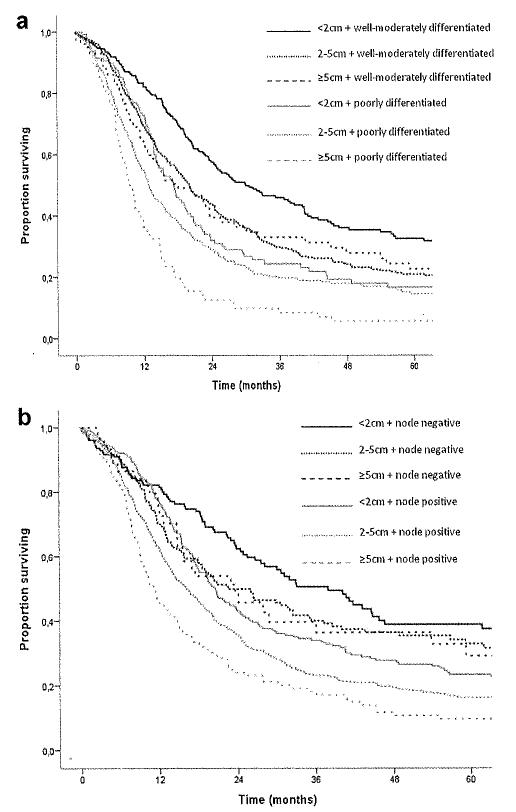
For the entire cohort of 1,697 patients, overall median survival was 18.3 months, and 5-year survival was 21.2% (Fig. 1). Descriptive survival statistics and Kaplan–Meier curves suggested that nodal status, histological differentiation, as well as resection margin all had prognostic significance (Table III). For example, lymph node metastasis was associated with a decrease in median survival from 28.2 to 16.6 months and in 5-year survival from 34.5% to 16.9% (HR 1.59 [1.37–1,83], P < 0,001) (Fig. 2a). Poor tumor differentiation was associated with a decrease in median survival from 22.3 to 13.7 months and in 5-year survival from 24.2% to 13.3% (HR 1.62 [1.43–1.82], P < 0.001) (Fig. 2b). Tumor size was also associated with long-term survival. When tumor size was examined as a continuous variable in univariate analyses, it was associated with survival (HR 1.11 [1.07–1.14]; P < 0.001). When examined as a categorical variable, tumor size ≤2 cm versus >2 cm was associated with a decrease in median survival from 23.9 to 16.3 months and in 5-year survival from 28.9% to 18.5% (HR 1.45 [1.26–1.671, P < 0.001). In fact, median and 5-year survival were both inversely proportional to the size of the tumor. Patients with a tumor ≤2 cm had a median and 5-year survival of 23.9 months and 28.8% compared with 17.5 months and 19.4% and 12.9 months and 14.2% for patients with tumors measuring 2–5 cm and ≥5 cm, respectively (P < 0.001) (Fig. 2c). On univariate, stratified analyses, tumor size was associated with prognosis regardless of lymph node status (Fig. 3a) or tumor grade (Fig. 3b).
Fig. 1.
Overall survival of patients who underwent curative intent surgery for pancreatic adenocarcinoma.
TABLE III.
Univariate and Multivariate Analyses of Factors Proposed to be Associated With Overall Survival
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Prognostic factor | Hazard ratio | 95% CI | p-value | Hazard ratio | 95% CI | p-value |
| Age at surgery | 1.01 | 1.00–1.02 | <0.001 | 1.01 | 1.00–1.02 | 0.02 |
| Male gender | 0.95 | 0.84–1.07 | 0.4 | — | — | — |
| Caucasian race | 1.19 | 1.04–1.36 | 0.01 | 1.39 | 0.92–2.09 | 0.1 |
| Size of tumor | ||||||
| ≤2 cm | Reference | Reference | ||||
| >2 to <5 cm | 1.39 | 1.20–1.60 | <0.001 | 1.27 | 0.95–1.71 | 0.1 |
| ≥5 cm | 1.83 | 1.50–2.22 | <0.001 | 1.58 | 1.03–2.44 | 0.04 |
| CA 19-9 >100 mg/dl | 1.46 | 1.19–1.80 | <0.001 | 1.21 | 0.95–1.55 | 0.13 |
| Extent of surgery | ||||||
| Classic pancreaticoduodenectomy | Reference | Reference | ||||
| Pylorus preserving pancreaticoduodenectomy | 0.93 | 0.81–1.05 | 0.2 | 0.95 | 0.74–1.23 | 0.7 |
| Total pancreatectomy | 1.70 | 1.23–2.33 | 0.001 | 1.44 | 0.77–2.70 | 0.3 |
| Receipt >2 PC, during surgery | 1.13 | 1.09–1.18 | <0.001 | 1.11 | 0.97–1.28 | 0.1 |
| AJCC T3/T4 status | 1.17 | 1.04–1.33 | 0.01 | 1.18 | 0.88–1.60 | 0.3 |
| Positive nodal status | 1.59 | 1.37–1.83 | <0.001 | 1.59 | 1.14–2.22 | 0.006 |
| Poor differentiation | 1.62 | 1.43–1.82 | <0.001 | 1.59 | 1.03–2.44 | 0.04 |
| Presence of vascular invasion | 1.26 | 1.10–1.44 | 0.001 | 1.04 | 0.82–1.32 | 0.76 |
| Presence of peri-neural invasion | 1.50 | 1.21–1.87 | <0.001 | 1.32 | 0.92–1.91 | 0.1 |
| Positive resection margin | 1.67 | 1.47–1.89 | <0.001 | 1.31 | 1.03–1.68 | 0.03 |
| Adjuvant chemotherapy | 0.84 | 0.73–0.97 | 0.01 | 0.80 | 0.62–1.04 | 0.1 |
| Adjuvant radiotherapy | 0.89 | 0.77–1.03 | 0.1 | — | — | — |
PC. packed cells; CA-19-9, pre-operative carbohydrate antigenic determinant 19-9,
Fig. 2.
Overall survival of patients who underwent curative intent surgery for pancreatic adenocarcinoma stratified by (a) lymph node status, (b) tumor differentiation, and (c) tumor size.
Fig. 3.
Impact of tumor size on overall survival of patients who underwent curative intent surgery for pancreatic adenocarcinoma stratified by (a) lymph node status and (b) tumor differentiation.
While tumor size was associated with survival on univariate analysis, after controlling for competing risk factors with multivariate analysis, pancreatic tumor size cut-off of 2 cm was no longer associated with overall survival (HR = 1.20 [0.86–1.77]; P = 0.30). Cut-off values of >3 and >4 cm were similarly not significant on multivariate analysis. In contrast, tumor size ≥5 cm remained associated with survival compared with tumor size ≤2 cm (P = 0.04) (Table III). Nodal status and tumor differentiation also remained associated with survival on multivariate analysis. Moreover, the relative impact of tumor size on prognosis was considerably less after controlling for other competing risk factors such as metastatic nodal disease (HR = 1.59 [1.14–2.22]; P = 0.006) and poor tumor differentiation (HR = 1.59 [1.03–2.44]; P = 0.04) (Table III). Therefore, further stratified analyses focusing on the impact of tumor size, nodal status, and tumor differentiation in relation to each other were performed. Among the 418 patients with tumor size ≤2 cm, 261 patients had lymph node metastasis and 129 patients had poor histological tumor differentiation, of whom 96 patients had both. Both lymph node metastasis (HR 1.62 [1.25–2.11], P < 0.001) and poor tumor differentiation (HR 1.74 [1.35–2.26], P < 0.001) predicted prognosis among patients with tumor size ≤2 cm. Among the 1,279 patients with tumor size >2 cm, 1,019 patients had lymph node metastasis and 520 patients had poor histological tumor differentiation, of whom 427 patients had both. As in patients with smaller tumors, both lymph node metastasis (HR 1.68 [1.41–2.02], P < 0.001) and poor histological tumor differentiation (HR 1.54 [1.35–1.77], P < 0.001) predicted prognosis among these patients with tumor size >2 cm. Importantly, among patients with tumor size ≥5 cm (n = 209; 12.3%) lymph node metastasis (HR 2.07 [1.37–3.13], P = 0.001) and poor histological tumor differentiation (HR 2.12 [1.52–2.95], P < 0.001) remained predictive of prognosis.
DISCUSSION
A number of previous studies, including data from our own institution, have reported on the prognostic factors associated with survival following resection of pancreatic adenocarcinoma [4,7,9,10,12-16,24-32]. Factors including lymph node status, peri-neural/vascular invasion, histological tumor grade, and margin status have been reported to affect outcome [4,10,12-16]. In the current study, we reviewed the largest single-institutional experience with resection of pancreatic adenocarcinoma to examine specifically the impact of tumor size on prognosis. Tumor size is a particularly important prognostic factor to examine as it is included in the AJCC staging of pancreatic adenocarcinoma [17]. In fact, tumor size is the sole factor determining whether a pancreatic tumor is staged as T1 or T2 disease. While in the current study we demonstrated that tumor size was associated with prognosis on univariate analysis, tumor size using the 2 cm cut-off value failed to remain statistically significant when competing risk factors were taken into account. Furthermore, we empirically noted that the majority of patients with pancreatic cancer had tumors >2 cm, with only about one-quarter of patients even having a tumor measuring ≥2 cm. Taken together, these data suggest that the use of a 2 cm tumor size cut-off in the AJCC staging may not be the most appropriate cut-off value to utilize for prognostic purposes. Rather, our data suggest that the effect of size on prognosis was largely attributable to other biologic factors rather than tumor size itself.
In the current study, the size of the pancreatic tumor was also noted to impact peri- and intra-operative management. Patients with larger tumors had longer operating times, a higher incidence of a classic pancreaticoduodenectomy, more intra-operative blood loss, and a greater need for packed red blood cell transfusion (Table II). Perhaps more importantly, tumor size was associated with the surgeon’s ability to achieve a microscopically (R0) negative surgical margin. In fact, while an R0 resection was achieved in 80% of patients with tumors measuring ≤2 cm, less than two-third of patients with a tumor ≥5 cm had an RO margin on final pathological analysis. While some data have suggested that surgical margin status may not be independently associated with long-term outcome [33], most studies [9,31,34] have indeed reported that achieving an R0 margin is an important factor associated with survival. We similarly noted that margin status was associated with long-term outcome, as the failure to achieve an RO resection margin was associated with a worse long-term outcome on both univariate and multivariate analyses (Table III). These data suggest that the inability to achieve a negative surgical margin in patients with large pancreatic tumors may contribute, in part, to the worse survival noted in this cohort.
While some have argued that smaller pancreatic tumors may have a better biologic behavior than tumors of greater size, the relationship of anatomic size, and other biologic determinants of outcome have not been well defined. Our data support the theory that tumor size was associated with an increased likelihood of having other adverse pathologic factors. Specifically, tumor size was predictive of both the risk of lymph node metastasis and poor tumor differentiation. In fact, among patients with tumors measuring ≥5 cm the risk of lymph node metastasis and poor tumor differentiation was 2.4- and 1.9-fold greater as compared with patients with tumors measuring ≤2 cm. Yamaguchi et al. [22] reported similar results, noting that pancreatic lesions measuring ≤2 cm were less likely to be associated with nodal metastasis and generally were better differentiated than tumors larger >2 cm. In turn, nodal status and tumor differentiation have both been well-established as factors that decrease overall survival [9,11,21,25]. Therefore, given the finding that tumor size was strongly associated with these adverse biologic factors, it was not surprisingly that tumor size would also be predictive of a worse overall survival. As noted in Figure 2c, increasing tumor size was indeed associated with an incrementally worse long-term survival on univariate log-rank analysis. On univariate analysis, tumor size remained associated with prognosis after stratifying based on lymph node status or tumor grade. However, interestingly, the 2 cm cut-off did not seem as prognostically relevant among patients with tumors characterized by worse biologic features (e.g., lymph node metastasis or poor tumor grade) (Fig. 3).
One important finding of the current study was that the tumor size cut-off of 2 cm was not statistically significant on multivariate survival analyses. Other authors [16] have similarly emphasized the paucity of previous empiric data to support the use of a 2 cm cut-off in the AJCC staging of pancreatic adenocarcinoma. While the 2 cm cut-off was associated with survival on univariate analyses, we noted that the 2 cm threshold failed to remain significant on multivariate analyses when other competing risk factors were considered (Table III). Interestingly, we also noted that a 3 and 4 cm cut-off threshold were similarly not significant on multivariate analyses, while ≥5 cm remained statistically significant (P = 0.04). Brennan et al. [35] had noted that tumor size was associated with outcome, but not in a mon-otonic fashion. Ferrone et al. [36] had similarly validated the use of tumor size in a nomogram to predict disease-free survival following resection of pancreatic adenocarcinoma. Again, in this nomogram, tumor size did not have a monotonic incremental effect on survival and was utilize in combination with a host of other clinico-pathological factors to predict outcome. Importantly, we similarly found that other factors such as the presence of lymph node metastasis and poor histological tumor differentiation were predictive of outcome among all patients regardless of tumor size. Furthermore, we noted that among patients with node negative disease and/or well-differentiated tumors overall survival was similar among patients regardless of tumor size. In contrast, among patients who did have either of these poor biologic characteristics, a tumor size of ≥5 cm conferred an even worse prognosis compared with patients who had lymph node metastasis/poor tumor differentiation and a tumor <5 cm. In aggregate, these data strongly suggest that the effect of tumor size on prognosis is largely mediated through other adverse biologic factors. Tumor size—independent of other biologic factors such as lymph node status and histologic differentiation—had little impact on long-term outcome especially when tumor size was <5 cm.
The current study has several limitations. In examining tumor size, we utilized the measurement as determined by final pathological assessment. Whether the findings from the current study can be extrapolated to tumor size as determined by pre-operative cross-sectional imaging remains undetermined. However, the purpose of the current study was to assess the role of tumor size in pathological staging as enumerated in the AJCC staging of pancreatic adenocarcinoma. Another limitation of the current study was the inclusion of patients with only head and uncinate lesions who underwent pancreaticoduodenectomy. We did not include patients with pancreatic tail lesions who underwent distal pancreatectomy. Patients with pancreatic tail lesions often present with a more advance stage of disease [37,38], probably due to later onset of symptoms. As such, patients with pancreatic tail lesions may present with larger tumors [27] and our data do not address the role of tumor size in predicting outcome in this particular subset of patients.
In conclusion, curative intent resection for pancreatic adenocarcinoma is associated with a survival benefit in a subset of patients. A number of prognostic factors are associated with survival following resection of pancreatic adenocarcinoma, however, tumor size has been singled out by its inclusion in the AJCC staging T classification system. We herein report that the cut-off value of 2 cm utilized in the AJCC staging is not independently associated with outcome. Rather tumor size was strongly associated with the risk of other adverse prognostic factors, In turn, the effect of tumor size on prognosis was largely attributable to these other biologic factors rather than tumor size itself. The use of the 2 cm cut-off in the current AJCC staging system is neither clinically applicable nor prognostically relevant in the majority of patients undergoing resection of pancreatic adenocarcinoma and therefore the use of the 2 cm cut-off should be reconsidered.
Footnotes
Mechteld C. de Jong and Fuyu Li contributed equally to this work.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Lowenfels AB, Maisonneuve P, Whitcomb DC. Risk factors for cancer in hereditary pancreatitis. International Hereditary Pancreatitis Study Group. Med Clin North Am. 2000;84:565–573. doi: 10.1016/s0025-7125(05)70240-6. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 4.Bouvet M, Gamagami RA, Gilpin EA, et al. Factors influencing survival after resection for periampullary neoplasms. Am J Surg. 2000;180:13–17. doi: 10.1016/s0002-9610(00)00405-0. [DOI] [PubMed] [Google Scholar]
- 5.Cress RD, Yin D, Clarke L, et al. Survival among patients with adenocarcinoma of the pancreas: A population-based study (United States) Cancer Causes Control. 2006;17:403–409. doi: 10.1007/s10552-005-0539-4. [DOI] [PubMed] [Google Scholar]
- 6.Cameron JL, Crist DW, Sitzmann JV, et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161:120–124. doi: 10.1016/0002-9610(91)90371-j. discussion 124-5. [DOI] [PubMed] [Google Scholar]
- 7.Han SS, Jang JY, Kim SW, et al. Analysis of long-term survivors after surgical resection for pancreatic cancer. Pancreas. 2006;32:271–275. doi: 10.1097/01.mpa.0000202953.87740.93. [DOI] [PubMed] [Google Scholar]
- 8.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: A population-based, linked database analysis of 396 patients. Ann Surg. 2003;237:74–85. doi: 10.1097/00000658-200301000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–1210. doi: 10.1016/j.gassur.2006.08.018. discussion 1210-1. [DOI] [PubMed] [Google Scholar]
- 10.Yeo CJ, Cameron JL, Lillemoe KD, et al. Pancreaticoduodenectomy for cancer of the head of the pancreas. 201 patients. Ann Surg. 1995;221:721–731. doi: 10.1097/00000658-199506000-00011. discussion 731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimada K, Sakamoto Y, Sano T, et al. Prognostic factors after distal pancreatectomy with extended lymphadenectomy for invasive pancreatic adenocarcinoma of the body and tail. Surgery. 2006;139:288–295. doi: 10.1016/j.surg.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Hirata K, Sato T, Mukaiya M, et al. Results of 1001 pancreatic resections for invasive ductal adenocarcinoma of the pancreas. Arch Surg. 1997;132:771–776. doi: 10.1001/archsurg.1997.01430310085018. discussion 777. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy EP, Yeo CJ. Pancreaticoduodenectomy with extended retroperitoneal lymphadenectomy for periampullary adenocarcinoma. Surg Oncol Clin N Am. 2007;16:157–176. doi: 10.1016/j.soc.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 14.Samra JS, Gananadha S, Hugh TJ. Surgical management of carcinoma of the head of pancreas: Extended lymphadenectomy or modified en bloc resection? ANZ J Surg. 2008;78:228–236. doi: 10.1111/j.1445-2197.2008.04426.x. [DOI] [PubMed] [Google Scholar]
- 15.Nitecki SS, Sarr MG, Colby TV, et al. Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg. 1995;221:59–66. doi: 10.1097/00000658-199501000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcea G, Dennison AR, Pattenden CJ, et al. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9:99–132. [PubMed] [Google Scholar]
- 17.Edge SB, Byrd DR, Compton CC, et al. AJCC cancer staging manual. 7th edition Springer; Chicago: 2010. [Google Scholar]
- 18.Shoup M, Cordon KC, Klimstra D, et al. Is extended resection for adenocarcinoma of the body or tail of the pancreas justified? J Ciastrointest Surg. 2003;7:946–952. doi: 10.1016/j.gassur.2003.08.004. discussion 952. [DOI] [PubMed] [Google Scholar]
- 19.Sperti C, Bonadimani B, Pasquali C, et al. Ductal adenocarcinoma of the pancreas: Clinicopathologic features and survival. Tumori. 1993;79:325–330. doi: 10.1177/030089169307900508. [DOI] [PubMed] [Google Scholar]
- 20.Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 21.Kuhlmann KF, de Castro SM, Wesseling JG, et al. Surgical treatment of pancreatic adenocarcinoma: Actual survival and prognostic factors in 343 patients. Eur J Cancer. 2004;40:549–558. doi: 10.1016/j.ejca.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi K, Mizumoto K, Noshiro H, et al. Pancreatic carcinoma: ≤2 cm versus >2 cm in size. Int Surg. 1999;84:213–219. [PubMed] [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Berger AC, Meszoely IM, Ross EA, et al. Undetectable preoperative levels of serum CA 19-9 correlate with improved survival for patients with resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2004;11:644–649. doi: 10.1245/ASO.2004.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Ferrone CR, Finkelstein DM, Thayer SP, et al. Perioperative CA19-9 levels can predict stage and survival in patients with resectable pancreatic adenocarcinoma. J Clin Oncol. 2006;24:2897–2902. doi: 10.1200/JCO.2005.05.3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takai S, Satoi S, Toyokawa H, et al. Clinicopathologic evaluation after resection for ductal adenocarcinoma of the pancreas: A retrospective, single-institution experience. Pancreas. 2003;26:243–249. doi: 10.1097/00006676-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 27.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: Results, outcomes, and prognostic-indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 28.Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: The Mayo Clinic experience (1975–2005) J Clin Oncol. 2008;26:3511–3516. doi: 10.1200/JCO.2007.15.8782. [DOI] [PubMed] [Google Scholar]
- 29.Miller RC, lott MJ, Corsini MM. Review of adjuvant radiochemotherapy for resected pancreatic cancer and results from Mayo Clinic for the 5th JUCTS symposium. Int J Radiat Oncol Biol Phys. 2009;75:364–368. doi: 10.1016/j.ijrobp.2008.11.069. [DOI] [PubMed] [Google Scholar]
- 30.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 31.Shimada K, Sakamoto Y, Nara S, et al. Analysis of 5-year survivors after a macroscopic curative pancreatectomy for invasive ductal adenocarcinoma. World J Surg. 2010;34:1908–1915. doi: 10.1007/s00268-010-0570-9. [DOI] [PubMed] [Google Scholar]
- 32.Vanderveen KA, Chen SL, Yin D, et al. Benefit of postoperative adjuvant therapy for pancreatic cancer: A population-based analysis. Cancer. 2009;115:2420–2429. doi: 10.1002/cncr.24269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg. 2007;246:52–60. doi: 10.1097/01.sla.0000259391.84304.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sierzega M, Popiela T, Kulig J, et al. The ratio of metastatic/resected lymph nodes is an independent prognostic factor in patients with node-positive pancreatic head cancer. Pancreas. 2006;33:240–245. doi: 10.1097/01.mpa.0000235306.96486.2a. [DOI] [PubMed] [Google Scholar]
- 35.Brennan MF, Kattan MW, Klimstra D, et al. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ferrone CR, Kattan MW, Tomlinson JS, et al. Validation of a postresection pancreatic adenocarcinoma nomogram for disease-specific survival. J Clin Oncol. 2005;23:7529–7535. doi: 10.1200/JCO.2005.01.8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lau MK, Davila JA, Shaib YIT. Incidence and survival of pancreatic head and body and tail cancers: A population-based study in the United States. Pancreas. 2010;39:458–462. doi: 10.1097/MPA.0b013e3181bd6489. [DOI] [PubMed] [Google Scholar]
- 38.Artinyan A, Soriano PA, Prendergast C, et al. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford) 2008;10:371–376. doi: 10.1080/13651820802291233. [DOI] [PMC free article] [PubMed] [Google Scholar]