Abstract
Cells decide to proliferate or remain quiescent using signaling pathways that link information about the cellular environment to the G1 phase of the cell cycle. Progression through G1 phase is controlled by pRB proteins, which function to repress the activity of E2F transcription factors in cells exiting mitosis and in quiescent cells. Phosphorylation of pRB proteins by the G1 cyclin-dependent kinases (CDKs) releases E2F factors, promoting the transition to S phase. CDK activity is primarily regulated by the binding of CDK catalytic subunits to cyclin partners and CDK inhibitors. Consequently, both mitogenic and antiproliferative signals exert their effects on cell proliferation through the transcriptional regulation and ubiquitin-dependent degradation of cyclins and CDK inhibitors.
Signaling pathways affecting G1 phase progression regulate CDKs (and thus, pRB phosphorylation) by controlling the abundance of cyclin partners and CDK inhibitors. For example, the MAPK pathway activates cyclin D.
1. INTRODUCTION
Control of cell proliferation generally occurs during the first gap phase (G1) of the eukaryotic cell division cycle (see Box 1). Multiple signals, ranging from growth factors to DNA damage to developmental cues, influence the decision to enter S phase, when DNA is replicated (Fig. 1). Hence, G1 phase cell cycle control is intrinsically linked with a diverse set of pathways controlling differentiation, stem and progenitor cell quiescence, senescence, and responses to a variety of stresses. The decision to enter S phase from G1 represents a point of no return that, in the absence of stress such as DNA damage, commits cells to complete the cell cycle and divide, and is therefore tightly controlled. This decision is made at what is called the “restriction point” in mammalian cells and “START” in yeast, after which cells become largely refractory to extracellular signals and will complete S phase and proceed through a second gap phase (G2 phase) and then mitosis. In multicellular organisms, most differentiated cells exit the active cell cycle during G1 phase and enter G0 phase, in which they remain metabolically active for days or even years, performing specialized functions. Postmitotic nerve and skeletal muscle cells provide good examples. Some G0 cells, such as quiescent T cells, can be stimulated by mitogenic signals to reenter the cell cycle.
BOX 1. THE EUKARYOTIC CELL CYCLE.
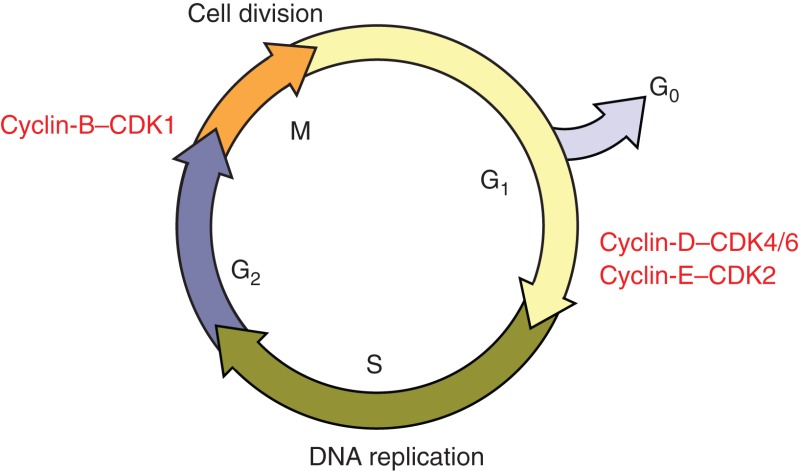
The classical cell cycle comprises four phases—G1, S, G2, and M—and is controlled by cyclin-dependent kinases (CDKs) and their cyclin partners. The commitment to divide occurs in G1 phase, which is controlled by cyclin-D–CDK4/6 and cyclin-E–CDK2 at the so-called G1/S transition. DNA is then replicated in S phase. This is followed by a second gap phase, G2, at the end of which cyclin-B–CDK1 controls entry into M phase (mitosis), when the cell divides. Cells can exit the cell cycle in G1 phase and enter G0 phase (quiescence). In some cases, they can reenter the cell cycle and begin dividing again (see main text).
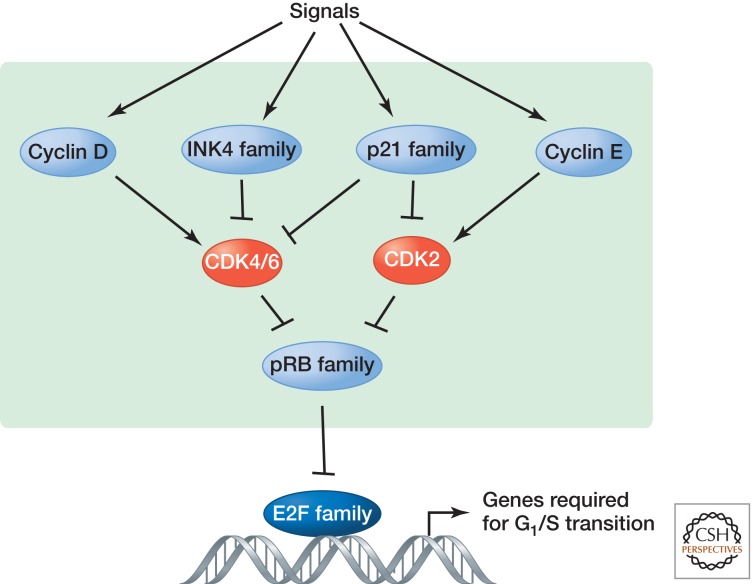
Figure 1.
G1 cell cycle control by the pRB pathway. Many cellular signaling events are intrinsically linked to G1 phase of the cell cycle, which is controlled by the RB pathway. Signaling to the RB pathway and thus G1 control by different cellular processes is achieved mainly through the regulation of cyclins and CDK inhibitors (CKIs). In mammalian cells, mitogenic signals first induce the synthesis of D-type cyclins, leading to activation of cyclin-D-dependent CDK4 and CDK6, and then induce E-type cyclins to activate CDK2. Cyclin-D–CDK4/6 and cyclin-E–CDK2 cooperatively phosphorylate RB-family proteins, derepressing E2F to allow transcription of E2F-target genes, thereby promoting the G1/S transition. The INK4 proteins specifically inhibit CDK4 and CDK6, whereas the p21 (CIP/KIP) family of CKIs inhibits multiple CDKs. Although the schematic illustration is based on mammalian cells, the regulation of both G1 cyclins and CDK inhibitors is evolutionarily conserved.
The restriction point is primarily controlled in mammalian cells by the RB pathway, named after the first tumor suppressor identified, the retinoblastoma protein (pRB) (Weinberg 1995). pRB is a member of a highly conserved family of proteins, encoded by a single gene in the single-celled green alga Chlamydomonas (MAT3), Caenorhabditis elegans (LIN-35), and Arabidopsis (RBR1); two genes in Drosophila (RBF1 and RBF2); and three genes in mammalian cells (RB1; p107, also known as RBL1; and p130, also known as RBL2) (Weinberg 1995; van den Heuvel and Dyson 2008). Budding yeast cells contain a protein (Whi5) that, although it does not share sequence similarity with pRB, functions at START in a similar manner (Costanzo et al. 2004; de Bruin et al. 2004). pRB proteins are present as hypophosphorylated, active forms in cells exiting mitosis and in quiescent cells, where they use a conserved pocket to bind to LxCxE motifs in numerous chromatin-associated proteins and transcription factors, particularly members of the E2F family. pRB proteins negatively regulate the expression of E2F-target genes, many of which are required for entry into and progression through S phase, by recruiting various repressive chromatin regulatory complexes and histone-modifying enzymes or by blocking the transactivation function of E2F proteins. Phosphorylation of the pRB family proteins by CDKs during G1 phase causes pRB to dissociate from E2Fs, allowing the transcription of target genes that stimulate progression into S phase (Fig. 1) (Dyson 1998).
The principal kinases that phosphorylate pRB family proteins during G1 phase in mammalian cells are three cyclin-dependent kinases (CDKs)5: cyclin-D-dependent CDK4 and CDK6 (Ewen et al. 1993; Kato et al. 1993) and cyclin-E-dependent CDK2 (Akiyama et al. 1992; Hinds et al. 1992). As many as eight distinct mammalian G1 CDK–cyclin complexes can be formed from combinatorial association of three D-type cyclins (cyclins D1, D2, and D3) with CDK4 and CDK6 and two E-type cyclins (cyclins E1 and E2) with CDK2, and these phosphorylate as many as 16 sites in pRB proteins (Akiyama et al. 1992; Kitagawa et al. 1996). Regulation of pRB-E2F by G1 CDKs has been evolutionarily conserved in plants, worms, flies, and mammals (Inze 2005; van den Heuvel and Dyson 2008). The complexity of the pRB pathway reflects the need to meet the demand to integrate diverse signals from different signaling pathways into a central G1 control mechanism. Disruption of this mechanism results in a wide range of developmental defects and human diseases, particularly cancer. Indeed, disruption of G1 control probably represents a common event in the development of most types of human cancer (Sherr 1996).
The critical role of pRB and G1 CDKs in controlling the G1/S transition is further illustrated by the studies of three DNA tumor viruses: adenovirus, human papilloma virus (HPV), and simian virus 40 (SV40). Although evolutionarily distant from each other, these viruses encode unrelated proteins (E1A in adenovirus, E7 in HPV, and large T in SV40) that bind to and inactivate pRB via an LxCxE motif to promote cell proliferation and viral replication. Primate herpesvirus saimiri and human Kaposi’s sarcoma virus encode cyclin D homologs (v-cyclins) that preferentially bind to and activate CDK6, creating complexes that are resistant to CDK inhibitors (CKIs; see below).
The steady-state levels of CDK2, CDK4, and CDK6 proteins remain relatively constant during the normal cell cycle and in quiescent, aging, and even terminally differentiated cells. Signaling pathways that affect G1 phase progression thus do not affect CDK levels and instead act mainly through regulation of CDK activity by controlling the abundance of their cyclin partners and a group of CKIs. Although both cyclins and CKIs can be regulated at the level of mRNA stability, translational control, and subcellular localization, the two major control mechanisms are transcriptional regulation and ubiquitin-dependent proteolysis. We discuss these mechanisms below, focusing on the regulation of expression and ubiquitylation of G1 cyclins and CKIs by different signal transduction pathways.
2. TRANSCRIPTIONAL REGULATION OF G1 CYCLINS BY MITOGENIC SIGNALS
2.1. D-Type Cyclins
D-type cyclins were simultaneously isolated initially from mammalian cells in a genetic screen for genes capable of complementing G1 cyclin deficiency in yeast, as the product of a gene whose expression is induced by colony-stimulating factor (CSF1), and as the product of the potential oncogene BCL1 that is clonally rearranged and overexpressed in a subset of parathyroid tumors (Matsushime et al. 1991; Motokura et al. 1991; Xiong et al. 1991). These findings provided early evidence linking the activation of a G1 cyclin with mitogenic growth factors and implicating abnormal expression of G1 cyclins in tumorigenesis. However, subsequent genetic analyses revealed only a relatively minor role of cyclin-D-dependent CDK activity in cell proliferation and development (Meyer et al. 2000; Kozar et al. 2004; Malumbres et al. 2004), although mouse embryonic fibroblasts (MEFs) from mice lacking CDK4 and CDK6 do have a reduced rate of exiting from quiescence in response to mitogenic stimulation. Hence, the D-type cyclins, although not an obligate component of the cell cycle machinery, couple extracellular mitogenic signals to the G1/S transition (Sherr and Roberts 2004).
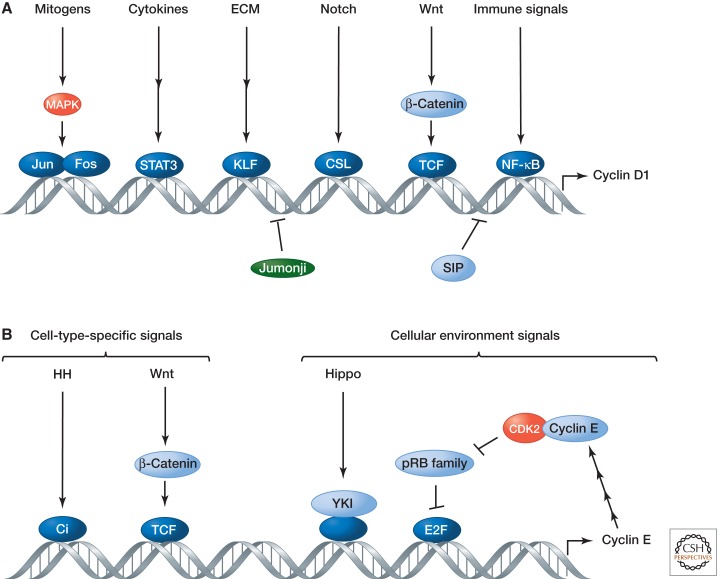
The canonical Ras–Raf–MEK–ERK mitogen-activated protein kinase (MAPK) pathway is the best characterized pathway for the activation of cyclin D transcription (Morrison 2012). It stimulates the expression of AP1 transcription factors such as the proto-oncogene products Jun and Fos, which bind directly to an AP1 site in the cyclin D1 promoter (Albanese et al. 1995). D-type cyclins can also be induced by other signaling pathways, including mitogen-stimulated Rac and NF-κB signaling, cytokine signaling, signaling by receptors for extracellular matrix (ECM) proteins (e.g., integrins), and the Wnt and Notch pathways (Kopan 2012; Nusse 2012). Multiple transcription factors directly regulate cyclin D genes, including Jun, Fos, STAT3, β-catenin, and NF-κB (Fig. 2A). Cyclin D genes are expressed at very low levels in most differentiated tissues, in part because of transcriptional repression by proteins such as Jumonji and SIP (Klein and Assoian 2008). Repression of G1 cyclin expression is an important part of cell cycle exit and terminal differentiation, and inappropriate reactivation of D- or E-type cyclins can drive differentiated cells back into S phase (Buttitta et al. 2007; Korzelius et al. 2011).
Figure 2.
Transcriptional regulation of G1 cyclins. (A) The expression of cyclin genes is tightly regulated at the level of transcription by different signals, including many mitogens. The figure uses human cyclin D1 as an example. (B) Cyclin E expression is also highly regulated and responds to two types of developmental signals, those that are cell-type specific and those that all cells use to control proliferation in response to their environment. MAPK, Mitogen-activated protein kinases; ECM, extracellular matrix; STAT, signal transducers and activators of transcription; KLF, Kruppel-like factor; CSL, CBF-1/suppressor of hairless/LAG-1; TCF, ternary complex factor; NF-κB, nuclear factor-κB; SIP1, SMAD interacting protein 1; HH, Hedgehog; Ci, Drosophila cubitus interruptus; YKI, Yorkie.
In contrast to cyclin D repression, inappropriate cyclin-D-dependent CDK4/6 activity represents the most frequent alteration of human cyclins in cancer and bears clear pathological significance. Human cyclin D1 is amplified in an estimated 13% of neoplasms of different types, including breast cancer, esophageal cancer, and lymphoma (Bates and Peters 1995). Mice transgenically expressing cyclin D1 develop mammary gland tumors and conversely are protected against mammary tumors if cyclin D1 is deleted (Wang et al. 1994; Yu et al. 2001). Likewise, CDK4 and CDK6 are also frequently amplified in diverse human cancers. Mouse cells lacking either combination of the three cyclin D proteins or CDK4/6 are more resistant to oncogenic transformation (Sherr and Roberts 2004; Malumbres and Barbacid 2009). These observations indicate that whereas a low level of G1 CDK activity is sufficient to support cell proliferation in response to normal physiological levels of mitogens, significantly higher levels of G1 CDK activity are required to sustain hyperproliferative stimulation, such as those elicited by activated oncogenes.
2.2. Cyclin E Expression
Cyclin E is encoded by a single gene in C. elegans (CYE-1) and Drosophila (CycE) and by two genes in mammalian cells (E1 and E2). The worm and fly cyclin E genes are essential for cell cycle progression and development (Knoblich et al. 1994; Fay and Han 2000). In contrast, mice lacking both cyclin E1 and E2 or CDK2 are viable and display relatively minor defects late in development, owing to compensation by other CDKs (Berthet et al. 2003; Geng et al. 2003; Ortega et al. 2003; Parisi et al. 2003). In well-fed proliferating cells, cyclin E expression is cyclical, peaking at the G1/S transition and being low or absent at other times in the cell cycle (Lew et al. 1991; Dulic et al. 1992; Koff et al. 1992). Conversely, MEFs lacking both cyclins E1 and E2 proliferate more slowly than normal cells and have a significantly reduced response to mitogenic stimulation, and cyclin E gene expression is repressed in serum-deprived cells, all of which suggest that cyclin E responds to growth factors (Herrera et al. 1996; Geng et al. 2003). This regulation is important, because forced overexpression of cyclin E can shorten G1 phase and drive cells into S phase, in part by causing phosphorylation of pRB family proteins (Hinds et al. 1992; Ohtsubo and Roberts 1993; Resnitzky et al. 1994). In vivo, transgenic expression of cyclin E under the control of the β-lactoglobulin promoter in mice results in mammary tumorigenesis (Smith et al. 2006), and overexpression of cyclin E is frequently observed in various human cancers and correlates with increased tumor aggression (Hwang and Clurman 2005). Hence, tight control of the levels of cyclin E is critically important for normal cell physiology and for preventing a neoplastic cell cycle. This notion is supported by biochemical and genetic analyses of the regulation of cyclin E by phosphorylation and by its regulatory protein FBW7 (see below).
Cyclin E transcription is directly controlled by E2F (Duronio and O’Farrell 1995; Ohtani et al. 1995; Geng et al. 1996). Thus, one important way that signaling regulates cyclin E is through the pRB/E2F pathway, which also integrates the output from the growth factor signals that control D-type-cyclin-dependent CDK activity. Indeed, if the mouse cyclin E gene is engineered to respond to the signals that control cyclin D1 gene expression, then cyclin D1 is no longer needed (Geng et al. 1999). Because cyclin-E–CDK2 can phosphorylate and inactivate pRB, resulting in E2F activity, a positive feedback amplification is an important part of G1/S control (Fig. 1B). This helps produce the switch-like behavior needed for unidirectional decisions like the G1/S transition (Xiong and Ferrell 2003; Ferrell et al. 2009).
Control of cyclin E transcription via E2F is a cornerstone of G1/S cell cycle control, but the cyclin E gene also responds directly to signaling pathways. This often occurs when developmental programs coordinate cell cycle progression with cell differentiation. In the Drosophila eye, for example, Hedgehog signaling induces cyclin E at the G1/S transition of the last cell cycle before differentiation of specialized cell types such as photoreceptors (Ingham 2012). The Drosophila CycE gene contains multiple enhancer elements that respond to and integrate various signals (Fig. 2B), including those from the pRB/E2F, Hedgehog and Wnt signaling pathways, in different cell types at different stages of development (Jones et al. 2000; Deb et al. 2008; Ingham 2012).
In Drosophila, CycE is also a target of the growth-inhibitory Hippo pathway (Harvey and Hariharan 2012), whose main target is the inactivation of the transcriptional coactivator Yorkie (YKI) (Huang et al. 2005). Tissue overgrowth upon disruption of the Hippo pathway is accompanied by increased expression of cyclin E, probably through direct regulation of CycE transcription by transcription factors associated with YKI. In vertebrates, the Hippo-pathway-mediated regulation of cell proliferation appears to be largely mediated by cyclin D1 (Cao et al. 2008).
Transcription of the cyclin E gene thus responds to two types of developmental signals: those that are cell type specific and essential for cell cycle progression (e.g., Hedgehog and Wnt signals), and those that are not cell type specific or strictly essential for cell cycle progression but instead modulate the rate of growth and cell proliferation in response to the cellular environment (e.g., E2F-mediated responses and Hippo) (Fig. 2B).
2.3. Posttranscriptional Regulation of CDKs
Posttranscriptional mechanisms also regulate CDK activity in response to various signals. The mitotic CDK, CDK1 (also known as CDC2), is inhibited during interphase by phosphorylation at two adjacent residues within its catalytic pocket, T14 and Y15, and is activated by CDC25-mediated dephosphorylation to bring about a sudden burst of CDK1 activity that triggers mitosis (Rhind and Russel 2012; Hariharan 2013). Both CDK2 and CDK4 are also phosphorylated at analogous residues to mediate the responses to different signals: phosphorylation of T14 and Y15 of CDK2 is important for regulating the timing of DNA replication and centrosome duplication (Zhao et al. 2012), and phosphorylation of Y17 of CDK4 is required for G1 arrest upon UV irradiation, which could cause DNA damage that should be repaired before entry into S phase (Terada et al. 1995).
3. TRANSCRIPTIONAL REGULATION OF CDK INHIBITORS
CKIs play an important role in arresting the cell cycle in G1 phase in response to a variety of stimuli, ranging from growth factor deprivation to DNA damage, cellular stress, differentiation, and senescence. Failure to arrest the cell cycle resulting from loss of function of a CKI can cause developmental defects or hyperplasia and tumorigenesis. The first CKI characterized was mammalian p21 (also known as CDKN1A, CIP1, or WAF1), which binds to and inhibits the activity of multiple CDK–cyclin complexes (Xiong et al. 1992, 1993a; Harper et al. 1993). The p21 family (also known as the CIP/KIP family) includes three related proteins: p21, p27 (also known as CDKN1B or KIP1), and p57 (also known as CDKN1C or KIP2). A distinct CKI, p16 (also known as INK4A), was isolated around the same time and is a specific inhibitor of CDK4 (Serrano et al. 1993). p16 is the founding member of a separate family of INK4 CKIs that includes three additional proteins: p15 (also known as INK4B), p18 (also known as INK4C), and p19 (also known as INK4D) (Sherr and Roberts 1995).
These two families of CKIs inhibit CDK via different mechanisms. The INK4 proteins bind selectively to the catalytic subunits of two CDKs, CDK4 and CDK6, preventing cyclin binding; and the p21 CKIs bind to the cyclin–CDK complex by contacting both subunits via different motifs to block kinase activity and substrate binding. CKIs of both families are localized predominantly in the nucleus in most tissues, but p21 family CKIs have also been frequently observed in the cytoplasm, where they have been linked to CDK-independent functions and tumor development. In particular, reduced nuclear p27 and accumulation of cytoplasmic p27 have been observed in multiple types of human cancers and are associated with poor prognosis of breast cancer (Wander et al. 2011).
The two separate families of multiple CDK inhibitors evolved to meet the increasing need to integrate numerous different antiproliferative signals that can arrest cells in G1 phase. Mice lacking CKI genes have various phenotypes, ranging from a compromised DNA damage response (p21 mutants) to widespread hyperplastic cell proliferation and organomegaly (p18- and p27-null mice), spontaneous tumor development (p16-null mice), and perinatal lethality and widespread developmental defects (in p57-null mice) (Ortega et al. 2002). Furthermore, genetic studies of p21-type CKIs in worms and flies have revealed various functions from control of cell cycle progression to cell cycle exit in specific cell types at various times in development (de Nooij et al. 1996; Lane et al. 1996; Hong et al. 1998; Firth and Baker 2005).
One major difference between the two CKI families is their stability. The p21 family inhibitors are intrinsically unstable (t1/2 < 30 min) as a result of ubiquitin-dependent, and in most cases phosphorylation-promoted, proteasomal degradation, and cause a rapid and transient cell cycle arrest, for example, following DNA damage. In contrast, the INK4 proteins are stable (t1/2 > 4–6 h) and are subject to minimal posttranslational regulation. INK4 proteins therefore maintain a long-term or permanent cell cycle arrest in stem, progenitor, senescent, and postmitotic cells. Accordingly, whereas p21 family CKIs are regulated both transcriptionally and posttranscriptionally, the INK4 members are regulated primarily at the level of transcription.
3.1. p21 Transcription Regulation by p53-Dependent and -Independent Mechanisms
Cells use signaling pathways to respond to a variety of exogenous and intrinsic stresses that have the potential to damage the genome. The tumor suppressor p53 functions as a transcription factor to activate the expression of many genes involved in stress responses, and defects in p53-mediated stress responses are associated with most types of human cancer. p53-mediated transcriptional activation of p21 following DNA damage was the first identified example of G1-phase regulation of a CKI gene (El-Deiry et al. 1993; Xiong et al. 1993b). Given that none of the other six CKI genes is a direct target of p53, the p53–p21–CDK regulatory module constitutes a major mechanism for DNA-damage-induced cell cycle arrest. Indeed, knocking out the p21 gene compromises the DNA damage response despite having little effect on overall mouse development (Brugarolas et al. 1995; Deng et al. 1995).
Transcriptional regulation of the p21 gene has also been linked to p53-independent cell cycle exit during development. In the Drosophila embryonic epidermis, activation of the dacapo (dap) gene, which encodes a p21-type CKI, triggers cell cycle exit (de Nooij et al. 1996; Lane et al. 1996). In Caenorhabditis elegans, the insulin-like growth factor signaling pathway similarly induces p21 expression in response to starvation, which results in cell cycle arrest in stem cells (Baugh and Sternberg 2006), and Ras/MAPK signaling activates p21 to control cell cycle exit in vulval precursor cells (Clayton et al. 2008). This diversity of responses probably relies on the existence of multiple, modular enhancers for the p21 gene that respond to different signaling pathways (Liu et al. 2002; Meyer et al. 2002).
3.2. INK4 Repression in Stem and Progenitor Cells
INK4 genes have distinct expression patterns during development in adult tissues and in response to different conditions (Roussel 1999). p16 is a target of Polycomb group (PcG) transcriptional repressors: deletion of the Polycomb gene Bmi1 retards cell proliferation, and this is associated with up-regulation of p16 and can be partially rescued by deletion of p16 (van Lohuizen et al. 1991; Jacobs et al. 1999). Furthermore, both PcG repression complexes (PRC1 and PRC2) collaborate with pRB proteins to bind to the p16 locus and trimethylate histone H3 lysine 27 (H3K27) to repress the expression of p16 (Bracken et al. 2007; Kotake et al. 2007). These findings explain how the up-regulation of p16 in aging stem cells results from decreased expression of Polycomb genes and reveal a negative-feedback loop between p16 and pRB.6 In many different types of human tumors, p16 expression is silenced by promoter DNA methylation (Merlo et al. 1995).
Unlike p16 mRNA, which is undetectable in young tissues and is induced during aging, p18 mRNA is present early in embryogenesis and maintains a high level throughout life in many adult tissues (Zindy et al. 1997). Deletion of p18 in mice results in spontaneous development of various tumors (Franklin et al. 1998; Pei et al. 2009) and increases self-renewing division of hematopoietic stem cells and expansion of mammary luminal progenitor cells (Yuan et al. 2004; Pei et al. 2009). p18 thus seems to suppress tumorigenesis by maintaining a quiescent state in stem and progenitor cells of different organs. GATA3, a transcription factor specifying mammary luminal cell fate, binds to the p18 locus and represses p18 transcription (Pei et al. 2009). It provides an example of a lineage-specifying factor that regulates cell differentiation in part by repressing the expression of an INK4 gene to allow quiescent progenitor cells to exit G0/G1 arrest, reenter the cell cycle, and proliferate.
4. CONTROL OF G1 CYCLINS BY THE UBIQUITIN–PROTEASOME SYSTEM
Like their mitotic counterparts (Hariharan 2013), G1 cyclins undergo rapid turnover and are degraded by the ubiquitin–proteasome pathway. This process is tightly regulated through the phosphorylation of cyclins and, in some cases, by proteins that target cyclins to E3 ubiquitin ligases, which provide mechanisms for extracellular factors to signal to the G1-phase cell cycle control machinery.
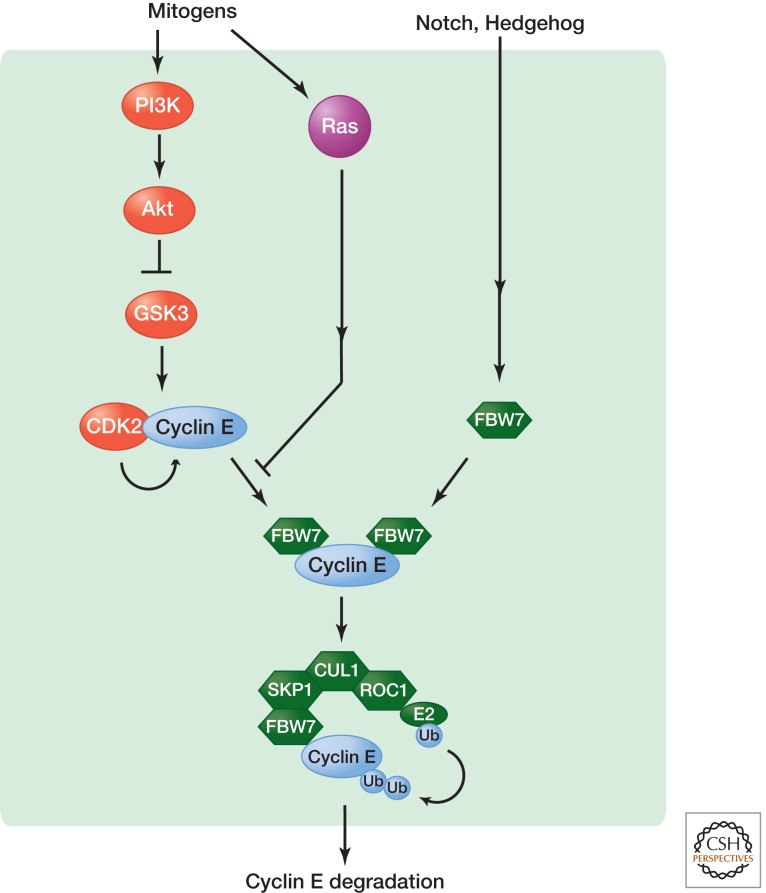
The level of cyclin E, and associated CDK2 activity, oscillates during the cell cycle (Dulic et al. 1992; Koff et al. 1992). Cyclin E begins to accumulate during the middle of G1 phase (as a result of E2F-mediated transcriptional activation), peaks at the G1/S transition, and then is destroyed during S phase following ubiquitylation. FBW7 (also known as Cdc4 or Ago) is an F-box protein that is the substrate-recognition component of the E3 ubiquitin ligase SCF (also known as CRL1) and recognizes two phosphodegrons in cyclin E: a carboxy-terminal degron centered on T380 and an amino-terminal degron centered on T62 (Fig. 3) (Welcker and Clurman 2008). Both cyclin E degrons are phosphorylated by GSK3 and CDK2 itself, creating two independent FBW7-binding sites. Cyclin-E–CDK2 is thought to phosphorylate cyclin E first at T384, creating a “priming phosphate” that is needed for GSK3 to phosphorylate T380 upstream, thus generating the doubly phosphorylated phosphodegron that is specifically recognized by the FBW7 targeting subunit of SCF-FBW7.
Figure 3.
Targeting ubiquitin-dependent degradation of cyclin E. F-box protein FBW7 specifically recognizes two separate phosphodegrons in cyclin E and targets cyclin E for ubiquitin-dependent proteasome degradation by the SCF-FBW7 E3 ligase complex. The phosphorylation of both amino- and carboxy-terminal degrons in cyclin E is catalyzed by GSK3 and CDK2 and creates two separate binding sites for FBW7. Both mitogenic and antiproliferative signals exert their effect on the cell cycle through cyclin E ubiquitylation by inhibiting the activity of GSK3 or stimulating the expression of FBW7, respectively.
Because GSK3 plays critical roles in diverse signals, including those activated by insulin, mitogenic growth factors, Wnts, Hedgehog, and cytokines, GSK3 activity can link the regulation of cyclin E and thus G1 progression to different signaling pathways. For example, GSK3 is regulated by the phosphoinositide 3 kinase (PI3K)–AKT pathway, which allows a major mitogen signaling pathway (Hemmings 2012) to couple cell growth to G1 regulation. Transgenic expression of mutant cyclin E (T380A) in mammary glands causes more widespread hyperplasia than that of wild-type cyclin E and promotes p53 loss of heterozygosity and tumorigenesis (Smith et al. 2006). Knock-in mutations that ablate both T62 and T380 result in disruption of cyclin E periodicity, increased cyclin E activity, and abnormal proliferation in multiple cell types (Minella et al. 2008).
Studies of Fbw7-mutant mice and loss-of-function mutations of FBW7 in human cancer support a role for SCF-FBW7 in negative regulation of cell proliferation by targeting cyclin E, as well as Myc, Notch, and Jun (Welcker and Clurman 2008). Mitogen signaling can also influence the activity of FBW7 itself. In mammalian cells, activated Ras increases cyclin E levels by inhibiting binding of cyclin E to FBW7 (Welcker and Clurman 2008), and Notch and Hedgehog signaling suppresses cyclin E accumulation by inducing FBW7 expression in Drosophila eye imaginal discs (Nicholson et al. 2011). Therefore, both oncogenic and developmental signals can control the level of cyclin E protein by regulating components of the E3 ubiquitin ligase that targets cyclin E for destruction (Fig. 3).
Cyclin D is phosphorylated at T286, a site analogous to T380 in cyclin E, and T286 phosphorylation promotes cyclin D destruction (Diehl et al. 1998). Multiple F-box proteins, such as Fbxo41, Fbxw8, SKP2, and Fbxo31, have been implicated in targeting cyclin D for destruction, but the E3 ligase responsible remains to be definitively identified (Kanie et al. 2012). Promoting the destruction of both D- and E-type G1 cyclins by GSK3-mediated phosphorylation, however, could allow cells to effectively couple the PI3K–AKT pathway to G1 cell cycle control. T286-phosphorylated cyclin D1 can also be recognized and stabilized in the nucleus by Pin1, a prolyl isomerase that regulates the function of proteins by causing conformational change of their S/T-phosphorylated forms (Liou et al. 2002).
Progression through G1 phase is also controlled by other E3 ligases. In particular, the anaphase-promoting complex (APC), which promotes the ubiquitin-dependent proteasomal degradation of multiple mitotic regulatory proteins, remains active in G1 phase to suppress accumulation of mitotic cyclins until cyclin-E–CDK2 is activated at the G1/S transition.
5. CONTROL OF G1 CDK INHIBITORS BY THE UBIQUITIN–PROTEASOME SYSTEM
Some CKIs are also regulated by the ubiquitin–proteasome pathway. Again, this regulation involves phosphorylation of these CKIs, which provides a mechanism linking extracellular signaling to the G1 cell cycle control machinery.
5.1. Phosphorylation-Dependent Ubiquitylation and Degradation of a Yeast CKI
In Saccharomyces cerevisiae, a single CDK, Cdc28, forms multiple B-type cyclin–CDK complexes to drive both S phase and mitosis. Cdc28 is inhibited by Sic1, a CKI that is unrelated in sequence to either the p21 or INK4 family of CKIs. Sic1 is targeted for ubiquitylation (Fig. 4) following phosphorylation by the G1 cyclin–CDK complex Cln–Cdc28 (Schwob et al. 1994). Inactivation of Sic1 rescues the inviability of yeast cells lacking the G1 cyclins Cln1, Cln2, and Cln3 (Schneider et al. 1996), and mutation of CDK phosphorylation sites in Sic1 causes stabilization of Sic1 and blocks DNA replication. These observations indicate that the primary function of these three G1 cyclins, once mitogenically activated, is to promote Sic1 ubiquitylation to bring about the G1/S transition. Phosphorylated, but not unmodified, Sic1 binds to the F-box protein Cdc4, which, through a linker protein, Skp1, brings Sic1 to the Cul1 (also known as Cdc53)–Roc1 (also known as Rbx or Hrt1) E3 ligase complex for ubiquitylation by the E2 enzyme Cdc34 (Feldman et al. 1997; Skowyra et al. 1997). Nine sites in Sic1 are phosphorylated, and each contributes to Cdc4 binding, with any six being required (Nash et al. 2001). This multisite phosphorylation requirement makes Sic1 ubiquitylation ultrasensitive to the level of G1 CDK activity, enabling cells to measure the strength of mitogens and set the level of CDK activity that determines the timing of DNA replication. It transforms a gradual accumulation process, such as protein synthesis during G1 phase, into an irreversible switch for the onset of DNA replication. Sic1 is also phosphorylated by its target, the B-type cyclin–CDK complex Clb5–CDK1, which may ensure irreversibility of the G1/S transition once DNA replication has been initiated.
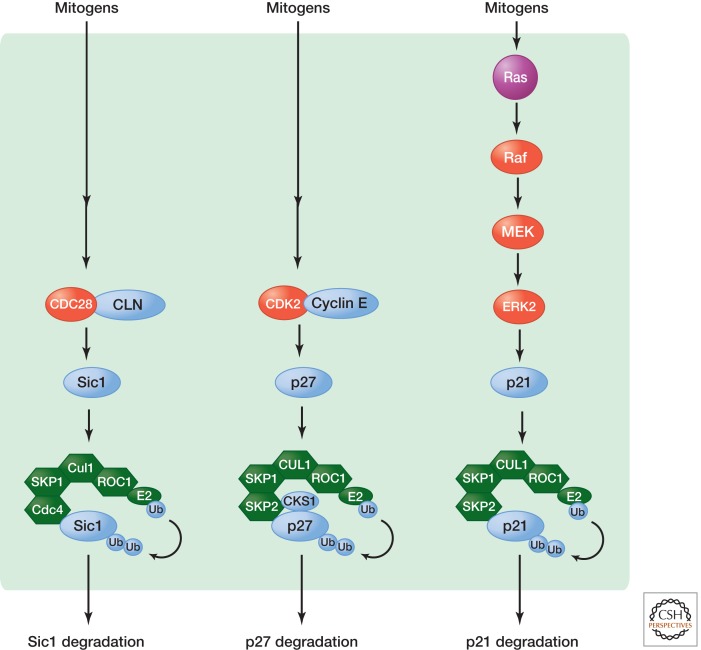
Figure 4.
Targeting ubiquitin-dependent degradation of CDK inhibitors. The p21 family of CKIs is regulated by the ubiquitin–proteasome pathway. In many cases, this involves phosphorylation of these CKIs. Phosphorylated CKIs are recognized by F-box proteins such as Cdc4 in budding yeast or SKP2 in human cells, which, through the SKP1 linker protein, recruits the CKI substrate to the SCF E3 ligase for ubiquitylation.
In response to mating pheromones, budding yeast cells arrest their cycle in G1 phase and fuse cytoplasms and nuclei to generate a diploid cell. This G1 cell cycle arrest is regulated by the Fus3 MAPK pathway, which leads to phosphorylation and activation of Far1, a second budding yeast CDK inhibitor that is unrelated to Sic1 and other CKIs in sequence. Far1 selectively inhibits G1 cyclin–Cdc28, leading to the inhibition of Cln–Cdc28-induced Sic1 degradation and G1 arrest.
The distantly related fission yeast, Schizosaccharomyces pombe, contains a single CKI, Rum1, that is unrelated to Sic1, p21, or INK4 CKIs in sequence. Rum1 inhibits the cyclin B–CDK complex Cdc13–Cdc2 and is an essential G1 regulator whose deletion causes premature S-phase initiation immediately after mitosis (Correa-Bordes and Nurse 1995). Rum1 is degraded following ubiquitylation by the SCF-Pop1 ligase, which uses Pop1, an ortholog of budding yeast Cdc4, to target Rum1 (Kominami and Toda 1997). Hence, the mechanism for targeting G1 CDK inhibitors for ubiquitylation has been conserved between two yeast species that are as evolutionarily divergent from each other as either is from animals.
5.2. Regulation of Mammalian CIP/KIP by E3 Ligases
The mammalian CKI p27 is also regulated by ubiquitin-dependent proteolysis (Pagano et al. 1995). p27 and its close relative p57 are phosphorylated by cyclin-E–CDK2 at analogous sites (T187 in p27 and T310 in p57), which promotes their binding to the F-box protein SKP2 and subsequent ubiquitylation by the SCF-SKP2 E3 ligase. The recognition of T187-phosphorylated p27 by SKP2 requires CKS1, a small evolutionarily conserved protein whose function is essential for yeast cell viability and normal mouse development (Fig. 3). A second p27 E3 ligase, KIP1-ubiquitylation-promoting complex (KPC), preferentially recognizes free p27 and is competed off by the binding of cyclin-E–CDK2 (Kamura et al. 2004). Mitogen-stimulated cyclin E expression and thus the formation of the cyclin-E–CDK2 complex may switch cells from KPC-mediated degradation of p27 during early G0/G1 transitions to SCF-mediated degradation at the G1/S transition. Likewise, p57, which plays important roles in development, is also ubiquitylated by the SCF-SKP2 E3 ligase and a second E3 ligase, SCF-FBL12, containing FBL12. FBL12 is induced by TGFβ1 and binds only to p57, providing a mechanism for TGFβ1-induced degradation of p57, but not p27 or p21 (Kim et al. 2008a).
p21 expression oscillates twice during each cell cycle: it is high in G1 phase, decreases during S phase, reaccumulates during G2 phase, and then decreases at early mitosis. The protein has a very short half-life (<30 min) and is rapidly turned over by ubiquitin-dependent proteolysis. Several E3 ligases can target p21 ubiquitylation at different phases of the cell cycle in both phosphorylation-dependent and phosphorylation-independent manners. During G1 phase, sustained activation of the ERK2 MAPK by mitogenic stimuli such as epidermal growth factor (EGF) results in T57 and S130 phosphorylation on p21, leading to its ubiquitin-dependent degradation (Fig. 3) (Hwang et al. 2009). During S phase, WD40 protein CDT2 and the F-box protein SKP2 target p21 for ubiquitylation by the CRL4-CDT2 and SCF-SKP2 E3 ligases to prevent DNA rereplication (Bornstein et al. 2003; Abbas et al. 2008; Kim et al. 2008b; Nishitani et al. 2008). The SCF-SKP2-mediated p21 ubiquitylation requires S130 phosphorylation by cyclin-E–CDK2 (Bornstein et al. 2003). During early mitosis, Cdc20 binds to p21 and targets it for ubiquitylation by APC. CRL4 also targets p21 for ubiquitylation after low-dose UV irradiation, to delay the cell cycle, allowing time for optimal DNA repair (Bendjennat et al. 2003; Havens and Walter 2011; Starostina and Kipreos 2012). Hence, the mechanism for targeting G1 CKIs for ubiquitylation has been conserved from yeast to animals and links the regulation of CKI stability to signals from different pathways via the phosphorylation of CKI proteins and their targeting molecules.
6. CONCLUDING REMARKS
Precise cell cycle regulation is an essential aspect of normal development and adult homeostasis. To achieve this, cells in G1 phase integrate inputs from major cellular signaling pathways to decide whether or not to enter S phase, which is an irreversible cell cycle step. This integration of signals is transformed into an appropriate level of CDK activity in large part via changes in the level of cyclins and CKIs achieved through the regulation of both transcription and protein stability. One challenge for the future is to understand how multiple signaling pathways cooperate to precisely regulate cyclin and CKI activity in various cell types, particularly stem cells, in intact tissues. Another is to use this information to develop novel therapeutics for the treatment of cancer, which arises in part because of disruptions to signaling pathways that affect cell cycle regulation.
ACKNOWLEDGMENTS
We thank Alan Diehl, Andrew Koff, Kun-Liang Guan, Michele Pagano, DJ Pan, and Xin-Hai Pei for discussions, and Tadashi Nakagawa and Ruiting Lin for helping with figure preparation.
CDKs are a family of kinases that regulate the cell cycle and that require binding to noncatalytic partner proteins termed cyclins for activity.
Linked to p16, both structurally in the genome and through regulation by Polycomb group proteins, is the product of the ARF tumor suppressor gene, which is transcribed from an alternative promoter and translated in an alternative reading frame from p16. ARF does not share any amino acid sequence similarity with INK4 proteins and instead acts as a p53 activator by binding to and inhibiting the activity of MDM2, the principle E3 ubiquitin ligase for and negative regulator of p53. As a result, any signal, such as oncogenic stimulation, that induces the expression of ARF will stabilize p53 and activate p21, leading to G1 cell cycle arrest.
Editors: Lewis Cantley, Tony Hunter, Richard Sever, and Jeremy Thorner
Additional Perspectives on Signal Transduction available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- Abbas T, Sivaprasad U, Terai K, Amador V, Pagano M, Dutta A 2008. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev 22: 2496–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Ohuchi T, Sumida S, Matsumoto K, Toyoshima K 1992. Phosphorylation of the retinoblastoma protein by cdk2. Proc Natl Acad Sci 89: 7900–7904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albanese C, Johnson J, Watanabe G, Eklund N, Vu D, Arnold A, Pestell RG 1995. Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J Biol Chem 270: 23589–23597 [DOI] [PubMed] [Google Scholar]
- Bates S, Peters G 1995. Cyclin D1 as a cellular proto-oncogene. Semin Cancer Biol 6: 73–82 [DOI] [PubMed] [Google Scholar]
- Baugh LR, Sternberg PW 2006. DAF-16/FOXO regulates transcription of cki-1/Cip/Kip and repression of lin-4 during C. elegans L1 arrest. Curr Biol 16: 780–785 [DOI] [PubMed] [Google Scholar]
- Bendjennat M, Boulaire J, Jascur T, Brickner H, Barbier V, Sarasin A, Fotedar A, Fotedar R 2003. UV irradiation triggers ubiquitin-dependent degradation of p21WAF1 to promote DNA repair. Cell 114: 599–610 [DOI] [PubMed] [Google Scholar]
- Berthet C, Aleem E, Coppola V, Tessarollo L, Kaldis P 2003. Cdk2 knockout mice are viable. Curr Biol 13: 1775–1785 [DOI] [PubMed] [Google Scholar]
- Bornstein G, Bloom J, Sitry-Shevah D, Nakayama K, Pagano M, Hershko A 2003. Role of the SCFSkp2 ubiquitin ligase in the degradation of p21Cip1 in S phase. J Biol Chem 278: 25752–25757 [DOI] [PubMed] [Google Scholar]
- Bracken AP, Kleine-Kohlbrecher D, Dietrich N, Pasini D, Gargiulo G, Beekman C, Theilgaard-Monch K, Minucci S, Porse BT, Marine JC, et al. 2007. The Polycomb group proteins bind throughout the INK4A-ARF locus and are disassociated in senescent cells. Genes Dev 21: 525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ 1995. Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377: 552–557 [DOI] [PubMed] [Google Scholar]
- Buttitta LA, Katzaroff AJ, Perez CL, de la Cruz A, Edgar BA 2007. A double-assurance mechanism controls cell cycle exit upon terminal differentiation in Drosophila. Dev Cell 12: 631–643 [DOI] [PubMed] [Google Scholar]
- Cao X, Pfaff SL, Gage FH 2008. YAP regulates neural progenitor cell number via the TEA domain transcription factor. Genes Dev 22: 3320–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton JE, van den Heuvel SJ, Saito RM 2008. Transcriptional control of cell-cycle quiescence during C. elegans development. Dev Biol 313: 603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa-Bordes J, Nurse P 1995. p25rum1 orders S phase and mitosis by acting as an inhibitor of the p34cdc2 mitotic kinase. Cell 83: 1001–1009 [DOI] [PubMed] [Google Scholar]
- Costanzo M, Nishikawa JL, Tang X, Millman JS, Schub O, Breitkreuz K, Dewar D, Rupes I, Andrews B, Tyers M 2004. CDK activity antagonizes Whi5, an inhibitor of G1/S transcription in yeast. Cell 117: 899–913 [DOI] [PubMed] [Google Scholar]
- Deb DK, Tanaka-Matakatsu M, Jones L, Richardson HE, Du W 2008. Wingless signaling directly regulates cyclin E expression in proliferating embryonic PNS precursor cells. Mech Dev 125: 857–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin RA, McDonald WH, Kalashnikova TI, Yates J 3rd, Wittenberg C 2004. Cln3 activates G1-specific transcription via phosphorylation of the SBF bound repressor Whi5. Cell 117: 887–898 [DOI] [PubMed] [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P 1995. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell 82: 675–684 [DOI] [PubMed] [Google Scholar]
- de Nooij JC, Letendre MA, Hariharan IK 1996. A cyclin-dependent kinase inhibitor, Dacapo, is necessary for timely exit from the cell cycle during Drosophila embryogenesis. Cell 87: 1237–1247 [DOI] [PubMed] [Google Scholar]
- Diehl JA, Cheng M, Roussel M, Sherr CJ 1998. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12: 3499–3511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulic V, Lees E, Reed SI 1992. Association of human cyclin E with a periodic G1–S phase protein kinase. Science 257: 1958–1961 [DOI] [PubMed] [Google Scholar]
- Duronio RJ, O’Farrell PH 1995. Developmental control of the G1 to S transition in Drosophila: Cyclin E is a limiting downstream target of E2F. Genes Dev 9: 1456–1468 [DOI] [PubMed] [Google Scholar]
- Dyson N 1998. The regulation of E2F by pRB-family proteins. Genes Dev 12: 2245–2262 [DOI] [PubMed] [Google Scholar]
- El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Lin DM, Mercer WE, Kinzler KWV, Vogelstein B 1993. WAF1, a potential mediator of p53 tumor suppression. Cell 75: 817–825 [DOI] [PubMed] [Google Scholar]
- Ewen ME, Sluss HK, Sherr CJ, Matsushime H, Kato J, Livingston DM 1993. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell 73: 487–497 [DOI] [PubMed] [Google Scholar]
- Fay DS, Han M 2000. Mutations in cye-1, a Caenorhabditis elegans cyclin E homolog, reveal coordination between cell-cycle control and vulval development. Development 127: 4049–4060 [DOI] [PubMed] [Google Scholar]
- Feldman RMR, Correll CC, Kaplan KB, Deshaies RJ 1997. A complex of Cdc4p, Skp1p, and Cdc53p/Cullin catalyzes ubiquitination of the phosphorylated CDK inhibitor Sic1p. Cell 91: 221–230 [DOI] [PubMed] [Google Scholar]
- Ferrell JE Jr, Pomerening JR, Kim SY, Trunnell NB, Xiong W, Huang CY, Machleder EM 2009. Simple, realistic models of complex biological processes: Positive feedback and bistability in a cell fate switch and a cell cycle oscillator. FEBS Lett 583: 3999–4005 [DOI] [PubMed] [Google Scholar]
- Firth LC, Baker NE 2005. Extracellular signals responsible for spatially regulated proliferation in the differentiating Drosophila eye. Dev Cell 8: 541–551 [DOI] [PubMed] [Google Scholar]
- Franklin DS, Godfrey VL, Lee H, Kovalev GI, Schoonhoven R, Chen-Kiang S, Su L, Xiong Y 1998. CDK inhibitors p18INK4c and p27KIP1 mediate two separate pathways to collaboratively suppress pituitary tumorigenesis. Genes Dev 12: 2899–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Eaton EN, Picon M, Roberts JM, Lundberg AS, Gifford A, Sardet C, Weinberg RA 1996. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene 12: 1173–1180 [PubMed] [Google Scholar]
- Geng Y, Whoriskey W, Park MY, Bronson RT, Medema RH, Li T, Weinberg RA, Sicinski P 1999. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell 97: 767–777 [DOI] [PubMed] [Google Scholar]
- Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, Rideout WM, Bronson RT, Gardner H, Sicinski P 2003. Cyclin E ablation in the mouse. Cell 114: 431–443 [DOI] [PubMed] [Google Scholar]
- *.Hariharan I 2013. Signaling pathways that regulate cell division. Cold Spring Harb Perspect Biol (to be published) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75: 805–816 [DOI] [PubMed] [Google Scholar]
- *.Harvey KF, Hariharan IK 2012. The Hippo pathway. Cold Spring Harb Perspect Biol 4: a011288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havens CG, Walter JC 2011. Mechanism of CRL4(Cdt2), a PCNA-dependent E3 ubiquitin ligase. Genes Dev 25: 1568–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hemmings BA, Restuccia DF 2012. PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol 4: a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera RE, Sah VP, Williams BO, Makela TP, Weinberg RA, Jacks T 1996. Altered cell cycle kinetics, gene expression, and G1 restriction point regulation in Rb-deficient fibroblasts. Mol Cell Biol 16: 2402–2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds PW, Mittnacht S, Dulic V, Arnold A, Reed SI, Weinberg RA 1992. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell 70: 993–1006 [DOI] [PubMed] [Google Scholar]
- Hong Y, Roy R, Ambros V 1998. Developmental regulation of a cyclin-dependent kinase inhibitor controls postembryonic cell cycle progression in Caenorhabditis elegans. Development 125: 3585–3597 [DOI] [PubMed] [Google Scholar]
- Huang J, Wu S, Barrera J, Matthews K, Pan D 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122: 421–434 [DOI] [PubMed] [Google Scholar]
- Hwang HC, Clurman BE 2005. Cyclin E in normal and neoplastic cell cycles. Oncogene 24: 2776–2786 [DOI] [PubMed] [Google Scholar]
- Hwang CY, Lee C, Kwon KS 2009. Extracellular signal-regulated kinase 2-dependent phosphorylation induces cytoplasmic localization and degradation of p21Cip1. Mol Cell Biol 29: 3379–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Ingham PW 2012. Hedgehog signaling. Cold Spring Harb Perspect Biol 4: a011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inze D 2005. Green light for the cell cycle. EMBO J 24: 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JJ, Kieboom K, Marino S, DePinho RA, van Lohuizen M 1999. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397: 164–168 [DOI] [PubMed] [Google Scholar]
- Jones L, Richardson H, Saint R 2000. Tissue-specific regulation of cyclin E transcription during Drosophila melanogaster embryogenesis. Development 127: 4619–4630 [DOI] [PubMed] [Google Scholar]
- Kamura T, Hara T, Matsumoto M, Ishida N, Okumura F, Hatakeyama S, Yoshida M, Nakayama K, Nakayama KI 2004. Cytoplasmic ubiquitin ligase KPC regulates proteolysis of p27Kip1 at G1 phase. Nat Cell Biol 6: 1229–1235 [DOI] [PubMed] [Google Scholar]
- Kanie T, Onoyama I, Matsumoto A, Yamada M, Nakatsumi H, Tateishi Y, Yamamura S, Tsunematsu R, Matsumoto M, Nakayama KI 2012. Genetic reevaluation of the role of F-box proteins in cyclin D1 degradation. Mol Cell Biol 32: 590–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato J-Y, Matsushime H, Hiebert SW, Ewen M, Sherr CJ 1993. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev 7: 331–342 [DOI] [PubMed] [Google Scholar]
- Kim M, Nakamoto T, Nishimori S, Tanaka K, Chiba T 2008a. A new ubiquitin ligase involved in p57KIP2 proteolysis regulates osteoblast cell differentiation. EMBO Rep 9: 878–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Starostina NG, Kipreos ET 2008b. The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev 22: 2507–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa M, Higashi H, Jung HK, Suzuki-Takahashi I, Ikeda M, Tamai K, Kato J, Segawa K, Yoshida E, Nishimura S, et al. 1996. The consensus motif for phosphorylation by cyclin D1–Cdk4 is different from that for phosphorylation by cyclin A/E–Cdk2. EMBO J 15: 7060–7069 [PMC free article] [PubMed] [Google Scholar]
- Klein EA, Assoian RK 2008. Transcriptional regulation of the cyclin D1 gene at a glance. J Cell Sci 121: 3853–3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoblich JA, Sauer K, Jones L, Richardson H, Saint R, Lehner CF 1994. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell 77: 107–120 [DOI] [PubMed] [Google Scholar]
- Koff A, Giordano A, Desai D, Yamashita K, Harper JW, Elledge S, Nishimoto T, Morgan DO, Franza BR, Roberts JM 1992. Formation and activation of a cyclin E–cdk2 complex during the G1 phase of the human cell cycle. Science 257: 1689–1694 [DOI] [PubMed] [Google Scholar]
- Kominami K-I, Toda T 1997. Fission yeast WD-repeat protein Pop1 regulates genome ploidy through ubiquitin–proteasome-mediated degradation of the CDK inhibitor Rum1 and the S-phase initiator Cdc18. Genes Dev 11: 1548–1560 [DOI] [PubMed] [Google Scholar]
- *.Kopan R 2012. Notch signaling. Cold Spring Harb Perspect Biol 4: a011213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzelius J, The I, Ruijtenberg S, Prinsen MB, Portegijs V, Middelkoop TC, Groot Koerkamp MJ, Holstege FC, Boxem M, van den Heuvel S 2011. Caenorhabditis elegans cyclin D/CDK4 and cyclin E/CDK2 induce distinct cell cycle re-entry programs in differentiated muscle cells. PLoS Genet 7: e1002362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotake Y, Cao R, Viatour P, Sage J, Zhang Y, Xiong Y 2007. pRB family proteins are required for H3K27 trimethylation and Polycomb repression complexes binding to and silencing p16INK4a tumor suppressor gene. Genes Dev 21: 49–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, Geng Y, Yu Q, Bhattacharya S, Bronson RT, et al. 2004. Mouse development and cell proliferation in the absence of D-cyclins. Cell 118: 477–491 [DOI] [PubMed] [Google Scholar]
- Lane ME, Sauer K, Wallace K, Jan YN, Lehner CF, Vaessin H 1996. Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell 87: 1225–1235 [DOI] [PubMed] [Google Scholar]
- Lew D, Dulic V, Reed SI 1991. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell 66: 1197–1206 [DOI] [PubMed] [Google Scholar]
- Liou YC, Ryo A, Huang HK, Lu PJ, Bronson R, Fujimori F, Uchida T, Hunter T, Lu KP 2002. Loss of Pin1 function in the mouse causes phenotypes resembling cyclin D1-null phenotypes. Proc Natl Acad Sci 99: 1335–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu TH, Li L, Vaessin H 2002. Transcription of the Drosophila CKI gene dacapo is regulated by a modular array of cis-regulatory sequences. Mech Dev 112: 25–36 [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M 2009. Cell cycle, CDKs and cancer: A changing paradigm. Nat Rev Cancer 9: 153–166 [DOI] [PubMed] [Google Scholar]
- Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M 2004. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 118: 493–504 [DOI] [PubMed] [Google Scholar]
- Matsushime H, Roussel MF, Ashmum RA, Sherr CJ 1991. Colony-stimulating factor 1 regulates a novel gene (CYL1) during the G1 phase of the cell cycle. Cell 65: 701–713 [DOI] [PubMed] [Google Scholar]
- Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, Burger PC, Baylin SB, Sidransky D 1995. 5′ CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med 1: 686–692 [DOI] [PubMed] [Google Scholar]
- Meyer CA, Jacobs HW, Datar SA, Du W, Edgar BA, Lehner CF 2000. Drosophila Cdk4 is required for normal growth and is dispensable for cell cycle progression. EMBO J 19: 4533–4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer CA, Kramer I, Dittrich R, Marzodko S, Emmerich J, Lehner CF 2002. Drosophila p27Dacapo expression during embryogenesis is controlled by a complex regulatory region independent of cell cycle progression. Development 129: 319–328 [DOI] [PubMed] [Google Scholar]
- Minella AC, Loeb KR, Knecht A, Welcker M, Varnum-Finney BJ, Bernstein ID, Roberts JM, Clurman BE 2008. Cyclin E phosphorylation regulates cell proliferation in hematopoietic and epithelial lineages in vivo. Genes Dev 22: 1677–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Morrison DK 2012. MAP kinase pathways. Cold Spring Harb Perspect Biol 4: 011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokura T, Bloom T, Kim HG, Juppner H, Ruderman JV, Kronenberg HM, Arnold A 1991. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature 350: 512–515 [DOI] [PubMed] [Google Scholar]
- Nash P, Tang X, Orlicky S, Chen Q, Gertler FB, Mendenhall MD, Sicheri F, Pawson T, Tyers M 2001. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature 414: 514–521 [DOI] [PubMed] [Google Scholar]
- Nicholson SC, Nicolay BN, Frolov MV, Moberg KH 2011. Notch-dependent expression of the archipelago ubiquitin ligase subunit in the Drosophila eye. Development 138: 251–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani H, Shiomi Y, Iida H, Michishita M, Takami T, Tsurimoto T 2008. CDK inhibitor p21 is degraded by a proliferating cell nuclear antigen-coupled Cul4–DDB1Cdt2 pathway during S phase and after UV irradiation. J Biol Chem 283: 29045–29052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Nusse R 2012. Wnt signaling. Cold Spring Harb Perspect Biol 4: a011163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani K, Degregori J, Nevins JR 1995. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci 92: 12146–12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo M, Roberts JM 1993. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science 259: 1908–1912 [DOI] [PubMed] [Google Scholar]
- Ortega S, Malumbres M, Barbacid M 2002. Cyclin D-dependent kinases, INK4 inhibitors and cancer. Biochim Biophys Acta 1602: 73–87 [DOI] [PubMed] [Google Scholar]
- Ortega S, Prieto I, Odajima J, Martin A, Dubus P, Sotillo R, Barbero JL, Malumbres M, Barbacid M 2003. Cyclin-dependent kinase 2 is essential for meiosis but not for mitotic cell division in mice. Nat Genet 35: 25–31 [DOI] [PubMed] [Google Scholar]
- Pagano M, Tam SW, Theodoras AM, Beer-Romero P, Del Sal G, Chau V, Yew PR, Draetta GF, Rolfe M 1995. Role of the ubiquitin–proteasome pathway in regulating abundance of the cyclin-dependent kinase inhibitor p27. Science 269: 682–685 [DOI] [PubMed] [Google Scholar]
- Parisi T, Beck AR, Rougier N, McNeil T, Lucian L, Werb Z, Amati B 2003. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J 22: 4794–4803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei XH, Bai F, Smith MD, Usary J, Fan C, Pai S-Y, Ho IC, Perou CM, Xiong Y 2009. CDK inhibitor p18INK4c is a downstream target of GATA3 and restrains mammary luminal progenitor cell proliferation and tumorigenesis. Cancer Cell 15: 389–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnitzky D, Gossen M, Bujard H, Reed S 1994. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol 14: 1669–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Rhind N, Russel P 2012. Signaling pathways that regulate cell division. Cold Spring Harb Perspect Biol 4: a005942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel MF 1999. The INK4 family of cell cycle inhibitors in cancer. Oncogene 18: 5311–5317 [DOI] [PubMed] [Google Scholar]
- Schneider BL, Yang QH, Futcher AB 1996. Linkage of replication to start by the Cdk inhibitor Sic1. Science 272: 560–562 [DOI] [PubMed] [Google Scholar]
- Schwob E, Bohm T, Mendenhall MD, Nasmyth K 1994. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell 79: 233–244 [DOI] [PubMed] [Google Scholar]
- Serrano M, Hannon GJ, Beach D 1993. A new regulatory motif in cell cycle control causing specific inhibition of cyclin D/CDK4. Nature 366: 704–707 [DOI] [PubMed] [Google Scholar]
- Sherr CJ 1996. Cancer cell cycle. Science 274: 1672–1677 [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM 1995. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev 9: 1149–1163 [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM 2004. Living with or without cyclins and cyclin-dependent kinases. Genes Dev 18: 2699–2711 [DOI] [PubMed] [Google Scholar]
- Skowyra D, Craig K, Tyers M, Elledge SJ, Harper JW 1997. F-box proteins are receptors that recruit phosphorylated substrates to the SCF ubiquitin-ligase complex. Cell 91: 209–219 [DOI] [PubMed] [Google Scholar]
- Smith AP, Henze M, Lee JA, Osborn KG, Keck JM, Tedesco D, Bortner DM, Rosenberg MP, Reed SI 2006. Deregulated cyclin E promotes p53 loss of heterozygosity and tumorigenesis in the mouse mammary gland. Oncogene 25: 7245–7259 [DOI] [PubMed] [Google Scholar]
- Starostina NG, Kipreos ET 2012. Multiple degradation pathways regulate versatile CIP/KIP CDK inhibitors. Trends Cell Biol 22: 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada Y, Tatsuka M, Jinno S, Okayama H 1995. Requirement for tyrosine phosphorylation of Cdk4 in G1 arrest induced by ultraviolet irradiation. Nature 376: 358–362 [DOI] [PubMed] [Google Scholar]
- van den Heuvel S, Dyson NJ 2008. Conserved functions of the pRB and E2F families. Nat Rev Mol Cell Biol 9: 713–724 [DOI] [PubMed] [Google Scholar]
- van Lohuizen M, Verbeek S, Scheijen B, Wientjens E, van der Gulden H, Berns A 1991. Identification of cooperating oncogenes in Eμ-myc transgenic mice by provirus tagging. Cell 65: 737–752 [DOI] [PubMed] [Google Scholar]
- Wander SA, Zhao D, Slingerland JM 2011. p27: A barometer of signaling deregulation and potential predictor of response to targeted therapies. Clin Cancer Res 17: 12–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang TC, Cardiff RD, Zukerberg L, Lees E, Arnold A, Schmidt EV 1994. Mammary hyperplasia and carcinoma in MMTV–cyclin D1 transgenic mice. Nature 369: 669–671 [DOI] [PubMed] [Google Scholar]
- Weinberg RA 1995. The retinoblastoma protein and cell cycle control. Cell 81: 323–330 [DOI] [PubMed] [Google Scholar]
- Welcker M, Clurman BE 2008. FBW7 ubiquitin ligase: A tumour suppressor at the crossroads of cell division, growth and differentiation. Nat Rev Cancer 8: 83–93 [DOI] [PubMed] [Google Scholar]
- Xiong W, Ferrell JE Jr 2003. A positive-feedback-based bistable “memory module” that governs a cell fate decision. Nature 426: 460–465 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Connolly T, Futcher B, Beach D 1991. Human D-type cyclin. Cell 65: 691–699 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Zhang H, Beach D 1992. D-type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 71: 505–514 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Hannon G, Zhang H, Casso D, Kobayashi R, Beach D 1993a. p21 is a universal inhibitor of the cyclin kinases. Nature 366: 701–704 [DOI] [PubMed] [Google Scholar]
- Xiong Y, Zhang H, Beach D 1993b. Subunit rearrangement of cyclin-dependent kinases is associated with cellular transformation. Genes Dev 7: 1572–1583 [DOI] [PubMed] [Google Scholar]
- Yu Q, Geng Y, Sicinski P 2001. Specific protection against breast cancers by cyclin D1 ablation. Nature 411: 1017–1021 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Shen H, Franklin DS, Scadden DT, Cheng T 2004. In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol 6: 436–442 [DOI] [PubMed] [Google Scholar]
- Zhao H, Chen X, Gurian-West M, Roberts JM 2012. Loss of cyclin-dependent kinase 2 (CDK2) inhibitory phosphorylation in a CDK2AF knock-in mouse causes misregulation of DNA replication and centrosome duplication. Mol Cell Biol 32: 1421–1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zindy F, Quelle DE, Roussel MF, Sherr CJ 1997. Expression of the p16INK4a tumor suppressor versus other INK4 family members during mouse development and aging. Oncogene 15: 203–211 [DOI] [PubMed] [Google Scholar]