Abstract
Connexin43 (Cx43) forms 22 s gap junctions that couple the granulosa cells of ovarian follicles. In Cx43 knockout mice, follicle growth is restricted due to impaired granulosa cell proliferation. We have used these mice to examine the importance of specific Cx43 phosphorylation sites in follicle growth. Serines at residues 255, 262, 279 and 282 are MAP kinase substrates that, when phosphorylated, reduce junctional conductance. Mutant forms of Cx43 were constructed with these serines replaced with amino acids that cannot be phosphorylated. These mutants were transduced into Cx43 knockout ovarian somatic cells which were combined with wildtype oocytes and grafted into immunocompromised female mice permitting follicle growth in vivo. Despite residues 255 or 262 being mutated to prevent their being phosphorylated, recombinant ovaries constructed with these mutants were able to rescue the null phenotype, restoring complete folliculogenesis. In contrast, Cx43 with serine to alanine mutations at both residues 279 and 282 or at all four residues failed to rescue folliculogenesis; the mutant molecules were largely confined to intracellular sites, with few gap junctions. Using an in vitro proliferation assay, we confirmed a decrease in proliferation of granulosa cells expressing the double mutant construct. These results indicate that Cx43 phosphorylation by MAP kinase at serines 279 and 282 occurs in granulosa cells of early follicles and that this is involved in regulating follicle development.
Keywords: Cx43, phosphorylation, folliculogenesis, oocyte growth, granulosa cell, proliferation
Introduction
Connexin43 (Cx43), the most widely expressed connexin, is differentially phosphorylated at a dozen or more serine residues throughout its life cycle (Lampe and Lau 2004; Solan and Lampe 2009). Protein kinase C (PKC) (Lampe et al. 2000; Saez et al. 1997), mitogen-activated protein kinase (MAPK) (Warn-Cramer et al. 1996), AKT (Park et al. 2007), casein kinase 1 (CK1) (Cooper and Lampe 2002), p34cdc2 (Kanemitsu et al. 1998; Lampe et al. 1998), SRC (Crow et al. 1990; Swenson et al. 1990) and likely other kinases can directly phosphorylate Cx43. Kinase activation can either effect an acute (within minutes) reduction of channel conductance and/or open probability, or over a somewhat longer time frame (tens of minutes to hours) can lead to compromised Cx43 targeting/retention at gap junctions and in some cases, altered Cx43 gene expression. Cx43 has an unusually short half-life in cultured cells and tissues (Beardslee et al. 1998; Crow et al. 1990; Laird et al. 1991; Lampe 1994; Musil et al. 1990). Cx43 turnover appears to be highly regulated with both MAPK- and PKC-mediated phosphorylation implicated in keeping Cx43 hemichannels closed (Goodenough and Paul 2003) and enhancing gap junction turnover (Leithe and Rivedal 2004).
Much of the work exploring the involvement of protein kinases in regulating gap junction assembly and function has come from the use of cultured cells, with little attention to in vivo models. To explore the involvement of Cx43 C-terminal tail phosphorylation in vivo, we have chosen the mouse ovarian follicle. In the mouse, Cx43 is expressed in all stages of follicle development from the primary through the pre-ovulatory stage where it forms gap junctions connecting granulosa cells (both cumulus and mural) with each other; another connexin, Cx37, forms gap junctions coupling the cumulus granulosa cells with the developing oocyte, thus linking the germline and somatic components of the follicle into a functional syncytium allowing diffusional transfer of small molecules throughout (reviewed by Kidder and Vanderhyden, 2010). Gene knockout studies have demonstrated that Cx43 is essential for follicle growth since, in its absence, granulosa cell proliferation is arrested or impaired, depending on strain background (Ackert et al. 2001; Tong et al. 2006). Furthermore, oocytes developing within Cx43-deficient follicles fail to grow properly and do not achieve meiotic competence (Ackert et al. 2001). This dependency of follicle and oocyte growth on Cx43 provides a testing ground for exploring the importance of C-terminal tail phosphorylation in connexin function.
In the present study, we have exploited the recombinant-reaggregated ovary technique (Eppig and Wigglesworth, 2000) to evaluate the importance of specific Cx43 phosphorylation sites in oocyte and follicle g rowth. Cx43 knockout granulosa cells were transduced with wildtype Cx43 or Cx43 in which specific serines, known to be phosphorylated by MAP kinases, had been replaced with alanine, to prevent phosphorylation, or aspartic acid, to mimic phosphorylation. The transduced granulosa cells were then aggregated with wildtype oocytes to form recombinant ovaries that were grafted under the kidney capsules of immunocompromised adult females to allow for in vivo follicle growth; previous work using this technique had confirmed the ability of transduced wildtype Cx43 to rescue folliculogenesis when Cx43-deficient granulosa cells were combined in grafts with wildtype oocytes (Gittens and Kidder, 2005). Using this approach, we were able to identify serines whose phosphorylation is involved in regulating Cx43 function in support of oocyte and follicle growth.
Materials and Methods
Ovary Collection
Ovaries lacking Cx43 were obtained from matings of heterozygous (Gja1+/Gja1−) C57BL/6 male and female mice. Immunocompromised adult SCID females (CB17/IcrPrkdcscid/IcrIcoCrl, originally obtained from Charles River, St-Constant, QC and maintained in the barrier facility of the Robarts Research Institute at the University of Western Ontario) were used as graft recipients. Fetuses were obtained from pregnant dams at day 17.5–18.5 of gestation after CO2 anesthesia and cervical dislocation. Fetuses were removed from the uteri and decapitated. A tail snip was collected from each female fetus for genotyping. Ovaries were removed and cleaned of surrounding tissue, then cultured on a 3 µm membrane cell culture insert (VWR USA, Radnor, PA) for 8–10 h in Waymouth’s MB 752/1 medium (Invitrogen Canada, Burlington, ON) supplemented with 10% fetal bovine serum and 1X antibiotic/antimycotic (both from Invitrogen) while the fetuses were genotyped.
Genotyping
The polymerase chain reaction (PCR) was applied to proteinase K-digested 104 tail snips to determine the genotypes of the fetal ovary donors. PCR was carried out utilizing two separate reactions that shared a downstream primer (5′-ACTTTTGCCGCCTAGCTATCCC-3′) specific for a part of the Cx43 C-terminal coding region which is retained in the null allele; seeReaume et al. (1995). To detect the presence of the wildtype Gja1 allele, an upstream primer (5′-CCCCACTCTCACCTATGTCTCC-3′) was used in conjunction with the downstream primer whereas to detect the null allele, an upstream primer located in the neo cassette (5′-GCTTGCCGAATATCATGGTGGA-3′) was used with the downstream primer. One µl of the diluted digestions were used per PCR. PCR was carried out using a “touch down” (65–58 °C) protocol for a total of 40 cycles. PCR products were visualized on a 1% agarose gel containing ethidium bromide and documented using a Bio-Rad imaging system and Quantity One software (Bio-Rad USA, Hercules, CA).
Retroviral Vector Construction
Cloned cDNAs encoding Cx43 with C-terminal mutations at various serine residues were constructed as described (Solan et al. 2007). Serine-to-alanine and serine-to-aspartate mutations were made using the GeneTailor (Invitrogen) or In-Fusion (Clontech Laboratories, Inc. Mountain View, CA) site-directed mutagenesis system applied to full-length Cx43 cDNA that had been cloned into the mammalian expression vector pIREShyg (Clonetech). The mutant cDNAs were inserted into the AP2 retroviral vector that contains an internal ribosomal entry site (IRES) that permits independent translation of the connexin and enhanced green fluorescent protein (EGFP; see Tong et al. 2007 for vector design). Vector lacking connexin cDNA served as negative control and vector encoding wildtype Cx43 served as positive control. The vector was packaged using 293 GPG packaging cells to produce active virus which was concentrated using Amicon Ultra Centrifugal Filter Devices (Millipore USA, Billerica, MA) according to the manufacturer’s protocol.
Construction of Chimeric Ovaries
Chimeric ovaries combining wildtype oocytes with Cx43-deficient (Gja1−/−), retrovirally transduced ovarian somatic cells were constructed essentially as described by Gittens and Kidder (2005) with minor modifications. Ovaries were grouped based on genotype (four to six per group), washed briefly in HBSS (Invitrogen) supplemented with 1 mg/ml bovine serum albumin (BSA; Sigma, Oakville, ON), and dissociated in trypsin-EDTA (0.05% trypsin, 0.53 mM EDTA, Invitrogen) for 45 minutes (Eppig and Wigglesworth, 2000). The dissociated cells were plated in TCM199 (Invitrogen) supplemented with 10% fetal bovine serum (M199-FBS) and antibiotic-antimycotic (Invitrogen) on cell culture-treated 3.5 cm dishes (Falcon) or glass coverslips and cultured overnight at 37°C in 5% CO2/5% O2/90% N2. After removal of the oocytes, the remaining somatic cells were infected for 48 h with one of the retroviral expression vectors. To prepare reaggregated ovaries, oocytes obtained from wildtype ovaries were washed with M199-FBS and placed in a 15 ml conical tube. The retrovirally-transduced somatic cells were trypsinized, washed with M199-FBS, and pelleted by centrifuging at 500 × g for 5 min. The pelleted cells were then resuspended in 500 µl M199-FBS and added to the washed oocytes at an equal ratio (e.g. wildtype oocytes from four ovaries were combined with the somatic cell pellet from four Cx43-deficient ovaries) along with 7 µl/ml phytohemagglutinin (0.05%, Sigma) in a 1.5 ml tube, followed by pelleting at 500 × g for 5 min. The pellets were carefully dislodged from the tubes and cultured overnight on 3 µm cell culture inserts at 37°C in 5% CO2 in air. The reaggregated ovaries were transplanted under the kidney capsules of ovariectomized SCID mice (one graft per mouse) and incubated 21–28 days as described previously (Gittens and Kidder 2005; Tong et al. 2007).
Histology
Reaggregated ovaries were recovered from the host kidneys and fixed in Bouin’s solution, then embedded in paraffin and sectioned at 6 µm onto Superfrost Plus slides (Fisher Canada, Ottawa, ON). The slides were dried, deparaffinized and stained with hematoxylin and eosin using a standard protocol. Measurements of oocyte diameters were taken using an Olympus inverted microscope and Open Lab software (Leica Microsystems Canada, Concord, ON). Three sections were analyzed from each of at least three recombinant ovary grafts.
Immunolocalization of Total and pS279/282 Cx43 in Natural Ovaries
Ovaries from 3 to 4 week old female C57BL/6 mice were placed into cryomolds containing O.C.T. (Tissue Tek), and were frozen on an aluminum block cooled in liquid nitrogen. Eight to 10 µm thick sections were cut and left at room temperature to dry 24–48 h before fixing. Sections were fixed in chilled 50:50 methanol/acetone at 4°C for 10–15 minutes then dried for 5 min or less. Sections were rinsed twice with 1x PBS to remove excess O.C.T. and blocked in 1% BSA in PBS with 5% normal goat serum (Sigma) for 30 min. Sections were outlined with a PAP pen to minimize the volume of blocking buffer and antibody needed. They were treated for 1 hour at room temperature with two primary antibodies: 1 µg/ml CT1 anti-Cx43 (Sosinsky et al. 2007) and 1:5000 anti-phospho-S279/282 Cx43 prepared at the Fred Hutchinson Cancer Research Center against peptide S279/282 (CAPL(pS)PM(pS)PPGY-amide (Solan and Lampe 2008). Secondary antibody treatment was also carried out for 1 hour at room temperature using Alexafluor 488-conjugated goat anti-mouse and Alexafluor 546-conjugated goat anti-rabbit, both from Invitrogen. Nuclei were labeled with DAPI (300 nM, Invitrogen). Sections were imaged using a Nikon E400 epifluorescence microscope with a Nikon DS-Qi1Mc camera.
Immunolocalization of Total Cx43 in Recombinant Ovaries
Grafts were recovered into TCM-FBS and follicles were liberated using 25 gauge needles. The follicles were plated on glass coverslips and cultured 24 to 48 h at 37°C in M199-FBS in 5% CO2/5% O2/90% N2. The cells were washed with PBS, fixed with 4% paraformaldehyde (PFA) for 20 min then blocked in PBS containing 5% BSA (PBS-BSA) for 1 h at room temperature before immunolabeling with Cx43 primary antibody (Sigma cat. no. C6219; 1/5000 in PBS-BSA) overnight at 4°C. Excess primary antibody was removed by washing three times with PBS-BSA before the secondary (goat anti-rabbit Alexafluor 488, 1/1000, Invitrogen) was added for 1 h at room temperature. Secondary antibody was removed by washing three times in PBS-BSA, then the nuclei were labelled with Hoechst 33342 (1 µg/ml in PBS) for 8 min at room temperature. A final PBS wash was performed before the coverslips were mounted on slides with Airvol. The cells were imaged using a Zeiss LSM 510 META confocal microscope and ZEN imaging software (Carl Zeiss Canada, Toronto, ON).
Cell Proliferation Assay
Cx43-deficient ovarian somatic cells, devoid of oocytes, were plated directly onto 13 mm Thermanox plastic coverslips (VWR Canada, Mississauga, ON). The Click-iT EdU Imaging Kit (Invitrogen) was used to measure cell proliferation. Following 48 h viral vector infection, cells were cultured with 10 µM EdU (5-ethynyl-2’-deoxyuridine) for 12 h then fixed in 4% PFA for 20 min. The fixative was washed off using PBS containing 3% BSA (PBS-BSA) and then the cells were incubated for 20 min with 0.5% Triton X-100, washed once with PBS-BSA and incubated 30 min with the Click-iT reaction cocktail followed by washing with PBS-BSA. Nuclei were labelled using the Hoechst dye provided in the kit and according to the manufacturer’s protocol. The coverslips were mounted onto slides using Airvol and documented using a fluorescence photomicroscope (Leica Microsystems Canada, Concord, ON). The ratio of EdU positive cells to total cells was determined by counting five fields per coverslip and each experiment was conducted three times.
Statistical Analyses
Statistical analysis of follicle stage distributions was performed using a chi-square test and analysis of oocyte size and granulosa cell proliferation rate data was conducted by one way ANOVA followed by Tukey’s post test using Graphpad Prism software (La Jolla, CA). Results were considered significant if P < 0.05.
Results
Specific Serines in the C-terminal Tail of Cx43 Are Phosphorylated during Follicle Growth
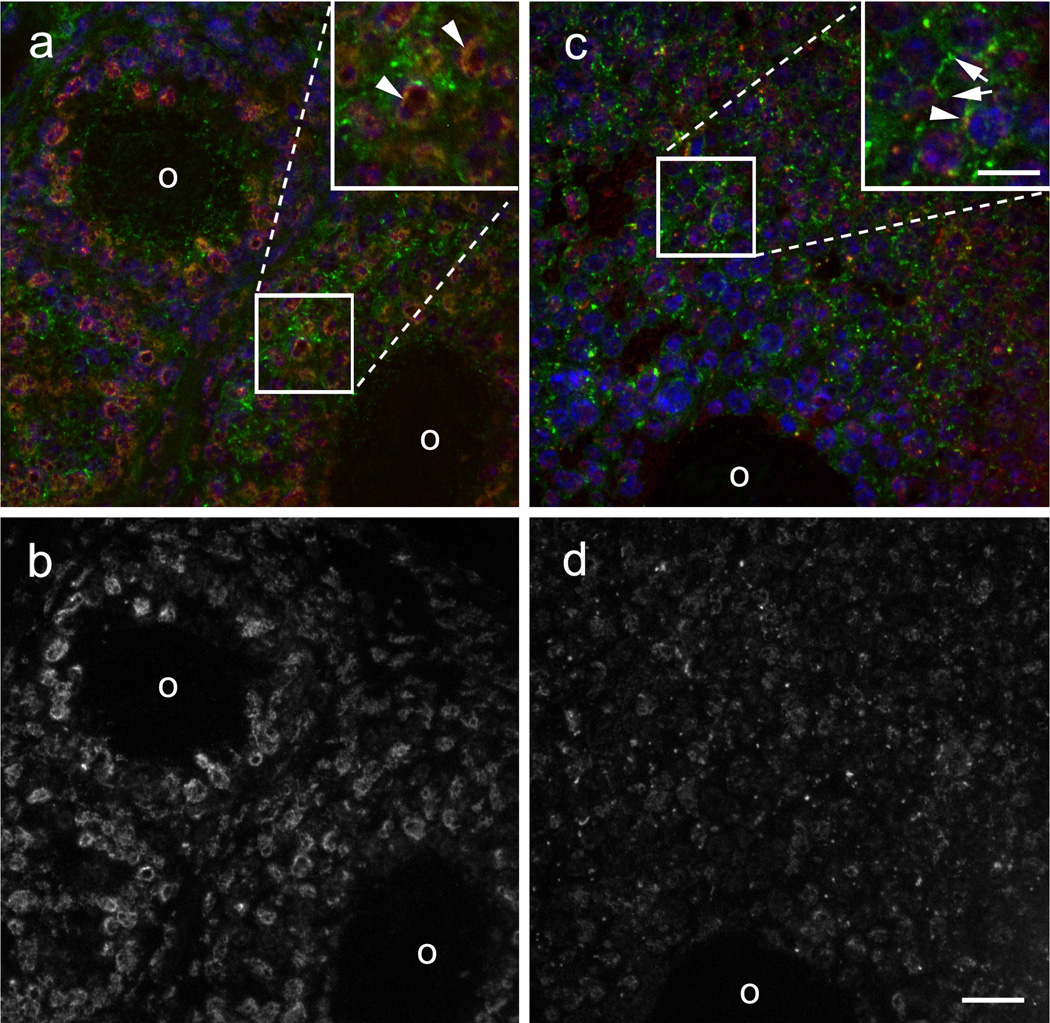
Cx43 phosphorylated at serines 279/282 is located primarily at perinuclear sites in small primary and secondary follicles (top left and bottom right, respectively, of Fig. 1a), overlapping to some extent with total Cx43 (arrowheads). In larger follicles (Fig. 1c), there is increased expression of total Cx43 in plaque-like structures at cell borders (arrows) but reduced expression of Cx43 phosphorylated at serines 279/282, some of which continues to exhibit perinuclear co-localization with Cx43 (arrowhead). This difference in level and localization of Cx43 phosphorylated at serines 279/282 in different follicle stages is clearer when its immunostaining is shown alone as in Fig. 1b and 1d. We conclude that the relative level of Cx43 phosphorylated at serines 279 and 282 is higher in earlier follicle stages where there are fewer gap junctions.
Fig. 1.
Cx43 in primary ovarian follicles is mainly perinuclear and phosphorylated at S279/282 whereas secondary follicles show more junctional staining. (a) A primary follicle (upper left) and a small secondary follicle (lower right) show perinuclear and junctional localization of total Cx43 (green) and predominantly perinuclear localization of Cx43 phosphorylated at S279/282 (red). The inset shows a magnified view of the colocalization of total Cx43 and Cx43 phosphorylated at S279/282, indicated by arrowheads. (b) A single channel image of the field in panel A shows more clearly the perinuclear localization of Cx43 phosphorylated at S279/282. (c) In a larger secondary follicle, a greater proportion of total Cx43 is in junctional plaques and less is perinuclear. The magnified inset shows an example of total Cx43 in plaques at a cell-cell interface (arrows) and colocalization of total Cx43 with Cx43 phosphorylated at S279/282 (arrowhead). (d) A single channel image of the field in panel C reveals that there is less perinuclear localization of Cx43 phosphorylated at S279/282 and an overall lower level of immunostaining, presumably reflecting a reduced level of phosphorylation. O, oocyte. Scale bars: c 10 µm; d 20 µm.
Mutation of Phosphorylation Sites S279 and S282 Abrogates the Ability of Cx43 to Rescue Folliculogenesis in Cx43-deficient Ovaries
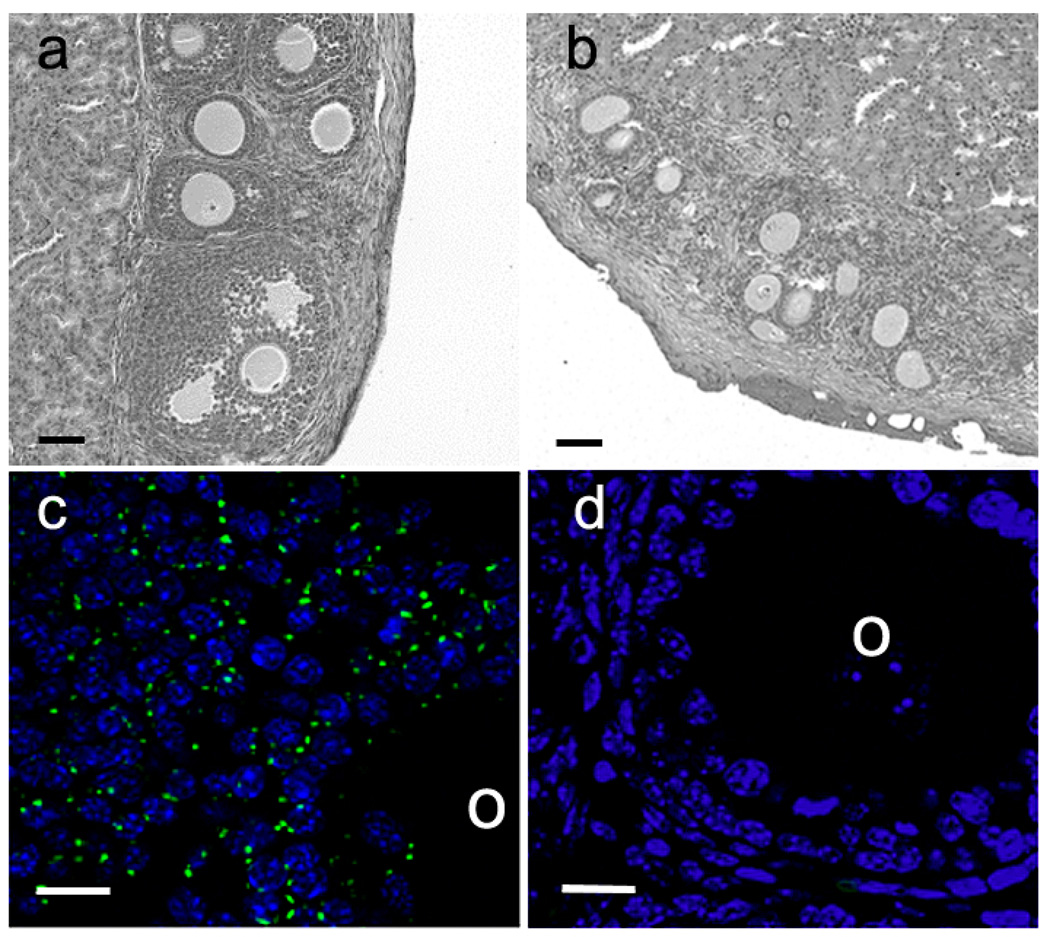
To confirm that the recombinant re-aggregated ovary system can be used to identify follicle growth differences in the presence or absence of Cx43, reaggregated ovaries were constructed with wildtype oocytes combined with Cx43-deficient somatic cells that had been transduced with either wildtype Cx43 or the empty vector control construct. These were transplanted under the kidney capsules of ovariectomized immunodeficient adult females and allowed to develop for 21–25 days. Ovaries containing Cx43-deficient somatic cells transduced with wildtype Cx43 produced follicles representing all stages of development, from primary to late antral (Fig. 2a, Table 1) demonstrating the ability of the transduced connexin to rescue the Cx43-deficient follicles from growth arrest. Conversely, in ovaries made with Cx43-deficient somatic cells transduced with the empty vector control, only primary stage follicles were observed (Fig. 2b). To confirm that the rescue of folliculogenesis after transduction with wildtype Cx43 did not result from contamination with wildtype granulosa cells, follicles were recovered from the grafts and immunostained for Cx43. Follicles derived from Cx43-transduced somatic cells (Fig. 2c) showed typical punctate immunostaining between granulosa cells whereas follicles derived from empty vector-transduced somatic cells did not show any Cx43 immunofluorescence (Fig. 2d). These results confirmed the validity of the recombinant-reaggregated ovary system for testing the ability of phosphorylation site mutants to support oocyte and follicle growth.
Fig. 2.
Folliculogenesis can be rescued by the transduction of wildtype Cx43 into Cx43-deficient ovarian somatic cells and aggregation with wildtype oocytes. (a) Section of a grafted recombinant ovary made with wildtype Cx43-transduced somatic cells. A full range of follicle stages is present in the ovary. (b) Section of a grafted recombinant ovary made with somatic cells transduced with the empty vector. Follicle development was limited to the primary and early secondary stages in these ovaries. (c) Immunofluorescence revealed robust Cx43 expression in gap junction plaques in the cumulus cells of recombinant ovaries made with wildtype Cx43- transduced somatic cells. (d) Cx43 was undetectable in recombinant ovaries made with somatic cells transduced with the empty vector. O, oocyte. Scale bars: a,b 50 µm; c,d 10 µm.
Table 1.
Connexin PCR primers
| Connexin | Upstream/Downstream Sequences | Annealing Temp. (°C) |
Amplicon Length (bp) |
|---|---|---|---|
| Cx26 | 5'-CGGAAGTTCATGAAGGGAGAGAT-3' | 60 | 365 |
| 5'-GGTCTTTTGGACTTTCCTGAGCA-3' | |||
| Cx29 | 5'-GCTCATGGGATTCCGTCTC-3' | 55 | 460 |
| 5'-GGTTGTGCTGCCAATACAAGG-3' | |||
| Cx30 | 5'-AATGTGGCCGAGTTGTGTTA-3' | 53 | 399 |
| 5'-CCAAGGCCCAGTTGTCAC-3' | |||
| Cx30.2 | 5'-TCATGCTGATCTTCCGCATC-3' | 55 | 218 |
| 5'-GGCCTGGTGCATGGAGTAGAT-3' | |||
| Cx30.3 | 5'-TCAAACATGGGCCCAATG-3' | 60 | 182 |
| 5'-GGGAGTCACAGAGCAAGC-3' | |||
| Cx31 | 5'-AGAAGCACGGGGAGCAAT-3' | 60 | 182 |
| 5'-TACTATGCTGGCGCACTG -3' | |||
| Cx31.1 | 5'-ATATACCCTCCCTTCTATGGT-3' | 55 | 357 |
| 5'-TCACAGAATGGTTTTCTTCA-3' | |||
| Cx32 | 5'-CTGCTCTACCCCGGCTATGC-3' | 60 | 386 |
| 5'-CAGGCTGAGCATCGGTCGCTCTT-3' | |||
| Cx33 | 5'-GAGGCAGATTGCTGCTAACC-3' | 56 | 323 |
| 5'-AGGGCATGGTAGCTCATCAC-3' | |||
| Cx36 | 5'-GGGGTGCTGCAGAACACAGAGA-3' | 60 | 344 |
| 5'-ACCACACAAATGCCGCTCACA-3' | |||
| Cx37 | 5'-GGCTGGACCATGGAGCCGGT-3' | 60 | 422 |
| 5'-TTTCGGCCACCCTGGGGAGC-3' | |||
| Cx39 | 5'-TTGCTCGCATGTACCCTG-3' | 55 | 265 |
| 5'-CACCTGACCTTGGCTAAGATA-3' | |||
| Cx40 | 5'-CTGTCCCCACCCAGTCAACT-3' | 57 | 460 |
| 5'-CGGTTTGTCACTATGGTAGC-3' | |||
| Cx43 | 5'-TACCACGCCACCACCGGCCCA-3' | 60 | 294 |
| 5'-GGCATTTTGGCTGTCGTCAGGGAA-3' | |||
| Cx45 | 5'-TTCCAAGTCCACCCATTTTAT-3' | 55 | 444 |
| 5'-ATCGTTCCTGAGCCATTCTGA-3' | |||
| Cx46 | 5'-GGAAAGGCCACAGGGTTTCCTGG-3' | 60 | 332 |
| 5'-GGGTCCAGGAGGACCAACGG-3' | |||
| Cx47 | 5'-ATGGGATTGCTTCGTGTCGC-3' | 60 | 256 |
| 5'-ACGCACCACCAGGCTGTAGTCTG-3' | |||
| Cx50 | 5'-CTTTGTATCCCGGCCTACTGA-3' | 60 | 466 |
| 5'-CTTCTCTCCCACTTCCGGTTCCA-3' | |||
| Cx57 | 5'-CCACATGACCGCAGTG-3' | 60 | 392 |
| 5'-GGTGATGGGCTGTTTTCT-3' |
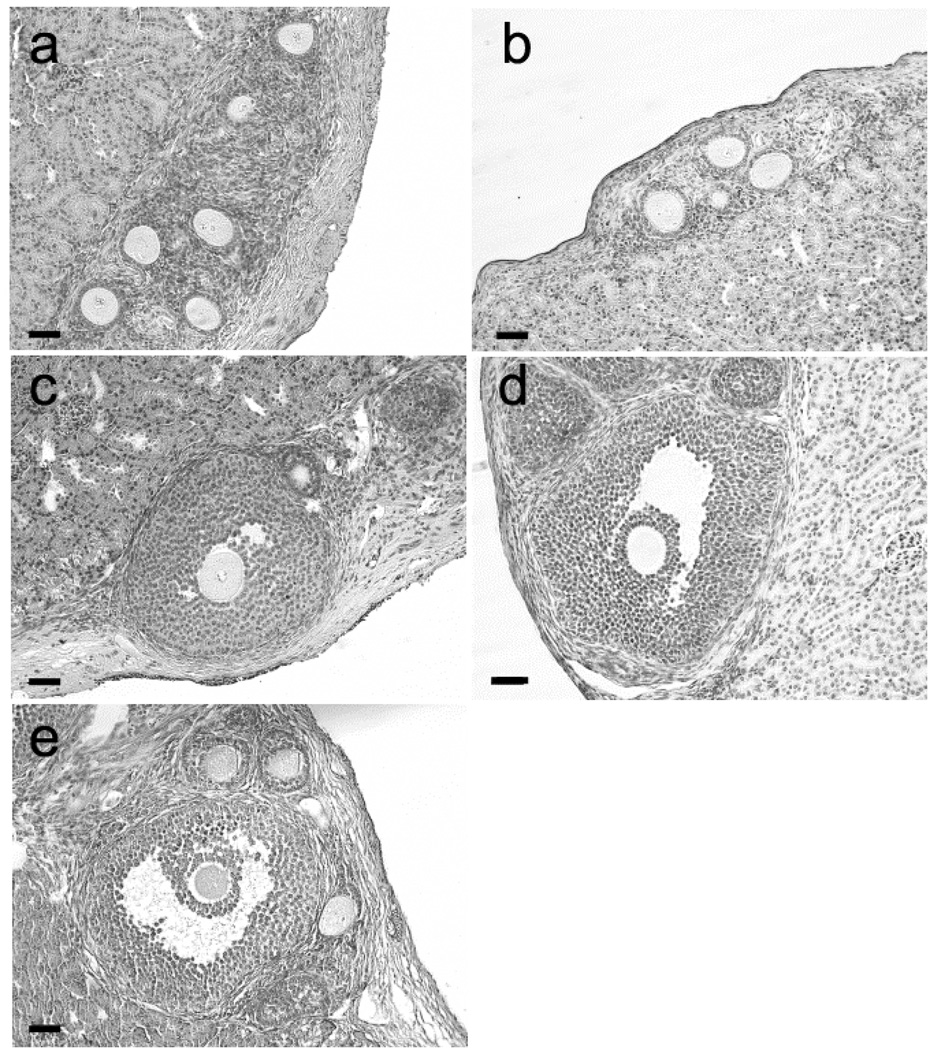
The first mutant phosphorylation site construct tested contained all four MAPK sites in the C-terminal tail (S255, S262, S279, and S282) mutated from serine to alanine. When this construct was used to transduce Cx43-deficient somatic cells for recombinant ovary construction, the resulting follicles were restricted to a single layer of granulosa cells, similar to the results seen in the empty vector controls (Fig. 3a, Table 1). Likewise, mutating both serine 279 and serine 282 to alanine resulted in the majority of follicles remaining restricted to the primary stage with only a few reaching the two layer early secondary stage (Fig. 3b, Table 1). We then tested individual sites for their ability to rescue folliculogenesis. When the transduced connexin contained a single serine to alanine mutation at residue 255, folliculogenesis proceeded to the antral stage suggesting phosphorylation of that residue is not critical in this context (Fig. 3c). When the same serine was mutated to aspartic acid (S255D), the same result was seen (Fig. 3d). Likewise, the S262D mutation supported folliculogenesis to the antral stage, rescuing the null mutant phenotype (Fig. 3e, Table 1). Thus phosphorylation at neither the S255 nor the S262 site is critical for folliculogenesis. Furthermore, the follicle stage distributions in the mutant-expressing recombinant grafts that exhibited limited follicle growth were significantly different from the distribution in grafts expressing Cx43.
Fig. 3.
Folliculogenesis is impaired in recombinant ovaries constructed with somatic cells expressing Cx43 with two or more MAPK site mutations. (a) In ovaries constructed with somatic cells expressing the quadruple mutant (S255A + S262A + S279A + S282A), folliculogenesis was restricted to the primary stage. (b) When constructed with somatic cells expressing the double mutant (S279A + S282A), ovaries were devoid of 514 follicles beyond the primary/early secondary stage. (c, d, e) In ovaries constructed with somatic cells expressing the single MAPK site mutations S255A (c), S255D (d), or S262D (e), folliculogenesis reached the antral stage. Scale bars: 50 µm
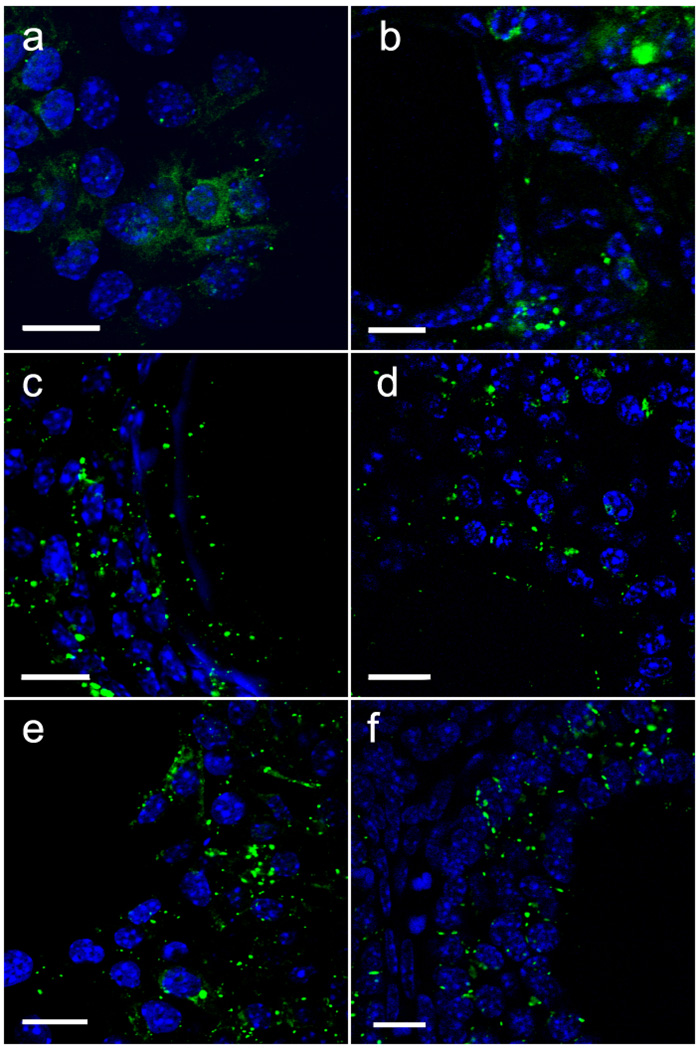
The localization of Cx43 within the recovered follicles was examined to determine if the phosphorylation mutants had an effect on gap junction plaque formation within the grafted recombinants. Plaques containing Cx43 were found at the junctional membranes of some granulosa cells in all groups tested, although follicles from reaggregated ovaries expressing the quadruple mutant (S255A + S262A + S279A + S282A) exhibited few gap junction plaques and more intracellular Cx43 (Fig. 4a). Similarly, follicles expressing the double mutant (S279A + S282A), while having more membrane plaques than ovaries constructed with the quadruple mutant, also exhibited cytoplasmic immunostaining (Fig. 4b). In contrast, typical punctate membrane immunostaining and little cytoplasmic immunostaining was observed in reaggregated ovaries expressing the single mutants S255A, S255D, and S262D that supported folliculogenesis (Fig. 4c–e).
Fig. 4.
Immunofluorescence analysis reveals altered Cx43 subcellular distribution in granulosa cells expressing mutants with two or more MAPK site mutations. (a) Ovaries made with somatic cells expressing the quadruple mutation (S255A + S262A + S279A + S282A) exhibited very few membrane plaques in the granulosa cells but ample intracellular staining. (b) Intracellular Cx43 immunostaining was also seen in follicles expressing the double mutant (S279A + S282A), but membrane plaques were more numerous than with the quadruple mutant. (c–f) Granulosa cells in recombinant ovaries expressing the single mutations S255A (c), S255D (d), and S262D (e) all displayed numerous membrane plaques with little intracellular immunoreactivity as in ovaries made with somatic cells expressing wildtype Cx43 (f). Scale bars: 10 µm
Oocyte Growth is Retarded in Reaggregated Ovaries Where Follicle Growth is Impaired
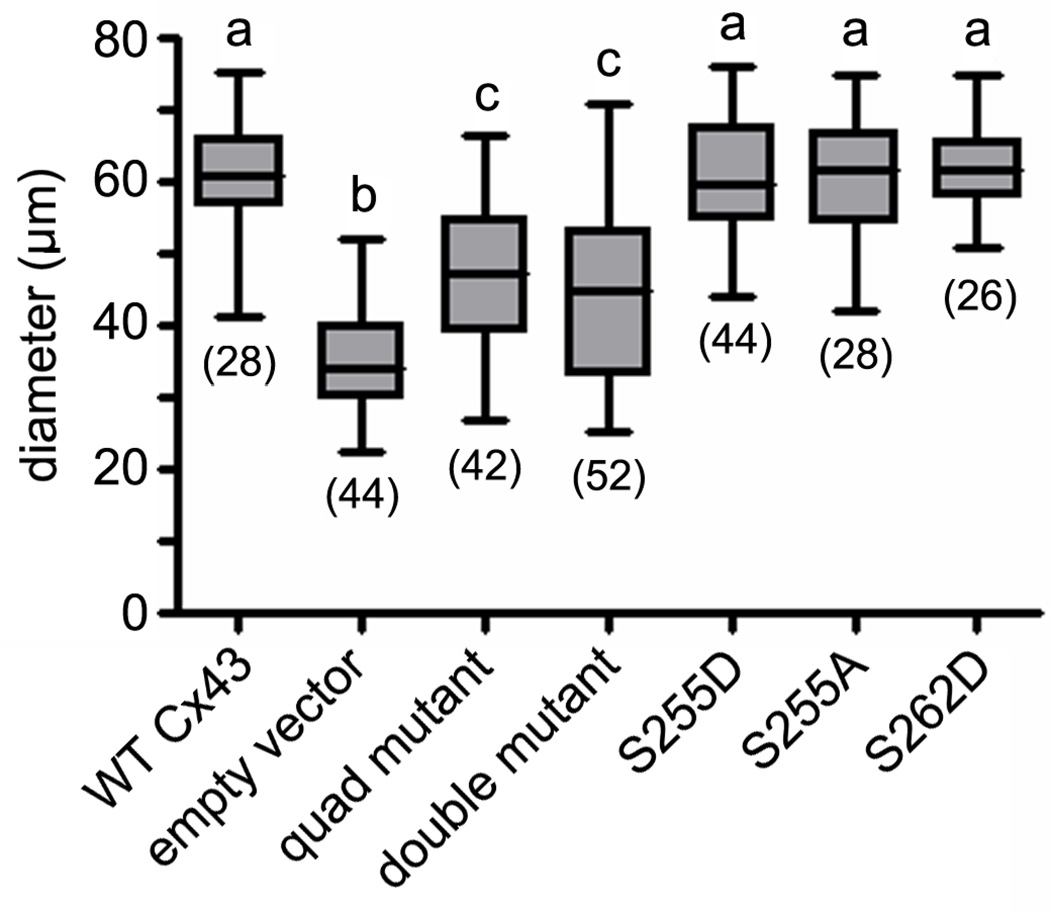
In order to determine if the inability to phosphorylate the selected MAPK sites in Cx43 had an effect on oocyte growth, oocyte diameters were measured. Ovaries constructed of wildtype oocytes and Cx43-deficient somatic cells transduced with wildtype Cx43 had a mean oocyte diameter of 61.34 ± 1.47 µm (Fig. 5). This was significantly (P < 0.01) greater than the mean diameter (35.47 ± 1.12 µm) of oocytes obtained from reaggregated ovaries constructed with wildtype oocytes and empty vector-transduced somatic cells. Likewise, the follicles of reaggregated ovaries expressing the quadruple mutant contained oocytes that were significantly smaller (at 47.05 ± 1.63 µm mean diameter) than those expressing wildtype Cx43 (P < 0.001), however, they were significantly larger than the oocytes in the empty vector control ovaries (P < 0.01) The single mutation S255A resulted in oocytes whose mean diameter (60.43 ± 1.69 µm) was not significantly different from that of follicles transduced with wildtype Cx43. Similarly, oocytes averaged 60.96 ± 1.22 µm and 62.11 ± 1.18 µm in diameter when combined with somatic cells expressing the S255D and S262D mutants, respectively, neither of which was significantly different from oocytes combined with wildtype Cx43-transduced somatic cells. Oocytes within follicles transduced with the double mutant S279A and S282A were significantly (P < 0.01) smaller (at 43.99 ± 1.64 µm) than those in follicles expressing wildtype Cx43 indicating that the ability to phosphorylate one or both of those sites is essential for oocyte growth (Fig. 5).
Fig. 5.
Oocyte growth was diminished in recombinant follicles that failed to develop beyond the early secondary stage. Oocyte diameters were measured in follicles constructed from ovarian somatic cells transduced with (left to right) wildtype Cx43, empty vector, the quadruple mutant (S255A + S262A + S279A + S282A), the double mutant (S279A + S282A), S255D, S255A, and S262D. The number of oocytes measured for each experimental group is shown in parentheses. Mean diameters with different letters above them differ significantly (P < 0.05).
Inability to Phosphorylate S279 and S282 Restricts Proliferation of Granulosa Cells in Vitro
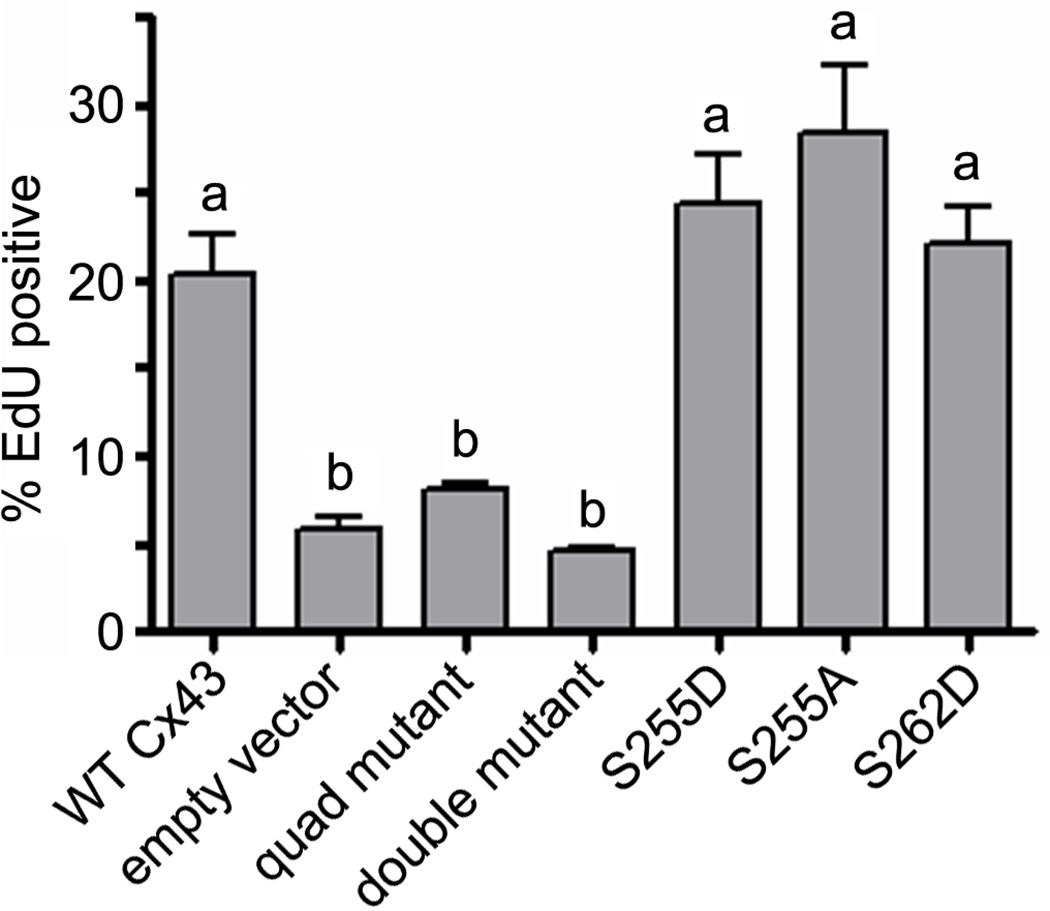
The inability of the transduced S279A/S282A double mutant construct to rescue folliculogenesis in Cx43-deficient reaggregated ovaries suggested failure of the mutant to support granulosa cell proliferation. To test this hypothesis directly, we infected Cx43-deficient ovarian somatic cells with the various wildtype and mutant vector constructs and after 48 h, added EdU and allowed the cells to incorporate this fluorescent nucleoside analog for a further 12 h in vitro. Cx43-deficient cells transduced with the quadruple mutant proliferated at a significantly lower rate (8.02 ± 0.35%; P < 0.05) when compared with cells expressing wildtype Cx43 (20.29 ± 2.38%); indeed, the quadruple mutant construct was not significantly better than the empty vector, which supported a proliferation rate of only 5.74 ± 0.84% (Fig. 6). Similar results were seen with the S279A/S282A double mutant-transduced ovarian somatic cells: the proliferation rate was significantly lower (4.49 ± 0.30%) when compared to the wildtype Cx43-transduced cells (Fig. 6, P < 0.01) and not significantly different from the empty vector control. The single mutants supporting rescue of folliculogenesis were not significantly different from wildtype Cx43 with S255D proliferating at 24.34 ± 2.82%, S255A at 28.46 ± 3.70%, and S262D at 22.07 ± 2.16%, indicating that the inability of the double and quadruple mutants to rescue folliculogenesis in Cx43-deficient reaggregated ovaries results from diminished granulosa cell proliferation.
Fig. 6.
Failure of recombinant follicles to develop beyond the early secondary stage is due at least in part to reduced granulosa cell proliferation. A 12 h in vitro EdU incorporation assay was used to measure proliferation of Cx43-deficient granulosa cells transduced with (left to right) wildtype Cx43, empty vector, the quadruple mutant (S255A + S262A + S279A + S282A), the double mutant (S279A + S282A), S255D, S255A, and S262D. Mean EdU incorporation frequencies with different letters above them differ significantly (P < 0.05).
Discussion
During the development of ovarian follicles, Cx43 expression in granulosa cells is required for both follicle and oocyte growth (Kidder and Vanderhyden 2010). The results of this study allowed us to conclude that phosphorylation of serine residues in the C-terminal cytoplasmic tail of Cx43, known to be targets for MAP kinases, occurs in the early stages of folliculogenesis and is involved in regulating granulosa cell proliferation. To our knowledge, this is the first demonstration that phosphorylation of specific Cx43 serines plays a role in cell proliferation in vivo. Previous work had demonstrated that phosphorylation of Cx43 in mature, pre-ovulatory follicles by MAP kinases occurs in response to luteinizing hormone (LH) (Sela-Abramovich et al. 2005, 2008). LH treatment results in phosphorylation of Cx43 at S255, S262, S279, and S282 (Norris et al. 2008), causing the attenuation of gap junctional communication among the cumulus cells that triggers oocyte maturation. The present work extends to earlier stages of folliculogenesis the importance of MAP kinases in the life cycle of ovarian follicles.
The majority of connexin proteins have been shown to be phosphorylated, with most of the phosphorylated residues residing in the cytoplasmic C-terminal tail (reviewed by Lampe and Lau 2000). However, given that not all connexins have a C-terminal tail long enough to include those sites and that truncated forms of Cx43 lacking the C-terminal tail are still capable of forming functional gap junctions (Maass et al. 2007), it is clear that phosphorylation of the C-terminal tail is not required for the establishment of intercellular coupling. It has been clear for some time, however, that C-terminal phosphorylation of Cx43 is involved in the regulation of gap junctional communication. For example, S255, S279 and S282 are phosphorylated directly by activated MAPK in EGF-treated cells with phosphorylation of one or both of the S279/S282 sites being adequate to disrupt gap junctional communication (Warn-Cramer et al. 1998). It was further reported in that study that mutating S255, S279, and S282 to alanine abolished the ability of EGF to disrupt communication. In addition to channel activity, EGF treatment leads to internalization of Cx43 (Leithe and Rivedal 2004), potentially in a direct manner through phosphorylation of Cx43 at serines 279 and 282 (Leykauf et al. 2003).
In the present work, Cx43 in primary and early secondary follicles, in contrast to that of more advanced follicles, was found to be predominately intracellular and relatively highly phosphorylated at serines 279 and 282. This is similar to the situation in cells undergoing mitosis, where Cx43 is redistributed to an intracellular location in association with an increase in its phosphorylation (Boassa et al. 2010). Possibly, phosphorylation at serines 279 and 282 in granulosa cells limits the amount of Cx43 within gap junctions and/or decreases the level of gap junctional communication, thereby allowing their entry into M phase.
Our results are consistent with the work ofMaass et al. (2004) who found that surviving knock-in female mice homozygous for a truncation mutant of Cx43 (Cx43K258stop), one that lacks most of the C-terminal cytoplasmic tail, are infertile due to impaired folliculogenesis. The mice analyzed in that study lacked three of the four serines (S262, S279 and S282) that, when collectively mutated to alanine in our study, failed to support folliculogenesis. However, our results must also be considered in the context of the preliminary (unpublished) finding that female knock-in mice homozygous for the same quadruple mutant used in the present study can produce apparently healthy litters. An analysis of follicle growth, ovulation rate, and litter size in those mice has not yet been carried out to determine if they may in fact be subfertile as recently determined in the case of apparently fertile females carrying the dominant loss-of-function G60S substitution in Cx43 (Tong et al. 2009). One possible reason for the apparent discrepancy with the present results is a difference in strain background. It is known that the importance of Cx43-mediated intercellular communication for folliculogenesis in mice is influenced by genetic background: the Gja1 null mutation in the homozygous state causes follicle development to arrest in the primary stage on the C57BL/6 background (Ackert et al. 2001) whereas some follicles can develop into antral stages when the same mutation is crossed into the CD1 background (Tong et al. 2006). The quadruple mutant mice have to this point been maintained on a mixed C57BL/6 × 129S6SvEV genetic background and thus their continued fertility could be explained by strain-specific genetic modifiers that do not come into play in recombinant ovaries when both oocytes and ovarian somatic cells are of the inbred C57BL/6 strain.
In conclusion, the results presented here add yet another level of regulation- that by MAP kinases acting on Cx43- to the list of factors regulating the early stages of folliculogenesis and oocyte growth, raising the possibility that interference with that regulation may contribute to female infertility.
Acknowledgements
This work was supported by an operating grant to G.M.K. and a Postdoctoral Fellowship to P.W.D. from the Canadian Institutes of Health Research and grant GM55632 to P.D.L. from the U.S. National Institutes of Health. We thank Kevin Barr, Dan Li, and Tony Y. Li for their expert technical assistance and the University of Western Ontario Health Sciences Animal Facility staff for providing mouse care.
References
- Ackert CL, Gittens JEI, O'Brien MJ, Eppig JJ, Kidder GM. Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol. 2001;233:258–270. doi: 10.1006/dbio.2001.0216. [DOI] [PubMed] [Google Scholar]
- Beardslee M, Laing J, Beyer E, Saffitz J. Rapid turnover of connexin43 in the adult rat heart. Circ Res. 1998;83:629–635. doi: 10.1161/01.res.83.6.629. [DOI] [PubMed] [Google Scholar]
- Boassa D, Solan JL, Papas A, Thornton P, Lampe PD, Sosinsky GE. Trafficking and recycling of the connexin43 gap junction protein during mitosis. Traffic. 2010;11:1471–1486. doi: 10.1111/j.1600-0854.2010.01109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper CD, Lampe PD. Casein kinase 1 regulates connexin43 gap junction assembly. J Biol Chem. 2002;277:44962–44968. doi: 10.1074/jbc.M209427200. [DOI] [PubMed] [Google Scholar]
- Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol. 1990;10:1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppig JJ, Wigglesworth K. Development of mouse and rat oocytes in chimeric reaggregated ovaries after interspecific exchange of somatic and germ cell components. Biol Reprod. 2000;63:1014–1023. doi: 10.1095/biolreprod63.4.1014. [DOI] [PubMed] [Google Scholar]
- Gittens JEI, Kidder GM. Differential contributions of connexin37 and connexin43 to oogenesis revealed in chimeric reaggregated mouse ovaries. J Cell Sci. 2005;118:5071–5078. doi: 10.1242/jcs.02624. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol. 2003;4:285–94. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Kanemitsu MY, Jiang W, Eckhart W. Cdc2-mediated phosphorylation of the gap junction protein, connexin43, during mitosis. Cell Growth Differ. 1998;9:13–21. [PubMed] [Google Scholar]
- Kidder GM, Vanderhyden BC. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can J Physiol Pharmacol. 2010;88:99–413. doi: 10.1139/y10-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW, Puranam KL, Revel JP. Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J. 1991;273:67–72. doi: 10.1042/bj2730067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD. Analyzing phorbol ester effects on gap junction communication: a dramatic inhibition of assembly. J Cell Biol. 1994;127:1895–1905. doi: 10.1083/jcb.127.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, Kurata WE, Warn-Cramer B, Lau AF. Formation of a distinct connexin43 phosphoisoform in mitotic cells is dependent upon p34cdc2 kinase. J Cell Sci. 1998;111:833–841. doi: 10.1242/jcs.111.6.833. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. Regulation of gap junctions by phosphorylation. Arch Biochem Biophys. 2000;384:205–215. doi: 10.1006/abbi.2000.2131. [DOI] [PubMed] [Google Scholar]
- Lampe PD, Lau AF. The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol. 2004;36:1171–1186. doi: 10.1016/S1357-2725(03)00264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF. Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol. 2000;126:1503–1512. doi: 10.1083/jcb.149.7.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leithe E, Rivedal E. Epidermal growth factor regulates ubiquitination, internalization and proteasome-dependent degradation of connexin43. J Cell Sci. 2004;117:1211–1220. doi: 10.1242/jcs.00951. [DOI] [PubMed] [Google Scholar]
- Leykauf K, Dürst M, Alonso A. Phosphorylation and subcellular distribution of connexin43 in normal and stressed cells. Cell Tiss Res. 2003;311:23–30. doi: 10.1007/s00441-002-0645-5. [DOI] [PubMed] [Google Scholar]
- Maass K, Ghanem A, Kim J-S, Saathoff M, Urschel S, Kirfel G, Grümmer R, Kretz M, Lewalter T, Tiemann K, Winterhager E, Herzog V, Willecke K. Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol Biol Cell. 2004;15:4597–4608. doi: 10.1091/mbc.E04-04-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maass K, Shibayama J, Chase SE, Willecke K, Delmar M. C-terminal truncation of connexin43 changes number, size, and localization of cardiac gap junction plaques. Circ Res. 2007;101:1283–1291. doi: 10.1161/CIRCRESAHA.107.162818. [DOI] [PubMed] [Google Scholar]
- Musil LS, Beyer EC, Goodenough DA. Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol. 1990;116:163–175. doi: 10.1007/BF01868674. [DOI] [PubMed] [Google Scholar]
- Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA. Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development. 2008;135:3229–38. doi: 10.1242/dev.025494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DJ, Wallick CJ, Martyn KD, Lau AF, Jin C, Warn-Cramer BJ. Akt phosphorylates Connexin43 on Ser373, a "mode-1" binding site for 14-3-3. Cell Commun Adhes. 2007;14:211–226. doi: 10.1080/15419060701755958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaume AG, De Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Saez JC, Nairn AC, Czernik AJ, Fishman GI, Spray DC, Hertzberg EL. Phosphorylation of connexin43 and the regulation of neonatal rat cardiac myocyte gap junctions. J Mol Cell Cardiol. 1997;29:2131–2145. doi: 10.1006/jmcc.1997.0447. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Chorev E, Galiani D, Dekel N. Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology. 2005;146:1236–1244. doi: 10.1210/en.2004-1006. [DOI] [PubMed] [Google Scholar]
- Sela-Abramovich S, Galiani D, Nevo N, Dekel N. Inhibition of rat oocyte maturation and ovulation by nitric oxide: mechanism of action. Biol.Reprod. 2008;78:1111–1118. doi: 10.1095/biolreprod.107.065490. [DOI] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin43 in LA-25 cells with active v-src is phosphorylated on Y247, Y265, S262, S279/282 and S368 via multiple signaling pathways. Cell Comm Adhes. 2008;15:75–84. doi: 10.1080/15419060802014016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan JL, Lampe PD. Connexin43 phosphorylation: structural changes and biological effects. Biochem J. 2009;419:261–272. doi: 10.1042/BJ20082319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD. Phosphorylation of Cx43 at S365 is a gatekeeper event that changes the structure of Cx43 and prevents downregulation by PKC. J Cell Biol. 2007;179:1301–1309. doi: 10.1083/jcb.200707060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosinsky GE, Solan JL, Gaietta GM, Ngan L, Mackey M, Lampe PD. The C-terminus of connexin43 adopts different conformations in the Golgi and gap junction as detected with structure specific antibodies. Biochem J. 2007;408:375–385. doi: 10.1042/BJ20070550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson KI, Piwnica-Worms H, McNamee H, Paul DL. Tyrosine phosphorylation of the gap junction protein connexin43 is required for pp60src-induced inhibition of communication. Cell Regul. 1990;1:989–1002. doi: 10.1091/mbc.1.13.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong D, Gittens JEI, Kidder GM, Bai D. Patch clamp study reveals that the importance of connexin43-mediated gap junctional communication for ovarian folliculogenesis is strain-specific in the mouse. Am J Physiol -Cell Physiol. 2006;290:290-C297. doi: 10.1152/ajpcell.00297.2005. [DOI] [PubMed] [Google Scholar]
- Tong D, Li TY, Naus KE, Bai D, Kidder GM. In vivo analysis of undocked connexin43 gap junction hemichannels in ovarian granulosa cells. J Cell Sci. 2007;120:4016–4024. doi: 10.1242/jcs.011775. [DOI] [PubMed] [Google Scholar]
- Tong D, Colley D, Thoo R, Li TY, Plante I, Laird DW, Bai D, Kidder GM. Oogenesis defects in a mutant mouse model of oculodentodigital dysplasia. Dis Models Mech. 2009;2:157–167. doi: 10.1242/dmm.000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LWM, Eckhart W, Lau AF. Characterization of the MAP kinase phosphorylation sites on the connexin43 gap junction protein. J Biol Chem. 1996;271:3779–3786. doi: 10.1074/jbc.271.7.3779. [DOI] [PubMed] [Google Scholar]
- Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF. Regulation of connexin43 gap junctional intercellular communication by mitogen-activated protein kinase. J Biol Chem. 1998;273:9188–9196. doi: 10.1074/jbc.273.15.9188. [DOI] [PubMed] [Google Scholar]