Abstract
BACKGROUND
Prognosis after surgery for pancreatic ductal adenocarcinoma (PDAC) is typically reported from the date of surgery. Survival estimates, however, are dynamic and may change based on the time already survived. The authors sought to assess conditional survival among a large cohort of patients who underwent resection of PDAC.
METHODS
Between 1970 and 2008, 1822 patients who underwent resection for PDAC with curative intent were identified. Kaplan-Meier and Cox regression analyses were performed to validate established predictors of survival, and results were compared with 2-year conditional survival.
RESULTS
Actuarial survival was 18% at 5 years, with a median survival of 18 months. Multivariate analysis revealed that tumor size, lymph node ratio, and positive margins were associated with worse survival (all P < .001). Differences in actuarial versus conditional survival estimates were greater the more years already survived by the patient. The 2-year conditional survival at 3 years—the probability of surviving to postoperative year 5 given that the patient had already survived 3 years—was 66% versus a 5-year actuarial survival calculated from the time of surgery of 18%. Stratification of 2-year conditional survival by lymph node ratio and margin status revealed that patients with high lymph node ratio or positive margins saw the greatest increase in 2-year conditional survival as more time elapsed (both P ≤ .01).
CONCLUSIONS
Differences in actuarial versus conditional survival estimates were more pronounced based on the additional years already survived by the patient. Conditional survival may be a helpful tool in counseling patients with PDAC, as it is a more accurate assessment of future survival for those patients who have already survived a certain amount of time.
Keywords: pancreatic cancer, conditional survival, surgery, Whipple
INTRODUCTION
In 2010, the American Cancer Society reported that pancreatic cancer was the fourth leading overall cause of cancer-related death, with 43,140 new cases and 38,400 deaths.1 For patients with pancreatic ductal adenocarcinoma (PDAC), resection remains the only potentially curative therapeutic option. Prognosis after surgery, however, remains poor, with 5-year survival ranging from 15% to 25%.2-6 Although some population-based studies7,8 have reported a worse 5-year survival of 10%, several small series have noted a subset of patients who may survive long-term beyond 5 years.9-12 In one study from the Mayo Clinic, Schnelldorfer et al12 reported a 5-year and 10-year survival of 18% and 13%, respectively. The authors suggested that long-term survival may be possible in a small subset of patients after surgery for PDAC.
Several clinicopathological factors have been reported to be associated with prognosis after surgery for PDAC. In turn, these factors have been incorporated into the American Joint Committee on Cancer (AJ CC) staging system for pancreatic cancer.13 Standard prognostic scoring systems are based, however, on data derived exclusively from the time of surgery and therefore may be increasingly inaccurate as time elapses from the date of surgery. In effect, survival probability may change for patients who have survived a period of time after surgery. Rather than being a static probability, the chance of survival is dynamic. Given that survival probabilities are likely to change over time, conditional survival estimates have been proposed as a useful adjunct to traditional measures of survival probability.14 Conditional survival is defined as the survival probability that is calculated after a given length of survival and includes only individuals who have survived to a predefined period of time of interest.15-17 Conditional survival may be particularly relevant when assessing patients with cancers traditionally associated with a very poor prognosis, as standard prognostic schemas may be too negative and be disproportionately influenced by the large number of patients who die within the first few years. 16, 18
Our group previously reported on conditional survival after surgical resection of colorectal liver metasta-sis.16 Other groups have similarly published on conditional survival for other anatomic sites; however, conditional survival after surgical resection of PDAC remains poorly defined. To date, only 1 subset analysis of conditional survival for PDAC reported in a larger population-based study using aggregate data from the Surveillance, Epidemiology, and End Results (SEER) program has been published.14 Given this, we sought to assess conditional survival relative to actuarial survival among a large cohort of patients who underwent curative intent surgical resection for PDAC at a single major hepatopancreatobiliary center. In addition, the impact of certain PDAC prognostic factors on relative conditional survival was examined.
MATERIALS AND METHODS
Patient Population and Data Collection
Between January 1970 and December 2008, 1822 patients treated with curative intent surgery for PDAC were identified from the Johns Hopkins Hospital pancreatic surgery database. Clinicopathologic data were collected, including age, sex, race, presenting symptoms, year of operation, location of the PDAC within the pancreas, type of cancer-directed operation performed, tumor size and grade, lymph node metastasis, and surgical margin status. Tumor, nodal, and metastasis status was classified according to the AJCC staging system.13 Because the delta spanned several editions of the AJCC staging system, no attempt was made to reclassify past cases to the most current seventh edition; instead, tumor size and the presence of lymph node metastasis were used. Only cases with confirmed PDAC on final pathologic analysis were included in the study. Lymph node ratio19 was determined by dividing the number of metastatic lymph nodes by the total number of lymph nodes examined in the pathologic specimen. Patients were stratified as lymph node positive versus negative, as well as into lymph node ratio tertiles. Date of last follow-up and vital status were collected on all patients. The study was approved by the institutional review board of the Johns Hopkins Medical Institutions.
Statistical Analyses
Overall survival time was calculated from the date of PDAC resection to the date of last follow-up/death. Cumulative event rates were calculated using the method of Kaplan and Meier.20 Univariate analyses were performed using the log-rank test to compare differences between categorical groups. Cox proportional hazards models21 were developed using relevant clinicopathologic variables in a direct enter fashion (univariate inclusion criteria of P < .25 by the Wald statistic) to determine the association of each with overall survival. The overall fit of the multivariate models was assessed using the likelihood ratio test. Relative risks were expressed as hazard ratios (HRs) with a 95% confidence interval (CIs). The final model was evaluated for goodness of fit using the method proposed by May and Hosmer.22, 23 Multicollinearity was assessed using matrices of Pearson correlation coefficients, and when 2 variables with high collinearity (such as lymph node ratio and positive nodal status) were considered for multivariate modeling, the covariate with the better discriminative ability was chosen for inclusion in the model. Significance levels were set at P < .05; all tests were 2-sided.
Conditional survival represents the probability that a patient will survive an additional amount of time based on the finding that the patient has already survived a given amount of time. Because the median survival after surgical resection of PDAC is about 18 to 24 months,2-6 we calculated the conditional survival at 2 years for patients given that they had survived to a specific point in time. For example, we assessed the probability of surviving an additional 2-years, given that the patient had already survived x period of time. Mathematically, 2-year conditional survival for a patient surviving (S) x period of time is represented as 2-year conditional survival = S(2 + x)/S(2). Conditional survival was stratified by several clinical variables of interest, including tumor size, lymph node ratio, and surgical margin status. The rate of change in 2-year conditional survival over time was evaluated using linear regression. All statistical analyses were performed using SPSS version 18.0 (SPSS Inc., Chicago, Ill).
RESULTS
Patient and Tumor Characteristics
Table 1 shows the clinicopathologic features of the 1822 patients included in the study. The median patient age was 67.0 years (standard deviation [SD], 10.8 years), and there was a slight predominance of male patients (n = 961; 52.7%). The most common presenting symptom was jaundice (n = 760; 41.7%) followed by preoperative weight loss (n = 693; 38.0%) and abdominal pain (n = 479; 26.0%). The majority of PDAC lesions were located in the pancreatic head (n 1600; 87.8%). At the time of surgery, 81. 5% (n = 1485) of patients underwent a pancreaticoduodenectomy (PD), with 2/3, being pylorus-preserving (n = 996; 67.1 % of PD). Resections of the distal pancreas (n = 155; 8.5% and total pancreatectomy (n = 111; 6.1%) were performed less frequently. The median length of hospital stay was 9.0 days (SD 15.0 days). Patients undergoing a PD had a greater median hospital stay after operation (9.0 days; SD, 15.5) compared with patients who underwent distal/other pancreatic resections (7.0 days; SD, 7.4; P = .004).
Table 1.
Demographic, Clinical, and Operative Characteristics of Patients With Pancreatic Ductal Adenocarcinoma Resected With Curative Intent
| Variable | Patients, No. (%), N = 1822 |
|---|---|
| Demographics | |
| Median age at operation, y ± SD; range |
67.0 ± 10.8; 25-93 |
| Male | 961 (52.7) |
| Race | |
| White | 1587 (87.1) |
| Black | 136 (7.5) |
| Other | 99 (5.4) |
| Preoperative weight loss | 693 (38.0) |
| Abdominal pain at presentation | 479 (26.0) |
| Jaundice at presentation | 760 (41.7) |
| Operative details | |
| Year of operation | |
| 1970-2001 | 816 (44.8) |
| 2001-2008 | 1006 (55.2) |
| Location | |
| Head of pancreas | 1600 (87.8) |
| Body/Distal/Multifocal | 222 (12.2) |
| Type of operation | |
| Classic pancreaticoduodenectomy (PD) | 489 (26.8) |
| Pylorus-preserving PD | 996 (54.7) |
| Distal pancreatectomy | 155 (8.5) |
| Total pancreatectomy | 111 (6.1) |
| Other | 71 (3.9) |
| Tumor characteristics at pathology | |
| Mean tumor size, cm ± SEM | 3.1 ± 0.1 |
| Grade | |
| Well differentiated | 64 (3.5) |
| Moderately differentiated | 1007 (55.3) |
| Poorly differentiated | 704 (38.6) |
| Undifferentiated | 4 (0.2) |
| Unknown | 28 (1.5) |
| Mean number of lymph nodes examined ± SD |
18.0 ± 0.2 |
| Mean number of lymph node metastases ± SD |
3.3 ± 0.1 |
| Lymph node ratio | |
| 0 | 437 (24.0) |
| 0,01-0,132 | 439 (24.1) |
| 0.133-0.307 | 462 (25.4) |
| >0.307 | 449 (24.6) |
| Missing | 35 (1.9) |
| Microscopically negative | 1095 (60.1) |
| [R0] resection margins | |
| Perineural invasion | 1250 (68.6) |
Abbreviation: SD, standard deviation; SEM, standard error of the mean.
On final pathologic analysis of the resected specimen, the mean tumor size was 3.1 cm (standard error of the mean [SEM], 0.1). Overall, the mean number of lymph nodes examined was 18.0 (SEM, 0.2). The majority of patients (n = 1354; 74.3%) had lymph node metastasis (ie, N1 disease), with larger tumor size being associated with an increased risk of lymph node metastasis (N1 disease: ≤2 cm, 58.7% vs >2 cm, 80.7%; P < .001). Most PDAC tumors were moderately differentiated (n = 1007; 55.3%) and had perineural invasion present (n = 1250; 68.6%). On final pathologic examination, the margin status was microscopically negative (R0) in 1095 (60.1%) patients.
Factors Associated With Survival
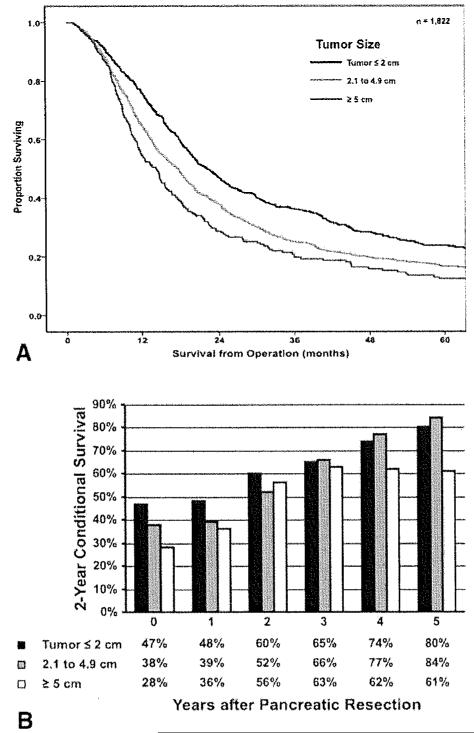
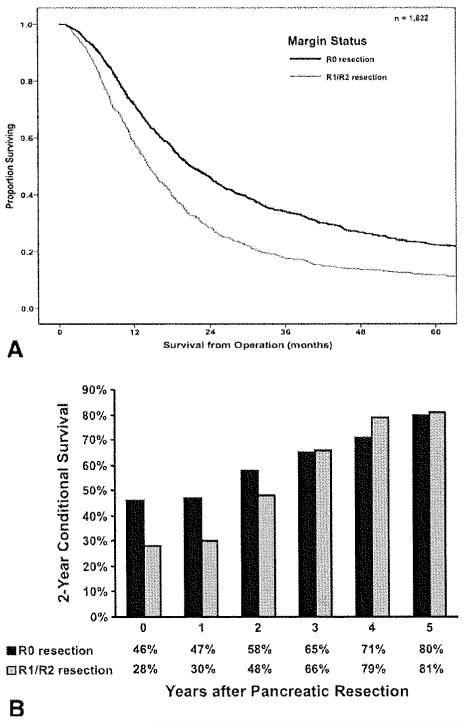
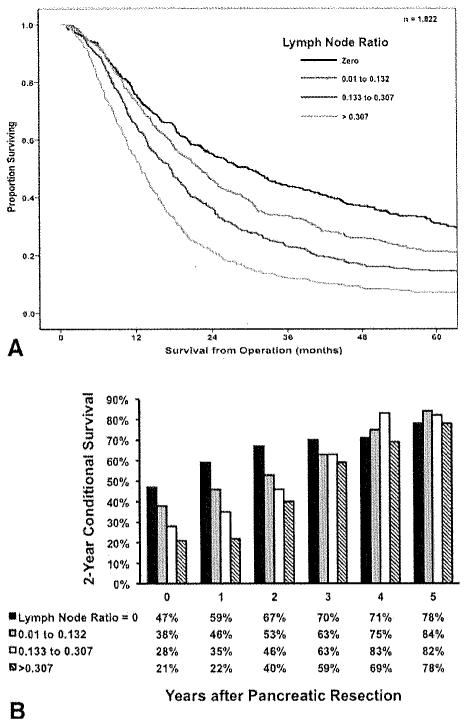
Median survival from the time of PDAC operation for all patients was 18.0 months, with a 1-year, 3-year, and 5-year overall survival of 68.3%, 38.9%, and 18.0%, respectively. On univariate analyses, several clinicopathologic factors were associated with survival, including tumor size (Fig. 1, Top), lymph node status (Pig. 2, Top), tumor grade, and margin status (Fig. 3, Top; all P < .05). Other factors, including year of surgery (P = .20) and location ofPDAC within the pancreas (P = .76) were not associated with survival. On multivariate analysis, after controlling for competing risk factors, tumor size, tumor grade, lymph node status, and surgical margin status remained independently associated with outcome (Table 2). Specifically, the presence of lymph node metastasis was associated with worse survival (HR, 1.38; 95% CI, 1.21-1.57; P < .00l), with a higher lymph node ratio strongly correlated with worsening survival (Fig. 2, Top). In addition, a positive margin of resection was also associated with worse survival (HR, 1.46; 95% CI, 1.31-1.63; p < .001).
Figure 1.
(Top) Overall actuarial survival is stratified by tumor size (P < .01), (Bottom) Two-year conditional survival is stratified by tumor size as a function of time elapsed since surgery (P = 54).
Figure 3.
(Top) Overall actuarial survival is stratified by surgical margin status (P < .001). (Bottom) Two-year conditional survival is stratified by surgical margin status as a function of time elapsed since surgery (P = .01).
Table 2.
Cox Regression Analyses of Variables Associated With Survival in Patients With Pancreatic Ductal Adenocarcinoma Resected With Curative Intent
| Prognostic Factor | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Year of operation 1970-2001 | 1.07 | 0.97-1.19 | .197 | 1.03 | 0.92-1.16 | .583 |
| Age ≥68 years | 1.27 | 1.15-1.41 | <.001 | 1.32 | 1.19-1.46 | <.001a |
| Male | 1.00 | 0.90-1.11 | .963 | – | – | – |
| White race | 1.25 | 1.06-1.48 | .007 | 1.23 | 1.05-1.44 | .012a |
| Preoperative weight loss | 1.25 | 1.03-1.25 | .028 | 1.01 | 0.89-1.15 | .899 |
| Preoperative abdominal pain | 1.10 | 0.98-1.24 | .116 | 1.08 | 0.96-1.23 | .212 |
| Presentation with jaundice | 1.10 | 1.99-1.22 | .069 | 1.03 | 0.91-1.17 | .663 |
| Tumor size at pathology, cm | ||||||
| ≤2 | Reference | |||||
| 2.1-4.9 | 1.28 | 1.13-1.44 | <.001 | 1.14 | 1.00-1.30 | .044a |
| ≥5 | 1.59 | 1.34-1.89 | <.001 | 1.47 | 1.23-1.75 | <.001a |
| Location in head of pancreasb | 1.03 | 0.87-1.20 | .759 | – | – | – |
| Grade | ||||||
| Unknown | Reference | |||||
| Well differentiated | 1.71 | 0.72-4.03 | .222 | 1.50 | 0.63-3.55 | .356 |
| Moderately differentiated | 2.99 | 1.34-6.67 | .050 | 2.59 | 1.04-6.45 | .041a |
| Poorly differentiated | 4.42 | 1.98-9.89 | <.001 | 3.75 | 1.67-8.43 | .001a |
| Undifferentiated | 9.26 | 2.61-32.85 | .001 | 7.95 | 2.23-28.38 | .001a |
| Lymph node metastasis | 1.54 | 1.36-1.74 | <.001 | 1.38 | 1.21-1.57 | <.001a |
| Positive margin of resection (R1/R2) | 1.51 | 1.36-1.68 | <.001 | 1.46 | 1.31-1.63 | <.001a |
| Perineural invasion | 1.11 | 0.99-1.24 | .07 | 0.95 | 0.84-1.07 | .379 |
Abbreviations: CI, confidence interval.
Statistically significant.
Compared to cancers in the body or tail, or multifocal pancreatic distribution.
Figure 2.
(Top) Overall actuarial survival is stratified by lymph node status (P < .01), (Bottom) Two-year conditional survival is stratified by lymph node status as a function of time elapsed since surgery (P < .001).
Overall and Conditional Survival
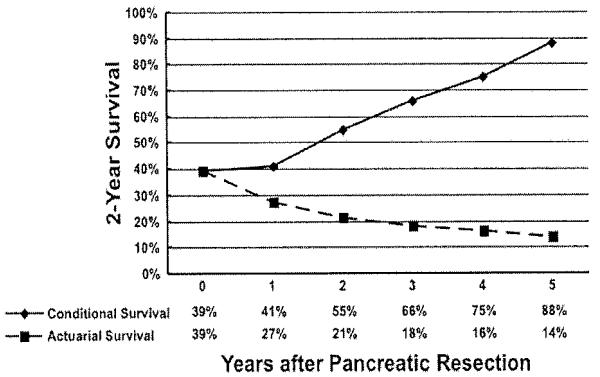
The 2-year survival probability estimates at the time of surgery and conditional on having survived 1 to 5 years after surgery are presented in Figure 4. As noted, the 2-year conditional survival probabilities increased as a function of time already survived. Whereas the actuarial survival at 4 years after pancreatic resection was 21 %, the 2-year conditional survival at 2 years—the probability of surviving an additional 2 years given that the patient has already survived 2 years (ie, postoperative year 4)—was 54.9%. The difference in relative survival probabilities comparing actuarial versus conditional survival estimates was more pronounced based on the more years already survived by the patient (Fig. 4). Specifically, the 2-year conditional survival at 3 years—the probability of surviving to postoperative year 5 given that the patient has already survived 3 years—was 65,7%, compared with a 5-year actuarial survival calculated from the time of surgery of 18.0%. To better assess the impact of time already survived on future survival, survival data were further stratifled. Table 3 details the probability of patients with resected PDAC surviving to a future point in time based on how long the patient had already survived. In each case, the survival estimates increased per time already survived relative to the predicted actuarial survival. For example, based on actuarial estimates from the time of surgery, the 5-year survival probability was only 18.0%. However, the probability of surviving to postoperative year 5 incrementally and dramatically increased as time already survived increased (ie, if the patient was alive at year 3 or 4, the probability of surviving to year 5 was 65.7% and 84.1%, respectively; Table 3). Similarly, whereas the actuarial probability of surviving to year 3 after surgety was < 50%, this probability increased to 54.7% and 70.3% if the patient was alive at 18 months and 2 years, respectively.
Figure 4.
Difference in overall relative survival probabilities compares actuarial versus conditional survival estimates as a function of time elapsed since surgery (eg. time 0 corresponds to the actuarial survival at 2 years). The 2-year conditional survival at 1 year—the probability of surviving to postoperative year 3 given that the patient has already survived 1 year—is 41%. whereas the actuarial survival at 3 years is 27%.
Table 3.
Proportion of Patients With Resected Pancreatic Ductal Adenocarcinoma Who Reach a Certain Survival Time Point Given That They Have Already Survived a Certain Amount of Timea
| Total Time of Survival |
If the Patient Has Survived to… | ||||||
|---|---|---|---|---|---|---|---|
| 6 Months | 12 Months | 18 Months | 2 Years | 3 Years | 4 Years | 5 Years | |
| 6 months | |||||||
| 12 months | 74.7 | ||||||
| 18 months | 56.3 | 75.3 | |||||
| 2 years | 43.8 | 58.6 | 77.8 | ||||
| 3 years | 30.8 | 41.2 | 54.7 | 70.3 | |||
| 4 years | 24.0 | 32.1 | 42.7 | 54.9 | 78.1 | ||
| 5 years | 20.2 | 27.1 | 35.9 | 46.2 | 65.7 | 84.1 | |
For example, if a patient has survived to 6 months, their chance of reaching 12 months of total survival time is 74.7%.
Two-year conditional survival was also calculated and stratified by those clinicopathological variables that were noted to be particularly associated with a poor prognosis: tumor size, lymph node ratio, and surgical margin status. Two-year conditional survival estimates remained stable over time within strata of primary tumor size, with patients who had tumors ≤2 cm (47%-80%; Δ, 33%) having a similar change in conditional survival compared with patients who had tumors >5 cm (28%-61 %; Δ, 33%; Fig. 1, Bottom; P = .54). In contrast, 2-year conditional survival estimates for patients with other adverse clinicopathological features increased as a function of the time elapsed since surgery. Specifically, when 2-year conditional survival estimates were stratified by lymph node ratio, there was a more notable increase in 2-year conditional survival over time when comparing patients with N0 disease (47%-78%; Δ, 31 %) or a low lymph node ratio (lymph node ratio 0.01-0.132: 38% to 84%; Δ, 46%) versus patients with a high lymph node ratio (lymph node ratio >0.307: 21 % to 78%; Δ, 57%; Fig. 2, Bottom; P < .001). Similarly, whereas patients with R1/R2 disease had the worst prognosis as estimated from the time of surgery, these patients saw the greatest increases in 2-year conditional survival as more time elapsed. Whereas patients who underwent an R0 resection experienced an increase in 2-year conditional survival from 46% to 80% (Δ, 34%), those patients who underwent an R1/R2 resection had an increase in 2-year conditional survival from 28% to 81 % (Δ, 53%; Fig. 3, Bottom; P= .01).
DISCUSSION
Pancreatic cancer is associated with a poor prognosis, even among patients who undergo a potentially curative resection. Survival estimates for patients with pancreatic cancer who have undergone surgical resection are traditionally reported in the literature as survival from the time of surgery, with 5-year survival ranging from 15% to 25%.2-6 These survival projections may, however, not necessarily be accurate for patients who have survived an initial period of time. That is, patients who have survived a period of time may have a different future prognosis. As such, conditional survival may provide a more useful and clinically relevant estimate of survival probability for patients who have survived a period of time beyond surgery. This may be particularly true for patients with malignancies that have a very poor prognosis, as survival curves estimated from the date of surgery may be overly influenced by the large number of patients who die within the first few years. 16,18 In the current study, we present conditional survival probability estimates for patients with PDAC who underwent curative intent surgery. We found that differences in relative survival probabilities comparing actuarial versus conditional survival estimates were more pronounced based on the additional years already survived by the patient (Fig. 4). Speciflcally, whereas the actuarial survival at 4 years after pancreatic resection was 21 %, the 2-year conditional survival at 2 years—the probability of surviving an additional 2 years given that the patient has already survived 2 years (ie, postoperative year 4)—was 54.9%. These data are important, as the reported conditional survival may provide more clinically relevant prognostic information to patients who have already survived a period of time after surgery for pancreatic cancer.
Several factors have been noted to be associated with survival after surgety for pancreatic cancer. Previous studies have reported that patients with large tumors,5,24 lymph node metastasis,19 or R1 surgical margins5 have a significantly worse long-term prognosis after surgical resection. In fact, in aggregate, patients who have any of tllese associated risk factors have a reported 5-year survival of <10% to 15% as a group. In the current study, we similarly found that tumor size, lymph node status, and margin status were associated with outcome on both univariate and multivariate analyses (Table 2). However, perhaps more importantly, we found that certain subsets of patients with these adverse prognostic factors, but who did survive for a period of time, had a higher probability of future survival (Figs, 1-3, Bottom). Although prognostic factors such as tumor size, lymph node, and margin status were important, prognosis based on these factors was misleading, as it was limited to the estimation of initial risk around the time of surgery.16
Conditional survival may be a better alternative to staging systems or isolated static prognostic factors to estimate survival probability over time. Rather than basing prognostic estimations solely on initial risk factors determined at the time of surgery, conditional survival provides a more dynamic assessment of survival probability.16, 25 Exemplified by data in the current study, survival was not only dependent on initial prognostic factors, but was also strongly dependent on time elapsed since surgery. Conditional survival may be particularly germane to inform the prognosis of patients anticipated to have a very poor prognosis, but who have survived a certain amount of time and therefore have beaten the initial odds.17,26,27 For example, 2-year conditional survival estimates for patients with adverse clinicopathological features such as a high lymph node ratio or R1 surgical margin increased the most as a function of the time elapsed since surgery. Specifically, when 2-year conditional survival estimates were stratified by lymph node ratio and margins status, there was a more notable increase in 2-year conditional survival over time when comparing patients with high lymph node ratio or R1 disease versus patients with N0 disease or R0 disease (Figs 2, 3, Bottom; both P < .05). Data such as these can provide more useful prognostic information that is tailored not only to the clinicopathological characteristics of the tumor, but also to the time a person has already survived after surgery for pancreatic cancer.
Long-term outcomes of patients with a range of malignancies have been examined using conditional survival, although conditional survival for pancreatic Cancer remains ill-defined. Choi et al26 reported conditional survival for patients with ovarian cancer extracted from the SEER dataset. The authors noted that 5-year conditional survival improved over time up to 5 years after diagnosis for ovarian cancer, with the largest gains in conditional survival over time being seen for patients with advanced stage disease, poor grade, and undifferentiated epithelioid histologies. In a separate SEER analysis, Chang et al15 examined conditional survival among colon cancer patients. Chang and colleagues found that conditional survival improved particularly among patients initially predicted to have the worse prognosis. Adjusted 5-year conditional survival improved from 42% to 80% for stage IIIC cancers and 5% to 48% for stage IV cancers. In a larger population-based study using aggregate data from SEER, Merrill et al14 examined 11 different cancers, 1 of which was pancreatic cancer, and reported similar trends. Because pancreas cancer was not a focus of the Merrill et al14 study, interpretation and extrapolation of the data to patients having undergone resection for PDAC was limited. As sllch, we chose to focus exclusively on pancreatic cancer and conditional survival after surgical resection of PDAC. Similar to previous data based on other malignancies,15,26 we noted that conditional survival probablities increased as a function of time already survived (Fig. 4). Specifically, the 2-year conditional survival at 3 years—the probability of surviving to postoperative year 5 given that the patient has already survived 3 years—was 65.7%, compared with a 5-year actuarial survival calculated from the time of surgery of 18.0%. In addition, similar to the findings of Choi et al26 and Chang et al15 we noted that conditional survival improved the most among patients initially predicted to have the worse prognosis (eg, larger tumors, high grade, high lymph node ratio, R1 margin). These data would suggest that standard 5-year survival estimates are not optimal, but also have marginal clinical utility when trying to provide future survival estimates for patients who present with more advanced stages of disease, but who remain alive and continue to be followed months to years later in the clinic.
The current study had several limitations. Due to of the constraints of data collection, we were unable to assess the interaction of the Memorial Sloan Kettering Cancer Center pancreatic survlval nomogram28 and conditional survival. Although we attempted to examine the nomogram, it became evident that some data necessary for tne nomogram calculation were not routinely available in the Johns Hopkins pancreatic database (eg, back pain). However, as our group has previously reported,16 prognostic scoring systems and nomograms frequently suffer from the same shortcomings inherent in an over-reliance on individual prognostic factors. Specifically, nomograms that are based on data exclusively from the time of surgery can be increasingly inaccurate for patients as time elapses.
In conclusion, we demonstrate that survival estimates after surgical resection of PDAC changed as a function of time survived since surgery. Specifically, although the 5-year survival probability was only 18.0% as measured from the time of surgery, the probability of surviving to postoperative year 5 incrementally and dramatically increased as time already survived increased. Factors such as tumor size, as well as nodal and margin status, were associated with worse survival, but relative conditional survival improved the most among those patients who were predicted to have the worse survival at the initial time of surgery. Conditional survival therefore may provide more accurate and useful prognostic information about how prognosis and risk of death change over time for cancer survivors. The data herein presented should help better inform discussions about prognosis among clinicians treating patients who have survived an initial period of time after surgical resection of PDAC.
Acknowledgments
FUNDING SOURCES
No specific funding was disclosed.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
REFERENCES
- 1.Jemal A, Siegel R, Xu J. Ward E. Cancer statistics. CA Cancer J Clin. 2010;2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 3.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 4.Ueno H, Kosuge T, Matsuyama Y, et al. A. randomised phase III trial comparing gemcitabine with surgery-only in patients with resected pancreatic cancer: Japanese Study Group of Adjuvant Therapy for Pancreatic Cancer. Br J Cancer. 2009;101:908–915. doi: 10.1038/sj.bjc.6605256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas—616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 6.Herman JM, Swam MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008;26:3503–3510. doi: 10.1200/JCO.2007.15.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slidell MB, Chang DC, Cameron JL, et al. Impact of total lymph node count and lymph node ratio on staging and survival after pancreatectomy for pancreatic adenocarcinoma: a large, population-based analysis. Ann Surg Oncol. 2008;15:165–174. doi: 10.1245/s10434-007-9587-1. [DOI] [PubMed] [Google Scholar]
- 8.Mayo SC, Austin DF, Sheppard BC, et al. Adjuvant therapy and survival after resection of pancreatic adenocarcinoma: a population-based analysis. Cancer. 2010;116:2932–2940. doi: 10.1002/cncr.25082. [DOI] [PubMed] [Google Scholar]
- 9.Adham M, Jaeck D, Le Borgne J, et al. Long-term survival (5-20 years) after pancreatectomy for pancreatic ductal adenocarcinoma: a series of 30 patients collected from 3 institutions. Pancreas. 2008;37:352–357. doi: 10.1097/MPA.0b013e31818166d2. [DOI] [PubMed] [Google Scholar]
- 10.Conlon KC, Klimstra D$, Brennan MF. Long-term survival after curative resection for pancreatic ductal adenocarcinoma. Clinicopathoiogic analysis of 5-year survivors. Ann Surg. 1996;223:273–279. doi: 10.1097/00000658-199603000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naseef O, Adham M, Hervieu V, Le Borgne J, Partensky C. Long-term survival (superior to 20 years) after pancreaticoduodenectomy for pancreatic duct adenocarcinoma: report of 2 cases. Hepatogas-troenterology. 2008;55:1110–1111. [PubMed] [Google Scholar]
- 12.Schnelldorfer T, Ware AL, Sarr MG, et al. Long-term survival after pancreatoduodenectomy for pancreatic adenocarcinoma: is cure possible? Ann Surg. 2008;247:456–462. doi: 10.1097/SLA.0b013e3181613142. [DOI] [PubMed] [Google Scholar]
- 13.American joint Commission on Cancer, American Cancer Society . Cancer Staging Manual. 7th ed. Springer; New York, NY: 2010. [Google Scholar]
- 14.Merrill RM, Henson DE, Ries LA. Conditional survival estimates in 34,963 patients with invasive carcinoma of the colon. Dis Colon Rectum. 1998;41:1097–1106. doi: 10.1007/BF02239430. [DOI] [PubMed] [Google Scholar]
- 15.Chang GJ, Hu CY, Eng C, Skibber JM, Rodriguez-Bigas MA. Practical application of a calculator for conditional survival in colon cancer. J Clin Oncol. 2009;27:5938–5943. doi: 10.1200/JCO.2009.23.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nathan H, de Jong MC, Pulitano C, et al. Conditional survival after surgical resection of colorectal liver metastasis: an international multi-institutional analysis of 949 patients. J Am Coll Surg. 2010;210:755–766. doi: 10.1016/j.jamcollsurg.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 17.Henson DE, Ries LA, Carriaga MT. Conditional survival of 56,268 patients with breast cancer. Cancer. 1995;76:237–242. doi: 10.1002/1097-0142(19950715)76:2<237::aid-cncr2820760213>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 18.Janssen-Heijnen ML, Houterman S, Lemmens VE, et al. Prognosis for long-term survivors of cancer. Ann Oncol. 2007;18:1408–1413. doi: 10.1093/annonc/mdm127. [DOI] [PubMed] [Google Scholar]
- 19.Pawlik TM, Gleisner AL, Cameron JL, et al. Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery. 2007;141:610–618. doi: 10.1016/j.surg.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Star Assoc. 1958;53:457–481. [Google Scholar]
- 21.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 22.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. 2nd. ed. John Wiley & Sons; New York, NY: 1999. [Google Scholar]
- 23.May S, Hosmer DW. A simplified method of calculating an overall goodness-of-fir test for the Cox proportional hazards model. Life-time Data Anal. 1998;4:109–120. doi: 10.1023/a:1009612305785. [DOI] [PubMed] [Google Scholar]
- 24.Cameron JL, Crist DW, Sitzmatm JV, et al. Factors influencing survival after pancreaticoduodenectomy for pancreatic cancer. Am J Surg. 1991;161:120–124. doi: 10.1016/0002-9610(91)90371-j. discussion 124-125. [DOI] [PubMed] [Google Scholar]
- 25.Bleyer A, Choi M, Fuller CD, Thomas CR, Jr, Wang SJ. Relative lack of conditional survival improvement in young adults with cancer. Semin Oncol. 2009;36:460–467. doi: 10.1053/j.seminoncol.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 26.Choi M, Fuller CD, Thomas CR, Jr, Wang SJ. Conditional survival in ovarian cancer: results from the SEER dataset 1988-2001. Gynecol Oncol. 2008;109:203–209. doi: 10.1016/j.ygyno.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Kato I, Severson RK, Schwartz AG. Conditional median survival of patients with advanced carcinoma: Surveillance, Epidemiology, and End Results data. Cancer. 2001;92:2211–2219. doi: 10.1002/1097-0142(20011015)92:8<2211::aid-cncr1565>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 28.Brennan MF, Kattan MW, Klimstra D, Conlon K. Prognostic nomogram for patients undergoing resection for adenocarcinoma of the pancreas. Ann Surg. 2004;240:293–298. doi: 10.1097/01.sla.0000133125.85489.07. [DOI] [PMC free article] [PubMed] [Google Scholar]