Abstract
Objectives. To evaluate hospital and outpatient pharmacists’ pharmacogenomics knowledge before and 2 months after participating in a targeted, case-based pharmacogenomics continuing education program.
Design. As part of a continuing education program accredited by the Accreditation Council for Pharmacy Education (ACPE), pharmacists were provided with a fundamental pharmacogenomics education program.
Evaluation. An 11-question, multiple-choice, electronic survey instrument was distributed to 272 eligible pharmacists at a single campus of a large, academic healthcare system. Pharmacists improved their pharmacogenomics test scores by 0.7 questions (pretest average 46%; posttest average 53%, p=0.0003).
Conclusions. Although pharmacists demonstrated improvement, overall retention of educational goals and objectives was marginal. These results suggest that the complex topic of pharmacogenomics requires a large educational effort in order to increase pharmacists’ knowledge and comfort level with this emerging therapeutic opportunity.
Keywords: pharmacogenomics, continuing education, genetic testing, survey, personalized medicine
INTRODUCTION
Since the human genome project was completed in 2003, pharmacogenomics has become a rapidly expanding area of science that blends molecular pharmacology and genomics.1 This burgeoning field investigates the important contribution of genetic inheritance in the variability of drug response. 2,3 A wide range of medication effects can be explained by genomic differences, which include but are not limited to toxic adverse effects and lack of efficacy.
The United States Food and Drug Administration (FDA) added pharmacogenomics testing requirements for several drugs, including cetuximab, dasatinib, maraviroc, and trastuzumab, and has recommended testing for abacavir, carbamazepine, mercaptopurine, and irinotecan, among others.4 In 2007, the FDA changed warfarin labeling to include pharmacogenomics testing considerations, and in 2010, announced the addition of a black-box warning for clopidogrel based on pharmacogenomics metabolism concerns.5,6 The FDA also has updated the pimozide drug label to include dosing information for CYP2D6 poor metabolizers.7 These labeling changes are among the first tangible steps toward individualized medication dosing based on a patient’s genetic information.
Rapid technological advancements in molecular pharmacology and genetics are facilitating the push to translate laboratory discoveries to the patient bedside. As drug experts and point-of-care providers, pharmacists are well-positioned to lead this new era of individualized medicine.8,9 Pharmacogenomics has moved beyond the limited confines of a laboratory environment and now represents an opportunity for clinical pharmacists to deliver enhanced patient care at the bedside and at the outpatient pharmacy window in an integrated clinical manner.10-12
An assessment of the pharmacogenomics educational needs of hospital and outpatient pharmacists was performed to facilitate the development of a pharmacogenomics educational program.13 Results of the needs assessment showed that although pharmacists believed that pharmacogenomics knowledge was important to the pharmacy profession, they lacked the knowledge and self-confidence to make therapeutic recommendations based on pharmacogenomics test results.13 Similarly, a community pharmacy survey found that pharmacists were not confident in their knowledge of genetic testing and pharmacogenomics.14 These results suggest that pharmacists would benefit from a clinically relevant pharmacogenomics educational program. Using this information as background, the objective of this study was to measure the impact of a case-based pharmacogenomics education program for pharmacists at 1 campus of a large, academic, multicampus healthcare system.
DESIGN
A previously conducted needs-assessment survey guided the development and delivery strategies for a case-based educational program.13 The pharmacy department at Mayo Clinic, Rochester, Minnesota, mandated the educational program for inpatient, outpatient, and administration pharmacists located on the study campus. The required educational program consisted of a 1-hour, case-based presentation focusing on fundamental principles of pharmacogenomics, including metabolic, labeling, and genomic considerations. The learning objectives for the fundamentals-of-pharmacogenomics education program included that participants be able to justify the importance of pharmacogenomics in pharmacy practice; explain pharmacogenomics concepts; evaluate pharmacokinetic alterations caused by polymorphisms; describe tests for pharmacogenomics issues; and specify FDA labeling requirements for pharmacogenomics. The concepts of pharmacogenomics were explored through patient cases based on drug-gene pairs including: abacavir (HLA-B*5701), mercaptopurine (thiopurine methyltransferase), warfarin (CYP2C9 and VKORC1), and codeine and tamoxifen (CYP2D6).
The Accreditation Council for Pharmacy Education (ACPE)-accredited educational program was presented live on 3 different occasions at various campus locations. The educational program was Web-cast by means of the campus intranet and was recorded and Web-archived for pharmacists who were unable to attend the live presentations because of schedule conflicts. Six additional nonmandatory 1-hour specialty lectures accredited by the Minnesota State Board of Pharmacy were subsequently offered to the pharmacists. The topics of these pharmacogenomics presentations included psychiatry, anesthesiology/pain, critical care, infectious diseases, hematology/oncology, and cardiology. The lectures (live and Web-archived) were available to pharmacists during the 2-month window between the mandatory lecture and administration of the post-education survey instrument.
EVALUATION AND ASSESSMENT
As part of an ACPE-accredited continuing education program, pharmacists provided an evaluation of the fundamental pharmacogenomics education program to fulfill their educational requirement. These results were collected and evaluated to ascertain the pharmacists’ perceptions of the quality of the program as well as their acceptance of it.
Prior to initiating the study, the institutional review board granted the study exempt status. An 11-question, multiple-choice, electronic survey instrument was developed in collaboration with the institutional survey research support center. The survey was composed of 2 sections. The first section, which was administered to both pre- and post-education groups, contained 10 knowledge questions about fundamental pharmacogenomics concepts, metabolism, FDA pharmacogenomics testing and labeling, and 1 yes/no question regarding provision of pharmacogenomics information to healthcare professionals or patients in the previous 2-3 months. Each knowledge question had 4 possible answers. The second section of the survey instrument, which was administered only to the post-education group, assessed demographic information, including primary pharmacy practice setting (hospital/inpatient, ambulatory/outpatient, administration, other), amount of time hospital/inpatient spent at the patient bedside (less than 25%, 25%-50%, 51%-75% or more than 75%), pharmacy practice years, attendance at additional expertise pharmacogenomics lectures (yes/no), and whether education was considered a valuable opportunity (yes/no). The survey was reviewed and critiqued by the institutional survey research support center. Six pharmacists reviewed and field-tested the survey instrument for question refinement and enhancement of content validity.
The survey instrument was administered in a matched fashion at baseline prior to pharmacogenomics education and 2 months after delivery of pharmacogenomics education to a target population of hospital, outpatient, and administrative pharmacists on 1 campus of a large, academic, multicampus healthcare system. The study campus included 2 hospitals with approximately 2,000 beds and a network of 6 outpatient pharmacies, and served more than 318,000 patients in 2010.15
E-mail addresses for all pharmacists on the study campus (N=272) were verified and collated into a distribution list. The internal survey research support center administered the matched survey instruments to maintain participant anonymity. Pharmacists received an e-mail invitation to participate that included a Web link to the survey instrument. Pharmacists who did not participate in the pre-education survey were removed from the post-education survey e-mail distribution list to ensure that only matched pre- and post-education survey instruments were evaluated for results. Two reminder e-mails were sent to nonrespondents, and the survey was closed 13 days after the initial survey invitation was issued. A 2-month period was selected as the timeframe to be evaluated for retention of pharmacogenomics education. After delivery of a case-based educational program, pharmacists who had participated in the pre-education survey were invited to complete the post-education survey instrument. Similarly, 2 reminder e-mails were sent to nonrespondents, and the survey was closed 14 days after the postsurvey invitation was sent. Pharmacists who did not respond to the post-educational survey instrument were excluded from data analyses.
Each of the 10 knowledge items on the pre- and post-education survey instruments was scored as correct or incorrect, with missing items scored as incorrect. The overall score was calculated for each respondent and summarized over all respondents. To investigate the degree of improvement among the respondents, McNemar’s test was used to compare the percentage of correct responses for each item on the pre-education survey instrument compared with that of the post-education instrument. The difference in the average score between pre- and post-education survey instruments was analyzed with the Wilcoxon signed-rank test. The average scores at each time point as well as the difference in scores between pre- and post-education survey instruments were compared between practice setting groups using Kruskal-Wallis tests. Cochran-Armitage tests for trend were performed to observe whether the scores increased or decreased over years of experience or by amount of bedside time (ordinal). P values of less than 0.05 were considered significant. All analyses were conducted using SAS version 9 (Cary, NC).
Two hundred seventy-two electronic survey instruments containing 11 multiple-choice test questions were distributed prior to the pharmacy department-required educational program on pharmacogenomics. Two hundred thirty-three pharmacists participated in the program during the study period and were eligible to participate in the matched surveys. One hundred eighty-six pharmacists (68%) completed the pre-education electronic survey instrument and thus were eligible to participate in the post-education survey 2 months later. Eighty-six pharmacists completed the paired survey instruments; however, 2 pharmacists’ responses were removed from the analyses because 1 of the pharmacists did not answer any of the scored questions on 1 survey instrument and the other’s e-mail/employee identification could not be verified. Therefore, 84 pharmacists (31% of the original 272) completed pre- and post-education survey instruments that could be matched.
Of those who completed both survey instruments, pharmacist practice years varied: 38 (46%) had been in practice for 5 or fewer years; 16 (19%) for 6 to 10 years; and 29 (35%) for more than 10 years. A majority (62%) of survey respondents were hospital pharmacists. Baseline characteristics are shown in Table 1. Survey items and quantified responses are listed in Table 2.
Table 1.
Demographic Information for Pharmacists on a Single Campus of a Large, Academic Multicenter Healthcare System
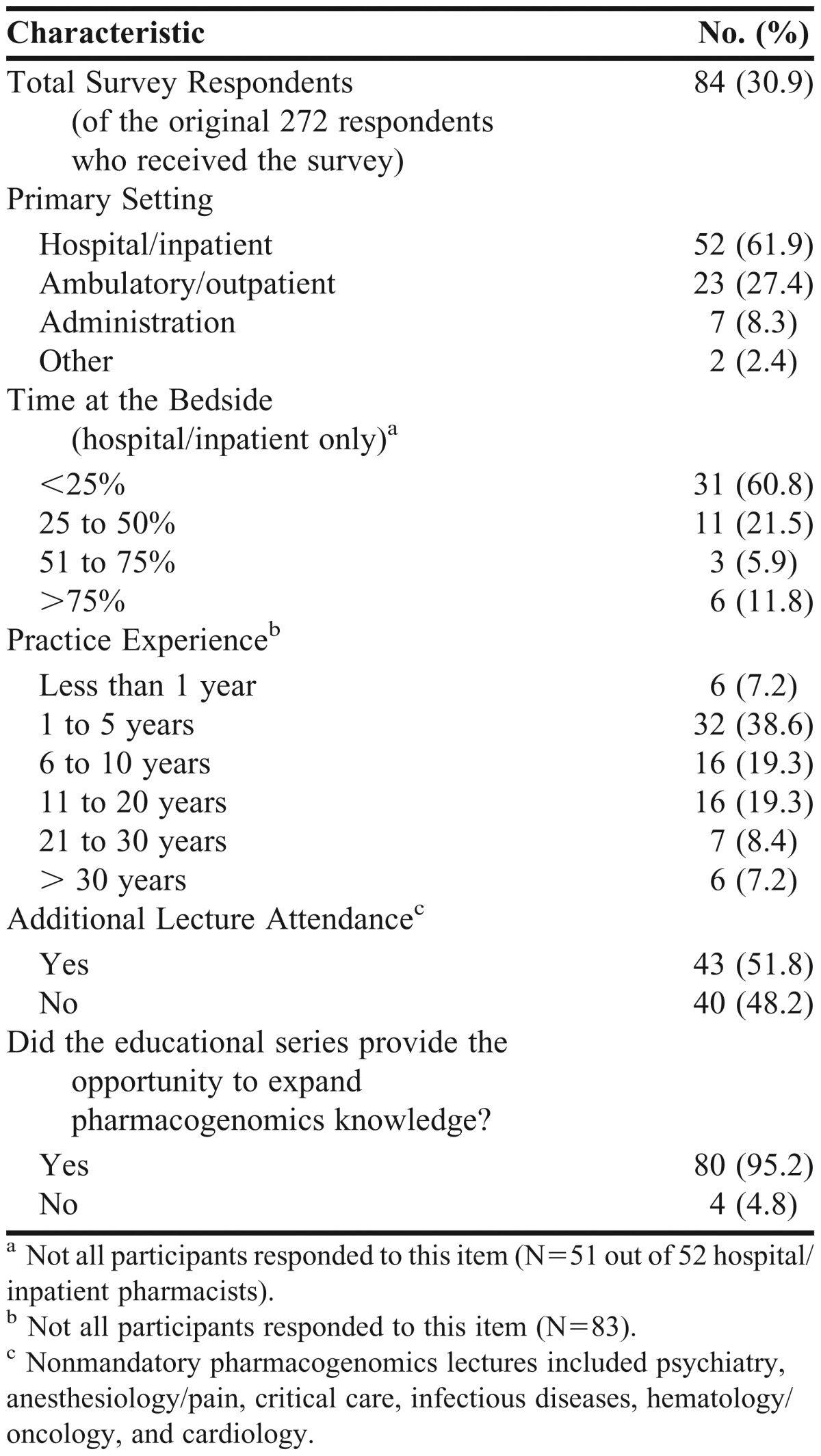
Table 2.
Respondents’ Cumulative Correct Responses to Educational Survey Items (N=84) Comparing the Pre-Education (Baseline) vs Post-education (2 Months Later) Results and Application of Pharmacogenomic Knowledge in Practice
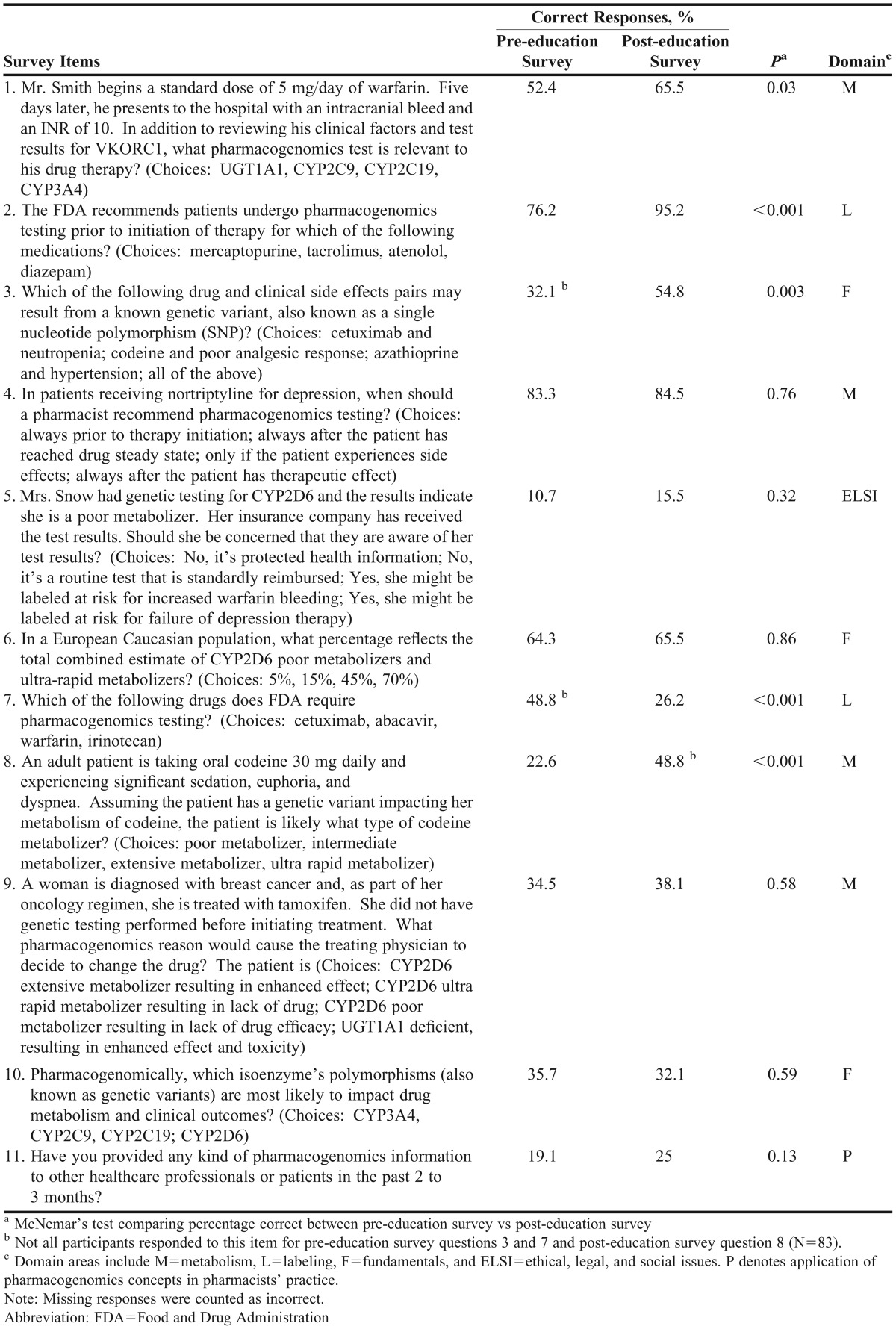
Three questions on the educational survey instrument probed pharmacist knowledge of pharmacogenomics fundamentals; 4 questions assessed concepts relating to genetic variability of metabolism; 2 questions explored knowledge of FDA pharmacogenomics testing and labeling requirements; and 1 question assessed ethical, social, and legal implications of pharmacogenomics testing. On average, pharmacists improved their correct response rate by 0.7 from the pre-education survey instrument to the post-education survey instrument (46% vs 53%, p=0.0003). When pharmacists were asked about providing clinical pharmacogenomics information to other professionals, 16 pharmacists reported having provided pharmacogenomics information pre-education, and 21 pharmacists reported having provided information post-education; there was no statistical difference between groups (19% vs 25%, p=0.13). Test scores showed a trend toward improvement based on attendance of additional nonrequired pharmacogenomics education lectures topics, but significance was not reached (p=0.056). Subgroup analyses were performed to evaluate survey differences for pharmacy practice setting, hospital/inpatient pharmacist bedside time, and pharmacy practice years.
The scores on the pre- and post-education survey instruments as well as the degree of difference in scores prior to and following the educational program were similar (p=0.75, p=0.75, and p=0.31, respectively) among pharmacy practice settings. Table 3 presents a comparison of pre- and post-education survey results by percentage of time spent at patient bedside among hospital/inpatient pharmacists. Prior to the educational program, the hospital/inpatient pharmacists with the most bedside time performed the best (p=0.008); however, the group with the least bedside time improved the most between the 2 surveys (the percentage of correct responses increased by an average of 8.4), whereas the other groups did not improve (p=0.055). Table 4 shows pre- and post-education survey comparisons by years of practice.
Table 3.
Subgroup Analyses for Comparison of Pre-education and Post-education Correct Responses, by Percentage of Time Spent at Bedside Among Hospital/Inpatient Pharmacists
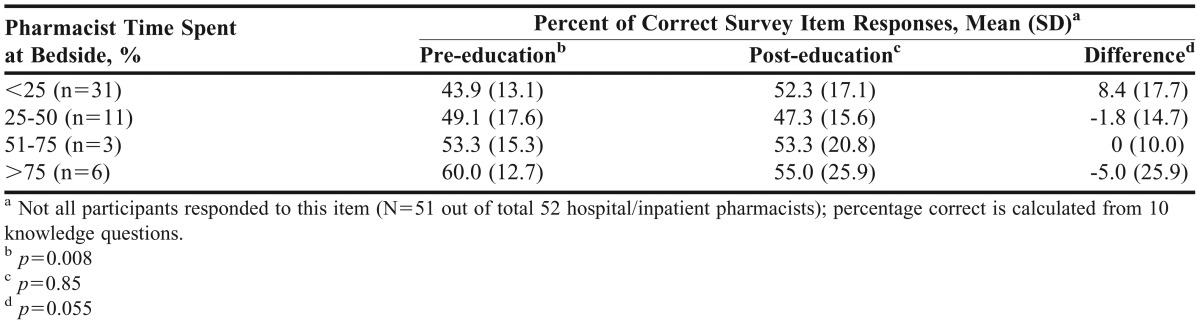
Table 4.
Subgroup Analyses for Comparison of Pre-education and Post-education Correct Responses, by Years of Practicea
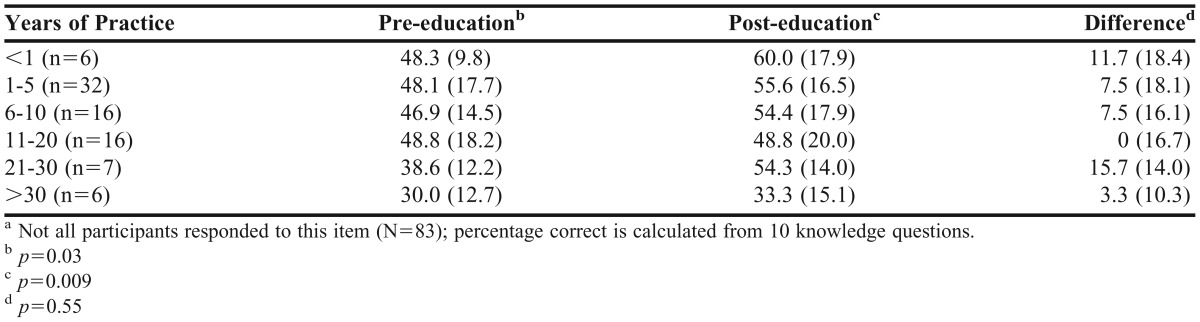
There was an inverse association between pharmacists’ years of experience and performance on the pre- and post-education survey instruments. With increasing years of experience, pharmacists demonstrated poorer scores on both the pre-education survey instrument (p=0.03) and the post-education survey instrument (p=0.009); however, all groups showed similar overall improvement on survey performance (p=0.55) after completing the program.
Finally, satisfaction with the mandatory pharmacogenomics educational program was evaluated as part of the process to award ACPE continuing education credit. Of the 233 pharmacists who participated in the educational program, a majority agreed or strongly agreed with the statements that pharmacogenomics was relevant to what pharmacists do (69%); the program increased pharmacists’ knowledge in this area (85%); the program justified the importance of pharmacogenomics in pharmacy practice (84%); and the program explained pharmacogenomics concepts (86%). Of the 84 pharmacists who participated in the matched educational surveys, 95% (n=80) indicated that they felt the pharmacogenomics educational series provided them the opportunity to expand their pharmacogenomics knowledge.
DISCUSSION
Pharmacogenomics has a growing role in drug development and clinical therapeutics. This emerging field of individualized medicine is based on an individual’s unique genetic composition and aims to reduce adverse drug effects, optimize drug therapeutics, and trim drug costs through cost-effective therapeutic management.16 Federal agencies such as the Department of Health and Human Services and FDA are incorporating pharmacogenomic dosing guidance into the labeling, development, and approval of drugs in a manner that supports gene-based care. Among the barriers to full clinical translation of pharmacogenomics is the need for evidence-based algorithms that guide clinicians toward optimal delivery of gene-based care.17 While there are well-developed electronic alert systems for drug interactions and allergies, informational technology systems are being developed to address the need for evaluation of drug and pharmacogenomics interactions.
The results of the needs assessments performed by McCullough and colleagues and Sansgiry and colleagues spurred our creation of a pharmacogenomics education program for pharmacists to support their professional pharmacogenomics interest and belief that pharmacists should lead the pharmacogenomics revolution toward personalized medicine.13,14 This task was achieved by addressing an educational gap that was identified as a result of the needs assessment for inpatient and outpatient pharmacists.
The educational program approach was to educate pharmacist clinicians regarding pharmacogenomics fundamentals and therapeutically relevant medications for use at the patient bedside or in the ambulatory/outpatient setting. The strategy was to prepare 1 mandatory continuing education lecture for all pharmacists and 6 additional nonmandatory pharmacogenomics specialty topics, including psychiatry, anesthesiology/pain, critical care, infectious diseases, hematology/oncology, and cardiology. The 6 nonmandatory lectures were developed to enhance the fundamental education and reduce pharmacogenomic complexity by breaking the information into smaller, more manageable educational segments. The small, long-term educational gains of the study may support a different educational delivery strategy, ie, establishing a minimum competency requirement for each segment to increase knowledge retention.18 Limited knowledge retention may have been attributable to the complex nature of pharmacogenomics or delivery of too much information in a short timeframe, preventing mastery of the topic. Other strategies to increase pharmacogenomics knowledge retention might include taking smaller educational steps with less information, providing annual re-education on fundamental concepts, and incorporating familiar pharmacology and pharmacokinetics concepts.19
The matched educational survey results were significantly different over a time period of 2 months, although pharmacists demonstrated marginal overall improvement. This finding may be a result of a low response rate (31%) for the matched survey instruments. We analyzed a subset of responders (n=181) from our original needs assessment to determine if the 2 groups were similar and found that the demographics of the large group were not different from those of the postsurvey data (n=84).13 Although we had a lower-than-expected matched-survey response rate, the responders to the post-education survey instrument were representative of those who responded to an earlier survey instrument with respect to work setting, percent of time spent at bedside, and years in practice. The results of this study, therefore, are generalizable to the large population of health system pharmacists who we previously assessed.13
There was a trend toward improved test scores for pharmacists who attended additional pharmacogenomics education (p=0.056); however, the small sample size of the subgroup analysis may not have provided enough power to detect any differences that may have existed. While a positive relationship between education and survey performance might be expected, the trend suggests that the subgroup analysis was too small to detect an improvement in survey performance with additional education, or that the period of 2 months between surveys resulted in poor knowledge retention for a complex topic. The results suggest that a strategy different than the one used for this particular study is needed for pharmacists to improve their retention of information and performance on pharmacogenomics knowledge survey instruments over time.
Contributing factors that may have impacted pharmacists’ retention of pharmacogenomics knowledge over the 2-month period between administration of the 2 survey instruments include the possibility that pharmacists did not use their newly acquired knowledge because of their general pharmacy practice areas. Pharmacists self-reported that their delivery of pharmacogenomics information to healthcare professionals or patients did not change over time (19% pre-education vs 25% post-education; p=0.13). The results of this study are similar to those of other studies, which have shown that over time, knowledge and skill retention are reduced in complex pharmaceutical and therapeutic skill sets, such as pharmaceutical drug compounding, delivery of advanced cardiovascular life support, use of automated external defibrillators, and use of metered-dose inhalers.20-24
The pharmacogenomics education program was attended by 233 pharmacists and was well-received, based on the overwhelmingly positive evaluations. Despite the positive reception, however, the program did not translate into overwhelming improvements on the matched survey instruments. Other educational programs that have been received positively have also demonstrated marginally effective long-term educational results.21,24
Our study has several possible limitations. The 31% matched survey response rate may not reflect the true overall effect of the pharmacogenomics education program; ie, the program may have had a larger impact if the remaining 70% of respondents had participated in the matched survey. A differently constructed educational program with a more focused approach toward competency attainment may be needed to enhance pharmacists’ performance on the matched survey instrument.
As this study was conducted in a large institutional setting, survey fatigue may have contributed to the poor matched-survey response. Our attempt to minimize fatigue by limiting the survey instrument to 11 multiple-choice items may not have fully compensated for the number of different survey instruments pharmacists in our study setting may have been subjected to on an institutional basis. Online internal department of pharmacy competencies and external survey requests also may have negatively impacted our response rate, resulting in nonrespondent bias.
Further, there may have been an educational bias in the study. The individuals who participated in the matched survey may have been more knowledgeable about pharmacogenomics and thus more likely to participate and perform well in the matched surveys. However, it does not appear that educational bias was a major factor considering that respondents’ average test scores demonstrated only modest improvement after a 2-month period (46% vs 53% correct, respectively). Additionally, response bias does not appear to have been a contributing factor. Although the 31% survey response rate was low, respondents appeared to be representative of the diverse pharmacist population that was previously studied and found to be generalizable to pharmacists in other institutions.13
Finally, there may have been exposure bias in this study, considering that the matched survey instruments were conducted in hospital and outpatient pharmacies on 1 campus of a large, academic, multicampus healthcare system. Pharmacists in this system may have greater exposure to pharmacogenomics tests and interpretations compared with those in other settings; however, our results suggest that this factor did not impact the overall results. Thus, the study results may be generalizable to pharmacists in other healthcare settings.
SUMMARY
To educate pharmacists about pharmacogenomics, an ACPE-accredited continuing education program was developed. The results of this pharmacogenomics education study were positive and significant, but showed limited retention of educational goals and objectives after 2 months, suggesting that pharmacogenomics education of pharmacist clinicians requires more effort. A large investment in pharmacogenomics education may be needed to increase pharmacists’ knowledge and comfort level with this emerging therapeutic opportunity.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the Mayo Clinic Eisenberg Genomics Educational Program for providing an educational grant to support this research. The authors thank Sarah Jenkins for outstanding statistical support, and thank the pharmacists who participated in this project.
The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health (NIH), through Grant Number 1 UL1 RR024150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- 1.Guttmacher AE, Collins FS. Realizing the promise of genomics in biomedical research. JAMA. 2005;294(11):1399–402. doi: 10.1001/jama.294.11.1399. [DOI] [PubMed] [Google Scholar]
- 2.Evans WE, McLeod HL. Pharmacogenomics-drug disposition, drug targets, and side effects. N Engl J Med. 2003;348(6):538–549. doi: 10.1056/NEJMra020526. [DOI] [PubMed] [Google Scholar]
- 3.Evans WE, Johnson JA. Pharmacogenomics: the inherited basis for interindividual differences in drug response. Annu Rev Genomics Hum Genet. 2001;2:9–39. doi: 10.1146/annurev.genom.2.1.9. [DOI] [PubMed] [Google Scholar]
- 4.Shin J, Kayser SR, Langaee TY. Pharmacogenetics: from discovery to patient care. Am J Health-Syst Pharm. 2009;66(7):625–637. doi: 10.2146/ajhp080170. [DOI] [PubMed] [Google Scholar]
- 5.Gage BF, Lesko LJ. Pharmacogenetics of warfarin: regulatory, scientific, and clinical issues. J Thromb Thrombolysis. 2008;25(1):45–51. doi: 10.1007/s11239-007-0104-y. [DOI] [PubMed] [Google Scholar]
- 6.Holmes DR, Dehmer GJ, Kaul S, Leifer D, O'Gara PT, Stein CM. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on clinical expert consensus documents and the American Heart Association endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J Am Coll Cardiol. 2010;56(4):321–341. doi: 10.1016/j.jacc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 7.U.S. Food and Drug Administration website. Drugs at FDA. FDA approved drug products. September 27, 2011 Drug labeling revision. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#labelinfo Accessed December 26, 2012. [Google Scholar]
- 8.Lee KC, Ma JD, Kuo GM. Pharmacogenomics: bridging the gap between science and practice. J Am Pharm Assoc. 2010;50(1):e1–e14. doi: 10.1331/JAPhA.2010.09124. [DOI] [PubMed] [Google Scholar]
- 9.Ellingrod VL, Moline J. Incorporating pharmacogenomics into practice. J Pharm Pract. 2007;20(3):277–282. [Google Scholar]
- 10.El-Ibiary SY, Cheng C, Alldredge B. Potential roles for pharmacists in pharmacogenetics. J Am Pharm Assoc. 2008. 48(2):e21–e32. doi: 10.1331/JAPhA.2008.07050. [DOI] [PubMed] [Google Scholar]
- 11.Brock TP, Valgus JM, Smith SR, Summers KM. Pharmacogenomics: implications and consideration for pharmacists. Pharmacogenomics. 2003;4(3):321–330. doi: 10.1517/phgs.4.3.321.22698. [DOI] [PubMed] [Google Scholar]
- 12.Streetman DS. Emergence and evolution of pharmacogenetics and pharmacogenomics in clinical pharmacy over the past 40 years. Ann Pharmacother. 2007;41(12):2038–2041. doi: 10.1345/aph.1K273. [DOI] [PubMed] [Google Scholar]
- 13.McCullough KB, Formea CM, Berg KD, et al. Assessment of the pharmacogenomics needs of pharmacists. Am J Pharm Educ. 2011;75(3):Article 51. doi: 10.5688/ajpe75351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sansgiry SS, Kulkarni AS. The Human Genome Project: assessing confidence in knowledge and training requirements for community pharmacists. Am J Pharm Educ. 2003;67(2):Article 39. [Google Scholar]
- 15. Mayo School of Health Sciences, College of Medicine, Mayo Clinic. http://www.mayo.edu/mshs/mcr.html. Accessed November 24, 2011. [Google Scholar]
- 16.American Pharmacists Association. Integrating pharmacogenomics into pharmacy practice via medication therapy management. J Am Pharm Assoc. 2011;51(6):e64–e74. doi: 10.1331/JAPhA.2011.11543. [DOI] [PubMed] [Google Scholar]
- 17.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med. 2011;364(12):1144–1153. doi: 10.1056/NEJMra1010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crews KR, Cross SJ, McCormick JN, et al. Development and implementation of a pharmacist-managed clinical pharmacogenetics service. Am J Health-Syst Pharm. 2011;68(2):143–150. doi: 10.2146/ajhp100113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleason BL, Peeters MJ, Resman-Targoff BH, et al. An active-learning strategies primer for achieving ability-based educational outcomes. Am J Pharm Educ. 2011;75(9):Article 186. doi: 10.5688/ajpe759186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eley JG, Birnie C. Retention of compounding skills among pharmacy students. Am J Pharm Educ. 2006;70(6):Article 132. doi: 10.5688/aj7006132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mieure KD, Vincent WR, Cox MR, Jones MD. A high-fidelity simulation mannequin to introduce pharmacy students to advanced cardiovascular life support. Am J Pharm Educ. 2010;74(2):Article 22. doi: 10.5688/aj740222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. doi: 10.1177/106002809202600723. O’Connell MB, Hewitt JM, Lackner TE, Pastor JD 3rd, Wong MT, Bishop AL. Short- and long-term retention of a nursing home education program on metered-dose inhaler technique. Ann Pharmacother. 1992;26(7-8):980-984. [DOI] [PubMed] [Google Scholar]
- 23.Fjortoft NF. Learning outcomes and behavioral changes with a pharmacy continuing professional education program. Am J Pharm Educ. 2006;70(2):Article 24. doi: 10.5688/aj700224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopacek KB, Dopp AL, Dopp JM, Vardeny O, Sims JJ. Pharmacy students’ retention of knowledge and skills following training in automated external defibrillator use. Am J Pharm Educ. 2010;74(6):Article 109. doi: 10.5688/aj7406109. [DOI] [PMC free article] [PubMed] [Google Scholar]


