Abstract
BACKGROUND
Recent experimental studies suggest that hierarchical expansion from a minor population of cancer cells with an unlimited self-renewal capacity, termed cancer initiating cells (CICs), drives both lethality and heterogeneity of prostate cancer. Human prostate CICs have been established from only two primary prostate cancer patients, with the remaining established CIC lines being derived from metastatic sites from <10 patients. This suggests that the established CIC lines are significant “outliers” and may not be representative of the prostate CICs seen clinically. Thus, there is an urgent need to develop new approaches to achieve the “routine” establishment of CIC containing lines, particularly derived from primary prostate cancers.
METHODS
In the present studies, we confirmed that in serum free, high Ca2+ (i.e., DMEN: F12) growth factor defined (GFD) media plus androgen, a large (n = 10) series of established human prostate cancer cell lines derived from both localized and metastatic sites characteristically self-associate in suspension and grow as unattached spheroids, termed prostaspheres which contain CICs based upon their self-renewal in vitro and tumorigenicity in vivo.
RESULTS
Unfortunately, however, while dissociated single cells from human primary prostate cancer tissues are viable, contain CICs as documented by their ability to take and proliferate as xenografts, and produce prostaspheres when plated with serum free, high Ca2+/GFD-media plus androgen onto standard tissue culture flask, these prostasphere do not contain CICs.
CONCLUSION
The development of reproducibly methods to culture CICs isolated directly from localized cancers is still an urgent unmeet need of the prostate cancer research community.
Keywords: human localized prostate cancer, cancer initiating cells, prostaspheres
INTRODUCTION
Prostate cancer is notoriously heterogeneous even when diagnosed initially as localized disease, being composed of phenotypically diverse malignant cell populations. Indeed, this tumor cell heterogeneity is the basis for the Gleason Grading system which combines the scores of the degree of morphological abnormalities of the most common, as well as the second most common, population of malignant cells within the primary prostate cancer lesion. Besides morphological heterogeneity, individual prostate cancer sites are also characteristically heterogeneous for the cellular expression of a series of differentiation marker [i.e., androgen receptor (AR), prostate specific antigen (PSA), prostate specific membrane antigen, and lineage specific cytokeratins]. While it is clear that prostate cancer is derived from the glandular epithelial compartment, this compartment is likewise composed of a heterogeneous mixture of morphological distinct cells types, including basal, intermediate, and luminal-secretory cells [1].
While the normal prostatic epithelial compartment is phenotypically heterogeneous, these cells are genetically identical being derived from the hierarchical expansion of a series of progenitors derived from a normal parental epithelial stem cell [1,2]. These stem cells reside in a tissue specific microenvironmental stem cell niche which allows them to maintain self-renewal ability and also generate a hierarchically expanding cascade of phenotypically diverse progeny having only limited self-renewal ability [1,2]. Based upon a growing knowledge of the role of normal prostate stem cells in both tissue renewal and the development of normal phenotypic heterogeneity, the characteristic tumor cell heterogeneity is consistent with the lethality of prostate cancers being the result of the hierarchical expansion from a minor population of cancer cells with an unlimited self-renewal capacity, termed cancer initiating cells (CICs). Recent experimental studies have documented that even though CICs are a minor population of cancer cells, they drive both the lethality and heterogeneity of the prostate cancer [3–11]. This is because these CICs have unlimited self-renewal ability while also giving rise to a hierarchically expanding cascade of phenotypically diverse malignant progeny which have only a limited proliferative ability even though they share the malignant genotype inherited from their CICs parents [3–11].
During prostate carcinogenesis, AR is transformed from a growth suppressive into a ligand dependent oncogenic protein directly stimulating the growth of prostate cancer cells [3]. This is because AR is a ligand dependent transcription factor for the expression of malignancy associated genes, like ETS fusion genes [12]. In addition, AR protein also gains the ability to be a licensing factor for DNA replication in prostate cancer cells [13,14]. Initially, this malignant growth stimulation requires a physiological level of androgen [i.e., testosterone and dihydrotestosterone (DHT)] providing the rationale for why androgen ablation (i.e., castration) is standard therapy for metastatic prostate cancer [3]. Unfortunately, after a variable period of response, there is progression to a castrate resistant state which despite secondary approaches to further lower androgen, eventually kills the patient [15]. Thus, prostate cancer will kill 30,000 US males this year alone [16]. The progression to this castration resistant state is due to CICs acquiring the novel ability to generate survival and proliferation signaling without requiring full occupancy of the AR by its normal cognate ligands [17]. Defining the mechanism for how CICs capture AR as a ligand dependent oncogenic protein during prostate carcinogenesis and how it eventually acquires ligand independent signaling during malignant progression is required to develop successful therapies for both the prevention and treatment of this devastating disease. Thus, isolating and propagating human prostate CICs from the full spectrum of clinical stages is more than an academic exercise; it is mandatory so that these cells can be used for such mechanistic studies.
Attempts to develop methods to isolate and propagate CICs have relied upon using established human prostate cancer cell lines since these clearly contain CICs based upon their lethal growth as xenografts in immune-deficient mice. Presently, prostate cancer cell lines have been established from only one primary prostate cancer from a patient with localized disease (i.e., E006AA), with the remaining established lines being derived from one primary prostate cancer (i.e., CWR-22) and from distant sites from <10 patients with metastatic disease. Thus, there is an urgent need for obtaining additional CIC containing lines particularly derived from primary prostate cancers. The major limitation for establishing new CIC driven human prostate cancer cell lines is not in dissociating these cells from patient specimens in a viable form. Instead the limitations are: (i) inability to prevent overgrowth of normal prostatic epithelial and mesenchymal cells also present within the starting clinical sample, and (ii) the inability to propagate the self-renewing CICs, as opposed to their more differentiated progeny which have only limited proliferative potential. Thus more refined methods are needed to propagate prostate CICs from a large series of prostate cancers patients.
One such refinement is based upon the fact that in serum free-growth factor defined (SFD) media containing high Ca2+ (i.e., >1 mM), normal human prostate epithelial and mesenchymal cells attach to standard tissue culture plastic [18,19], but CICs from human prostate cancer cell lines and from early passage cultures derived from chemotherapy/radiation recurrent prostate patients do not and instead self-associate in suspension and grow as non-adherent spheroids, termed prostaspheres [9–11]. Growth of such non-adherent prostaspheres documents that the CICs have lost contact inhibition which is a fundamental characteristic of cancer versus normal cells. In the present study, CICs from seven additional human prostate cancer lines were documented to form prostaspheres when plated onto standard tissue culture flask in high Ca2+/SFD-media supplemented with androgen. Based upon these facts, single cell suspensions derived from a large series (n = 72) of radical prostatectomy specimen were plated onto standard tissue culture flask in high Ca2+/SFD-media supplemented with androgen in order to fractionate normal contaminating host cells from human cancer cells by allowing the latter to form prostaspheres. These prostaspheres were then tested to determine whether they contained self-renewing CICs as documented by their ability to be continuously propagated in vitro.
METHODS
Cell Lines, Primary Cell Suspensions, and Materials
The history, characteristics, and growth media of all the human prostate cancer lines used in this study are as reported previously [8][20–23]. Primary prostate cells were isolated from patients undergoing radical prostatectomy at our institution according to an Institutional Review Board approved protocol. Briefly, 18-gauge biopsy needle cores (C. R. Bard, Inc., Tempe, AZ) of prostate tissue were obtained and a portion of the cores is fixed, paraffin embedded, sectioned and H&E stained and the stained slides read by Dr. Angelo De Marzo for pathological confirmation of cancer and for determination of its Gleason Score. The remaining portion of the cores is initially digested overnight at 37°C in collagenase solution [0.28% collagenase I (Sigma–Aldrich, St. Louis, MO), 1% DNase I (Sigma), 10% FCS, 1× antibiotic/antimycotic (Life Technologies-Invitrogen), in RPMI 1640] and then the mixture centrifuged at 250 × g. The pellet is then re-suspended in PBS containing dithiothreitol (DTT, 1 mmol/L) and then incubated for 30 min at 37°C (i.e., this step is to reduce the disulfide bonds of extra-cellular portion of E-cadherin proteins preventing its homotypic binding between epithelial cells). The mixture is then centrifuged, and the pellet re-suspended in PBS containing 0.25% trypsin/1 mM EDTA for 30 min at 37°C. The trypsin is neutralized with 10% FCS, and the cells washed twice in PBS. Cells are subsequently passed through a cell strainer to ensure a single-cell suspension (BD Falcon, NJ). Ten biopsy cores yield 2–4 million single cells with >90% cell viability as scored by try-pan blue exclusion. 500,000 viable cells were plate into standard tissue culture plastic dishes with either; (i) RPMI-1640 plus 10% FBS (HyClone, Logan, UT) containing 0.1 nM R1881 [i.e., non-metabolized synthetic androgen obtained from Perkin-Elmer (Boston, MA)] or (ii) 1:1 mixture of Dulbecco’s Modified Earle’s Media/F12 media (i.e., DMEM/F12) containing 1 mmM Ca2+ supplemented with 500 ng/ml of bovine pituitary extract (BPE), 20 ng/ml of epidermal growth factor (EGF), and B27 [i.e., which contains insulin, tri-iodothyronine, corticosterone, retinol, alpha-tocopherol, transferrin, linoleic acid, linolenic acid, selenite, putrescine, carnitine, glutathione, ethanol-amine, galactose, catalase, albumin, and super oxide dismutase] (i.e., all from Invitrogen, Carlsbad, CA). Where indicated, cells which attached using serum free high Ca2+ DMEM/F12 media with DGFs were replated into low (i.e., <100 μM) Ca2+ serum free keratinocyte-media containing defined growth factors as described previously [5].
In Vivo Tumor Assays
All animal studies were performed according to animal protocol MO09M434 approved by the Johns Hopkins Animal Care and Use Committee specifically for this study. In vivo growth assays were performed as previously described [22]. For subcutaneous injections, one million cells in 100 μl of 80% Matrigel (BD Biosciences, Sparks, MD) were injected into the flanks of male NOG-SCID mice which were implanted in the flank with a 1 cm silastic capsule filled with testosterone as described previously [23]. This size implant maintains serum testosterone of 2–3 ng/ml [23]. For CWR22 and PC-82 xenograft inoculations, 20 mg of tissue was injected per mouse. Surgical castration was done as previously described [23]. Passage of tumor tissue was conducted by mincing the tumor tissue, passing it through a sterile tissue strainer, washing it in PBS, and re-injecting 50 mg of tumor in 100 μl of 80% Matrigel.
Cytochemical Staining
Immunocytochemistry for AR, p63, smooth muscle actin, and vimentin was preformed as described previously [5]. Senescence associated β-galactosidase staining was performed as described previously [24]. Immunocytochemistry for alpha methyl Co-A racemase (i.e., AMCAR) was performed as described previously [25].
RESULTS
Tissue Dissociation Protocol Liberates Proliferation Competent Localized Prostate Cancer Initiating Cells Plus an Admixture of Normal Cells
Prostate tissue obtained under an IRB approved protocol was harvested from hormonally naïve patients undergoing prostatectomy for localized prostate cancer. Such tissue harvest was performed under sterile conditions and multiple cores from the area of cancer taken using a biopsy needle to increase overall cancer cell yield. A portion of the tissue cores was processed for pathological and immune-cytochemical analyses to document the extent of cancer and allow Gleason grading. The remaining tissue was dissociated into single cells using collagenase digestion, DTT exposure, and then treatment with trypsin/EDTA. Using these methods, 2–4 million viable cells are routinely harvested from 10 biopsy punches. An aliquot of these cells was used to determine viability via trypan blue exclusion and to produce cytospin slides which are then formalin fixed and immunocytochemically stained for detection of p63 and alpha methyl Co-A racemase (i.e., AMCAR). AMCAR was used because it is highly detectable only in prostate cancer cells which do not express p63 but not in normal prostate epithelial, mesenchymal, or infiltrating host cells [25]. Based upon trypan blue exclusion and lack of p63 expression coupled with positive AMCAR staining (Fig. 1) more than 80% of the total dissociated cells were viable and 20–90% were prostate cancer cell. The remaining fraction was composed of a variable admixture of normal prostate epithelial and mesenchymal cells.
Fig. 1.
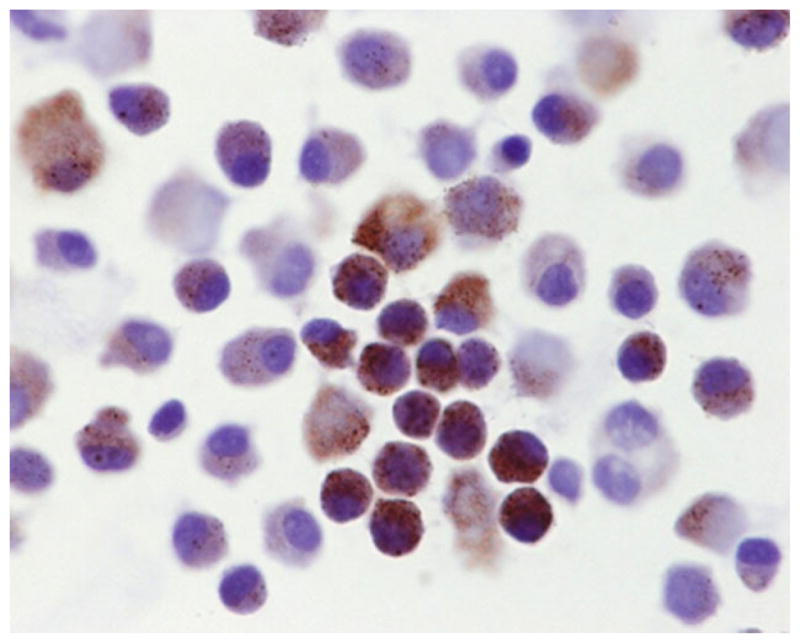
Alpha methyl Co-A racemase immunocytochemical staining of a representive cytospin from a dissociated single cell suspension from a Gleason 7 localized prostate cancer.[Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/pros]
To document that this cell dissociation protocol liberates not only viable but proliferation competent prostate CICs, dissociated single cells from seven independent patients with varying Gleason scores from 6 to 9 were mixed with Matrigel. One hundred microliters of each of these preparations containing one million cells was inoculated into three triple (i.e., T, B, and NK cell) immune-deficient adult NOG male mice which were implanted subcutaneously with a testosterone filled silastic capsule to maintain the serum testosterone at a level comparable to that in an intact adult human male [i.e., 3–10 ng/ml] [26]. This latter point is critical since serum testosterone in the un-supplemented intact adult male NOG mice varies diurnally from 0.1 to 1 ng/ml (Sedelaar and Isaacs, unpublished data) which is a range only slightly higher than the serum testosterone (i.e., 0.2 ng/ml) present in an androgen ablated human male [26]. None of the animals inoculated with any of these primary prostate cancers developed macroscopically detectable tumors within 6 months. However, in animals inoculated with dissociated cells from Gleason score 7 or higher primary cancers, these prostate cancer cells did “take” and survive as documented by histological analysis of the Matrigel plug. Such histological analysis documented the presence of viable cancer cells (i.e., AR and PSA positive, but p63 negative), which were surviving and proliferating (i.e., Ki67 positive) in NOG mice inoculated with Gleason score 7 or higher cells (Fig. 2). Interestingly, the growth fraction (i.e., determined as the percent of cancer cells Ki67 positive) was between 2% and 5% in these non-palpable lesions, which is very similar to the Ki67 index of primary prostate cancer determined on prostatectomy tissue directly from patients [27]. The lack of detection of macroscopically detectable tumor growth is explainable if within these histological lesions, the cancer cell death rate is close to that of its proliferation, such that net growth (i.e., tumor doubling time) is longer than 1 months. This is because the starting volume of the one million cells inoculated is only 1 μl and thus it takes at least seven population doublings (i.e., >7 months) for these cancer cells to grow to a size (i.e., >100 μl) larger than the original volume of the Matrigel plug.
Fig. 2.
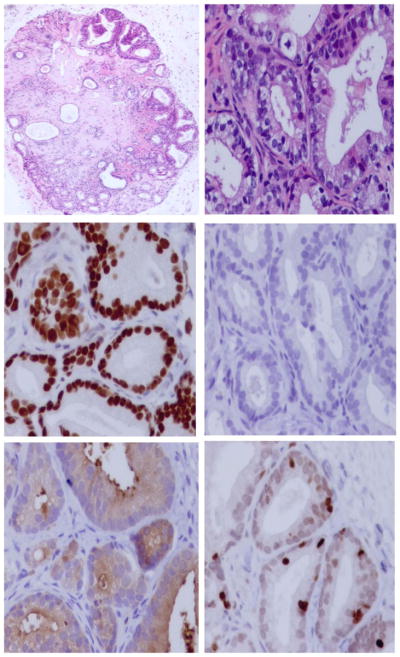
H&E histology (upper panels), AR (middle-left panel), p63 (middle-right panel), PSA (lower-left panel), and Ki67 (lower-right panel) immunocytochemical staining of xenograft tissue at 4 month post inoculation of a Gleason 7 human prostate cancer. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/pros]
These results document that the protocol used dissociates Gleason score 7 or higher primary prostate cancer cells into a single cell suspension containing CICs as determined by their take and proliferation when inoculated into testosterone supplemented NOG male mice. The characteristically slow net growth rate of these inoculated cells, however, makes using these xenograft tumors for experimental studies extremely difficult.
Culturing of Unfractionated Localized Prostate Cancer Cell Suspensions in Serum Containing Media Results in Over growth of Normal Mesenchymal Cells
Based upon this validation of the dissociation protocol and the fact that all of the established human prostate cancer cell lines containing CICs were established and are maintained in media supplemented with fetal bovine serum [i.e., FBS] [20], the dissociated admixture of normal and malignant single cell was resuspended in RPMI-1640 media containing 10% FBS and placed in standard tissue culture plastic flasks. Over the first several days in such cultures, cancer cells as well as normal prostate epithelial and mesenchymal cells attach to the flasks. Within 1–2 weeks, these primary cultures become confluent. Representative flasks containing such confluent cultures were fixed and immunocytochemically stained for expression of markers of normal epithelial (i.e., p63+) versus normal mesenchymal cells (i.e., vimentin+) versus prostate cancer cells (i.e., AMCAR+). These results document that these culture are composed of islands of malignant cells surrounded by normal epithelial cells and mesenchymal cells. When unfixed confluent primary cultures containing this admixture of cell types are treated with Trypsin/EDTA and the resulting single cell mixture replated into standard tissue culture plastic flasks with RPMI-1640 media containing 10% FBS, the mesenchymal cells rapidly attach, grow, and become confluent over the next week (Fig. 3-upper panel). By this second passage, these cultures are composed of >95% mesenchymal cells based upon the cells being vimentin positive, and p63/AMCAR negative. Such pure mesenchymal cultures can be serially propagated for 10–12 passages in this RPMI-1640-10% FBS containing media before undergoing cellular senescence as determined by expression of senescence associated β-galactosidase.
Fig. 3.
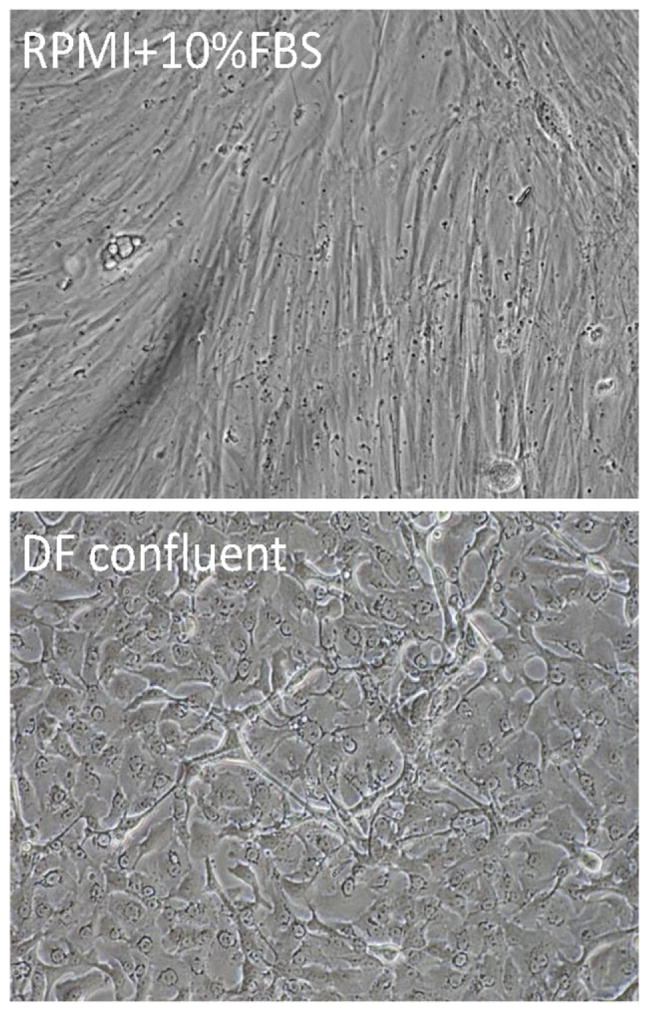
Morphology of dissociated single cells from radical prostatectomy specimen growing in RPMI-1640 plus 10% FBS (upper panel) versus cells growing in serum free high calcium DF media plus defined growth factors (lower panel).
Since the tissue used to initiate these cultures were all obtained from hormonally naïve adult male patients and since RPMI-1640 media containing 10% FBS only supplies androgen at the level of a castrated male [26], this raised the issue of whether the inability to propagate proliferation competent cancer cells is due to an insufficient level of androgen. Therefore, 0.1 nM R1881, which is a synthetic non-metabolized androgen, was added to the initial dissociation media to raise its androgen level to that of an intact male and 0.1 nM R1881 added to the RPMI-1640 plus 10% FBS culture media to test whether this would stimulate sufficient growth of the malignant cells to prevent mesenchymal cells overgrowth. Unfortunately, adding androgen to the culture media did not prevent mesenchymal cells overgrowth.
Established Human Prostate Cancer Cell Lines Characteristically Form Prostaspheres With Self-Renewal Capacity in High Ca2+/SFD-Media
One approach to prevent overgrowth of the CICs by either normal prostatic epithelial and/or mesenchymal cells is to fractionate these cells in primary culture based upon their differential ability to adhere to standard tissue culture plastic when plated in high Ca2+/SFD-media. This is based upon the demonstration that CICs from human LNCaP, PC-3, and Du145 prostate cancer cell lines and early passage chemotherapy/radiation recurrent prostate cancer strains form prostaspheres when plated onto standard tissue culture plastic flasks in high Ca2+/SFD-media [9–11]. Since these previously tested cell lines/strains were derived from advanced stages cancers, this raises the issue of whether prostasphere formation also occurs with CICs from localized cancers. Therefore, five additional advanced stage and two localized human prostate cancer cell lines were tested for their response to exposure to high Ca2+/SFD-media [i.e., DMEM/F12 media containing BPE/EGF/B27 supplement] plus androgen (i.e., 0.1 nM R1881). These lines were chosen because: (i) they have variable phenotypic and genetic characteristics [20–22] and (ii) all contain CICs as documented by their tumorigenic ability when xenografted into mice [21,22,27]. As a control for comparison, the LNCaP, PC-3, and DU145 were also tested. For all of the new lines tested, approximately 1 in 1,000 cells developed prostaspheres when plated in high Ca2+/SFD-media plus androgen on standard tissue culture flasks, as did the LNCaP and DU145 (Table I). By 2 weeks, these non-adherent prostaspheres contained between 50 and 100 cells which is consistent with a average doubling time of about 48 hr, which is essentially identical to the doubling time for these human prostate cancer cell lines growing as adherent cultures in standard serum containing media [28]. Only PC-3 did not form prostaspheres (Table I). Instead PC-3 cells attached to the flasks. This may be significant since all the other lines which formed non-adherent prostaspheres are characteristically adenocarcinomas while PC-3 is characteristic a small cell neuroendocrine carcinoma [29].
TABLE I.
Characteristics of Human Prostate Cancer Cell Lines Tested for Ability to Form Prostaspheres in High Calcium/SFD-Media
| Human prostate cancer cell lines | Cell line site of origin | Characteristic
|
Initial formation of prostasphere | Serial propagation of prostasphere | ||
|---|---|---|---|---|---|---|
| Androgen receptor | PSA | Androgen growth response | ||||
| DU-145 | Brain | − | − | − | + | + |
| LNCaP | Lymph node | + (Mutated) | + | Sensitive | + | + |
| C4-2B | Lymph node | + (Mutated) | + | Sensitive | + | + |
| LAPC-4 | Lymph node | + | + | Sensitive | + | + |
| PacMetUTI | Lymph node | + | − | − | + | + |
| PC-3 | Bone | − | − | − | − | − |
| MDA-PC-2B | Bone | + (Mutated) | + | Sensitive | + | + |
| VCap | Bone | + | + | Sensitive | + | − |
| CWR22Rv1 | Primary | + (Mutated) | + | Sensitive | + | + |
| E006AA | Primary | + (Mutated) | − | − | + | + |
When these non-adherent prostaspheres (i.e., termed p1-prostaspheres) were harvested, enzymatically digested into single cells, and replated, they regenerated non-adherent prostaspheres in secondary culture (Fig. 4) in all of the adenocarcinomas lines, except VCap (Table I). In fact, single cells dissociated from previous prostaspheres regenerated prostasphere formation for more than five serial passages, documenting their self-renewal capacity. When single cells dissociated from these p5-prostaspheres were replated in RPMI-1640 media containing 10% FBS, the cells attached to the standard tissue culture plastic flasks. These attached cells could be serially propagated in the RPMI-1640 media containing 10% FBS. When such attached cells were harvested and subsequently inoculated into triple immune (i.e., T, B, and NK cell) deficient NOG mice, all of the lines produced growing lethal tumors. These combined results document that when placed in SFD-media with androgen, CICs from established human prostate cancer cell lines derived from all clinical stages characteristically produce prostaspheres which can be serially propagated without their loss of self-renewal in vitro or tumorigenic ability in vivo.
Fig. 4.
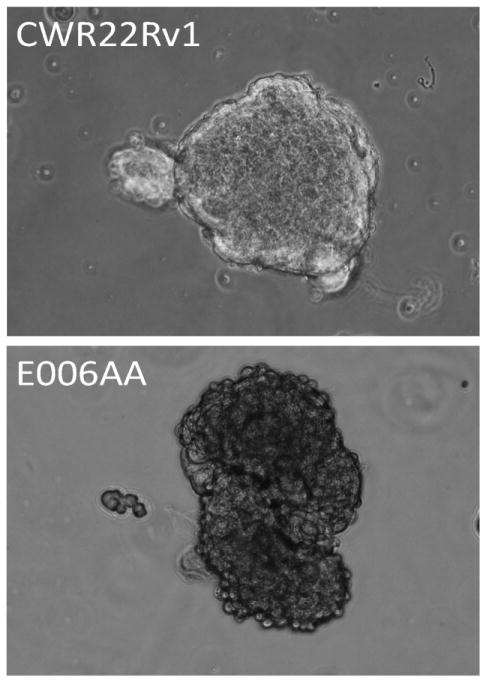
Morphology of prostaspheres formed by CWR22Rv1 cells (upper panel) and E006AA cells (lower panel) in serum free high calcium DF-media containing defined growth factors and androgen.
Dissociated Cells From a Subset of Localized Prostate Cancers Form Prostaspheres With Only Limited Self-Renewal Capacity in Primary Culture in High Ca2+/SFD-Media Containing Androgen
Based upon the positive previous results with the established human prostate cancer cell lines, dissociated single cells from a total of 72 Gleason score 6–9 primary prostate cancer specimens were plated in high Ca2+/SFD-media containing 0.1 nM R1881 into standard tissue culture flasks. Androgen was added since these cells were derived from hormonally naïve patients. Within the first several days, a subset of cells attached to the plastic surface in all 72 primary cultures. Morphologically, the attached cells were of two major types. The first type of cells were small and more rounded in morphology with the characteristics of normal human basal epithelial cells [5,8] while the second type of attached cell was elongated with a fibroblastoid morphology. Over the first 2 weeks in primary culture, only the attached cells with epithelial morphology grew into discreet adherent colonies. During this same 2 week period, cells from 43% of the localized prostate cancer patients (i.e., 31 out of 72) formed unattached prostaspheres in primary culture, with the proportion being more than twofold greater for Gleason Score 8 and higher cancers (Table II). For the positive samples, approximately one out of 20,000 cancer cells formed prostaspheres (Fig. 5-upper panels). These prostaspheres are composed of cancer cells and not normal prostate epithelial or mesenchymal cells as documented by their lack of expression of p63 (Fig. 5-lower panel) smooth muscle actin, or vimentin (data not shown).
TABLE II.
Percentage of Dissociated Single Cells From Radical Prostatectomy Samples Which Formed Prostaspheres in High Calcium/SFD-Media Based Upon Gleason Score
| Gleason score of sample | 6 | 7 | 8 | 9 |
| Number of samples | 19 | 21 | 17 | 15 |
| Number forming spheroids | 4 (20%) | 6 (29%) | 11 (65%) | 10 (67%) |
| Racemase statining | 20–90% Positve staining | |||
Fig. 5.
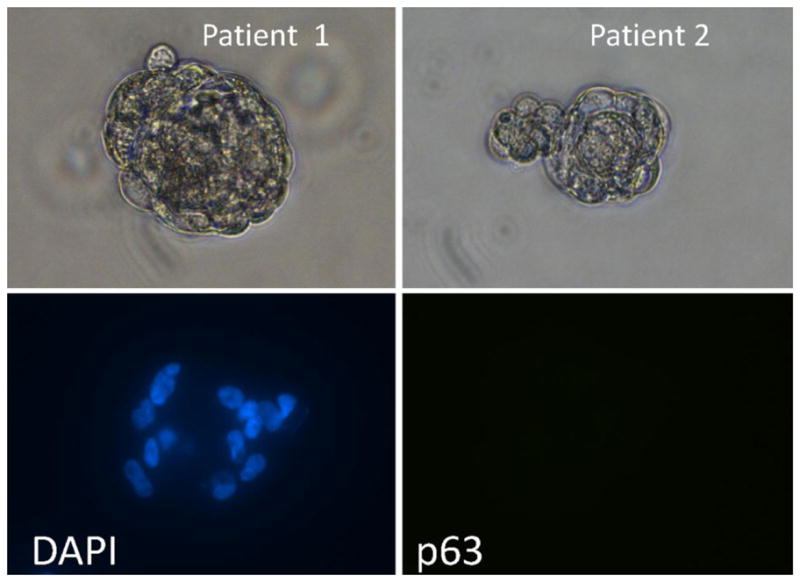
Morphology of prostaspheres (upper panels) formed in primary culture of dissociated single cells from two different radical prostatectomy specimens in serum free high calcium DF-media containing defined growth factor plus androgen. Prostaspheres were dual stained with DAPI (i.e., DNA stain) to identify cell nuclei (lower-left panel) and rabbit anti-p63 antibody/alexa red labeled anti-rabbit antibody to detect p63 expression (lower-right panel). [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/pros]
After 2 weeks, the media was harvested and in the 43% of samples which produced prostaspheres, these were isolated from the cells attached to the flask. The attached cells were trypsinized, and an aliquot replated in high Ca2+/SFD-media containing 0.1 nM R1881 into standard tissue culture flasks. When these p-2 attached cells grew to confluence (Fig. 3-lower panel) they were analyzed for AR, p63, and AMCAR expression. From all of the samples even if they did not produce prostaspheres, these p-2 attached cells were >90% p63 positive but AR and AMCAR negative documenting that they are normal basal epithelial cells. This conclusion is also supported by the fact that these p-2 attached normal basal cells could be passaged only once more at a 1:4 split ratio when maintained in high Ca2+/SFD-media containing 0.1 nM R1881 into standard tissue culture flasks, but 8–10 times, if shifted to a low (i.e., <100 μM) Ca2+/SFD-media (i.e., serum free keratinocyte media plus added DGF) even without added androgen. Such extensive growth before senescing when placed in low Ca2+/SFD-media is characteristic of normal human prostate basal cells since in this low Ca2+ media, Notch-1 is constitutively active while E-cadherin is inactivated preventing contact inhibition [30].
With regard to the cancer p-1 prostaspheres initially isolated after 2 weeks of primary culture, these were dissociated into single cells and replated in high Ca2+/SFD-media containing 0.1 nM R1881 into standard tissue culture plastic flasks. Dissociated single cells regenerated unattached growing prostaspheres in such secondary cultures in 10 of 31 (1:3 of patient samples which formed prostaspheres in initial culture or 13% of all patient samples tested). The p2-prostaspheres from four patients with Gleason 9 cancers were dissociated into single cells and these single cells were re-plated in standard tissue culture plastic flasks containing either in high Ca2+/SFD-media containing 0.1 nM R1881 or growth factor defined (GFD) media containing 10% FBS plus androgen. In the high Ca2+/SFD-media containing 0.1 nM R1881, these single cells regenerated unattached p3-prostaspheres. In contrast in the GFD media containing 10% FBS plus androgen, single cells from p-2 prostaspheres attached to the culture dish and initially grew. When these attached p-3 cells were trypsinized and re-plated in GFD-media containing 10% FBS plus androgen, they did not attach nor form prostaspheres. Likewise, when dissociated cells from the p3-prostaspheres were re-plated in high Ca2+/SFD-media containing 0.1 nM R1881, they did not regenerate p4 prostaspheres. These results document that the cells in primary prostaspheres derived from localized prostate cancers have only a limited self-renewal capacity (i.e., they are not CICs).
Dissociated Cells From Prostate Cancers Xenografts Also Form Prostaspheres in Primary Culture in High Ca2+/SFD-Media Containing Androgen With Only Limited Self-Renewal Capacity
It is unexpected that when dissociated cancer cells from 72 localized prostate cancers were tested, none formed prostaspheres in primary culture containing self-renewal CICs. This raises the issue of whether this is due to the fact that none of these cancers contained CICs and thus are “incidental” cancers with no lethal potential. Along these lines, in one screening study, 48 men had to be treated to save one man with locally detected prostate cancer [31]. On the other hand, in appropriately one-third of radical prostatectomized patients, the cancer recurs documenting its tumorigenic behavior [32]. Combining these clinical data suggests that in at least a subset (i.e., 1:3) of the localized prostate cancers tested, CICs are present but are not incorporated into prostaspheres using the high Ca2+/SFD-media protocol. To resolve this possibility, PC-82 and CWR-22 human prostate cancer xenografts derived from localized prostate cancer from two independent hormonally naïve patients were used as the test tissue specimens. These cancers are not established cell lines but are serially passageable as tumorigenic xenografts in mice documenting that they contain self-renewing tumorigenic CICs. Both lines are androgen responsive, growing when initially inoculated into androgenized, but not castrated adult male mice [33,34].
Enzymatically dissociated single cells from these PC-82 and CWR-22 xenografts were separately plated in standard tissue culture plastic flasks with high Ca2+/SFD-media containing 0.1 nM R188. Androgen was added since the xenografts are highly androgen dependent for their take and subsequent growth in vivo. In this serum free-androgen containing media, one in 10,000 of unattached malignant cells formed prostaspheres. After 2 weeks in culture, these primary p-1 prostaspheres (Fig. 6-upper panels) were isolated, dissociated, and replated in serum free-androgen containing media where they again regenerated unattached p-2 prostaspheres. These p-2 prostaspheres were dissociated into single cells and replated in serum containing media to allow the attachment of the cells. The results of these studies documented that in the presence of serum, the cells attached and grew (Fig. 6-lower panels). These adherent cells were passaged and cells from each of these passage two adherent cultures were co-inoculated with Matrigel into androgen supplemented immune-deficient NOG male mice, but no palpable cancers developed even when followed for more than a year. At that time, histological examination of the residual Matrigel plug did not document even microscopically detectable prostate cancer cells surviving. This in vivo lack of tumorigenicity by the p-2 adherent cells was likewise coupled with an inability to serial passage these cells in vitro (i.e., cancer cells attached to the plastic in the third passage but the attached cells did not proliferate). These results documented that primary prostaspheres lack self-renewal capacity and thus do not contain CICs even when starting with a single cell suspension documented to contain CICs based upon their ability to produce lethally growing cancers when xenografted in mice.
Fig. 6.
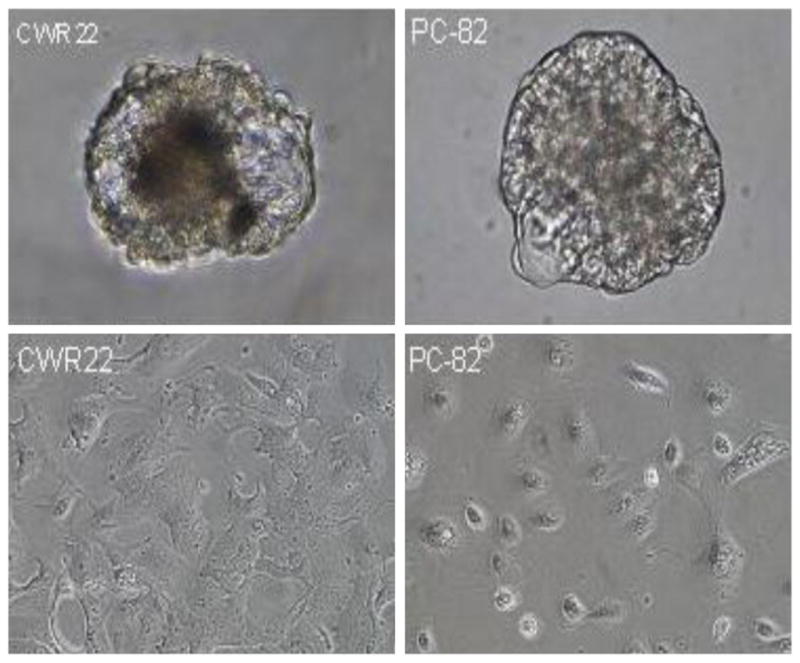
Morphology of prostaspheres formed when dissociated single cell from CWR22 (upper-left panel) and PC-82 (upper-right panel) xenograft tissue was plated into serum free high calcium DF-media containing defined growth factors plus androgen. Morphology of CWR22 (lower-left panel) and PC-82 (lower right panel) which attached to flask when single cells were dissociated from prostaspheres and replated in serum containing media. [Color figure can be seen in the online version of this article, available at http://wileyonlinelibrary.com/journal/pros]
DISCUSSION
Despite large numbers of tissue specimens processed (i.e., >several hundred) by several dozen independent groups over the last 30 years, human prostate cancer cell lines have been established from only two primary prostate cancer patients (i.e., CWR-22 and E006AA), with the remaining established lines being derived from metastatic sites from <10 patients. All of the presently available established human prostate cancer cell lines were originally established with and are maintained in 10% FBS containing medium whose Ca2+ is between 650 and 1,860 μmol/L [5,20–22]. When such 10% FBS containing media is used to culture dissociated single cells from primary human prostate cancer specimens, there is a rapid overgrowth of normal mesenchymal cells which nearly universally prevents successful establishments of new cell lines. This suggests that the cancer cell lines which have been established are significant “outliers” and may not be representative of the prostate adenocarcinomas seen clinically. Thus, there is an urgent need to develop new approaches to achieve the “routine” establishment of CIC containing lines, particularly derived from primary prostate cancers.
One approach that has become popular for culturing dissociated single cells from human prostate cancer tissue is to use a serum free-growth factor defined (SFG)-media containing a low (i.e., <260 μmol/L) level of calcium since such media prevents growth of mesenchymal cells [5,30,35,36]. When single cell suspensions of malignant human prostate tissue are culturing in such low Ca2+/SFD-medium, however, only normal basal epithelial cells grow producing adherent basal cell cultures which can be serially passaged for 8–10 times before undergoing proliferative senescence [5,30,35,36]. In contrast, this low Ca2+/SFD-medium prevents adherence to the tissue culture surface of prostate cancer cells and thus these cells undergo anoikis [5,30]. Importantly, however, if under such serum free conditions the Ca2+ level is high enough (i.e., >1 mM), CICs from human prostate cancer cell lines and from early passage cultures derived from chemotherapy/radiation recurrent prostate patients do not attach when plated onto standard tissue culture plastic flasks, but instead self-associate in suspension and grow as unattached prostaspheres [9–11]. The presence of CICs in these prostaspheres is documented by their self-renewal capacity in vitro and their tumorigenicity in vivo [9–11].
In the present studies, we confirmed that in such serum free, high Ca2+ (i.e., DMEN/F12) GFD media plus androgen, a large series of human prostate cancer cell lines established from both localized and metastatic sites characteristically form prostaspheres which contain CICs based upon their self-renewal in vitro and tumorigenicity in vivo. Unfortunately, however, while dissociated single cells from human primary prostate cancer tissues are viable, contain CICs as documented by their ability to take and proliferate as xenografts, and produce prostaspheres when plated onto standard tissue culture flask in serum free, high Ca2+/GFD-media plus androgen, these prostasphere do not contain CICs. Thus, the development of reproducibly methods to culture CICs isolated directly from localized cancers is still an urgent unmeet need of the prostate cancer research community.
Acknowledgments
We wish to acknowledge the expert technical assistance of Susan Dalrymple, Lizamma Antony, and Georges H. Ndikuyeze in performing these experiments and Lillian Dasko-Vincent of the Comprehensive Cancer Center Cell Imaging Core Facility of the Johns Hopkins School of Medicine, and Lee Blosser and Ada Tam of Comprehensive Cancer Center Flow Sorting Facility of the Johns Hopkins School of Medicine for their assistance. We also wish to thank Dr. Angelo DeMarzo, Department of Pathology-The Johns Hopkins School of Medicine and Helen Fedor and the Brady Urological Institute Prostate Specimen Repository supported by the Prostate SPORE grant for Tissue Microarrays and Jessica Hicks of the Comprehensive Cancer Center Histology Core of the Johns Hopkins School of Medicine for immunocytochemical staining.
Grant sponsor: The Maryland Stem Cell Research Fund; Grant number: MSCRFII-0428-00.
Abbreviations
- AR
androgen receptor
- DHT
dihydrotestosterone
- FBS
fetal bovine serum
- PSA
prostate specific antigen
- SFD-media
serum free-growth factor defined media
References
- 1.Isaacs JT. Prostate stem cells and benigh prostatic hyperplasia. Prostate. 2008;68:1025–1034. doi: 10.1002/pros.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldstein AS, Stoyanova T, Witte ON. Primative origins of prostate cancer: In vivo evidence for prostate-regenerating cells and prostate cancer-initiating cells. Mol Oncol. 2010;4:385–396. doi: 10.1016/j.molonc.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litvinov IV, De Marzo AM, Isaacs JT. Is the Achilles’ heel for prostate cancer therapy a gain of function in androgen receptor signaling? J Clin Endocrinol Metab. 2003;88:2972–2982. doi: 10.1210/jc.2002-022038. [DOI] [PubMed] [Google Scholar]
- 4.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 5.Litvinov IV, Vander Griend DJ, Xu Y, Antony L, Dalrymple SL, Isaacs JT. Low-calcium serum-free media selects for growth of normal prostate epithelial stem cells. Cancer Res. 2006;66:8598–8607. doi: 10.1158/0008-5472.CAN-06-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gu G, Yuan J, Wills M, Kasper S. Prostate cancer cells with stem cell characteristics reconstitute the original human tumor in vivo. Cancer Res. 2007;67:4807–4815. doi: 10.1158/0008-5472.CAN-06-4608. [DOI] [PubMed] [Google Scholar]
- 7.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: The CD44 + alpha2beta1 + cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 8.Vander Griend DJ, Karthaus WL, Dalrymple S, Meeker A, DeMarzo AM, Isaacs JT. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008;68:9703–9711. doi: 10.1158/0008-5472.CAN-08-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei C, Guomin W, Yujun L, Ruizhe Q. Cancer stem-like cells in human prostate carcimoma cells DU145-the seeds of the cell line? Cancer Biol Ther. 2007;6:e1–e6. doi: 10.4161/cbt.6.5.3996. [DOI] [PubMed] [Google Scholar]
- 10.Hurt EM, Kawaski BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24− prostate cells are early cancer progenitor/stem cells that provide a model for patients with pooor prognosis. Br J Cancer. 2008;98:756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duhagon MA, Hurt EM, Sotelo-Silveira JR, Zhang X, Farrar WL. Genomic profiling of tumor initiating prostaspheres. BMC Genomics. 2010;11:324. doi: 10.1186/1471-2164-11-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haffner MC, Aryee MJ, Toubaji A, Esopi DM, Albadine R, Gurel B, Isaacs WB, Bova GS, Liu W, Xu J, Meeker AK, Netto G, De Marzo AM, Nelson WG, Yegnasubramanian S. Androgen-induced TOP2B mediated double strand breaks and prostate cancer gene rearrangement. Nat Genet. 2010;42:668–675. doi: 10.1038/ng.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Litvinov IV, Vander Griend DJ, Antony L, Dalrymple S, De Marzo AM, Drake CG, Isaacs JT. Androgen receptor as a licensing factor for DNA replication in androgen-sensitive prostate cancer cells. Proc Natl Acad Sci USA. 2006;103:15085–15090. doi: 10.1073/pnas.0603057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vander Griend DJ, Litvinov IV, Isaacs JT. Stabilizing androgen receptor in mitosis inhibits prostate cancer proliferation. Cell Cycle. 2007;6:647–651. doi: 10.4161/cc.6.6.4028. [DOI] [PubMed] [Google Scholar]
- 15.deBono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB, Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI COU-AA-301 Investigators. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 17.Isaacs JT, Isaacs WB. Androgen receptor outwits prostate cancer drugs. Nat Med. 2004;10:26–27. doi: 10.1038/nm0104-26. [DOI] [PubMed] [Google Scholar]
- 18.Edick MJ, Tesfay L, Lamb LE, Knudsen BS, Miranti CK. Inhibition of integrin-mediated crosstalk with epidermal growth factor receptor/Erk or Src signaling pathways in autophagic prostate epithelial cells induces caspase-independent death. Mol Biol Cell. 2007;18:2481–2490. doi: 10.1091/mbc.E06-04-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peehl DM, Sellers RG, Wong ST. Defined medium for normal adult human prostatic stromal cells. In Vitro Cell Dev Biol Animal. 1998;34:555–560. doi: 10.1007/s11626-998-0115-9. [DOI] [PubMed] [Google Scholar]
- 20.van Bokhoven A, Varella-Garcia M, Korch C, Johannes WU, Smith EE, Miller HL, Nordeen SK, Miller GJ, Lucia MS. Molecular characteristization of human prostate carcinoma cell lines. Prostate. 2003;57:205–225. doi: 10.1002/pros.10290. [DOI] [PubMed] [Google Scholar]
- 21.Troyer DA, Tang Y, Bedolla R, Adhvaryu SG, Thompson IM, Abboud-Werner S, Sun LZ, Friedrichs WE, deGraffenried LA. Characterization of PacMetUT1, a recently isolated human prostate cancer cell line. Prostate. 2008;68:883–892. doi: 10.1002/pros.20758. [DOI] [PubMed] [Google Scholar]
- 22.D’Antonio JM, Vander Griend DJ, Antony L, Ndikuyeze G, Dalrymple SL, Koochekpour S, Isaacs JT. Loss of androgen receptor dependent growth suppression by prostate cancer cells can occur independently from acquiring oncogenic addition to androgen receptor signaling. PLoS One. 2010;5:e11475. doi: 10.1371/journal.pone.0011475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao J, Isaacs JT. Development of an androgen receptor-null model for identifying the initiation site for androgen stimulation of proliferation and suppression of programmed (apoptotic) death of PC-82 human prostate cancer cells. Cancer Res. 1998;58:3299–3306. [PubMed] [Google Scholar]
- 24.Jarrard DF, Sarkar S, Shi Y, Yeager TR, Magrane G, Kinoshita H, Nassif N, Meisner L, Newton MA, Waldman FM, Reznikoff CA. p16/pRb pathway alterations are required for bypassing senescence in human prostate epithelial cells. Cancer Res. 1999;59:2957–2964. [PubMed] [Google Scholar]
- 25.Luo J, Zha S, Gage WR, Dunn TA, Hicks JL, Bennett CJ, Ewing CM, Platz EA, Ferdinandusse S, Wanders RJ, Trent JM, Isaacs WB, De Marzo AM. Alpha-methylacyl-CoA racemase: A new molecular marker for prostate cancer. Cancer Res. 2002;62:2220–2226. [PubMed] [Google Scholar]
- 26.Sedelaar JP, Isaacs JT. Tissue culture media supplemented with 10% fetal calf serum contains a castrate level of testosterone. Prostate. 2009;69:1724–1729. doi: 10.1002/pros.21028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pienta KJ, Abate-Schen C, Agus DB, Attar RM, Chung LW, Greenberg NM, Hahn WC, Isaacs JT, Navone NM, Peehl DM, Simons JW, Solit DB, Soule HR, VanDyke TA, Weber MJ, Wu L, Vessella RL. The current state of preclinical prostate cancer animal models. Prostate. 2008;68:629–639. doi: 10.1002/pros.20726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berges RR, Vukanovic J, Epstein JI, CarMichel M, Cisek L, Johnson DE, Veltri RW, Walsh PC, Isaacs JT. Implication of cell kinetic changes during the progression of human prostatic cancer. Clin Cancer Res. 1995;1:473–480. [PMC free article] [PubMed] [Google Scholar]
- 29.Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang CZ, Huang J. PC3 is a cell line characteristic of prostate small cell carcinoma. Prostate. 2011;71:1668–1679. doi: 10.1002/pros.21383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalrymple S, Antony L, Xu Y, Uzgare AR, Arnold JT, Savaugeot J, Sokoll LJ, De Marzo AM, Isaacs JT. Role of notch-1 and E-cadherin in the differential response to calcium in culturing normal vs. malignant prostate cells. Cancer Res. 2005;65:9269–9279. doi: 10.1158/0008-5472.CAN-04-3989. [DOI] [PubMed] [Google Scholar]
- 31.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast T, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A ERSPC Investigators. Screening and prostate cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 32.Boorjian SA, Eastham JA, Graefen M, et al. A critical analysis of the long-term impact of radical prostatecomy on cancer control and function outcomes. Eur Urol. 2011 doi: 10.1016/j.eururo.2011.11.053. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Van Steenbrugge GJ, van Donger JJ, Reuvers PJ, de Jong FH, Schroeder FH. Transplantable human prostatic carcinoma (PC-82) in athymic nude mice. I. Hormone dependence and the concentration of androgens in plasma and tumor tissue. Prostate. 1987;11:195–210. doi: 10.1002/pros.2990110210. [DOI] [PubMed] [Google Scholar]
- 34.Nagabhushan M, Miller CM, Pretlow TP, Giaconia JM, Edgehouse NL, Schwartz S, Kung HJ, de Vere White RW, Gumerlock PH, Resnick MI, Amini SB, Pretlow TG. CWR22: The first human prostate cancer xenograft with strongly androgen dependent and relapsed strains both in vivo and in soft agar. Cancer Res. 1996;56:3024–3026. [PubMed] [Google Scholar]
- 35.Peehl DM, Stamey TA. Serum-free growth of adult human prostatic epithelial cells. In Vitro Cell Dev Biol. 1986;22:82–90. doi: 10.1007/BF02623537. [DOI] [PubMed] [Google Scholar]
- 36.Garraway IP, Sun W, Tran CP, Perner S, Zhang B, Goldstein AS, Hahm SA, Haider M, Head CS, Reiter RE, Rubin MA, Witte ON. Human prostate sphere-forming cells represent a subset of basal epithelial cells capable of glandular regeneration in vivo. Prostate. 2009;70:491–501. doi: 10.1002/pros.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]