Abstract
Objectives:
Patients with idiopathic REM sleep behavior disorder (IRBD) are at risk for developing Parkinson disease (PD) and dementia with Lewy bodies (DLB). We aimed to identify functional brain imaging patterns predicting the emergence of PD and DLB in patients with IRBD, using SPECT with 99mTc–ethylene cysteinate dimer (ECD).
Methods:
Twenty patients with IRBD were scanned at baseline during wakefulness using 99mTc-ECD SPECT. After a follow-up of 3 years on average, patients were divided into 2 groups according to whether or not they developed defined neurodegenerative disease (PD, DLB). SPECT data analysis comparing regional cerebral blood flow (rCBF) between groups assessed whether specific brain perfusion patterns were associated with subsequent clinical evolution. Regression analysis between rCBF and clinical markers of neurodegeneration (motor, color vision, olfaction) looked for neural structures involved in this process.
Results:
Of the 20 patients with IRBD recruited for this study, 10 converted to PD or DLB during the follow-up. rCBF at baseline was increased in the hippocampus of patients who would later convert compared with those who would not (p < 0.05 corrected). Hippocampal perfusion was correlated with motor and color vision scores across all IRBD patients.
Conclusions:
99mTc-ECD SPECT identifies patients with IRBD at risk for conversion to other neurodegenerative disorders such as PD or DLB; disease progression in IRBD is predicted by abnormal perfusion in the hippocampus at baseline. Perfusion within this structure is correlated with clinical markers of neurodegeneration, further suggesting its involvement in the development of presumed synucleinopathies.
Idiopathic REM sleep behavior disorder (IRBD)—a condition characterized by dream-enactment behaviors—is frequently associated with the subsequent development of other presumed synucleinopathies: Parkinson disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA).1 The physiopathology underlying this association remains unclear.
Some studies have attempted to identify biomarkers predictive of an evolution toward PD, DLB, or MSA. For instance, patients with IRBD who would evolve had worse baseline scores for olfaction and color vision than those who would remain stable.2 In addition, transcranial ultrasound of substantia nigra and dopaminergic imaging show promise as predictors of evolution.3
In this study, we aimed to characterize the brain perfusion patterns at baseline that predicted future evolution from isolated IRBD to other neurodegenerative diseases, using SPECT with 99mTc–ethylene cysteinate dimer (ECD). Because previous 99mTc-ECD SPECT studies in patients with IRBD highlighted perfusion changes in the pons, striatum, and hippocampus, as well as neocortical areas (frontal, temporoparietal),4 we hypothesized that patterns predictive of the conversion would be part of these structures.
METHODS
Subjects.
Patients had an interview with a sleep specialist and a polysomnographic recording at the Sacre-Coeur Hospital to confirm the diagnosis of REM sleep behavior disorder (RBD). All patients discontinued psychotropic medications for the study. Their neurologic evaluation included a motor assessment with the Unified Parkinson Disease Rating Scale (UPDRS)-III, color vision discrimination by the Farnsworth-Munsell (FM)-100 test, and olfactory identification by the University of Pennsylvania Smell Identification Test 12 items (UPSIT-12). Exclusion criteria were psychiatric condition or dementia according to DSM-IV-TR and neuropsychological assessment, Mini-Mental State Examination score <26, and other sleep and neurologic disorders. Twenty patients with IRBD met the criteria for inclusion; 12 of them were also part of a previous study.4 Neurologic, neuropsychological, and SPECT assessments were performed during the same week. Although all IRBD patients were free of PD, DLB, and MSA at the time of scanning (baseline), 13 of them had mild cognitive impairment (MCI). Patients were then followed up annually during an average period of 3 years for detection of signs suggestive of a conversion to PD, DLB, or MSA. Ten healthy subjects, matched for age and gender, were submitted to a similar experimental procedure.
Standard protocol approvals, registrations, and patient consents.
The protocol was approved by the Ethics Committee of the Sacre-Coeur Hospital–University of Montreal, and all participants signed a written consent form to participate.
SPECT data acquisition and analysis.
SPECT scans with 99mcTc-ECD were performed in a 3-headed SPECT scanner (PRISM system, Picker Co.) in the morning during resting wakefulness (40 projections by scanner head, matrix of 128 × 128).
After reconstruction (filtered back projection, Butterworth filter, order: 8, cut-off: 0.39 FWHM [full width at half maximum], subtraction of 40% of the Compton window from the peak window) and attenuation correction (noniterative Chang algorithm, 0.15 cm−1), SPECT data were processed in Statistical Parametric Mapping 8. All studies were registered and normalized to a SPECT template and smoothed with a 14-mm FWHM filter. Global normalization was performed using proportional scaling. Gray matter threshold was set at 1.0.5
Differences of regional cerebral blood flow (rCBF) between groups were assessed using a 2-sample t test. Correlations between rCBF and clinical scores (UPDRS-III, FM-100, and UPSIT-12; at baseline) were assessed across all included patients (except one, because of incomplete clinical scores). In contrast to a previous study,4 clinical scores were entered in the same design matrix to ensure correlations were independent of other variables. Age and disease duration were included in the regression model. Statistical thresholds were set at p < 0.05 after correction for multiple comparisons on small volumes (sphere, 10 mm). Significant results were overlaid on an MRI template for localization of rCBF changes.
RESULTS
Demographic and clinical variables of IRBD patients are presented in table e-1 on the Neurology® Web site at www.neurology.org. Of 20 patients, 10 showed clinical evolution within the follow-up period (RBDEv): 5 were diagnosed with PD, and the other 5 with DLB. The 10 other patients remained in a state of isolated RBD (RBDSt). Seven RBDEv (including all those evolving to DLB) and 6 RBDSt patients had MCI at baseline.
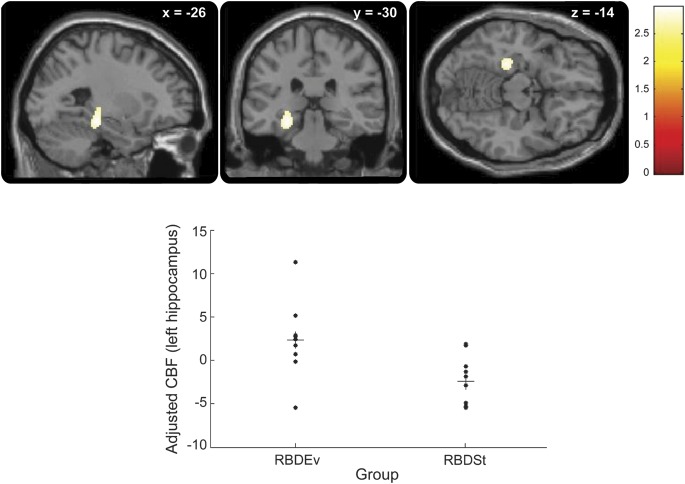
Group comparisons of SPECT data at baseline revealed increased rCBF in the hippocampus of RBDEv bilaterally as compared with RBDSt (figure 1 and table e-2). Within RBDEv, no significant difference was observed between those evolving to PD and those converting to DLB.
Figure 1. Hippocampal hyperperfusion in RBDEv compared with RBDSt.
(Upper panels) Brain perfusion increases at baseline in patients with idiopathic REM sleep behavior disorder who would convert to neurodegenerative disease (RBDEv), compared with those who would not (RBDSt), were located in the hippocampus. The panels show the peak hyperperfusion, centered on the left hippocampus, and represented on sagittal, coronal, and transverse sections (from left to right panels) (pcorr < 0.05). The level of section is indicated on the top of each panel (x, y, and z coordinates, in mm). The color scale indicates the range of t values for this contrast. (Lower panel) Plot of the adjusted rCBF responses (arbitrary units) in the left hippocampus (x = −30 mm, y = −30 mm, z = −14 mm) showing the distinct distribution in RBDEv and RBDSt patients. Each circle represents 1 subject. Horizontal bars represent mean values. RBDEv = REM sleep behavior disorder with clinical evolution; RBDSt = state of isolated REM sleep behavior disorder; rCBF = regional cerebral blood flow.
Correlations at baseline between rCBF and clinical markers associated with the neurodegenerative process were looked for across all patients. UPDRS-III positively correlated with rCBF in the right hippocampus (figure 2 and table e-2). UPSIT-12 positively correlated with rCBF in the medial frontal gyrus (table e-2). FM-100 positively correlated with rCBF in the pons and left hippocampus (figure 3 and table e-2). No significant negative correlation was observed.
Figure 2. Correlation between motor scores (UPDRS-III) and rCBF in patients with IRBD.
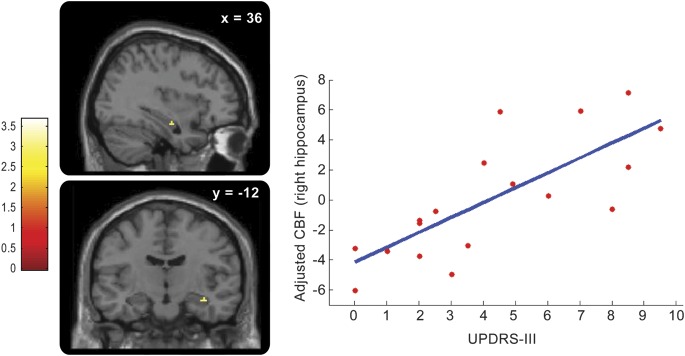
Brain perfusion in the right hippocampus is positively correlated at baseline with UPDRS-III scores across IRBD patients (pcorr < 0.05). (Left) The 2 panels show the location of this positive correlation in the right hippocampus, on sagittal and coronal sections (from top to bottom), respectively. The level of section is indicated on the top of each panel (x and y coordinates, in mm). The color scale indicates the range of t values. (Right) Plot of the adjusted rCBF responses (arbitrary units) in the right hippocampus (x = 36 mm, y = −14 mm, z = −18 mm) in relation to UPDRS-III scores: rCBF increases when UPDRS-III is higher. Each circle represents 1 subject. The blue line is the linear regression. IRBD = idiopathic REM sleep behavior disorder; rCBF = regional cerebral blood flow; UPDRS = Unified Parkinson Disease Rating Scale.
Figure 3. Correlation between color vision scores (FM-100) and rCBF in patients with IRBD.
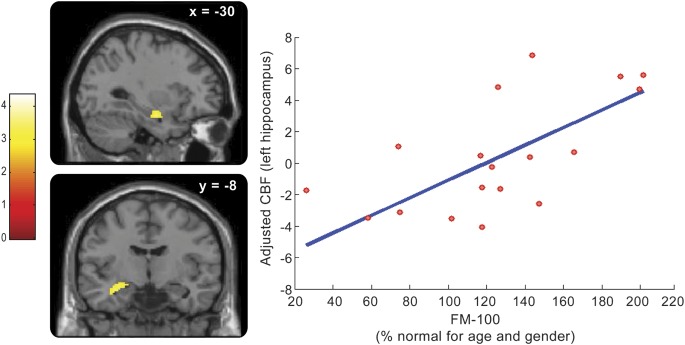
Brain perfusion in the left hippocampus is positively correlated at baseline with FM-100 scores across IRBD patients (pcorr < 0.05). (Left) The 2 panels show the location of this positive correlation in the left hippocampus, on sagittal and coronal sections (from top to bottom), respectively. The level of section is indicated on the top of each panel (x and y coordinates, in mm). The color scale indicates the range of t values. (Right) Plot of the adjusted rCBF responses (arbitrary units) in the left hippocampus (x = −32 mm, y = −10 mm, z = −16 mm) in relation to FM-100 scores (adjusted for age and sex, in %): rCBF increases when FM-100 is higher. Each circle represents 1 subject. The blue line is the linear regression. FM = Farnsworth-Munsell; IRBD = idiopathic REM sleep behavior disorder; rCBF = regional cerebral blood flow.
Compared with healthy controls, RBDEv showed increased perfusion in the hippocampus bilaterally as well as in the pons (table e-4 and figure e-1). No region displayed significant hypoperfusion in RBDEv compared with controls. Within RBDEv, those evolving to PD displayed increased perfusion in the hippocampus and pons, with additional hyperperfusion of the anterior cingulate cortex, whereas those evolving to DLB had only increased perfusion in the hippocampus. No significant difference of brain perfusion was found in either direction in a comparison of RBDSt with controls.
DISCUSSION
Our findings demonstrate brain perfusion patterns predicting the appearance of PD or DLB in patients with IRBD. The most consistent brain area associated with this process was the hippocampus. This structure showed increased perfusion in patients with impending conversion, not only when compared with nonevolving IRBD patients but also when compared with control subjects.
Involvement of the hippocampus in the pathophysiology of RBD is supported by both functional4 and structural brain imaging studies,6 which suggests a deep reorganization of the hippocampus in RBD. In this study, we demonstrated that hippocampal hyperperfusion was restricted to IRBD patients with future evolution to PD or DLB. Additionally, hyperperfusion of the hippocampus was correlated with worse scores on motor and color-discrimination assessments, 2 parameters associated with the transition to PD.2,7 Increased hippocampal perfusion is thus a harbinger of the neurodegenerative process leading isolated RBD to clinical PD or DLB. In line with this concept is the observation of hippocampal hyperperfusion in the initial stages of PD.8 Whether modifications of the hippocampus constitute a primary mechanism or merely reflect a corollary feature remains to be determined.
Besides the hippocampus, the pons also displayed increased perfusion in RBDEv compared with controls, in agreement with a possible dysfunction of pontine nuclei underlying the loss of REM sleep atonia.9 Correlation between decreased perfusion in the medial prefrontal cortex and impaired olfaction is in agreement with the association of olfactory disturbances with cognitive impairment observed in PD.10 It should be noted that most of our patients with RBD already had MCI at baseline, particularly those evolving to DLB, which concurs with a protracted course of cognitive impairment.
Our results thus provide the first whole-brain exploration of the functional changes associated with conversion of IRBD patients, using neuroimaging and a prospective design. The present findings converge to hippocampal hyperperfusion as a predictive imaging biomarker of the neurodegenerative evolution in RBD. 99mcTc-ECD SPECT should therefore be considered in the prognostic assessment of patients with IRBD, which might become of crucial importance in the light of future disease-modifying therapies. Although no clear difference emerged in our sample between RBD patients evolving to PD and those converting to DLB, comparison with controls suggested a more extended pattern of hyperperfusion for future PD patients. Further studies should extend these findings on larger cohorts of patients and during various states of vigilance.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Boualem Mensour for his advice on data analysis and Josie-Anne Bertrand for her help in data collection.
GLOSSARY
- DLB
dementia with Lewy bodies
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders, 4th edition, Text Revision
- ECD
ethylene cysteinate dimer
- FM
Farnsworth-Munsell
- FWHM
full width at half maximum
- IRBD
idiopathic REM sleep behavior disorder
- MCI
mild cognitive impairment
- MSA
multiple system atrophy
- PD
Parkinson disease
- RBD
REM sleep behavior disorder
- RBDEv
REM sleep behavior disorder with clinical evolution
- RBDSt
state of isolated REM sleep behavior disorder
- rCBF
regional cerebral blood flow
- UPDRS
Unified Parkinson Disease Rating Scale
- UPSIT-12
University of Pennsylvania Smell Identification Test 12 items
Footnotes
Editorial, page 2296
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Dang-Vu contributed to the study concept/design, conducted data analysis and interpretation, and wrote the manuscript. Drs. Montplaisir and Gagnon contributed to the study concept/design, fundraising, and critically reviewed the manuscript. Dr. Postuma contributed to the study concept/design, participated in data collection/analysis, and critically reviewed the manuscript. Mrs. Vendette and Dr. Soucy contributed to the study concept/design and critically reviewed the manuscript.
DISCLOSURE
T.T. Dang-Vu receives support from the Canadian Institutes of Health Research, the Belgian Fonds National de la Recherche Scientifique, the Fonds Léon Frédéricq, and the Belgian College of Neuropsychopharmacology and Biological Psychiatry; and received support from the European Sleep Research Society, the Belgian American Educational Foundation, the Belgian Neurological Society, the Horlait-Dapsens Medical Foundation, and Wallonie-Bruxelles International. J.-F. Gagnon receives research support from the Canadian Institutes of Health Research (MOP-84482 [PI], MOP-62955 [co-PI], MOP-191058 [co-I], and MOP-93802 [co-PI]) and Fonds de recherche du Québec–Santé (11834 [PI]). M. Vendette reports no disclosures. J.-P. Soucy receives grants from the Canadian Institutes of Health Research (173519 [co-I], 191058 [co-I], 97865 [co-I], 102631 [co-I], 245908 [co-I], 115125 [co-PI], 259005 [co-I], and 97970 [co-I]) and from a conjoint Canadian Institutes of Health Research/Natural Sciences and Engineering Research Council of Canada program (1004158 [co-I]). R.B. Postuma receives research funds from the Weston Foundation, the Webster foundation, the Canadian Institutes of Health Research (201317 [PI], 192205 [co-PI], and 166206 [co-I]), the Fonds de recherche du Québec–Santé (9965 [PI]), and the Parkinson Society of Canada; and received funds for travel from Allergan and Novartis Canada, and speaker fees from Teva Neuroscience and Novartis Canada. J. Montplaisir receives grants from GlaxoSmithKline Pharma (PI), from the Canadian Institute of Health Research (115125 [co-I]; 102631 [PI]; 106692 [PI]; 97865 [co-I]; 62955 [PI]; 173519 [co-PI]; and 64221 [co-I]), and from Government of Canada Senior Research Chair in Sleep Medicine (PI); and received research grants/support from Merck and GlaxoSmithKline, served as an advisor for Sanofi-Aventis, Servier, Merck, Jazz Pharmaceutical, Valeant Pharmaceutical, and Impax Laboratories, and received honoraria for speaking engagements from Valeant Pharmaceutical and Otsuka Pharmaceutical. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Iranzo A, Molinuevo JL, Santamaria J, et al. Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study. Lancet Neurol 2006;5:572–577 [DOI] [PubMed] [Google Scholar]
- 2.Postuma RB, Gagnon JF, Vendette M, Desjardins C, Montplaisir JY. Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder. Ann Neurol 2011;69:811–818 [DOI] [PubMed] [Google Scholar]
- 3.Iranzo A, Lomena F, Stockner H, et al. Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study. Lancet Neurol 2010;9:1070–1077 [DOI] [PubMed] [Google Scholar]
- 4.Vendette M, Gagnon JF, Soucy JP, et al. Brain perfusion and markers of neurodegeneration in rapid eye movement sleep behavior disorder. Mov Disord 2011;26:1717–1724 [DOI] [PubMed] [Google Scholar]
- 5.Cistaro A, Valentini MC, Chio A, et al. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging 2012;39:251–259 [DOI] [PubMed] [Google Scholar]
- 6.Scherfler C, Frauscher B, Schocke M, et al. White and gray matter abnormalities in idiopathic rapid eye movement sleep behavior disorder: a diffusion-tensor imaging and voxel-based morphometry study. Ann Neurol 2011;69:400–407 [DOI] [PubMed] [Google Scholar]
- 7.Postuma RB, Lang AE, Gagnon JF, Pelletier A, Montplaisir JY. How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder. Brain 2012;135:1860–1870 [DOI] [PubMed] [Google Scholar]
- 8.Imon Y, Matsuda H, Ogawa M, Kogure D, Sunohara N. SPECT image analysis using statistical parametric mapping in patients with Parkinson's disease. J Nucl Med 1999;40:1583–1589 [PubMed] [Google Scholar]
- 9.Boeve BF, Silber MH, Saper CB, et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007;130:2770–2788 [DOI] [PubMed] [Google Scholar]
- 10.Bohnen NI, Muller ML, Kotagal V, et al. Olfactory dysfunction, central cholinergic integrity and cognitive impairment in Parkinson's disease. Brain 2010;133:1747–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.