Summary
Background
Extracorporeal photopheresis (ECP) has been utilized for more than 20 years to treat cutaneous T-cell lymphoma (CTCL), but a clinical response can take up to 9 months to manifest. This study was undertaken to determine whether clinical features, laboratory values, cytokine levels, or gene expression levels of tumor markers are useful to predict the subsequent response to ECP in CTCL patients with blood involvement.
Methods
Twenty-one patients with CTCL treated with ECP as monotherapy for at least 6 months were retrospectively identified. Laboratory and clinical data and blood obtained at baseline, 3, and 6 months of treatment were used for analysis.
Results
In pretreatment blood specimens, a lower percentage of Sézary cells and a higher absolute eosinophil count were associated with a favorable clinical response. Clinical evidence of an early response after 3 months of ECP did not reliably predict a favorable response at 6 months or beyond. Comparison of cytokines, gene transcripts, and other laboratory measures of disease did not correlate with the subsequent clinical response, although lactate dehydrogenase levels tended to decrease progressively in ECP-responsive cases and increase progressively in ECP-non-responsive cases. Additionally, serum levels of TNF-α significantly increased from baseline to 6 months of ECP, but was not found to correlate with the clinical response.
Conclusions
Although we found that increased eosinophils and decreased percentage of Sézary cells were associated with a favorable clinical response to ECP, we were not able to identify the predictors of ECP response within the first 3 months of treatment.
Keywords: cutaneous lymphoma, mycosis fungoides, photopheresis, predictors of response, Sézary syndrome
Initially developed by Edelson in the 1970s, extracorporeal photopheresis (ECP) is a process by which peripheral blood mononuclear cells (PBMC) are isolated from the circulation by discontinuous leukapheresis, exposed ex vivo to pro-apoptotic doses of 8-methoxypsoralen and ultraviolet A radiation, and then reinfused to the patient (1). ECP was approved by the Food and Drug Administration for advanced cutaneous T-cell lymphoma (CTCL) in 1988 (1), and is now utilized for the treatment of various lymphocyte-mediated diseases, including graft-versus-host disease (GvHD) and solid organ transplant rejection (2–4).
Mycosis fungoides (MF) and Sézary syndrome (SS), the major variants of CTCL, are malignancies of clonal T lymphocytes that preferentially infiltrate the skin (5–7). Overt blood involvement is uncommon in clinically early MF, but may be encountered in advanced MF and in SS, which is currently defined as an erythrodermic and leukemic expression of CTCL (8). ECP is often used as a first-line therapy for advanced MF with blood involvement and SS, and the initial pivotal study in which two ECP treatments were administered on consecutive days at 4-week intervals demonstrated that ECP monotherapy induced a substantial improvement in 83% of erythrodermic patients, with 21% of patients eventually achieving a complete response (CR) (1, 9). Of note, in responding patients, the mean time to a positive response was 22.4±9.6 weeks, meaning that ECP must be administered for 32 weeks or more before its effectiveness can be properly assessed. Subsequent studies have shown that the overall response rates to ECP range widely from 31% to 86%, depending on patient selection, treatment frequency, and use of adjunct therapies (10, 11).
Despite its utilization for more than two decades, the precise mechanism(s) of action by which ECP exerts its therapeutic benefit on CTCL is not clearly understood nor have reliable predictors of response been identified (12–14). Possible and not necessarily mutually exclusive mechanisms of action include:
Induction of an anti-clonotypic immune response against the malignant clone (15).
Production of immunosuppressive or immunomodulatory cytokines [e.g. TNF-α, interleukin-10 (IL-10)] by treated mononuclear cells with normalization of the Th1/Th2 immune balance (16–20).
Induction of regulatory T (Treg) cells that inhibit neoplastic T-cell growth directly or indirectly via their effect on dendritic cells (21–24).
Clinical parameters that have been associated with sustained beneficial responses of CTCL to ECP include short disease durations (early treatment) and clinical improvement before 6 months (9, 25, 26). Laboratory parameters that have been reported to be correlated with favorable responses to ECP include (1) near-normal CD4/CD8 ratios or absolute number of CD8+ cells in the peripheral blood (9, 27), (2) the presence of modest numbers of Sézary cells (28), and (3) relatively low percentages of CD4+CD7− T cells, a phenotype that is often expressed by neoplastic T cells (25).
The primary goal of this study was to determine whether the cytokine profile in serum samples obtained at baseline and after 3 months of ECP monotherapy could be used as an early predictor of a clinical response to ECP monotherapy. Previously, we reported that baseline levels of soluble interleukin-2 receptor (sIL-2R) and lactate dehydrogenase (LDH) were not useful in this regard (29). However, sIL-2R and LDH levels did correlate with clinical and other laboratory parameters used to evaluate the response to ECP. A second objective was to evaluate the effect of ECP monotherapy on Th1 and Th2 cytokine levels in the serum. It is widely accepted that the neoplastic T cells of most cases of MF/SS are polarized to secrete Th2 cytokines, and that in advanced CTCL, a Th2 immune profile dominates and contributes to immunosuppression, eosinophilia, and increased IgE levels (30, 31). Our hypothesis was that a shift toward Th1 might occur as an early event in patients responding to ECP as reported for clinically early MF (19). The third goal of this study was to correlate usual clinical and laboratory measures of clinical response to ECP with possible molecular ‘tumor-associated markers’ (STAT3, MICB, and T-plastin/PLS3) in patients with blood involvement. Our hypothesis was that response to ECP monotherapy will result in an early decrease in the level of proposed tumor-associated marker message (mRNA) in PBMC samples obtained during the first 3 months of ECP monotherapy. Lastly, we planned to evaluate clinical and laboratory data during the initial 3 months of ECP treatment in order to identify potential predictors of response.
Materials and methods
This retrospective study utilized the CTCL tissue repository at Johns Hopkins School of Medicine and was approved by the Johns Hopkins Institutional Review Board. Twenty-one subjects were selected based on the diagnosis of advanced MF/SS with blood involvement and administration of ECP as the only treatment for at least 6 months.
Patients and treatment protocol
Three patients had the skin manifestations of widespread MF and the remaining 18 patients had generalized erythroderma. The histologic diagnosis of CTCL was confirmed on skin biopsy specimens in all cases, and all had negative serologic testing for human T-cell lymphoma virus, type 1 antibody. Blood studies included quantitative Sézary cell counts, flow cytometry, molecular analysis of the TCR-β chain by Southern blot or the TCR-γ chain by polymerase chain reaction (PCR) for T-cell clonality, and chromosome karyotyping as described previously (29, 32, 33). The blood findings were used to classify the 18 erythrodermic patients into SS (15 cases) and erythrodermic CTCL, not otherwise defined (three cases) subsets, and also for staging as recommended by the International Society for Cutaneous Lymphomas (8). The initial nine patients in this series were reported previously (29).
ECP was administered on 2 consecutive days at 4-week intervals starting between March 1991 and April 2001 (Tables 1 and 2). Before February 1999, methoxsalen was given orally at a dose of 0.6 mg/kg before ECP, but thereafter, methoxsalen solution was added directly to the lymphocyte collection bag. The basic approach was to utilize ECP as the only treatment for the initial 6 months, and thereafter continue ECP monotherapy if there was clinical evidence of improvement or add an adjunct treatment, most often interferon-α (IFN-α), if the response to ECP was deemed to be inadequate. The response to ECP was determined by one of us (E. C. V.) before each ECP cycle, and was based on clinical assessment of skin manifestations and lymphadenopathy relative to baseline status as described previously (29). If improvement on ECP was sustained for at least 4 weeks (two consecutive ECP cycles), the observed global clinical responses were scored as minor responses (MR) if there was an objective improvement but < 50% reduction in skin manifestations (usually diminished intensity of skin inflammation), partial responses (PR) if the skin improvement was > 50% of baseline scores, CR if all clinical manifestations of CTCL disappeared, worsening (usually increased intensity of skin inflammation), or disease progression (DP). Patients were evaluated before each ECP session and blood was obtained for special studies (Sézary cell counts, flow cytometry, LDH, complete blood counts with differential, and serum and lymphocyte storage) at 3-month intervals.
Table 1.
Patients with advance CTCL and favorable short term clinical response on extra corporeal photopheresis momotherapgy
| Patient/age (years)/sex | DX | TNBM* | Months | Response | %SC | SC/mm3 | %CD4/8/7 | CD4/8 | CD4P7M/mm3† | LDH |
|---|---|---|---|---|---|---|---|---|---|---|
| 1/73/F‡ | SS | T4(2)NXB2 | 0 | 44 | 2760 | 87/3/59 | 29.0 | 2070 | 172 | |
| 3 | NC | 52 | 2944 | 76/4/54 | 19.0 | 1698 | 153 | |||
| 6 | MR | 58 | 2127 | 89/4/72 | 22.3 | 734 | 155 | |||
| >6 | IFN-α: NR | |||||||||
| 2/48/M‡ | ECTCL, NOS | T4N1B1 | 0 | 17 | 317 | 54/24/68 | 2.3 | – | 184 | |
| 3 | MR | 15 | 288 | 48/18/63 | 2.7 | – | 162 | |||
| 6 | MR | 13 | 245 | 46/18/66 | 2.6 | – | 157 | |||
| >6 | IFN-α: NR | |||||||||
| 3/61/M‡ | ECTCL, NOS | T4N3B1 | 0 | 34 | 660 | 84/23/21 | 3.7 | 294 | ||
| 3 | PR | 39 | 452 | 70/24/35 | 2.9 | 200 | ||||
| 6 | MR | 32 | 300 | 47/27/44 | 1.7 | 256 | ||||
| >6 | IFN-γ: DP | |||||||||
| 4/69/M‡ | SS | T4N1B2 | 0 | 24 | 2359 | 86/1/9 | 86.0 | – | 193 | |
| 3 | NR | 38 | 2793 | 93/1/21 | 93.0 | – | 227 | |||
| 6 | MR | 30 | 2297 | 63/1/20 | 63.0 | – | 235 | |||
| 5/31/F‡ | SS | T4N3B2 | 0 | 50 | 653 | 82/8/32 | 10.3 | 717 | 214 | |
| 3 | NR | 52 | 835 | 84/7 | 12.0 | – | 203 | |||
| 6 | MR | 54 | 726 | 71/6/28 | 11.8 | 605 | 181 | |||
| >6 | IFN-α: PR | |||||||||
| 6/52/M‡ | SS | T4N3B2 | 0 | 50 | 1066 | 89/4/16 | 22.3 | 1556 | 203 | |
| 3 | NR | 39 | 2048 | 80/3/16 | 26.7 | 1523 | 224 | |||
| 6 | MR | 43 | 2839 | 89/3/12 | 29.7 | 5282 | 212 | |||
| >6 | IFN-α: PR | |||||||||
| 7/55/F | MF | T2NXB2 | 0 | 24 | 499 | 74/9/84 | 8.2 | – | 170 | |
| 3 | MR | 14 | 290 | 70/12/78 | 5.8 | – | 174 | |||
| 6 | PR | 22 | 537 | 69/8/80 | 8.6 | – | 197 | |||
| >6 | IFN-α: NR | |||||||||
| 8/54/F | SS | T4N3B2 | 0 | 31 | 695 | 86/4/62 | 21.5 | 627 | 263 | |
| 3 | PR | 25 | 374 | 83/3/51 | 27.7 | 524 | 260 | |||
| 6 | PR | 37 | 539 | 81/2/48 | 40.5 | 524 | 264 | |||
| >6 | IFN-α: NR | |||||||||
| 9/72/F | SS | T4N3B2 | 0 | 48 | 5576 | 98/0.5/5 | 196.0 | 10 919 | 336 | |
| 3 | MR | 43 | 5315 | 96/0.5/6 | 192.0 | 11 248 | 364 | |||
| 6 | MR | 41 | 5574 | 98/2/1 | 49.0 | 13 188 | 325 | |||
| >6 | IFN-α: NR | |||||||||
| 10/79/F | SS | T4N3B2 | 0 | 33 | 340 | 67/13/66 | 5.2 | – | 308 | |
| 3 | MR | 22 | 172 | 43/17/61 | 2.5 | – | 257 | |||
| 6 | NR | 24 | 133 | 56/8/57 | 7.0 | – | 241 | |||
| >6 | CR | |||||||||
| 11/77/M | ECTCL, NOS | T4N1B1 | 0 | 13 | 121 | 42/23/80 | 1.8 | – | 184 | |
| 3 | NR | 29 | 339 | 58/18/84 | 3.2 | – | 171 | |||
| 6 | MR | 15 | 171 | 59/20/83 | 3.0 | – | 191 | |||
| >6 | CR | |||||||||
| 12/72/F | SS | T4NXB2 | 0 | 0 | 0 | 73/5/35 | 14.6 | 899 | 212 | |
| 3 | PR | 0 | 0 | 69/5/33 | 13.8 | 776 | 194 | |||
| 6 | PR | 0 | 0 | 67/4/31 | 16.8 | 726 | 165 | |||
| >6 | CR |
TNBM, tumor-node-blood-visceral ratings at start of ECP (63); B rating as recommended by the ISCL (8). All cases were at M0.
The absolute number of CD4+CD7− cells for cases in which neoplastic cells have a reduced expression of CD7 estimated by subtracting absolute CD7 from absolute CD3 counts.
Patients 1–6 previously reported as cases 2, 16, 19, 21, 22, and 29, respectively (29).
DX, diagnosis; SS, Sézary syndrome; ECTCL, NOS, erythrodermic cutaneous T-cell lymphoma, not otherwise defined; MF, mycosis fungoides; IFN-α, interferon-α; CR, complete response; MR, minor response; NR, no response; SC, Sézary cells; LDH, lactate dehydrogenase; CD4P7M, CD4+CD7−, subset of T cells; PR, partial response; W, worse; DP, disease progression; M, male; F, female.
Table 2.
Patients with advanced CTCL and lack of short-term clinical responses on extracorporeal photopheresis momotherapgy (Group2)
| Patient/age (years)/sex | DX | TNBM | Months | Response | %SC | SC/mm3 | %CD4/8/7 | CD4/8 | CD4P7N/mm3† | LDH |
|---|---|---|---|---|---|---|---|---|---|---|
| 13/44/F* | SS | T4N1B2 | 0 | 70 | 2010 | 79/11/41 | 7.2 | 41 | 251 | |
| 3 | W | 75 | 2772 | 77/12/46 | 6.4 | <6 | 178 | |||
| 6 | NR | 55 | 2884 | 78/10/28 | 7.8 | 28 | 213 | |||
| >6 | IFN-α: NR | |||||||||
| 14/66/M* | SS | T4N3B2 | 0 | 57 | 614 | 85/5/87 | 17.0 | – | 225 | |
| 3 | MR | 68 | 799 | 86/4/85 | 21.5 | – | 224 | |||
| 6 | NR | <6 | 149 | – | – | – | 213 | |||
| 15/69/M* | SS | T4NXB2 | 0 | 52 | 860 | 88/3/17 | 29.3 | 1207 | 198 | |
| 3 | NR | 67 | 965 | 89/3/18 | 29.7 | 1051 | 175 | |||
| 6 | NR | 58 | 438 | 79/4/28 | 19.8 | 416 | 204 | |||
| >6 | IFN-α: MR | |||||||||
| 16/67/M | SS | T4N3B2 | 0 | 52 | 2677 | 92/3/94 | 30.7 | – | 300 | |
| 3 | PR | 47 | 2386 | 94/2/47 | 47.0 | – | 385 | |||
| 6 | W | 37 | 2174 | 95/3/95 | 31.7 | – | 347 | |||
| >6 | IFN-α: NR | |||||||||
| 17/50/M | MF | T3(2)N1B1 | 0 | 13 | 496 | 38/31/74 | 1.2 | – | 161 | |
| 3 | W | 36 | 242 | 43/27/75 | 1.6 | – | 184 | |||
| 6 | DP | 16 | 188 | – | – | – | 200 | |||
| 18/72/F | SS | T4NXB2 | 0 | 54 | 3558 | 89/6/12 | 14.8 | 5468 | 216 | |
| 3 | NR | 29 | 2212 | 87/7/14 | 12.4 | 6101 | 238 | |||
| 6 | W | 52 | 2777 | 86/7/11 | 12.3 | 4380 | 293 | |||
| >6 | IFN-α: NR | |||||||||
| 19/61/M | MF | T2NXB2 | 0 | 42 | 511 | 77/2/69 | 38.5 | – | 256 | |
| 3 | W | 56 | 511 | 72/2/80 | 36.0 | – | 275 | |||
| 6 | NR | 43 | 636 | 75/2/84 | 37.5 | – | 230 | |||
| >6 | IFN-α: PR | |||||||||
| 20/80/M | SS | T4N3B2 | 0 | 85 | 5039 | 88/1/77 | 88.0 | – | 602 | |
| 3 | NR | 80 | 7410 | 97/1/80 | 97.0 | – | 578 | |||
| 6 | NR | 95 | 5045 | 98/0.1/79 | 980.0 | – | 486 | |||
| 21/73/F | SS | T4NXB2 | 0 | 68 | 3225 | 79/10/32 | 7.9 | 1945 | 248 | |
| 3 | NR | 76 | 5194 | 83/8/27 | 10.4 | 4305 | 250 | |||
| 6 | NR | 84 | 4287 | 86/9/25 | 9.6 | 3471 | 285 |
RNA isolation
Immediately before the start of ECP and before the fourth and seventh ECP sessions (3-month intervals), 30 ml of peripheral blood was collected and PBMC were isolated using a Ficoll density gradient. These cells were cryopreserved in liquid nitrogen and were thawed immediately before RNA isolation. 1×106 cells were utilized for the trizol-based protocol.
cDNA preparation and real-time PCR
Determination of relative gene expression was performed using quantitative real-time PCR (qPCR) using an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA). cDNA was prepared from the isolated RNA using random hexamers to prime reverse transcription (Ready-to-Go You-Prime First-Strand Beads; GE Healthcare, Buckinghamshire, UK) following the manufacturer’s protocol. Multiplexed quantitative determination was carried out in triplicate wells using approximately 1 : 30 of the cDNA per well and primer/probe sets for the FAM-labeled target genes and VIC-labeled endogenous reference gene (GAPDH) using the standard ABI chemistry and reagents. Relative transcripts were determined using the formula: 1/2 (CTtarget−CTcontrol). Real-time PCR efficiencies of target genes and the reference gene were approximately equal over a concentration of 0.1–200 ng total cDNA. Three possible tumor-associated target genes (T-plastin/PLS3, STAT3, and MICB) were selected for study by qPCR. T-plastin has emerged as a useful molecular marker of neoplastic T cells in CTCL (34–38), and upregulation of STAT3 has been associated with neoplastic cell growth/survival in MF/SS (39–43). MICB might be another molecular marker of CTCL based on our previous study of leukemic CTCL cases (35). Primers for STAT3, T-plastin/PLS3, and MICB were purchased from ABI Corporation (Foster City, CA, USA).
Cytokines
Before the initial ECP and at 3-month intervals, 10 ml of peripheral blood was obtained and centrifuged to separate the serum. The serum was stored at −80 °C, and thawed immediately before use. We utilized the Bio-Plex multiplex assay system based on xMAP technology from Bio-Rad Laboratories (Hercules, CA, USA) to simultaneously measure cytokines possibly implicated in the Th1/Th2 and host immune response, including IL-2, IL-4, IL-5, IL-10, IL-12p70, IL-13, IFN-γ, granulocyte macrophage colony-stimulating factor (GM-CSF), and TNF-α.
Statistical analysis
The results of standard laboratory and cytokine studies were given as mean values and 1 standard deviation (SD) and/or median value with range. The Mann–Whitney (MW) U-test was used to test for differences in the laboratory test values for two patient groups. An exact Wilcoxon signed-rank (WSR) test was utilized to test for differences in the direction of cytokine and gene expression levels for paired baseline and later samples on the same patients. Spearman’s rank order test was used to test for correlation between two variables. Fisher’s (F) and Pearson’s χ2 exact tests were used to test categorical data in 2×2 and R×C tables, respectively. The difference in survival curves was tested using the Gehan–Breslow (GB) method in order to lend more weight to deaths at time points closer to ECP usage. The statistical packages used for data analysis were SYSTAT10 and SPSS 13.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Clinical response to ECP monotherapy
The overall response to ECP monotherapy was divided into two groups according to the overall assessment of response (Tables 1 and 2). Long-term follow-up indicates that 10 of 12 patients with responsive disease (group 1; Table 1) are currently deceased, with all but one attributed to CTCL. The overall disease-related median survival was 67 months. Conversely, nine patients failed to respond adequately to treatment (group 2; Table 2). At last contact, only one patient is alive with slowly progressing SS. The overall median disease-related survival of this group is 41 months. The difference in survival curves between group 1 and group 2 patients was significant (GB test, P = 0.039). The overall median survival for all patients was 64 months.
The status of patients after 3 and 6 months of ECP monotherapy was categorized as clinically improved (MR, PR, and CR), unchanged (NC), or worse (W, DP) to determine whether early response was a predictor of eventual response to ECP alone. After 3 months of treatment, clinical improvement, which was usually manifested by a reduced intensity of skin inflammation, was noted in nine patients (five MRs, four PRs) compared with baseline. Of these early responders, continued improvement occurred by 6 months for three patients (patient 7: MR to PR; patients 8 and 12: sustained PR), with patient 12 eventually achieving a CR on ECP monotherapy. Of the remaining six early responders, three patients were scored as MR at 6 months (one with PR at 3 months), and three patients had evidence of relapsing disease including patient 16 with PR at 3 months and DP thereafter. Of interest, patient 10, who had an MR at 3 months and an NC at 6 months compared with baseline, eventually had a CR after 13 months. By contrast, 12 patients did not show evidence of clinical improvement at 3 months. However, a delayed response was apparent at 6 months for five patients (NC to MR), with three ultimately achieving a PR (patients 5 and 6) or a CR (patient 11) with continued ECP monotherapy beyond 6 months. This experience indicates that responses to ECP alone are often slow to occur and early improvement after 3 months is not a reliable indicator of a subsequent response to treatment.
A number of clinical parameters available at the start of ECP were compared in patients who responded to ECP monotherapy (group 1) vs. those who did not respond adequately (group 2) (Table 3). Clinical parameters including the intensity of skin erythema (in Caucasian patients only) and T4 vs. T2/T3 skin rating did not correlate with subsequent response to ECP (data not shown).
Table 3.
Comparison of baseline parameters in favorable (Group 1) and poor (Group 2) response patients on extracorporeal photopheresis momotherapgy
| Parameters | Group 1 (n=12) | Group 2 (n=9) | P-value |
|---|---|---|---|
| Age (years) | 65 (31–79)* | 67 (44–80) | 0.859 |
| Duration of disease (months) | 23 (4–240) | 54 (8–207) | 0.394 |
| WBC/µl | 7.8 (5.2–17.6) | 8.7 (3.8–12.2) | 0.394 |
| Lymphocyte count/mm3 | 2062 (928–11 616) | 2871 (1078–6588) | 0.887 |
| Eosinophils/mm3 | 388 (61–1545) | 87 (0–351) | 0.008 |
| Sézary cells/100 lymphocytes | 32 (0–51) | 54 (36–85) | 0.001 |
| Sézary cells/mm3 | 657 (0–5576) | 2010 (496–5039) | 0.155 |
| %CD4+ cells | 83 (42–98) | 85 (38–92) | 0.499 |
| CD4+ (cells/mm3) | 1585 (390–11 384) | 2268 (523–5863) | 0.943 |
| %CD8+ cells | 6.5 (0.5–24) | 5 (1–45) | 0.887 |
| CD8+ (cells/mm3) | 118 (58–447) | 154 (24–1279) | 0.887 |
| CD4/CD8 ratio | 12.4 (1.8–196) | 17.0 (1.2–88) | 0.522 |
| LDH (U/l) | 208 (170–336) | 248 (161–602) | 0.355 |
| IgE (U/l) | 92 (2–2716) | 35 (5–630) | 0.434 |
| IL-5 (pg/ml) | 0.06 (0–0.18)† | 0 (0–0.21) | 0.345 |
| IL-10 (pg/ml) | 0.14 (0–30.02)† | 0.63 (0–3.21) | 0.504 |
| IL-12 (pg/ml) | 0.06 (0–12.69)† | 0.01 (0–3.20) | 0.720 |
| TNF-α (pg/ml) | 0.45 (0–1.34)† | 0.45 (0–4.78) | 0.626 |
Values given as median (range).
Patient 3 not studied.
LDH, lactate dehydrogenase; IL, interleukin.
The effect of ECP on clinical laboratory parameters
Pairwise comparisons of each clinical laboratory parameter were made after 3 and 6 months relative to the pretreatment baseline values. When all 21 cases were analyzed as a single group, the only quantitative change that was statistically significant was a decrease in absolute eosinophils at 3 months (median change, −29 cells/mm3 or −16%, WSR test, P = 0.049) but not at 6 months (median change, +1 cells/mm3 or +1.5%, P = 0.468). Additionally, absolute Sézary cell counts, absolute CD4+ counts, and LDH levels decreased a median of −2.6%, −14%, and −7% in responding patients compared with median increases of +18.8%, +17%, and +3% in non-responding patients, respectively.
The only baseline clinical laboratory parameters that were statistically different between the ECP-responsive and the non-responsive groups were the lower proportion of Sézary cells in the lymphocyte population (median, 32% vs. 54%, MW test, P =0.001) and the higher absolute eosinophil count (median, 388 vs. 87 cells/mm3 MW test, P= 0.008) in responding patients compared with non-responding patients (Table 3).
Baseline cytokine expression
At least one cytokine per growth factor had measurable values in 19 of the 20 pre-ECP serum samples that were studied. At least one Th2-type cytokine (IL-4, IL-5, IL-10, and IL-13) was present in 16 (80%) samples, but IL-4, the prototypic Th2 cytokine, was detected in only one sample (patient 16). Other Th2-type cytokines that were detectable were IL-5 (eight cases, median, 0.125, range, 0.06–0.21 pg/ml), IL-10 (12 cases, median, 0.85, range, 0.14–30.02 pg/ml), and IL-13 (four cases, median, 0.07, range, 0.01–0.54 pg/ml). In addition, at least one Th1-associated cytokine (IL-2, IL-12, TNF-α, and IFN-γ) was detected in 18 (90%) samples. The most commonly detected Th1-associated cytokines were IL-12 (11 cases, 0.14, range, 0.01–12.69 pg/ml) and TNF-α (15 cases, median, 0.48, range, 0.01–4.78 pg/ml). IFN-γ was present in only three cases (median, 11.95, range, 3.26–12.28 pg/ml) and IL-2 was found in five cases (median, 0.32, range, 0.01–0.63 pg/ml). Finally, GM-CSF was detected in six cases (median, 0.405, range, 0.01–3.53 pg/ml).
Effect of ECP on cytokine expression
Pairwise comparison of pre- and post-ECP samples indicated significant increases in IL-5 and TNF-α cytokine levels and after 6 months compared with baseline (median change, +0.11 and +2.33 pg/ml, respectively; both P < 0.001). IL-10 also tended to increase (median change, +0.25 pg/ml, P=0.091), whereas IL-12 levels remained unchanged. Changes in these cytokine levels were not apparent in 3-month samples (Data not shown). In 11 cases, the Th2 cytokine IL-5 was below the threshold of detection in pretreatment samples, and became measurable after 6 months of ECP. All four of the positive cases at baseline remained positive in subsequent samples, although the levels increased in only two instances. Similarly, IL-10 transformed from undetectable to detectable in three cases at 6 months or was present in both pre-and post-samples, and none reverted from positive to negative. However, a concurrent inhibitory effect of ECP on Th1 cytokines, IL-12, and TNF-α could not be shown. For example, none of the cases in which TNF-α was detected in pretreatment samples became negative at 6 months, whereas three TNF-α-negative cases at baseline subsequently became positive. Furthermore, TNF-α levels increased significantly after 6 months of ECP.
Correlation of cytokine measurements and clinical laboratory parameters
When correlated with clinical responses after 6 months of ECP, the absolute eosinophil counts tended to decrease in ECP-responsive group 1 cases (median change, −60 cells/mm3 or –14%) compared with an increase in ECP-non-responsive group 2 cases (median change, +23 cells/mm3 or +11%, MW test, P = 0. 0.255). By contrast, the increase in IL-5 levels in group 1 (median change, +0.21 ng/ml) was significantly higher than group 2 (+0.10 ng/ml, MW test, P = 0.02).
LDH levels in pretreatment samples did not correlate significantly with other measures of blood tumor burden, specifically, absolute Sézary cell count (n= 21, ρ= 0.411, P= 0.063) and the CD4/CD8 ratio (n= 21, ρ= 0.375, P= 0.092). Nevertheless, because LDH levels tended to decrease in responsive patients and increase in non-responsive patients, we analyzed the relationship between LDH and IL-10, IL-12, and TNF-α during treatment by categorizing the change relative to baseline values in levels in 3- and 6-month samples and then comparing the results in a two-way contingency table. LDH and TNF-α varied in the opposite direction in samples taken at 3 months (F test, P= 0.01). In six of 15 samples, LDH increased while TNF-α deceased at this time point, and conversely, in seven other samples, LDH decreased and TNF-α increased. In addition, a significant correlation was found between the percentage of change from baseline for each variable (n=15, ρ=−0.649, P= 0.009). However, this effect was not observed in samples taken at 6 months.
Correlations with tumor-associated gene expression levels
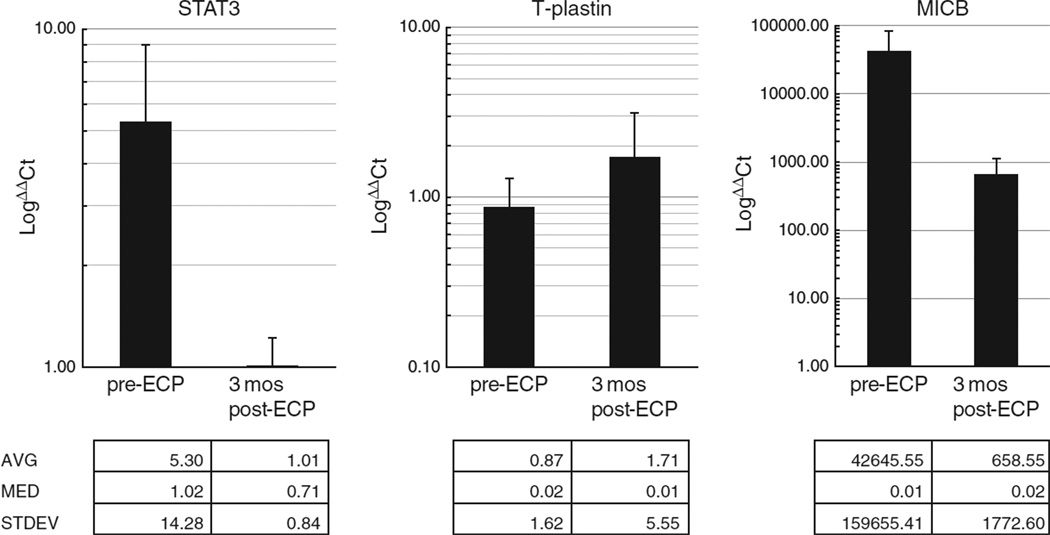
qPCR was utilized to measure the expression levels of STAT3, T-plastin, and MICB in PBMC collected immediately before and after 3 months of ECP in eight patients with favorable and seven with poor responses to ECP. Overall, RT-PCR demonstrated the presence of the three genes, STAT3, MICB, and T-plastin as compared with the control gene (GAPDH) (Fig. 1). There was no significant difference in expression at time point 0–3-month post-ECP treatment (STAT3, ΔΔCt 5.30±14.28 vs. 1.01±0.84; MICB, ΔΔCt 42645.55±159655.41 vs. 658.55±1772.60; T-plastin, ΔΔCt 0.87±1.62 vs. 1.71±5.55) (Fig. 1, Table 4). We did not have adequate sample numbers to evaluate the 6-month post-ECP treatment time point. Lastly, the levels of gene expression in baseline samples were not significantly different in ECP-responsive (group 1) vs. non-responsive (group 2) cases (data not shown).
Fig. 1.
Real time PCR of the genes STAT3 T-plastin, and MICB demonstrated measurable expression, but significant change was not detected between pre-ECP and 3 months post-ECP.
A significant positive correlation was found between T-plastin expression and TNF-α serum levels (n=9, ρ= 0.917, P < 0.001) and serum IL-12 levels (n=9, ρ = 0.730, P =0.02) in the pretreatment samples, and the absolute Sézary cell count was also correlated with the TNF-α serum levels in the pretreatment samples (ρ =0.438, P = 0.067).
Discussion
Our study indicates that baseline and changes in cytokine levels and gene expression levels of ‘tumor-associated’ molecular markers (T-plastin, STAT3, and MICB) in PBMCs, and clinical response after 3 months of ECP monotherapy in patients with advanced CTCL and blood involvement do not provide useful information about subsequent clinical responses. However, patients with a lower percentage of Sézary cells among lymphocytes and higher absolute eosinophil counts in the pretreatment samples were more likely to respond.
Gottlieb reported that patients with evidence of Sézary cells in the blood were more likely to respond to ECP than patients without these cells, and indeed, this was the best predictor of a good response in their experience (28). However, the percentage of Sézary cells among leukocytes did not correlate well with the observed response. Conversely, Evans found that a higher baseline Sézary cell count as a percentage of the total white cell count, but not absolute counts, predicted a favorable response after 6 months of ECP (14). Therefore, the clinical relevance of Sézary cells in the context of ECP is unclear, but could be related to the well-known subjectivity of Sézary cell counts and that Sézary cells can represent both reactive and neoplastic cells, especially in the setting of erythrodermic CTCL.
To our knowledge, the association of higher eosinophil counts with a better response to ECP has not been reported before, and is somewhat unexpected, considering that a high absolute eosinophil count (> 700 cells/mm3) has been reported to be indicative of a poor prognosis in CTCL (44, 45). In our cohort, four cases had absolute eosinophil counts exceeding 700/mm3 and all had a favorable response to ECP.
In GvHD and in type 1 diabetes, an ECP-mediated shift toward Th2 has been reported (46, 47). One potential mechanism that might underlie this observation is that ECP-treated dendritic cells can be primed to increase Th2-producing T cells (47, 48). Conversely, Di Renzo reported that PBMCs from patients with MF at stage Ib (presumably no blood involvement) before ECP produced significantly higher levels of IL-4 and lower levels of IFN-γ and IL-12 (i.e. a Th2 profile), compared with healthy volunteers, and that this reverted to normal after 1 year of ECP (19). A Th1-promoting effect by ECP has also been reported in the setting of GvHD (49, 50). The effect of ECP on the Th1/Th2 balance may depend on the disease setting, and it seems possible that normalization of a skewed Th1/Th2 immune balance in ECP-responsive cases is secondary to the improvement in the disease rather than directly modulating Th1 or Th2 cytokine production. We did not appreciate a clear-cut shift to either Th1 or Th2 when looking at the group as a whole or evaluating ECP-responsive vs. ECP-non-responsive. Overall, we found an increase in TNF-α and IL-5 over the 6 months of ECP treatment, with a predominance of IL-5 in those who responded to ECP.
Many genes have been identified as being up-regulated or altered in expression in CTCL.We highlighted three distinct genes, which we evaluated to look for a change in expression during ECP therapy. STAT3 is an acute-phase response factor within the STAT protein family and has been found to be constitutively phosphorylated in SS neoplastic cells and may play a role in their growth (51). Additionally, forced expression of a transfected dominant-negative STAT3 in CTCL cell lines led to a marked increase in apoptosis of cells, whereas transfection with normal, wild-type STAT3 did not alter the levels of apoptosis (40). Because of these data, STAT3 has been considered to be a ‘malignancy factor’ in CTCL (40). Plastins or fimbrins are a family comprised of three isoforms of actin-bundling proteins. Interestingly, although the T isoform (T-plastin) is generally expressed in all tissues, except leukocytes, it was recently demonstrated to be expressed in lymphocytes of SS and leukemic MF (35, 37). Lastly, MICB encodes a heavily glycosylated protein that is a ligand for the NKG2D type II receptor. The study by Capriotti demonstrated MICB transcript expression through RT-PCR in over 90% of ‘leukemic CTCL’ samples evaluated (35). They also noted 100% T-plastin expression in those same samples.
We did identify measurable transcript expression levels of STAT3, MICB, and T-plastin as compared with the expression of the housekeeping gene, GAPDH. We were not able to find significant changes in the expression of any of these genes between time point 0 and 3 months post-therapy. We feel that this lack of change may be secondary to the wide range of gene expression levels between the individual subjects and the fact that these methods may not be sensitive enough to detect changes in non-purified neoplastic cells in individual patients. We also feel that 3 months of ECP therapy may have simply been too early to detect a change in the expression of these markers. It is also important to note that these genes have not been fully established as tumor markers, and further studies are needed to better address this issue.
The presence of TNF-α in the serum of most of our cases is also worth commenting on because it has been called a sentinel cytokine that can augment host defense mechanisms at low concentrations. The observation that TNF-α, IL-10, and to a lesser degree, IL-12 are present in the sera of patients with advanced CTCL may have relevance to ECP. For example, TNF-α is released by ECP-treated monocytes (16), and if administered to patients with already high endogenous TNF-α levels, thismight account for post-treatment flares of erythema and/or fever observed in some patients, but might not affect tumor burden.
It is alsoworth noting thatMF and SS have developed in patients treated with the TNF antagonists (52–58). The commercially available TNF antagonists (infliximab, adalimumab, etenercept, certolizumab pegol, and golimumab) not only remove soluble TNF-α but also bind to its membrane-bound receptors (TNFR1 or TNFR2). This could either block the binding of soluble TNF-α to its receptors or could initiate signaling, resulting in cell activation or apoptosis depending on a complex interplay between the metabolic status and the microenvironment of the cell. Most of the TNF antagonist-associated cases of MF/SS have occurred in patients thought to have psoriasis, and the biologic behavior of the emergent lymphoma was often aggressive. This experience suggests either that soluble TNF-α was inhibiting neoplastic T-cell growth or that TNF antagonists were directly stimulating neoplastic T-cell growth (59). The former concept is supported by one study using a CTCL-derived cell line (SeAx), which had growth retarded through administration of TNF-α (60) and could explain some of the therapeutic effects of ECP (16). Additional in vitro studies on the effect of TNF antagonists on neoplastic T-cell growth might be illuminating.
Conversely, TNF-α can also lead to excess inflammation and organ injury at high concentrations. The positive correlation between TNF-α serum levels and T-plastin expression in PBMCs in our cases indicates that neoplastic T cells may actually be a major source of TNF-α. Interestingly, neoplastic cells of SS are capable of producing TNF-α when directly stimulated with bacterial products. Recent studies reveal that TNF-α may act as an autocrine growth factor for some cell lines as reported for the SS-derived HUT78 cell line (61, 62).
The mechanisms by which ECP exerts its effect are clearly complex and likely involve multiple pathways.We were unable to identify any clinical or laboratory findings after 3 months of ECP therapy that were ultimately associated with a clinical response to ECP at 6 months and beyond. We did document a positive association between a favorable ECP response and elevated eosinophil levels, as well as reduced Sézary cell numbers measured at the initiation of ECP treatment, suggesting that patients with less advanced disease were more likely to benefit. Based on this experience, we would advocate administration of adjunct therapies at the initiation of ECP to hopefully reduce the time to response. Lastly, we also found an increase in TNF-α in the serum of patients after 6 months of ECP treatment. Further investigation will be required to fully appreciate the therapeutic effect of ECP, and to identify early markers that may indicate eventual clinical response to therapy.
Acknowledgements
This study was supported by the American Skin Association Research Grant and the Leonard and Ruth Levine Skin Research Fund.
Footnotes
Conflicts of interest:
None declared.
References
- 1.Edelson R, Berger C, Gasparro F, et al. Treatment of cutaneous Tcell lymphoma by extracorporeal photochemotherapy. Preliminary results. N Engl J Med. 1987;316:297–303. doi: 10.1056/NEJM198702053160603. [DOI] [PubMed] [Google Scholar]
- 2.Oliven A, Shechter Y. Extracorporeal photopheresis: a review. Blood Rev. 2001;15:103–108. doi: 10.1054/blre.2001.0155. [DOI] [PubMed] [Google Scholar]
- 3.Babic AM. Extracorporeal photopheresis: lighting the way to immunomodulation. Am J Hematol. 2008;83:589–591. doi: 10.1002/ajh.21166. [DOI] [PubMed] [Google Scholar]
- 4.Dani T, Knobler R. Extracorporeal photoimmunotherapy–photopheresis. Front Biosci. 2009;14:4769–4777. doi: 10.2741/3566. [DOI] [PubMed] [Google Scholar]
- 5.Zinzani PL, Ferreri AJ, Cerroni L. Mycosis fungoides. Crit Rev Oncol Hematol. 2008;65:172–182. doi: 10.1016/j.critrevonc.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Lansigan F, Choi J, Foss FM. Cutaneous T-cell lymphoma. Hematol Oncol Clin North Am. 2008;22:979–996. doi: 10.1016/j.hoc.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Hwang ST, Janik JE, Jaffe ES, Wilson WH. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945–957. doi: 10.1016/S0140-6736(08)60420-1. [DOI] [PubMed] [Google Scholar]
- 8.Vonderheid EC, Bernengo MG, Burg G, et al. Update on erythrodermic cutaneous T-cell lymphoma: report of the International Society for Cutaneous Lymphomas. J Am Acad Dermatol. 2002;46:95–106. doi: 10.1067/mjd.2002.118538. [DOI] [PubMed] [Google Scholar]
- 9.Heald P, Rook A, Perez M, et al. Treatment of erythrodermic cutaneous T-cell lymphoma with extracorporeal photochemotherapy. J Am Acad Dermatol. 1992;27:427–433. doi: 10.1016/0190-9622(92)70212-x. [DOI] [PubMed] [Google Scholar]
- 10.McKenna KE, Whittaker S, Rhodes LE, et al. Evidence-based practice of photopheresis 1987-2001: a report of a workshop of the British Photodermatology Group and the U.K. Skin Lymphoma Group. Br J Dermatol. 2006;154:7–20. doi: 10.1111/j.1365-2133.2005.06857.x. [DOI] [PubMed] [Google Scholar]
- 11.Duvic M, Chiao N, Talpur R. Extracorporeal photopheresis for the treatment of cutaneous T-cell lymphoma. J Cutan Med Surg. 2003;7:3–7. doi: 10.1007/s10227-003-5001-1. [DOI] [PubMed] [Google Scholar]
- 12.Rao V, Ryggen K, Aarhaug M, Dai HY, Jorstad S, Moen T. Extracorporeal photochemotherapy in patients with cutaneous T-cell lymphoma: is clinical response predictable? J Eur Acad Dermatol Venereol. 2006;20:1100–1107. doi: 10.1111/j.1468-3083.2006.01745.x. [DOI] [PubMed] [Google Scholar]
- 13.Heshmati F. Mechanisms of action of extracorporeal photochemotherapy. Transfus Apher Sci. 2003;29:61–70. doi: 10.1016/S1473-0502(03)00103-4. [DOI] [PubMed] [Google Scholar]
- 14.Evans AV, Wood BP, Scarisbrick JJ, et al. Extracorporeal photopheresis in Sézary syndrome: hematologic parameters as predictors of response. Blood. 2001;98:1298–1301. doi: 10.1182/blood.v98.5.1298. [DOI] [PubMed] [Google Scholar]
- 15.Berger CL, Longley J, Hanlon D, Girardi M, Edelson R. The clonotypic T cell receptor is a source of tumor-associated antigens in cutaneous T cell lymphoma. Ann NY Acad Sci. 2001;941:106–122. doi: 10.1111/j.1749-6632.2001.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 16.Vowels BR, Cassin M, Boufal MH, Walsh LJ, Rook AH. Extracorporeal photochemotherapy induces the production of tumor necrosis factor-alpha by monocytes: implications for the treatment of cutaneous T-cell lymphoma and systemic sclerosis. J Invest Dermatol. 1992;98:686–692. doi: 10.1111/1523-1747.ep12499907. [DOI] [PubMed] [Google Scholar]
- 17.Klosner G, Trautinger F, Knobler R, Neuner P. Treatment of peripheral blood mononuclear cells with 8-methoxypsoralen plus ultraviolet A radiation induces a shift in cytokine expression from a Th1 to a Th2 response. J Invest Dermatol. 2001;116:459–462. doi: 10.1046/j.1523-1747.2001.01276.x. [DOI] [PubMed] [Google Scholar]
- 18.Di Renzo M, Rubegni P, Pasqui AL, et al. Extracorporeal photopheresis affects interleukin (IL)-10 and IL-12 production by monocytes in patients with chronic graft-versus-host disease. Br J Dermatol. 2005;153:59–65. doi: 10.1111/j.1365-2133.2005.06482.x. [DOI] [PubMed] [Google Scholar]
- 19.Di Renzo M, Rubegni P, De Aloe G, et al. Extracorporeal photochemotherapy restores Th1/Th2 imbalance in patients with early stage cutaneous T-cell lymphoma. Immunology. 1997;92:99–103. doi: 10.1046/j.1365-2567.1997.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Craciun LI, Stordeur P, Schandene L, et al. Increased production of interleukin-10 and interleukin-1 receptor antagonist after extracorporeal photochemotherapy in chronic graft-versus-host disease. Transplantation. 2002;74:995–1000. doi: 10.1097/00007890-200210150-00017. [DOI] [PubMed] [Google Scholar]
- 21.Maeda A, Schwarz A, Kernebeck K, et al. Intravenous infusion of syngeneic apoptotic cells by photopheresis induces antigenspecific regulatory T cells. J Immunol. 2005;174:5968–5976. doi: 10.4049/jimmunol.174.10.5968. [DOI] [PubMed] [Google Scholar]
- 22.Maeda A, Schwarz A, Bullinger A, Morita A, Peritt D, Schwarz T. Experimental extracorporeal photopheresis inhibits the sensitization and effector phases of contact hypersensitivity via two mechanisms: generation of IL-10 and induction of regulatory T cells. J Immunol. 2008;181:5956–5962. doi: 10.4049/jimmunol.181.9.5956. [DOI] [PubMed] [Google Scholar]
- 23.Lamioni A, Parisi F, Isacchi G, et al. The immunological effects of extracorporeal photopheresis unraveled: induction of tolerogenic dendritic cells in vitro and regulatory T cells in vivo. Transplantation. 2005;79:846–850. doi: 10.1097/01.tp.0000157278.02848.c7. [DOI] [PubMed] [Google Scholar]
- 24.Gatza E, Rogers CE, Clouthier SG, et al. Extracorporeal photopheresis reverses experimental graft-versus-host disease through regulatory T cells. Blood. 2008;112:1515–1521. doi: 10.1182/blood-2007-11-125542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stevens SR, Baron ED, Masten S, Cooper KD. Circulating CD4+CD7− lymphocyte burden and rapidity of response: predictors of outcome in the treatment of Sézary syndrome and erythrodermic mycosis fungoides with extracorporeal photopheresis. Arch Dermatol. 2002;138:1347–1350. doi: 10.1001/archderm.138.10.1347. [DOI] [PubMed] [Google Scholar]
- 26.Zic JA, Stricklin GP, Greer JP, et al. Long-term follow-up of patients with cutaneous T-cell lymphoma treated with extracorporeal photochemotherapy. J Am Acad Dermatol. 1996;35:935–945. doi: 10.1016/s0190-9622(96)90118-8. [DOI] [PubMed] [Google Scholar]
- 27.Jiang SB, Dietz SB, Kim M, Lim HW. Extracorporeal photochemotherapy for cutaneous T-cell lymphoma: a 9.7-year experience. Photodermatol Photoimmunol Photomed. 1999;15:161–165. doi: 10.1111/j.1600-0781.1999.tb00077.x. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb SL, Wolfe JT, Fox FE, et al. Treatment of cutaneous T-cell lymphoma with extracorporeal photopheresis monotherapy and in combination with recombinant interferon alfa: a 10-year experience at a single institution. J Am Acad Dermatol. 1996;35:946–957. doi: 10.1016/s0190-9622(96)90119-x. [DOI] [PubMed] [Google Scholar]
- 29.Vonderheid EC, Zhang Q, Lessin SR, et al. Use of serum soluble interleukin-2 receptor levels to monitor the progression of cutaneous T-cell lymphoma. J Am Acad Dermatol. 1998;38:207–220. doi: 10.1016/s0190-9622(98)70597-3. [DOI] [PubMed] [Google Scholar]
- 30.Chong BF, Wilson AJ, Gibson HM, et al. Immune function abnormalities in peripheral blood mononuclear cell cytokine expression differentiates stages of cutaneous T-cell lymphoma/ mycosis fungoides. Clin Cancer Res. 2008;14:646–653. doi: 10.1158/1078-0432.CCR-07-0610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim EJ, Hess S, Richardson SK, et al. Immunopathogenesis and therapy of cutaneous T cell lymphoma. J Clin Invest. 2005;115:798–812. doi: 10.1172/JCI24826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vonderheid EC, Bigler RD, Kotecha A, et al. Variable CD7 expression on T cells in the leukemic phase of cutaneous T cell lymphoma (Sézary syndrome) J Invest Dermatol. 2001;117:654–662. doi: 10.1046/j.1523-1747.2001.01456.x. [DOI] [PubMed] [Google Scholar]
- 33.Vonderheid EC, Pena J, Nowell P. Sézary cell counts in erythrodermic cutaneous T-cell lymphoma: implications for prognosis and staging. Leuk Lymphoma. 2006;47:1841–1856. doi: 10.1080/10428190600709655. [DOI] [PubMed] [Google Scholar]
- 34.Booken N, Gratchev A, Utikal J, et al. Sézary syndrome is a unique cutaneous T-cell lymphoma as identified by an expanded gene signature including diagnostic marker molecules CDO1 and DNM3. Leukemia. 2008;22:393–399. doi: 10.1038/sj.leu.2405044. [DOI] [PubMed] [Google Scholar]
- 35.Capriotti E, Vonderheid EC, Thoburn CJ, Wasik MA, Bahler DW, Hess AD. Expression of T-plastin, FoxP3 and other tumorassociated markers by leukemic T-cells of cutaneous T-cell lymphoma. Leuk Lymphoma. 2008;49:1190–1201. doi: 10.1080/10428190802064917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kari L, Loboda A, Nebozhyn M, et al. Classification and prediction of survival in patients with the leukemic phase of cutaneous T cell lymphoma. J Exp Med. 2003;197:1477–1488. doi: 10.1084/jem.20021726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su MW, Dorocicz I, Dragowska WH, et al. Aberrant expression of T-plastin in Sézary cells. Cancer Res. 2003;63:7122–7127. [PubMed] [Google Scholar]
- 38.Tiemessen MM, Mitchell TJ, Hendry L, Whittaker SJ, Taams LS, John S. Lack of suppressive CD4+CD25+FOXP3+T cells in advanced stages of primary cutaneous T-cell lymphoma. J Invest Dermatol. 2006;126:2217–2223. doi: 10.1038/sj.jid.5700371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fantin VR, Loboda A, Paweletz CP, et al. Constitutive activation of signal transducers and activators of transcription predicts vorinostat resistance in cutaneous T-cell lymphoma. Cancer Res. 2008;68:3785–3794. doi: 10.1158/0008-5472.CAN-07-6091. [DOI] [PubMed] [Google Scholar]
- 40.Sommer VH, Clemmensen OJ, Nielsen O, et al. In vivo activation of STAT3 in cutaneous T-cell lymphoma. Evidence for an antiapoptotic function of STAT3. Leukemia. 2004;18:1288–1295. doi: 10.1038/sj.leu.2403385. [DOI] [PubMed] [Google Scholar]
- 41.van Kester MS, Out-Luiting JJ, von dem Borne PA, Willemze R, Tensen CP, Vermeer MH. Cucurbitacin I inhibits Stat3 and induces apoptosis in Sézary cells. J Invest Dermatol. 2008;128:1691–1695. doi: 10.1038/sj.jid.5701246. [DOI] [PubMed] [Google Scholar]
- 42.Zhang C, Li B, Gaikwad AS, et al. Avicin D selectively induces apoptosis and downregulates p-STAT-3, bcl-2, and survivin in cutaneous T-cell lymphoma cells. J Invest Dermatol. 2008;128:2728–2735. doi: 10.1038/jid.2008.138. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Q, Nowak I, Vonderheid EC, et al. Activation of Jak/STAT proteins involved in signal transduction pathway mediated by receptor for interleukin 2 in malignant T lymphocytes derived from cutaneous anaplastic large T-cell lymphoma and Sézary syndrome. Proc Natl Acad Sci USA. 1996;93:9148–9153. doi: 10.1073/pnas.93.17.9148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tancrede-Bohin E, Ionescu MA, de La Salmoniere P, et al. Prognostic value of blood eosinophilia in primary cutaneous Tcell lymphomas. Arch Dermatol. 2004;140:1057–1061. doi: 10.1001/archderm.140.9.1057. [DOI] [PubMed] [Google Scholar]
- 45.Sausville EA, Eddy JL, Makuch RW, et al. Histopathologic staging at initial diagnosis of mycosis fungoides and the Sézary syndrome. Definition of three distinctive prognostic groups. Ann Intern Med. 1988;109:372–382. doi: 10.7326/0003-4819-109-5-372. [DOI] [PubMed] [Google Scholar]
- 46.Faresjo MK, Ernerudh J, Berlin G, Garcia J, Ludvigsson J. The immunological effect of photopheresis in children with newly diagnosed type 1 diabetes. Pediatr Res. 2005;58:459–466. doi: 10.1203/01.pdr.0000176906.42001.c3. [DOI] [PubMed] [Google Scholar]
- 47.Gorgun G, Miller KB, Foss FM. Immunologic mechanisms of extracorporeal photochemotherapy in chronic graft-versus-host disease. Blood. 2002;100:941–947. doi: 10.1182/blood-2002-01-0068. [DOI] [PubMed] [Google Scholar]
- 48.Holtick U, Marshall SR, Wang XN, Hilkens CM, Dickinson AM. Impact of psoralen/UVA-treatment on survival, activation, and immunostimulatory capacity of monocyte-derived dendritic cells. Transplantation. 2008;85:757–766. doi: 10.1097/TP.0b013e31816650f6. [DOI] [PubMed] [Google Scholar]
- 49.Darvay A, Salooja N, Russell-Jones R. The effect of extracorporeal photopheresis on intracellular cytokine expression in chronic cutaneous graft-versus-host disease. J Eur Acad Dermatol Venereol. 2004;18:279–284. doi: 10.1111/j.1468-3083.2004.00814.x. [DOI] [PubMed] [Google Scholar]
- 50.Silva MG, Ferreira Neto L, Guimaraes A, Machado A, Parreira A, Abecasis M. Long-term follow-up of lymphocyte populations and cellular cytokine production in patients with chronic graftversus- host disease treated with extracorporeal photopheresis. Haematologica. 2005;90:565–567. [PubMed] [Google Scholar]
- 51.Eriksen KW, Kaltoft K, Mikkelsen G. Constitutive STAT3-activation in Sézary syndrome: tyrphostin AG490 inhibits STAT3- activation, interleukin-2 receptor expression and growth of leukemic Sézary cells. Leukemia. 2001;15:787–793. doi: 10.1038/sj.leu.2402093. [DOI] [PubMed] [Google Scholar]
- 52.Adams AE, Zwicker J, Curiel C, et al. Aggressive cutaneous T-cell lymphomas after TNFalpha blockade. J Am Acad Dermatol. 2004;51:660–662. doi: 10.1016/j.jaad.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 53.Schmidt A, Robbins J, Zic J. Transformed mycosis fungoides developing after treatment with alefacept. J Am Acad Dermatol. 2005;53:355–356. doi: 10.1016/j.jaad.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 54.Chuang GS, Wasserman DI, Byers HR, Demierre MF. Hypopigmented T-cell dyscrasia evolving to hypopigmented mycosis fungoides during etanercept therapy. J Am Acad Dermatol. 2008;59:S121–S122. doi: 10.1016/j.jaad.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 55.Dalle S, Balme B, Berger F, Hayette S, Thomas L. Mycosis fungoides-associated follicular mucinosis under adalimumab. Br J Dermatol. 2005;153:207–208. doi: 10.1111/j.1365-2133.2005.06686.x. [DOI] [PubMed] [Google Scholar]
- 56.Dauendorffer JN, Rivet J, Allard A, Bachelez H. Sézary syndrome in a patient receiving infliximab for ankylosing spondylitis. Br J Dermatol. 2007;156:742–743. doi: 10.1111/j.1365-2133.2006.07713.x. [DOI] [PubMed] [Google Scholar]
- 57.Sanli H, Ataman S, Akay BN, Yilmaz A, Yildizlar D, Gurgey E. Mycosis fungoides in a patient with ankylosing spondylitis during infliximab therapy. J Drugs Dermatol. 2007;6:834–836. [PubMed] [Google Scholar]
- 58.Lafaille P, Bouffard D, Provost N. Exacerbation of undiagnosed mycosis fungoides during treatment with etanercept. Arch Dermatol. 2009;145:94–95. doi: 10.1001/archdermatol.2008.526. [DOI] [PubMed] [Google Scholar]
- 59.Kirchner S, Holler E, Haffner S, Andreesen R, Eissner G. Effect of different tumor necrosis factor (TNF) reactive agents on reverse signaling of membrane integrated TNF in monocytes. Cytokine. 2004;28:67–74. doi: 10.1016/j.cyto.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 60.Dobbeling U, Dummer R, Laine E, Potoczna N, Qin JZ, Burg G. Interleukin-15 is an autocrine/paracrine viability factor for cutaneous T-cell lymphoma cells. Blood. 1998;92:252–258. [PubMed] [Google Scholar]
- 61.O’Connell MA, Cleere R, Long A, O’Neill LA, Kelleher D. Cellular proliferation and activation of NF kappa B are induced by autocrine production of tumor necrosis factor alpha in the human T lymphoma line HuT 78. J Biol Chem. 1995;270:7399–7404. doi: 10.1074/jbc.270.13.7399. [DOI] [PubMed] [Google Scholar]
- 62.Ringrose A, Zhou Y, Pang E, et al. Evidence for an oncogenic role of AHI-1 in Sézary syndrome, a leukemic variant of human cutaneous T-cell lymphomas. Leukemia. 2006;20:1593–1601. doi: 10.1038/sj.leu.2404321. [DOI] [PubMed] [Google Scholar]
- 63.Lamberg SI, Bunn PA., Jr Cutaneous T-cell lymphomas. Summary of the Mycosis Fungoides Cooperative Group-National Cancer Institute Workshop. Arch Dermatol. 1979;115:1103–1105. doi: 10.1001/archderm.115.9.1103. [DOI] [PubMed] [Google Scholar]