Abstract
Unlike the polymorphic MHC class Ia molecules, MHC class Ib molecules are oligomorphic or nonpolymorphic. We recently discovered a protective CD8 T cell response to mouse polyomavirus (MPyV) in H-2b haplotype mice that is restricted by H2-Q9, a member of the Qa-2 MHC class Ib family. Here, we demonstrate that immunization with a peptide corresponding to a virus capsid-derived peptide presented by Q9 also elicits MHC class Ib-restricted MPyV-specific CD8 T cells in mice of H-2s and H-2g7 strains. These findings support the concept that immunization with a single MHC class Ib-restricted peptide can expand CD8 T cells in MHC class Ia allogeneic hosts.
Conventional αβ T cell receptor (TCR)-expressing CD8 T cells recognize antigens presented by polymorphic MHC class Ia molecules. This presents a challenge for peptide-based vaccine strategies to elicit pathogen-specific CD8 T cells (i.e., any single peptide will only bind a fraction of the MHC molecules in an outbred population). However, it is now well-established that αß TCR CD8 T cells also recognize microbial antigens in the context of MHC class Ib molecules. The limited polymorphism of MHC class Ib molecules raises the possibility that the same peptide can be used as an immunogen to recruit protective anti-pathogen CD8 T cells across MHC class Ia allogeneic hosts.
Polyomaviruses are ubiquitous viruses that persist in a smoldering infectious state in many vertebrate species, including humans (12,32,35). In healthy hosts, polyomavirus infection is clinically silent (6). However, immunosuppression resulting from HIV/AIDS, aging, and immunomodulatory agents may result in unchecked viral replication with life-threatening complications, such as nephropathy in kidney transplant patients, central nervous system demyelination, and cutaneous malignancies (2–4,26,28,37).
Using MHC class Ia-deficient C57BL/6 mice (B6.Kb-/-Db-/-), we recently discovered a class Ib-restricted CD8 T cell response that confers protection against MPyV infection (31). A peptide derived from amino acids 139–147 of the VP2 capsid protein (VP2.139; HALNVVHDW) binds Q9, a ß2m-associated MHC class I molecule encoded in the mouse Qa-2 locus. H2-Q9 is nonpolymorphic in mice (30). In addition, H2-Q9 shares the peptide binding specificity of the nearly identical Qa-2 family member H2-Q7 (29). In this study, we tested the hypothesis that immunization with the VP2.139 peptide will generate Q9:VP2.139-specific CD8 T cells in mice of non-H-2b haplotypes that express Q9 and/or Q7. H-2s and H-2g7 strains express Qa-2 (15,25). We confirmed Qa-2 expression in SJL (H-2s) and NOD (H-2g7) mice by positive staining of splenocytes with Qa-2 antibody (clone 1-1-2, BD Biosciences) which recognizes the Qa-2 antigen expressed by Q6, Q7, Q8, and Q9 (7, 27) (data not shown).
Adult (6–13 wk) female SJL and B6 mice, and male NOD mice, were injected in a hind footpad with 100 μg VP2.139 peptide emulsified in Complete Freund's Adjuvant (CFA) containing 1 mg/mL heat-killed Mycobacterium tuberculosis. Control mice received phosphate buffered saline (PBS) emulsified in CFA. Male NOD mice were used due to the lower incidence of a diabetogenic phenotype than observed in NOD females (1). Two weeks later, mice were boosted with 50 μg VP2.139 peptide emulsified in Incomplete Freund's Adjuvant (IFA) injected subcutaneously (s.c.) at the tail base. Control mice received PBS emulsified in IFA. Two–three weeks after the IFA boost, mice were infected with 2×105 PFU of MPyV (strain A2) intraperitoneally (i.p.). Six days later, samples of spleen, kidney, salivary gland, and heart were snap-frozen. DNA was isolated from these organs and Taqman-based quantitative (q)PCR performed as previously described (14). Single cell suspensions of RBC-lysed splenocytes prepared from each of these mice were incubated with or without 10 μM VP2.139 peptide for 5 h in the presence of Brefeldin A (GolgiPlug, BD Bioscience). Cells were surface stained, then permeabilized (Cytofix/Cytoperm, BD Bioscience) and stained intracellularly, as previously described (11). Alternatively, RBC-lysed splenocytes were treated with FcBlock (BD Biosciences) then co-stained with tetramers (described below) and antibodies, as previously described (11). Samples were run on BD FACSVerse (BD Biosciences). FACS data were analyzed using FlowJo (Tree Star) and statistical analyses were performed using Prism (GraphPad).
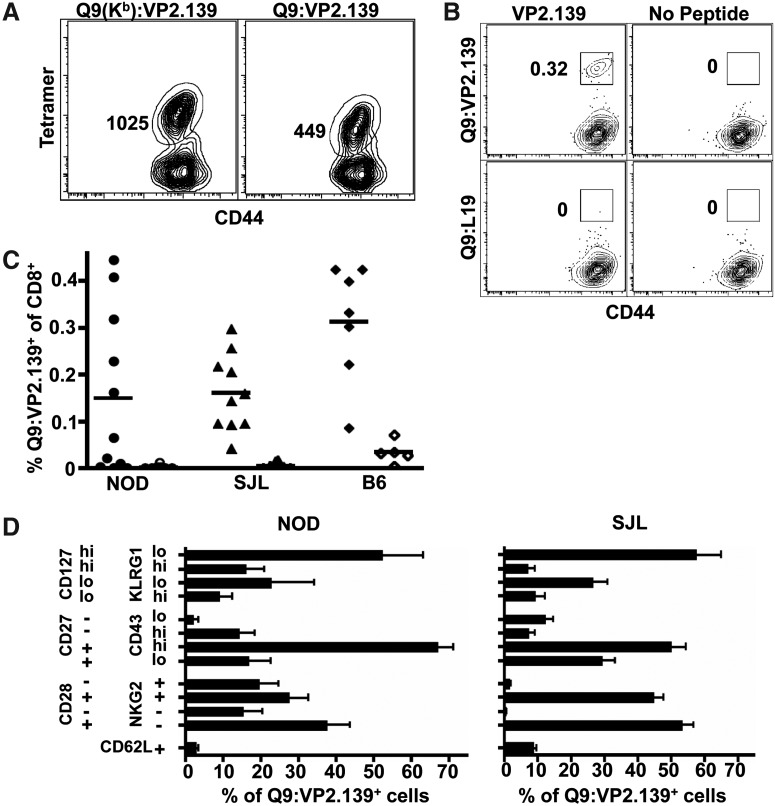
Engagement of CD8 coreceptors with Q9 is likely handicapped by an unusual structure and orientation of a CD8-binding loop of the Q9 α3 domain (8). Therefore, a novel Q9 tetramer having the α3 domain of H-2Kb, which efficiently binds CD8 (17), was constructed by The NIH Tetramer Core Facility. This chimeric Q9(Kb):VP2.139 tetramer stains CD8 T cells from MPyV-infected B6.Kb-/-Db-/- mice with 2-fold higher mean fluorescence intensity (MFI) than Q9:VP2.139 tetramers (Fig. 1A). To control for nonspecific tetramer binding, a chimeric Q9(Kb) tetramer containing a Q9-binding peptide from the L19 ribosomal protein (13) was also constructed by the NIH Tetramer Core Facility.
FIG. 1.
VP2.139 peptide-immunized NOD, SJL, and B6 mice generate Q9:VP2.139-specific CD8 T cell responses. (A) Representative tetramer staining of splenocytes from B6.Kb-/-Db-/- mice 3 wk after MPyV inoculation. RBC-lysed splenocytes were incubated with FcBlock, then surface stained with CD8α (clone 53–6.7), CD44, and either Q9:VP2.139 tetramer or a chimeric Q9(Kb):VP2.139 tetramer. Splenocytes gated on CD8+ CD44+ cells. Geometric MFI of the tetramer-associated fluorophore within the gated population is indicated. Data are representative of 10 mice pooled from two separate experiments. (B) Representative Q9:VP2.139 tetramer staining of splenocytes from VP2.139 peptide-immunized NOD mice at day 6 p.i. by MPyV. “VP2.139” indicates mice immunized with CFA+peptide. “No Peptide” indicates control mice receiving CFA+PBS. Q9:L19 is the control tetramer for Q9:VP2.139. Numbers indicate the frequency of tetramer+ CD8+ lymphocytes. Data are representative of 11 mice pooled from three separate experiments. (C) Frequency of Q9:VP2.139-specific CD8+ lymphocytes, with Q9:L19 tetramer staining subtracted, in VP2.139 peptide-immunized NOD (circles), SJL (triangles), and B6 (diamonds) mice at day 6 p.i. Horizontal lines indicate geometric mean. Symbols represent individual mice. Filled symbols indicate VP2.139-primed mice. Open circles indicate PBS-primed mice. Two NOD mice with no detectable Q9:VP2.139 tetramer staining were omitted from the phenotyping in Figure 1D. (D) Phenotyping of splenic Q9:VP2.139-specific CD8 T cells in NOD and SJL mice. Q9:VP2.139 tetramer+ CD8 T cells were surface-stained with antibodies to the indicated molecules. Data are clustered by costained markers, as indicated in the y axis. “+” indicates cells which are positive for the indicated molecule, and “−” indicates cells which are negative for the indicated molecule. “hi” indicates cells expressing a high level of the indicated molecule, and “lo” indicates cells expressing a low level of the indicated molecule. Data are representative of 9 mice (NOD) and 10 mice (SJL) pooled from two separate experiments.
As shown in Figure 1B and C, Q9:VP2.139-specific CD8 T cells were readily detected in VP2.139-immunized mice at d 6 p.i. PBS-immunized mice showed no tetramer binding above background staining by Q9:L19 tetramers (Fig. 1B). However, the frequency of responders varied among mice of these inbred strains. Approximately 60% of NOD mice, but >80% of SJL and B6 mice had Q9:VP2.139-specific CD8 T cell responses to acute MPyV infection (Fig. 1C). It is important to note that Q9:VP2.139-specific CD8 T cells are detected in only a third of B6 mice infected with MPyV, with Q9:VP2.139 tetramer+ CD8 T cells first detected at day 8 p.i. (31). In this experimental setup, most VP2.129 peptide-immunized mice produced a Q9:VP2.139-specific CD8 T cell response by 6 days after MPyV inoculation.
Q9:VP2.139-specific CD8 T cells in VP2.139 peptide-primed NOD and SJL mice express a phenotypic profile suggestive of a memory recall response after initial priming under high inflammatory conditions. The Q9:VP2.139-specific CD8 T cells were predominantly CD62L-, as expected for a secondary effector response (Fig. 1D). Most of the Q9:VP2.139 tetramer+ CD8 T cells expressed high levels of CD127, which may be a consequence of CFA-based immunization (10). In addition, 16% (SJL) or 25% (NOD) of the Q9:VP2.139-specific CD8 T cells were KLRG-1hi, which may similarly reflect the highly inflammatory CFA priming environment (20,22). The majority of the tetramer+ cells were CD27+CD43hi, which has been associated with potential for antigenic recall (10). The Q9:VP2.139-specific CD8 T cells were predominantly CD28+, as expected for activated T cells (34). Approximately half of the tetramer+ cells expressed CD94-NKG2A/C/E (clone 20d5). In B6 mice, NKG2A is the predominant NKG2 isoform expressed by this heterodimeric receptor (36), and has been interpreted as a T cell activation marker (16,18,19). These data are consistent with our previous observations that CD94-NKG2A expression increases while CD28 expression decreases on Q9:VP2.139-specific CD8 T cells over the course of MPyV infection (11).
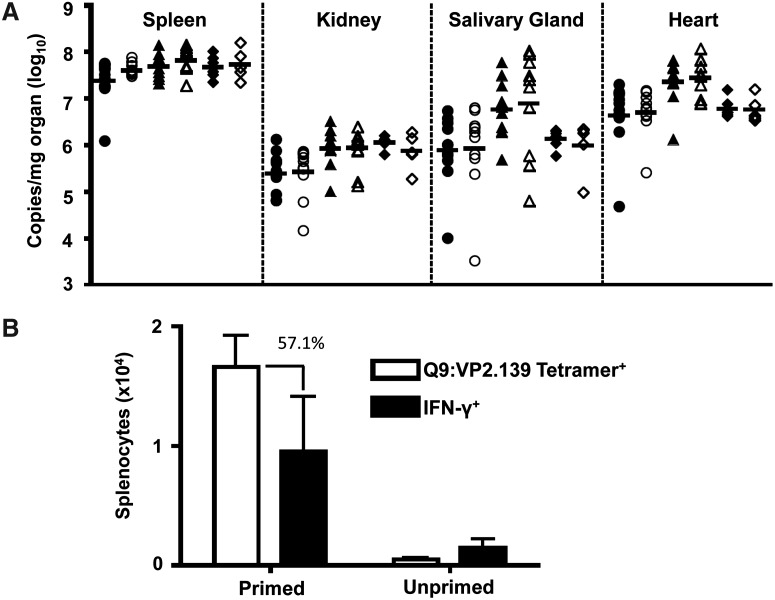
We previously observed that Q9:VP2.139-specific CD8 T cells mediate protection against MPyV infection in MHC class Ia knockout mice. However, viral titers were equivalent between control mice and those receiving VP2.139 peptide (Fig. 2A). Three explanations may account for this discrepancy. First, competition with conventional MHC class Ia-restricted antiviral CD8 T cells may curtail the magnitude of expansion of effector Q9:VP2.139-specific CD8 T cells needed to control acute MPyV infection. Approximately 2% of CD8 T cells in VP2.139-primed B6 mice were specific for the immunodominant Db:LT359 epitope (14) at day 6 p.i. (data not shown). Moreover, the immunization regimen used here may be insufficient to drive maximal expansion of these MHC Ib-restricted T cells. Third, Q9:VP2.139-specific CD8 T cells are defective in producing IFN-γ (31), which we recently demonstrated to be a central anti-MPyV effector activity (38). As shown in Figure 2B, over 50% of the Q9:VP2.139 specific CD8 T cells, based on tetramer staining, produce IFN-γ after VP2.139 peptide (10 μM) stimulation; this comprises just 10,000 cells in the spleen at day 6 p.i. The low magnitude Q9:VP2.139-specific CD8 T cell response elicited by the CFA-prime, IFA-boost strategy, compounded by this marked deficit in a critical antiviral cytokine, likely explains the inability of these cells to control MPyV infection.
FIG. 2.
VP2.139 peptide immunized NOD mice do not control acute MPyV infection. (A) MPyV genome copies from NOD (circles), SJL (triangles), and B6 (diamonds) mice in the indicated organs at day 6 p.i. with MPyV were determined by Taqman-based qPCR. Closed symbols: VP2.139 peptide-immunized mice. Open symbols: PBS control mice. Horizontal lines represent geometric mean of each group of samples. Symbols represent individual mice, pooled from two (B6 and SJL) or three (NOD) separate experiments. (B) Numbers of Q9:VP2.139 tetramer+ and VP2.139 peptide-stimulated IFN-γ+ CD8 splenic T cells in SJL mice. The percentage shown indicates the frequency of IFN-γ+ cells relative to the number of tetramer+ CD8 T cells in mice immunized with VP2.139 peptide. Data are representative of 10 mice per group, pooled from two separate experiments.
The diversity and level of expression of Qa-2 MHC class Ib molecules varies among inbred mouse strains due to differences in their germline representation of H2-Q6, -Q7, -Q8, and -Q9 (5). In addition, H2-Q7 and -Q9 differ by only a single amino acid outside their peptide-binding grooves and have overlapping peptide-binding repertoires (13,29,33). In contrast to B6 mice, which express both Q7 and Q9 (39), NODs express either Q7 or Q9 (15). There is also evidence that SJLs express less surface Qa-2 than B6 mice (23). It is possible that differences between B6, SJL, and NOD mice in the repertoire and expression of Qa-2 genes may affect the level of Q9:VP2.139 epitope density and explain the differences in magnitude of antigen-specific CD8 T cells observed in these mouse strains.
In summary, evidence is presented here that immunization with a single peptide presented by an MHC class Ib molecule can elicit a CD8 T cell response in mice of allogeneic MHC class Ia haplotypes. Although we were unable to demonstrate control of MPyV infection, this does not preclude the possibility that a more functionally competent and higher magnitude virus-specific CD8 T cell response restricted by other MHC class Ib molecules would confer protection against infection. In this connection, HLA-E-restricted CD8 T cells specific for cytomegalovirus, Mycobacterium tuberculosis and Salmonella typhi have been identified (9,21,24). Data described in this study support the concept that vaccines using single antigenic peptides may be useful in generating virus-specific, MHC class Ib-restricted CD8 T cell responses in individuals differing in HLA class Ia haplotypes. Work presented here underscores the need for further efforts to identify MHC class Ib-restricted CD8 T cells in humans.
Acknowledgments
This work was supported by National Institutes of Health Grant R01 CA139220. The authors would like to thank the NIH Tetramer Core for production of the Q9 tetramers.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Anderson MS. Bluestone JA. The NOD mouse: A model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 2.Bellizzi A. Nardis C. Anzivino E, et al. Human polyomavirus JC reactivation and pathogenetic mechanisms of progressive multifocal leukoencephalopathy and cancer in the era of monoclonal antibody therapies. J Neurovirol. 2012;18:1–11. doi: 10.1007/s13365-012-0101-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bennett SM. Broekema NM. Imperiale MJ. BK polyomavirus: Emerging pathogen. Microbes Infect. 2012;14:672–683. doi: 10.1016/j.micinf.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bloomgren G. Richman S. Hotermans C, et al. Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med. 2012;366:1870–1880. doi: 10.1056/NEJMoa1107829. [DOI] [PubMed] [Google Scholar]
- 5.Chiang EY. Stroynowski I. The role of structurally conserved class I MHC in tumor rejection: Contribution of the Q8 locus. J Immunol. 2006;177:2123–2130. doi: 10.4049/jimmunol.177.4.2123. [DOI] [PubMed] [Google Scholar]
- 6.Delbue S. Comar M. Ferrante P. Review on the relationship between human polyomaviruses-associated tumors and host immune system. Clin Dev Immunol. 2012:542092. doi: 10.1155/2012/542092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devlin JJ. Weiss EH. Paulson M. Flavell RA. Duplicated gene pairs and alleles of class I genes in the Qa2 region of the murine major histocompatibility complex: A comparison. EMBO J. 1985;4:3203–3207. doi: 10.1002/j.1460-2075.1985.tb04066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He X. Tabaczewski P. Ho J. Stroynowski I. Garcia KC. Promiscuous antigen presentation by the nonclassical MHC Ib Qa-2 is enabled by a shallow, hydrophobic groove and self-stabilized peptide conformation. Structure. 2001;9:1213–1224. doi: 10.1016/s0969-2126(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 9.Heinzel AS. Grotzke JE. Lines RA, et al. HLA-E-dependent presentation of Mtb-derived antigen to human CD8+ T cells. J Exp Med. 2002;196:1473–1481. doi: 10.1084/jem.20020609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hikono H. Kohlmeier JE. Takamura S. Wittmer ST. Roberts AD. Woodland DL. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J Exp Med. 2007;204:1625–1636. doi: 10.1084/jem.20070322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofstetter AR. Ford ML. Sullivan LC, et al. MHC class Ib-restricted CD8 T cells differ in dependence on CD4 T cell help and CD28 costimulation over the course of mouse polyomavirus infection. J Immunol. 2012;188:3071–3079. doi: 10.4049/jimmunol.1103554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johne R. Buck CB. Allander T, et al. Taxonomical developments in the family Polyomaviridae. Arch Virol. 2011;156:1627–1634. doi: 10.1007/s00705-011-1008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joyce S. Tabaczewski P. Angeletti RH. Nathenson SG. Stroynowski I. A nonpolymorphic major histocompatibility complex class Ib molecule binds a large array of diverse self-peptides. J.Exp.Med. 1994;179:579–588. doi: 10.1084/jem.179.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kemball CC. Lee ED. Vezys V. Pearson TC. Larsen CP. Lukacher AE. Late priming and variability of epitope-specific CD8+ T cell responses during a persistent virus infection. J Immunol. 2005;174:7950–7960. doi: 10.4049/jimmunol.174.12.7950. [DOI] [PubMed] [Google Scholar]
- 15.Lund T. Simpson E. Cooke A. Restriction fragment length polymorphisms in the major histocompatibility complex of the non-obese diabetic mouse. J Autoimmun. 1990;1990;3:289–298. doi: 10.1016/0896-8411(90)90147-k. [DOI] [PubMed] [Google Scholar]
- 16.Maris CH. Miller JD. Altman JD. Jacob J. A transgenic mouse model genetically tags all activated CD8 T cells. J Immunol. 2003;171:2393–2401. doi: 10.4049/jimmunol.171.5.2393. [DOI] [PubMed] [Google Scholar]
- 17.Moody AM. Xiong Y. Chang HC. Reinherz EL. The CD8αβ co-receptor on double-positive thymocytes binds with differing affinities to the products of distinct class I MHC loci. Eur J Immunol. 2001;31:2791–2799. doi: 10.1002/1521-4141(200109)31:9<2791::aid-immu2791>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 18.Moser JM. Byers AM. Lukacher AE. NK cell receptors in antiviral immunity. Curr Opin Immunol. 2002;14:509–516. doi: 10.1016/s0952-7915(02)00357-6. [DOI] [PubMed] [Google Scholar]
- 19.Moser JM. Gibbs J. Jensen PE. Lukacher AE. CD94-NKG2A receptors regulate antiviral CD8+ T cell responses. Nat Immunol. 2002;3:189–195. doi: 10.1038/ni757. [DOI] [PubMed] [Google Scholar]
- 20.Obar JJ. Jellison ER. Sheridan BS, et al. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pietra G. Romagnani C. Mazzarino P, et al. HLA-E-restricted recognition of cytomegalovirus-derived peptides by human CD8+ cytolytic T lymphocytes. Proc Natl Acad Sci USA. 2003;100:10896–10901. doi: 10.1073/pnas.1834449100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prlic M. Sacks JA. Bevan MJ. Dissociating markers of senescence and protective ability in memory T cells. PLoS One. 2012;7:e32576. doi: 10.1371/journal.pone.0032576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rucker J. Horowitz M. Lerner EA. Murphy DB. Monoclonal antibody reveals H-2-linked quantitative and qualitative variation in the expression of a Qa-2 region determinant. Immunogenetics. 1983;17:303–316. doi: 10.1007/BF00364414. [DOI] [PubMed] [Google Scholar]
- 24.Salerno-Goncalves R. Fernandez-Vina M. Lewinsohn DM. Sztein MB. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2004;173:5852–5862. doi: 10.4049/jimmunol.173.9.5852. [DOI] [PubMed] [Google Scholar]
- 25.Sandrin MS. Hogarth PM. McKenzie IF. Two “Qa” specificities: Qa-m7 and Qa-m8 defined by monoclonal antibodies. J Immunol. 1983;131:546–547. [PubMed] [Google Scholar]
- 26.Schrama D. Ugurel S. Becker JC. Merkel cell carcinoma: Recent insights and new treatment options. Curr Opin Oncol. 2012;4:141–149. doi: 10.1097/CCO.0b013e32834fc9fe. [DOI] [PubMed] [Google Scholar]
- 27.Sharrow SO. Arn JS. Stroynowski I. Hood L. Sachs DH. Epitope clusters of Qa-2 antigens defined by a panel of new monoclonal antibodies. J Immunol. 1989;142:3495–3502. [PubMed] [Google Scholar]
- 28.Siguier M. Sellier P. Bergmann JF. BK-virus infections: A literature review. Med Mal Infect. 2012;42:181–187. doi: 10.1016/j.medmal.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 29.Soloski MJ. Hood L. Stroynowski I. Qa-region class I gene expression: Identification of a second class I gene, Q9, encoding a Qa-2 polypeptide. Proc Natl Acad Sci USA. 1988;85:3100–3104. doi: 10.1073/pnas.85.9.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stroynowski I. Tabaczewski P. Multiple products of class Ib Qa-2 genes: Which ones are functional? Res Immunol. 1996;147:290–301. doi: 10.1016/0923-2494(96)89642-8. [DOI] [PubMed] [Google Scholar]
- 31.Swanson PA., II Pack CD. Hadley A, et al. An MHC class Ib-restricted CD8 T cell response confers antiviral immunity. J Exp Med. 2008;205:1647–1657. doi: 10.1084/jem.20080570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swanson PA., II Lukacher AE. Szomolanyi-Tsuda E. Immunity to polyomavirus infection: The polyomavirus-mouse model. Semin Cancer Biol. 2009;19:244–251. doi: 10.1016/j.semcancer.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tabaczewski P. Chiang E. Henson M. Stroynowski I. Alternative peptide binding motifs of Qa-2 class Ib molecules define rules for binding of self and nonself peptides. J Immunol. 1997;159:2771–2781. [PubMed] [Google Scholar]
- 34.Turka LA. Ledbetter JA. Lee K. June CH. Thompson CB. CD28 is an inducible T cell surface antigen that transduces a proliferative signal in CD3+ mature thymocytes. J Immunol. 1990;144:1646–1653. [PubMed] [Google Scholar]
- 35.Van Ghelue M. Khan MT. Ehlers B. Moens U. Genome analysis of the new human polyomaviruses. Rev Med Virol. 2012;22:354–377. doi: 10.1002/rmv.1711. [DOI] [PubMed] [Google Scholar]
- 36.Vance RE. Jamieson AM. Cado D. Raulet DH. Implications of CD94 deficiency and monoallelic NKG2A expression for natural killer cell development and repertoire formation. Proc Natl Acad Sci USA. 2002;99:868–873. doi: 10.1073/pnas.022500599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wanat KA. Holler PD. Dentchev T, et al. Viral-associated trichodysplasia: characterization of a novel polyomavirus infection with therapeutic insights. Arch Dermatol. 2012;148:219–223. doi: 10.1001/archdermatol.2011.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilson JJ. E Lin E. CD Pack CD, et al. Gamma interferon controls mouse polyomavirus infection in vivo. J Virol. 2011;85:10126–10134. doi: 10.1128/JVI.00761-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu L. Exley GE. Warner CM. Differential expression of Ped gene candidates in preimplantation mouse embryos. Biol Reprod. 1998;59:941–952. doi: 10.1095/biolreprod59.4.941. [DOI] [PubMed] [Google Scholar]