Abstract
Purpose
To assess the diagnostic performance of signal changes in Hoffa's fat pad (HFP) assessed on non-contrast-enhanced (CE) MRI in detecting synovitis, and the association of pain with signal changes in Hoffa’s fat pad on non-CE MRI and peripatellar synovial thickness on CE MRI.
Methods
The Multicenter Osteoarthritis (MOST) Study is an observational study of individuals who have or are at high risk for knee OA. All subjects with available non-CE and CE MRIs were included. Signal changes in HFP were scored from 0 to 3 in 2 regions using non-CE MRI. Synovial thickness was scored from 0 to 2 on CE MRI in 5 peripatellar regions. Sensitivity, specificity and accuracy of HFP signal changes were calculated considering synovial thickness on CE MRI as the reference standard. We used logistic regression to assess the associations of HFP changes (non-CE MRI) and synovial thickness (CE MRI) with pain from walking up or down stairs, after adjusting for potential confounders.
Results
A total of 393 subjects were included. Sensitivity of infrapatellar and intercondylar signal changes in HFP was high (71% and 88%), but specificity was low (55% and 30%). No significant associations were found between HFP changes on non-CE MRI and pain. Grade 2 synovial thickness assessed on CE MRI was significantly associated with pain after adjustments for potential confounders.
Conclusion
Signal changes in HFP detected on non-CE MRI are a sensitive but non-specific surrogate for the assessment of synovitis. CE MRI identifies associations with pain better than non-CE MRI.
Keywords: Knee osteoarthritis, synovitis, magnetic resonance imaging, knee pain
INTRODUCTION
Synovitis is an important feature of knee osteoarthritis (OA), and may manifest directly as thickening of the synovial membrane or indirectly as joint effusion due to synovial activation [1, 2]. Direct imaging assessment of synovitis is probably ideally performed using contrast-enhanced (CE) magnetic resonance imaging (MRI) [1, 3–5]. Because administration of gadolinium-based contrast agents has potential hazardous side effects and is costly, CE MRI is rarely applied in large epidemiological OA studies or clinical trials. Assessment of synovitis in knee OA studies is usually performed on non-CE MRI using signal alterations detected in Hoffa’s fat pad as a synovitis surrogate [6–9]. In a radiological-pathologic correlation study conducted by Fernandez-Madrid et al., it was shown that such signal changes correlate with mild chronic synovitis [10]. This work led to the assumption that synovitis may be assessed on non-CE MRI, mainly on fat-suppressed proton density- or T2-weighted sequences, using signal alterations in the Hoffa’s fat pad as a surrogate for whole-knee synovitis. Furthermore, such signal changes detected in Hoffa’s fat pad on non-CE MRI have shown clinically relevant associations in subjects with knee OA [6, 7, 11].
While signal alterations in Hoffa’s fat pad detected on non-CE MRI sequences are a sensitive sign of peripatellar synovitis, they are non-specific as has recently been shown in a small study comparing non-CE and CE MRI [12], and present a multitude of possible diagnoses [13]. It is important to confirm whether such signal alterations in Hoffa’s fat pad truly correspond with synovitis as detected by synovial thickening and enhancement on CE MRI, as the role of synovitis in regard to symptoms and progression of disease is still not fully understood. To our knowledge, the associations of synovitis assessed on both non-CE and CE MRI with peripatellar pain have never been evaluated and compared using a large study sample.
The aims of this study were: 1) to evaluate the diagnostic performance of signal changes in Hoffa’s fat pad on non-CE MRI for the detection of synovitis using synovial thickness on CE MRI as the reference standard; and 2) to assess the associations of signal changes in Hoffa’s fat pad (non-CE MRI) and peripatellar synovial thickness (CE MRI) with pain on walking up or down stairs.
MATERIAL AND METHODS
Study Design and Subjects
Subjects were participants in the Multicenter Osteoarthritis (MOST) Study, a prospective epidemiological study of 3,026 people aged 50 to 79 years with a goal of identifying risk factors for incident and progressive knee OA in a population either with or at high risk of developing OA. They were recruited from two US communities, Birmingham, Alabama and Iowa City, Iowa through mass mailing of letters and study brochures, supplemented by media and community outreach campaigns. MOST subjects were recruited and enrolled between June 2003 and March 2005. The Health Insurance Portability and Accountability Act-compliant study protocol was approved by the Institutional Review Boards at the University of Iowa, University of Alabama at Birmingham, University of California San Francisco and Boston University School of Medicine. We obtained written informed consent from all patients. Subjects considered at high risk for knee OA included those who were overweight or obese, those with knee pain, aching or stiffness on most of the last 30 days, a history of knee injury that made it difficult to walk for at least one week, or previous knee surgery. Subjects were not eligible to participate in MOST if they screened positive for rheumatoid arthritis [14], had ankylosing spondylitis, psoriatic arthritis, reactive arthritis, renal insufficiency that required hemo- or peritoneal dialysis, a history of cancer (except for non-melanoma skin cancer), had or planned to have bilateral knee replacement surgery, were unable to walk without assistance, or were planning to move out of the area in the next 3 years.
In the present study, an unselected subset of MOST subjects who volunteered to undergo CE MRI of one knee at the 30-month follow-up clinic visit was evaluated. CE MRI scans were obtained on one knee only. The knee with lower Kellgren–Lawrence (KL) grade was selected to avoid choosing knees with severe OA and decrease the likelihood of co-occurrence of other structural features that are potentially associated with pain. If the grade was the same for both knees, the dominant leg was chosen. The CE MRI was performed on the same day or within 30 days of non-CE MRI obtained in all MRI eligible subjects in the parent study (95% of knees were assessed with CE MRI on the same day as the non-CE MRI). For persons with renal disease, diabetes or over the age of 65, serum creatinine was determined and the glomerular filtration rate calculated before intravenous gadolinium administration. Persons with renal insufficiency (glomerular filtration rate <30 ml/min) were excluded from the present study.
Altogether 1295 subjects were approached at the two clinical centers (624 at Birmingham and 671 at Iowa City). Of these, 336 participants refused to undergo the 1.5 T CE MRI. Documented reasons were “unwillingness to receive injection” (n=121)"no time/too busy” (n=169) and “other reason” (n=46). 256 subjects were excluded because they reported kidney disease, had an elevated serum creatinine level or did not receive a 1.0T MRI at 30 months. Further, 157 subjects that were approached and scheduled missed the 30-month visit for other reasons leaving 546 subjects that were examined with 1.5T CE MRI. Finally, other 153 were excluded from the analysis due to incomplete MRI readings for peripatellar synovitis (CE MRI) and signal changes in Hoffa’s fat pad (non CE MRI), leaving 393 subjects (1 knee per subject) included in the analysis.
Radiographs
At the 30-month follow-up visit, all subjects underwent weight-bearing postero-anterior (PA) fixed flexion knee radiographs using the protocol by Kothari et al. and a Plexiglas positioning frame (SynaFlexer™) [15]. A musculoskeletal radiologist and a rheumatologist who were not authors, both with over 10 years experience reading study radiographs and blinded to clinical data, graded the x-rays independently according to the Kellgren- Lawrence (KL) scale [16]. Radiographs were presented sequentially with readers blinded to all clinical data and to MRI. Whole knee radiographic OA was considered present if KL grade ≥2 or patellofemoral OA occurred on lateral view radiographs, which was defined by the presence of a definite osteophyte in the patellofemoral joint. If readers disagreed on the presence of radiographic OA, readings were adjudicated by a panel of 3 readers (2 non-authors and DTF).
MRI Acquisition
For the MOST parent study, MRIs were obtained with a 1.0 T dedicated extremity unit (ONI MSK Extreme 1.0T, GE Healthcare, Wilmington, MA) with a circumferential extremity coil using fat-suppressed (FS) fast spin-echo proton density-weighted (PDw) sequences in two planes, sagittal (TR = 4800 ms, TE = 35 ms, 3 mm slice thickness, 0 mm interslice gap, 32 slices, 288×192 matrix, 2 excitations (NEX), 140×140 mm field of view (FOV), echo train length (ETL) = 8) and axial (TR = 4680 ms, TE = 13 ms, 3 mm slice thickness, 0 mm interslice gap, 20 slices, 288×192 matrix, 2 NEX, 140×140 mm FOV, ETL = 8), and a short tau inversion-recovery (STIR) sequence in the coronal plane (TR = 6650 ms, TE = 15 ms, TI = 100 ms, 3 mm slice thickness, 0 mm interslice gap, 28 slices, 256×192 matrix, 2 NEX, 140×140 mm FOV, ETL = 8).
Additionally, CE MRIs were obtained with a 1.5 T system (Siemens MAGNETOM Symphony™, Malvern, PA) with a circumferential extremity coil. Axial and sagittal FS T1-weighted CE sequences were acquired (TR = 600 ms, TE = 13 ms, 3.0 mm slice thickness, 0.3 mm interslice gap, 160 × 160 mm FOV, 512 × 512 matrix, ETL = 1). Intravenous gadolinium (Magnevist™ [gadopentetate dimeglumine; Bayer HealthCare Pharmaceuticals, Bayer Schering PharmaAG, Berlin, Germany] or Omniscan™ [gadodiamide; GE Healthcare, New Jersey, USA]) was administered at a dose of 0.2 ml (0.1 mmol) kg body weight. Two minutes after completing the injection of the gadolinium, sagittal sequences were obtained followed immediately by the axial sequences. Two minutes was chosen to depict optimal synovial enhancement and complete acquisition of images before relevant diffusion of the contrast agent into the joint cavity occurred [17].
MRI Interpretation
MRI readings were performed independently by two musculoskeletal radiologists (AG, FWR), with 9 and 7 years of experience in semiquantitative MRI assessment of knee OA. Readers were blinded to radiographic data, as well as to the subjects’ pain status. MRIs were assessed using eFilm™ software (Version 2.0.0, Merge Healthcare, Milwaukee, WI). On sagittal non-CE MRI PDw FS images, signal alterations in the infrapatellar and intercondylar regions of Hoffa’s fat pad were assessed and semiquantitatively scored from 0 to 3, as a surrogate for synovitis as previously reported [6, 7, 10]. Changes in Hoffa’s fat pad considered in the present study represented areas of ill-defined high signal intensity in Hoffa’s fat pad seen on sagittal proton-density fat-suppressed images, which were lining the synovial membrane in the infrapatellar and intercondylar regions (Figure 1). The extent of signal changes was subjectively evaluated regarding the area of interest of Hoffa’s fat pad assessed (infrapatellar vs. intercondylar) that is affected by such signal changes, according to the following scale: 0 (normal), 1 (mild), 2 (moderate), and 3 (severe). The inter-reader reliability (weighted kappa) for the readings of signal alterations in Hoffa’s fat pad on non-CE MRI was 0.65.
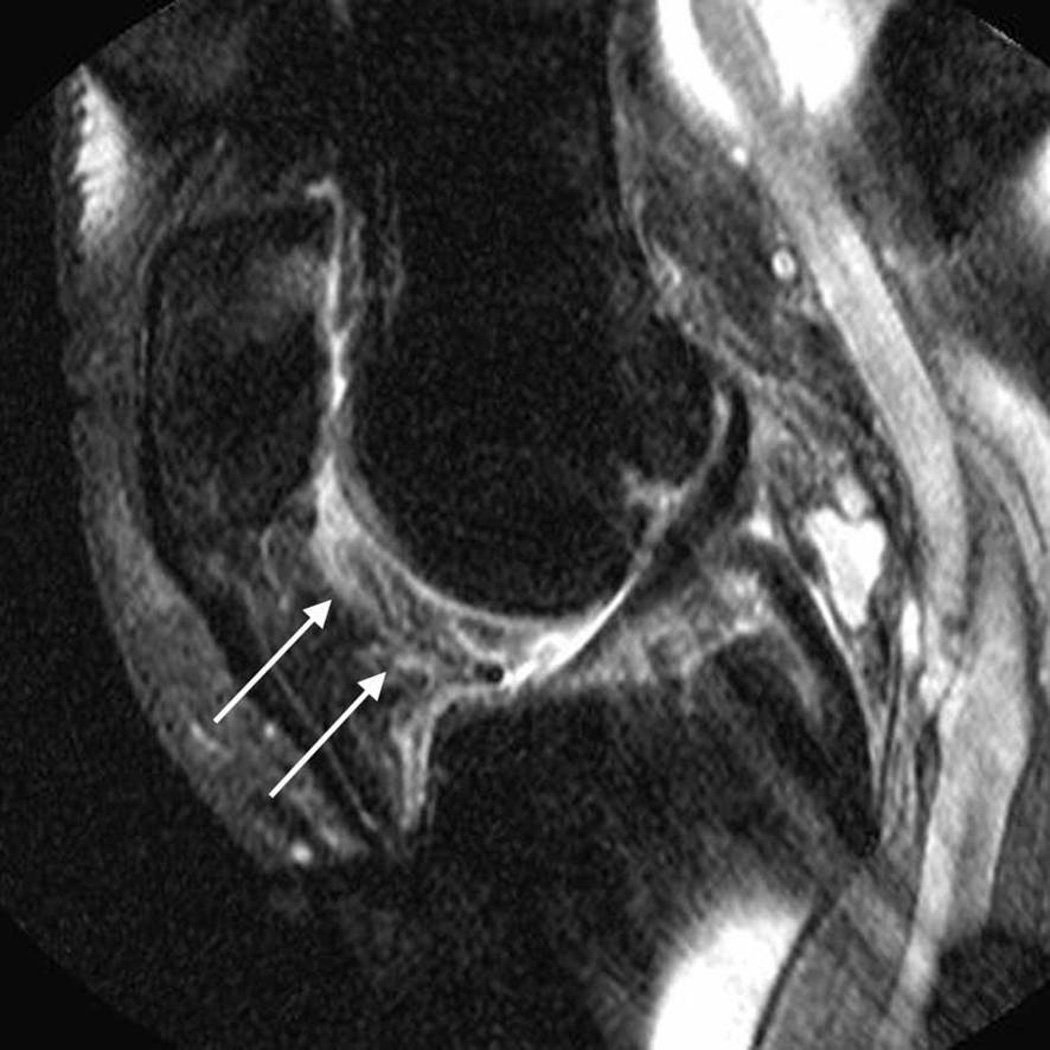
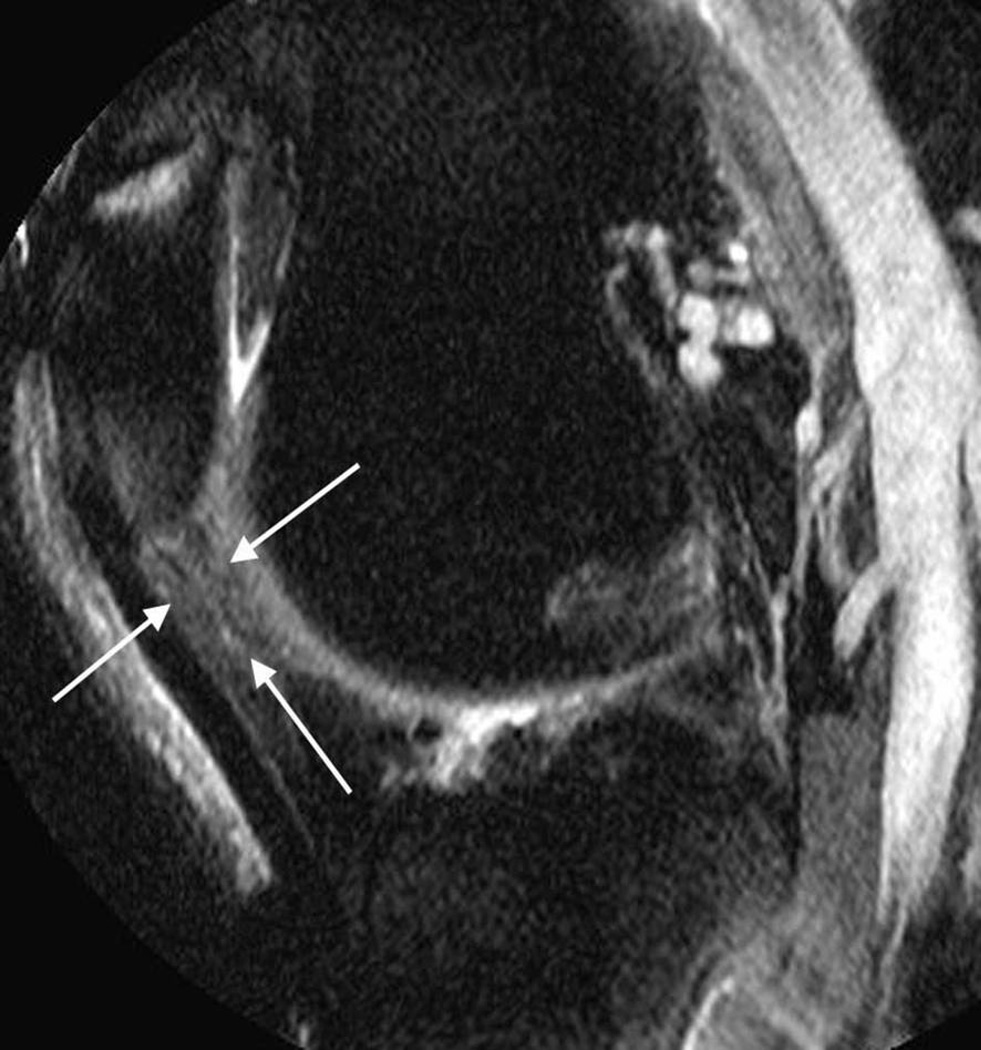
Figure 1.
Sagittal fat-suppressed proton density-weighted (non-contrast-enhanced MRI) shows signal changes in Hoffa’s fat pad depicted at the intercondylar (arrows, A) and the infrapatellar (arrows, B) regions.
On CE MRI, synovitis was defined as enhancing thickened synovium (>2 mm). We assessed the synovium in five peripatellar regions: the suprapatellar, infrapatellar, and intercondylar regions – evaluated with sagittal CE MRI; and the medial and lateral parapatellar regions – evaluated with axial CE MRI images. Synovial thickness was scored using a whole-joint semiquantitative scoring system for the assessment of synovitis in CE MRI [5]: grade 0 if <2 mm, grade 1 if 2–4 mm, and grade 2 if >4 mm, based on the maximal thickness at each site (Figure 2). The inter-reader reliability (weighted kappa) for the readings of synovial thickness on CE MRI varied from 0.67 to 0.83.
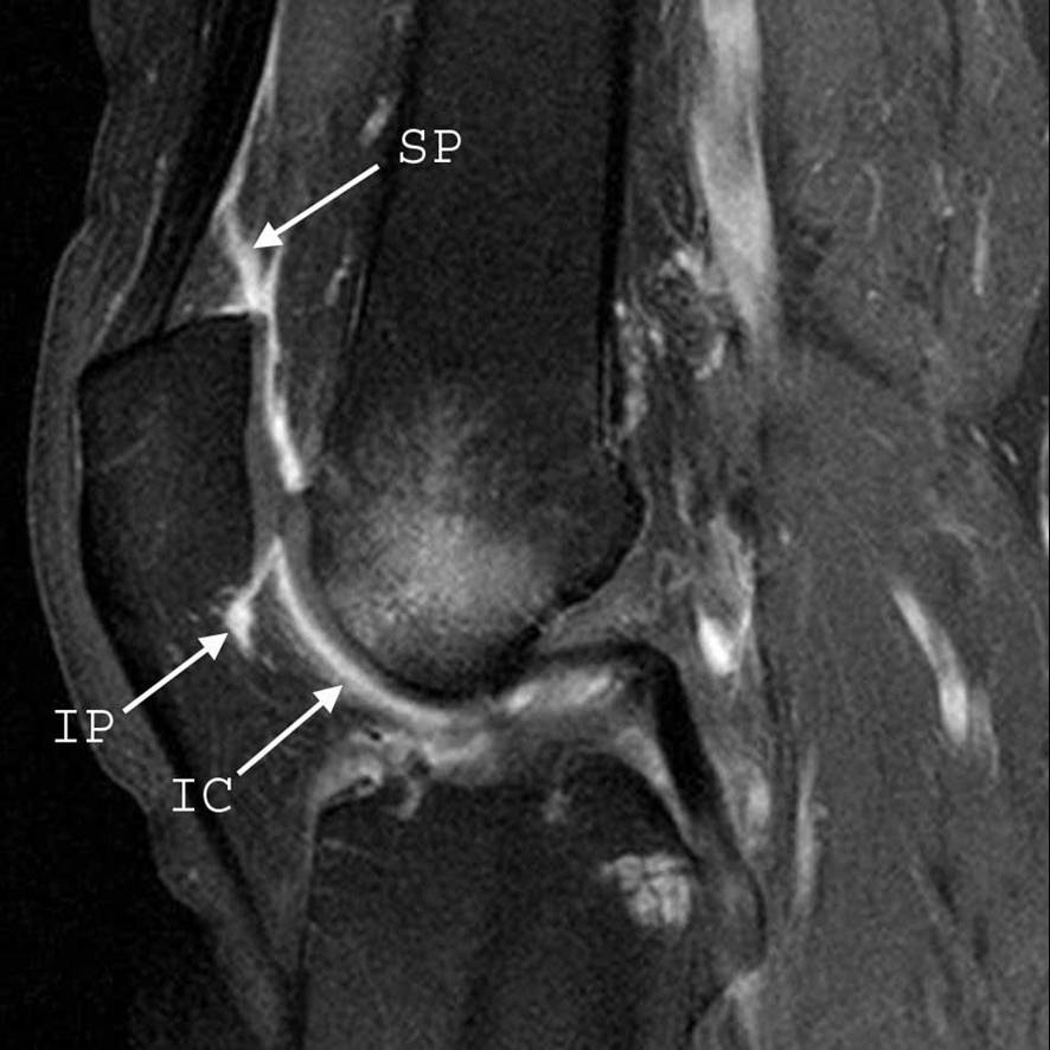
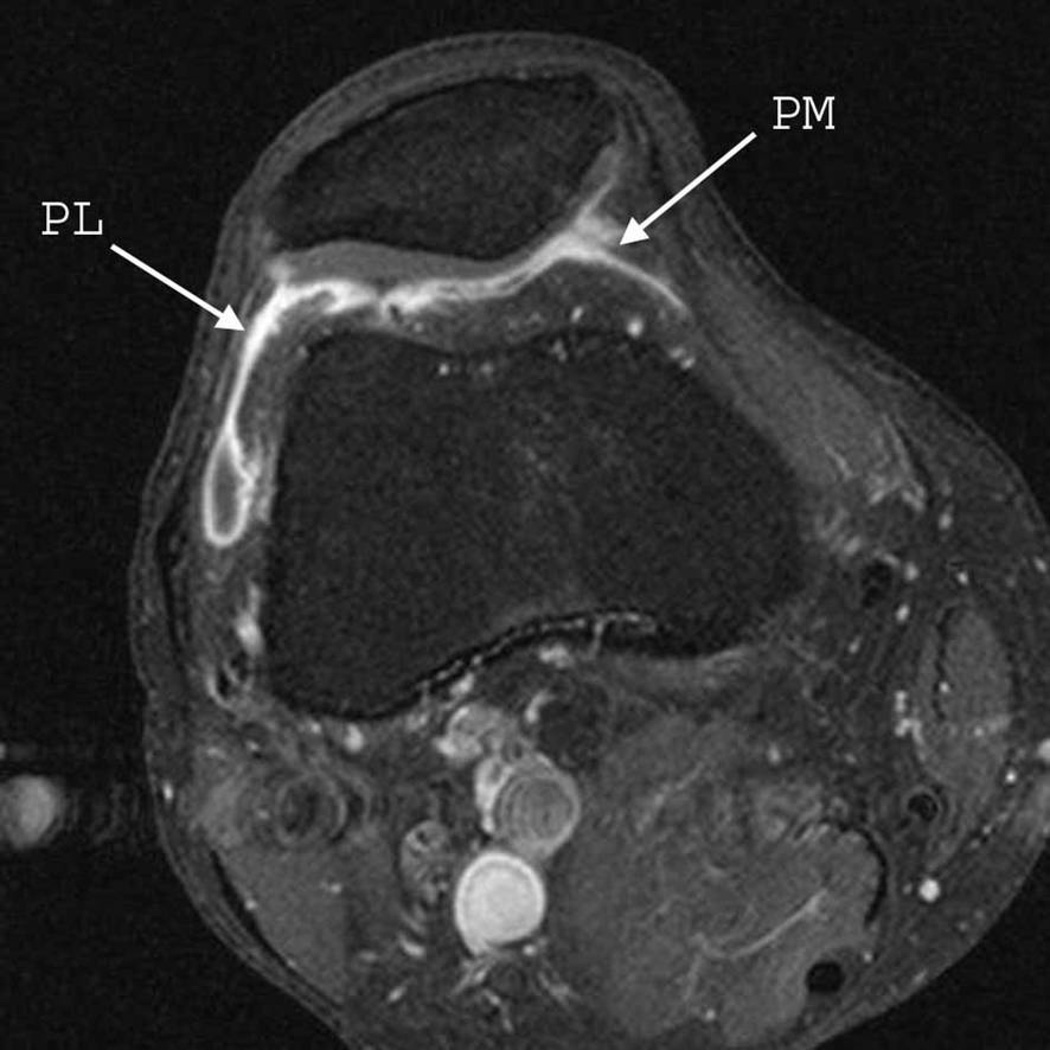
Figure 2.
Sagittal (A) and axial (B) fat-suppressed T1-weighted MRI after intravenous gadolinium injection (contrast-enhanced MRI) demonstrate pathological synovial enhancement and thickening (synovitis) detected at the suprapatellar (SP), infrapatellar (IP), intercondylar (IC), medial parapatellar (PM), and lateral parapatellar (PL) regions.
On non-CE MRI, we also assessed the presence of bone marrow lesions (BMLs) in knees using the WORMS scoring system [18]. BMLs were assessed in this study since such lesions are associated with knee pain [11,19]. BMLs were evaluated in all 15 subregions of the knee as defined in WORMS, and scored from 0–3 based on the extent of regional involvement: 0 = none; 1 = <25% of the subregion, 2 = 25–50% of the subregion; 3 = >50% of the subregion. BMLs were considered as “present” when any of the 15 subregions of the knee had a BML score ≥ 1.
Knee pain assessment
The 3.0 Likert version of the Western Ontario and McMaster Osteoarthritis Index (WOMAC) was applied at the 30-month clinic visit. For each of the five WOMAC pain items subjects rated their pain from 0 (no pain) to 4 (extreme pain). As we evaluated peripatellar synovitis and pain on walking up or down stairs is commonly considered to be present in subjects with patellofemoral pain syndrome [20], we retained only the WOMAC score for pain on walking up or down stairs in our analyses.
Statistical analyses
To test the diagnostic performance of signal changes depicted in Hoffa’s fat pad (infrapatellar and intercondylar regions) on non-CE MRI, we calculated the sensitivity, specificity, and accuracy of any signal change detected on non-CE MRI (grade ≥1), referring to the synovial thickness measurements in the infrapatellar and intercondylar regions on CE MRI as the reference standard, in which synovitis was defined as any grade ≥1. Infrapatellar and intercondylar synovitis were analyzed separately.
To assess the cross-sectional associations of signal changes in Hoffa’s fat pad depicted on non-CE MRI and abnormal synovial thickening on CE MRI (the predictors) with pain on walking up or down stairs (the outcome), we considered only the maximum score of signal changes on non-CE MRI (2 locations) or synovial thickening on CE MRI (5 locations). Patients with no signal changes on non-CE MRI (grade 0) and no abnormal synovial thickening (grade 0) in any assessed region formed the reference group. The presence of pain on walking up or down stairs (outcome) was defined as WOMAC grade ≥1.
The associations were evaluated using logistic regression, adjusting for age, gender, body mass index (BMI), whole knee radiographic OA, and the presence of BMLs in any of the 15 subregions of the knee (adjusted model). We further examined the incremental utility of each of the predictors by evaluating discrimination using c-statistic analysis. We further compared c-statistics using the Chi-square test to evaluate whether values for both non-CE and CE MRI were significantly different. Finally, we included synovitis measurements performed on both non-CE and CE MRI in the same model to assess their association with pain (fully adjusted model). All analyses were performed using SAS 9.1 (SAS Institute, Cary, North Carolina, USA).
RESULTS
A total of 393 subjects (one knee per subject) were included. At baseline, the mean age (standard deviation) was 58.8 ± 7.0 years, mean BMI 29.5 ± 4.8, and 46.1% were women. The prevalence of whole knee radiographic OA was 26.2%. There were significant differences for the same characteristics when comparing to subjects from MOST not included in the present analysis (n=2633): mean age 63.1 ± 8.1 years (p-value < 0.001), mean BMI 30.9 ± 6.1 (p-value < 0.001), 62.3% women (p-value < 0.001), and prevalence of whole knee radiographic OA of 31.7% (p-value < 0.001).
The prevalence of knee pain on walking up or down stairs was 52.9%. Signal changes (grade ≥ 1) in Hoffa’s fat pad on non-CE MRI were detected in 312 knees (79.4%). Abnormal synovial thickening (grade ≥ 1) in at least 1 peripatellar region on CE MRI was detected in 200 knees (50.9%). From 393 knees included, 358 were completely evaluated for the presence of BMLs at 30 months (no missing values in any of the 15 subregions), and 287 (80.2%) had BMLs in at least 1 subregion of the knee. Missing BML data in 35 knees was mainly due to the presence of susceptibility artifacts in the field of view affecting at least 1 of the 15 subregions assessed. The subjects’ characteristics and prevalence of each grade of signal changes and synovial thickening are presented in Table 1.
Table 1.
Characteristics of 393 knees (1 knee per subject) used for knee pain analysis.
| N= 393 knees | ||
|---|---|---|
| Age (year) | 58.8 + 7.0 | |
| Women | 181 (46.1%) | |
| BMI kg/ m2 | 29.5 + 4.8 | |
| Whole knee radiographic OA | 103 (26.2%) | |
|
Knees with pain when climbing up or down stairs |
208 (52.9%) | |
|
Maximal signal change in Hoffa fat pad on PDwFS |
||
| Normal (grade 0) | 81 (20.6%) | |
| Mild (grade 1) | 154 (39.2%) | |
| Moderate (grade 2) | 109 (27.7%) | |
| Severe (grade 3) | 49 (12.5%) | |
|
Maximal synovial thickness on T1w post-gadolinium of 5 sites |
||
| <2 mm thickness (grade 0) | 193 (49.1%) | |
| 2–4 mm thickness (grade 1) | 118 (30.0%) | |
| >4 mm enhancement (grade 2) | 82 (20.9%) | |
BMI= body mass index; PDwFS= proton density-weighted fat-suppressed; T1w= T1-weighted.
With CE MRI as the reference standard, the sensitivity of infrapatellar and intercondylar signal changes in Hoffa’s fat pad on non CE MRI was high (71% and 88%, respectively), but specificity was low (55% and 30%, respectively) (Table 2).
Table 2.
Sensitivity, specificity, accuracy of signal changes in Hoffa’s fat pad on non-contrast-enhanced MRI (proton density-weighted fat-suppressed), considering synovial thickness measurements on contrast-enhanced MRI as the reference standard.
| INFRAPATELLAR | |||||
|---|---|---|---|---|---|
|
Synovial thickness on contrast- enhanced MRI (reference) |
Signal changes in HFP on non- contrast-enhanced MRI (test) |
Sensitivity (95% CI) |
71% (60%. 81%) |
||
| Grade 0 (normal) |
Grades 1–3 (abnormal) |
Total |
Specificity (95% CI) |
55% (49%. 60%) |
|
| Grade 0 (normal) | 174 (TN) |
143 (FP) |
317 | ||
| Grade ≥ 1 (abnormal) |
22 (FN) |
54 (TP) |
76 |
Accuracy (95% CI) |
58% (53%. 63%) |
| INTERCONDYLAR | |||||
|
Synovial thickness on contrast- enhanced MRI (reference) |
Signal changes in HFP on non- contrast-enhanced MRI (test) |
Sensitivity (95% CI) |
88% (79%. 95%) |
||
| Grade 0 (normal) |
Grades 1–3 (abnormal) |
Total |
Specificity (95% CI) |
30% (25%. 35%) |
|
| Grade 0 (normal) | 93 (TN) |
222 (FP) |
315 | ||
| Grade ≥ 1 (abnormal) |
9 (FN) |
69 (TP) |
78 |
Accuracy (95% CI) |
41% (36%. 46%) |
HFP= Hoffa’s fat pad. TN = true negatives; FN = false negatives; FP = false positives; TP = true positives; CI = confidence intervals; Sensitivity = TP/TP+FN; Specificity = TN/TN+FP; Accuracy = TP+TN/TP+FP+FN+TN
In the adjusted model, a significant association with pain on walking up and down stairs was demonstrated only when synovitis was assessed with CE MRI for grade 2 peripatellar synovial thickness (adjusted OR of 4.1; 95% confidence interval (CI) 2.0, 8.1) (Table 3). The assessment of synovitis on CE MRI discriminated pain from non-pain better than the assessment on non-CE MRI (c-statistics 0.66 vs. 0.59 respectively, p-value = 0.03). In the fully adjusted model in which we included both non-CE and CE synovitis, a significant association of synovitis with pain was also found only when the synovitis was assessed with CE MRI for grade 2 synovial thickness (adjusted OR of 3.2; 95% CI 1.5, 6.7) (Table 3). No significant associations were found between signal changes in Hoffa’s fat pad assessed using non-CE MRI and pain in both models. The lack of BML data in 35 knees did not significantly affect the results in the fully adjusted model when considering only the 358 knees completely evaluated for the presence of BMLs (data not shown).
Table 3.
Association between maximum signal change in Hoffa’s fat pad (proton density-weighted fat-suppressed) and maximum synovial peripatellar thickness on contrast-enhanced MRI with peripatellar knee pain (WOMAC score climbing up and down stairs was dichotomized into pain or no pain).
| Signal changes on non-contrast- enhanced MRI in HFP |
Number of knees (%) with pain climbing stairs |
Adjusted OR[1] (95% confidence intervals) |
Adjusted OR[2] (95% confidence intervals) |
|---|---|---|---|
| Grade 0 | 41/81 (51%) | 1.0 (reference) | 1.0 (reference) |
| Grade 1 | 65/154 (42%) | 0.6 (0.3, 1.1) | 0.6 (0.3, 1.1) |
| Grade 2 | 66/109 (61%) | 1.4 (0.7,2.7) | 1.2 (0.6,2.4) |
| Grade 3 | 36/49 (74%) | 2.2 (0.9, 5.4) | 1.3 (0.5,3.4) |
|
Synovial thickness on contrast- enhanced MRI |
Number of knees (%) with pain climbing stairs |
Adjusted OR[1] (95% confidence intervals) |
Adjusted OR[2] (95% confidence intervals) |
| Grade 0 | 78/193 (40%) | 1.0 (reference) | 1.0 (reference) |
| Grade 1 | 65/118(55%) | 1.3 (0.7,2.2) | 1.2 (0.7,2.1) |
| Grade 2 | 65/82 (79%) | 4.1 (2.0, 8.1)* | 3.2 (1.5, 6.7)* |
HFP= Hoffa’s fat pad.
Statistically significant defined as p<0.05.
Adjusted model (age, sex, BMI, whole-knee radiographic OA, and presence of BMLs).
Fully adjusted model (age, sex, BMI, whole-knee radiographic OA, and presence of BMLs), and measurements of synovitis on both contrast-enhanced and non-contrast-enhanced MRI.
DISCUSSION
Our study tested the diagnostic performance of signal changes detected in Hoffa’s fat pad on non-CE MRI in the detection of peripatellar synovitis, and demonstrated that such signal changes are sensitive but not specific for synovitis (as assessed on CE MRI). In the adjusted model of our analysis, synovial thickening (grade 2) assessed using CE MRI showed a significant association with peripatellar pain, with CE MRI identifying associations with pain better than non-CE MRI, as demonstrated by a significant difference between both c-statistics values. In the fully adjusted model, only synovitis assessed on CE MRI showed a significant association with peripatellar pain.
Signal changes in Hoffa’s fat pad detected on non-CE MRI have been used as a surrogate for whole-knee synovitis in several clinical and epidemiologic knee OA studies [6–9, 11, 21], and have demonstrated associations with knee pain [6, 7, 11]. Our study confirmed previous work by Roemer et al., which showed that, although sensitive, signal changes in Hoffa’s fat pad detected on non-CE MRI are not specific for synovitis, as represented by thickening and enhancement of the peripatellar synovium membrane on CE MRI [12]. The same study also found that in several cases signal changes in Hoffa’s fat pad depicted on non-CE MRI did not enhance at all after intravenous contrast administration [12]. Along with our results, we may assume that signal changes seen in Hoffa’s fat pad do not always reflect peripatellar synovitis; such changes may reflect the late sequelae of active synovial inflammation. Further, such signal changes might represent a multitude of conditions other than acute or chronic peripatellar synovitis [13].
We also assessed both the associations of signal changes in Hoffa’s fat pad (non-CE MRI) and of peripatellar synovial thickening (CE MRI) with knee pain. In a recent study using a sample of 30 subjects with ACR criteria for knee OA, Loeuille et al. compared non-CE and CE MRIs for the assessment of synovitis and did not find significant correlations of any of the 2 methods (non-CE and CE MRIs) with knee pain, assessed using the 0–100 pain visual analog scale (VAS) [4]. In our study sample, pain was evaluated using the WOMAC questionnaire, which includes assessment of pain in 5 different situations. Of those 5 items, only assessment of pain when walking up or down stairs is part of the clinical criteria of peripatellar pain syndrome [20]. For this reason we decided to analyze only this feature when evaluating the associations of peripatellar signal changes (non-CE MRI) and synovial thickening (CE MRI) with pain. After adjusting for age, gender, BMI, whole-knee radiographic OA, and BMLs (adjusted model) we demonstrated that only grade 2 synovitis assessed using CE MRI was significantly associated with pain. Furthermore, by comparing c-statistics (the area under the ROC curve), we could demonstrate that synovial thickening detected on CE MRI discriminates patients with pain from those without pain better than signal changes depicted on non-CE MRI. After including the measurements of both non-CE and CE MRI in the model (fully adjusted model), the association of grade 2 synovial thickening on CE MRI with pain remained significant. In both models, signal changes in Hoffa’s fat pad detected using non-CE MRI was not significantly associated with pain. However, the use of a higher threshold of signal changes in Hoffa’s fat pad on non-CE MRI might increase not only specificity in the detection of synovitis [12], but also its association with knee pain.
There are limitations to our study. First, we compared non-CE vs. CE MRI using different magnetic field strengths, and one could argue that this might lead to bias, especially as the assessment of signal changes (non-CE) was performed at a lower magnetic field strength (1.0 T) than the CE MRI (1.5 T). Second, no histological correlation was performed in our study. However, previous studies on synovitis in rheumatoid arthritis and osteoarthritis demonstrated that CE MRI is an accurate tool for the assessment of synovitis [1, 21, 22]. Further, we considered only the presence of pain on walking up or down stairs for the analyses. Even though the presence of pain on walking up or down stairs is described as a feature of the patellofemoral pain syndrome [20], there is no evidence that such pain is associated only with patellofemoral disease. Also, there is no evidence that the presence of pain in other situations as assessed using the WOMAC questionnaire is not associated with patellofemoral disease. Finally, we did not control for subject selection bias regarding the participants who volunteered to undergo CE MRI, and this could potentially affect the homogeneity of subjects included in the present analysis compared to the whole MOST study sample.
In summary, we confirmed that signal changes in Hoffa’s fat pad detected on non-CE MRI are a sensitive but non-specific surrogate for the assessment of peripatellar synovitis. Our data suggests that CE MRI identifies associations of peripatellar synovitis with pain better than non-CE MRI. Assessment of synovitis should ideally be performed on CE MRI when possible.
ACKNOWLEDGEMENTS
We would like to thank all staff at the Coordinating Center at the University of California at San Francisco. We would also like to thank all staff at the clinical sites in Iowa and Alabama. Finally, we would like to express our thanks to all participants of the MOST study.
FUNDING SOURCE
Supported by NIH grants from the National Institute of Aging to Drs. Lewis (U01-AG-18947), Torner (U01-AG-18832), Nevitt (U01-AG-19069), and Felson (U01-AG-18820) and NIH AR47785.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHORS CONTRIBUTIONS
Conception and design: all authors; Analysis and interpretation of the data: MDC, DTF, FWR, JN, YZ, JAL, AG; Drafting of the article: all authors; Critical revision of the article for important intellectual content: all authors; Final approval of the article: all authors; Provision of study materials or patients: DTF, JAL, GYE, CEL; Statistical expertise: DTF, JN, YZ, JAL, MCN; Obtaining of funding: DTF,CEL.
CONFLICT OF INTERST
Michel D. Crema, Frank W. Roemer, and Monica D. Marra are stockholders of Boston Imaging Core Lab (BICL), LLC. Ali Guermazi is president of BICL, LLC. He is also a consultant for MerckSerono, Genzyme, Novartis, Stryker, and AstraZeneca.
REFERENCES
- 1.Loeuille D, Rat AC, Goebel JC, Champigneulle J, Blum A, Netter P, et al. Magnetic resonance imaging in osteoarthritis: which method best reflects synovial membrane inflammation? Correlations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2009;17:1186–1192. doi: 10.1016/j.joca.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Rhodes LA, Grainger AJ, Keenan AM, Thomas C, Emery P, Conaghan PG. The validation of simple scoring methods for evaluating compartment-specific synovitis detected by MRI in knee osteoarthritis. Rheumatology (Oxford) 2005;44:1569–1573. doi: 10.1093/rheumatology/kei094. [DOI] [PubMed] [Google Scholar]
- 3.Roemer FW, Kassim Javaid M, Guermazi A, Thomas M, Kiran A, Keen R, et al. Anatomical distribution of synovitis in knee osteoarthritis and its association with joint effusion assessed on non-enhanced and contrast-enhanced MRI. Osteoarthritis Cartilage. 2010;18:1269–1274. doi: 10.1016/j.joca.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Loeuille D, Sauliere N, Champigneulle J, Rat AC, Blum A, Chary-Valckenaere I. Comparing non-enhanced and enhanced sequences in the assessment of effusion and synovitis in knee OA: associations with clinical, macroscopic and microscopic features. Osteoarthritis Cartilage. 2011;19:1433–1439. doi: 10.1016/j.joca.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Guermazi A, Roemer FW, Hayashi D, Crema MD, Niu J, Zhang Y, et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: the MOST study. Ann Rheum Dis. 2011;70:805–811. doi: 10.1136/ard.2010.139618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill CL, Gale DG, Chaisson CE, Skinner K, Kazis L, Gale ME, et al. Knee effusions, popliteal cysts, and synovial thickening: association with knee pain in osteoarthritis. J Rheumatol. 2001;28:1330–1337. [PubMed] [Google Scholar]
- 7.Hill CL, Hunter DJ, Niu J, Clancy M, Guermazi A, Genant H, et al. Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis. 2007;66:1599–1603. doi: 10.1136/ard.2006.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roemer FW, Kwoh C, Hannon MJ, Green SM, Jakicic JM, Boudreau R, et al. Risk factors for MRI-detected patellofemoral and tibiofemoral cartilage loss over a 6-month period: the JOG study. Osteoarthritis Cartilage. 2010;18(Suppl 2):S180–S181. [Google Scholar]
- 9.Roemer FW, Guermazi A, Felson DT, Niu J, Nevitt MC, Crema MD, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follw-up: the MOST study. Ann Rheum Dis. 2011;70:1804–1809. doi: 10.1136/ard.2011.150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Madrid F, Karvonen RL, Teitge RA, Miller PR, An T, Negendank WG. Synovial thickening detected by MR imaging in osteoarthritis of the knee confirmed by biopsy as synovitis. Magn Reson Imaging. 1995;13:177–183. doi: 10.1016/0730-725x(94)00119-n. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Nevitt M, Niu J, Lewis C, Torner J, Guermazi A, et al. Fluctuation of knee pain and changes in bone marrow lesions, effusions, and synovitis on magnetic resonance imaging. Arthritis Rheum. 2011;63:691–699. doi: 10.1002/art.30148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roemer FW, Guermazi A, Zhang Y, Yang M, Hunter DJ, Crema MD, et al. Hoffa's Fat Pad: Evaluation on Unenhanced MR Images as a Measure of Patellofemoral Synovitis in Osteoarthritis. AJR Am J Roentgenol. 2009;192:1696–1700. doi: 10.2214/AJR.08.2038. [DOI] [PubMed] [Google Scholar]
- 13.Saddik D, McNally EG, Richardson M. MRI of Hoffa's fat pad. Skeletal Radiol. 2004;33:433–444. doi: 10.1007/s00256-003-0724-z. [DOI] [PubMed] [Google Scholar]
- 14.Karlson EW, Sanchez-Guerrero J, Wright EA, Lew RA, Daltroy LH, Katz JN, et al. A connective tissue disease screening questionnaire for population studies. Ann Epidemiol. 1995;5:297–302. doi: 10.1016/1047-2797(94)00096-c. [DOI] [PubMed] [Google Scholar]
- 15.Kothari M, Guermazi A, von Ingersleben G, Miaux Y, Sieffert M, Block JE, et al. Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004;14:1568–1573. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- 16.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Østergaard M, Klarlund M. Importance of timing of post-contrast MRI in rheumatoid arthritis: what happens during the first 60 minutes after IV gadolinium-DTPA? Ann Rheum Dis. 2001;60:1050–1054. doi: 10.1136/ard.60.11.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterfy C, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 19.Felson DT, Chainsson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–549. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 20.Thomeé R, Augustsson J, Karlsson J. Patellofemoral pain syndrome: a review of current issues. Sports Med. 1999;28:245–262. doi: 10.2165/00007256-199928040-00003. [DOI] [PubMed] [Google Scholar]
- 21.Loeuille D, Chary-Valckenaere I, Champigneulle J, Rat AC, Toussaint F, Pinzano-Watrin A, et al. Macroscopic and microscopic features of synovial membrane inflammation in the osteoarthritic knee: correlating magnetic resonance imaging findings with disease severity. Arthritis Rheum. 2005;52:3492–3501. doi: 10.1002/art.21373. [DOI] [PubMed] [Google Scholar]
- 22.Ostergaard M, Hansen M, Stoltenberg M, Gideon P, Klarlund M, Jensen KE, et al. Magnetic resonance imaging-determined synovial membrane volume as a marker of disease activity and a predictor of progressive joint destruction in the wrists of patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:918–929. doi: 10.1002/1529-0131(199905)42:5<918::AID-ANR10>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]