Abstract
Preclinical studies suggest that cost/benefit decision-making involves interactions between adenosine and dopamine (DA). In rats, DA depletion decreases willingness to incur effort costs, while adenosine antagonism reverses these effects, likely by increasing DA transmission. Caffeine is a non-selective adenosine antagonist commonly used to facilitate effortful tasks, and thus may affect decisions involving effort costs in humans. The current study examined acute effects of 200 mg of caffeine on willingness to exert effort for monetary rewards at varying levels of reward value and reward probability, in young adult light caffeine users. Based on previous findings with amphetamine, we predicted that caffeine would increase willingness to exert effort. At separate sessions, 23 healthy normal adults received placebo or 200 mg caffeine under counterbalanced double-blind conditions, then completed the effort expenditure for rewards task (EEfRT). Measures of subjective and cardiovascular effects were obtained at regular intervals. Caffeine produced small but significant subjective and cardiovascular effects, and sped psychomotor performance on the EEfRT. Caffeine did not alter willingness to exert effort, except in high cardiovascular responders to caffeine, in whom it decreased willingness to exert effort. These results were contrary to our predictions, but consistent with rodent studies suggesting that moderate doses of caffeine alone do not affect effort, but rather only influence effort in the context of DA antagonism. Our results demonstrate that psychomotor speeding and decisional effects on the EEfRT are dissociable, providing additional evidence for the EEfRT as a specific measure of effort-based decision-making. This study provides a starting point for exploring contributions of the adenosine system to motivation in humans.
Keywords: Caffeine, Adenosine, Dopamine, Effort-based decision-making
1. Introduction
In order to survive, organisms must choose adaptively between opportunities that vary in both reward and cost. Costs can include effort required (effort costs) and likelihood of failing to obtain the reward (probability costs). Pre-clinical studies indicate that nucleus accumbens dopamine (DA) is crucially involved in valuing effort and probability costs. In these studies, animals choose between a response that requires high effort (high effort cost) or has a high probability of failure (high probability cost) combined with a high reward (called “high cost/high reward” options, “HC/HR”) and a response that requires less effort or less uncertainty, combined with a lower reward (low cost/low reward options, “LC/LR”; summarized in Salamone et al., 2007). Drugs or lesions that decrease DA function shift the animals' preferences away from ‘high cost, high reward’ options toward ‘low cost, low reward’ options. In contrast, drugs that increase DA function, such as stimulants, generally appear to increase animals' preferences for HC/HR options, although this may depend on the dose, task design, type of cost, and individual differences (Bardgett et al., 2009; Cocker et al., 2012; Floresco et al., 2008; Simon et al., 2011; St. Onge et al., 2010). These findings suggest that DA may mediate the ability of the organism to weigh the consequences of effort versus reward.
We recently extended some of these pre-clinical findings to humans by demonstrating that a moderate dose of the DA stimulant d-amphetamine increases willingness to exert effort in healthy young adults, particularly when reward probability is low (Wardle et al., 2011). In the present study, we examined effort-based decision-making with another stimulant drug, caffeine. Caffeine has milder stimulant effects than d-amphetamine, and it exerts its effects on DA indirectly, by antagonizing adenosine receptors. Adenosine and DA receptors co-localize on medium spiny neurons in striatum and nucleus accumbens, where adenosine agonists inhibit DA transmission, and adenosine antagonists potentiate DA transmission (Ferré, 2008). Congruent with this neural mechanism, pre-clinical studies suggest that adenosine receptors interact with DA receptors to influence both the reinforcing effects of psychostimulants (Chen et al., 2000; Soria et al., 2005) and effort exerted for rewards (Salamone et al., 2010). Therefore, we examined effects of caffeine, a widely used minor stimulant and non-selective adenosine A1/A2A antagonist, on effort-based decision-making in humans.
Caffeine may be expected to increase willingness to incur effort costs, considering its widespread use to facilitate effortful tasks. Although a large exercise psychology literature indicates that caffeine decreases the perception of effort in humans (Doherty and Smith, 2005), its effects on willingness to exert effort in the preclinical literature are mixed. On one hand, adenosine A2A agonists decrease willingness to exert effort in rats, just as DA antagonists do (Font et al., 2008; Mingote et al., 2008), and A2A antagonists ameliorate the effects of DA antagonists (Salamone et al., 2010). However, A2A antagonists alone (either caffeine or the more specific A2A antagonists KW6002 and MSX-3) do not increase willingness to exert effort, even at doses that reverse DA antagonist effects (Farrar et al., 2007; Salamone et al., 2009). Thus the preclinical literature suggests that moderate doses of A2A antagonists may not increase effortful responding directly, but instead may only reverse the effects of DA depletion. Adenosine A1 antagonists do not appear to affect effort-based decision-making (Nunes et al., 2010; Salamone et al., 2009). Thus, the preclinical literature is mixed, and the human literature is limited to self-reports of ease of engaging in effortful tasks. To date, no human studies have directly tested effects of adenosine antagonists like caffeine on effort-based decision-making.
In the current study we examine the relationship between caffeine and willingness to exert effort in humans by testing the effects of 200 mg of caffeine vs. placebo on the effort expenditure for rewards task (EEfRT; Treadway et al., 2009), in healthy light caffeine users. The EEfRT is modeled on the concurrent choice paradigm of Salamone et al. (1994). Participants are presented with a series of HC/HR vs. LC/LR choices that vary in both amount of reward for the HC/HR option, and reward probability. Reward amount and probability are systematically varied to increase task sensitivity. We predicted that caffeine would increase HC/HR choices (indicating increased willingness to expend effort), similar to d-amphetamine (Wardle et al., 2011).
2. Material and methods
2.1. Study design
The EEfRT was administered to a subset of participants (N = 23) from a larger study (N = 82). The study utilized a counterbalanced, double-blind, within-subject protocol with five separate testing sessions during which participants received capsules containing placebo, 200 mg caffeine, or one of three doses of an additional test substance (not reported here). At each session participants completed the EEfRT during the expected window of peak drug effect, based on previous data on caffeine blood levels and subjective effects (Liguori et al., 1997; Mumford et al., 1996). Sessions were separated by at least 2 days but no more than 21 days.
2.2. Participants
Healthy participants (N = 23; 13 male) ages 18–35 (M = 23.4, SD = 3.4) were recruited through flyers and online advertisements as part of a larger study (N = 82). Participants completed a screening consisting of physical examination, electrocardiogram, modified Structured Clinical Interview for DSM-IV (SCID; First et al., 1996) and self-reported health and drug use history. Inclusion criteria were: Body Mass Index between 19 and 26, no medical or psychiatric contraindications, no prescription medication or herbal supplement use, not pregnant, nursing, or trying to become pregnant, not smoking in the last 6 months, alcohol consumption below 21 units/week for females or 28 units/week for males, no history of adverse reactions to caffeine, fluency in English and reported caffeine consumption of <500 mg/week, including all dietary sources. Estimated average caffeine consumption (based on self-report of types/quantities of caffeinated beverages) was 227 mg/week (SD = 139, range = 25 mg–500 mg), or around two cups of drip coffee per week. Participants were all Caucasian, as genetic analysis was a primary aim of the larger study. Two participants were missing EEfRT data from one session due to computer malfunction. Three further participants did not complete the task as directed on at least one session, and were removed from all analyses, leaving n = 18 participants with complete data for primary analyses.
Participants were instructed to refrain from recreational and over-the-counter drugs and herbal supplements 24 h before and 10 h after sessions. Compliance was verified using breath alcohol (Alcosensor III, Intoximeters Inc., St. Louis, MO) and urine tests for commonly used drugs (ToxCup, Branan Medical Corporation, Irvine, CA). Subjects were instructed to refrain from food and drinks containing caffeine (coffee, tea, chocolate, etc.) for 24 h prior to the session. Female participants were urine tested for pregnancy before each session (AimStrip, Germaine Laboratories, San Antonio, TX). Participants were informed that they might receive a stimulant/appetite suppressant, a sedative/tranquilizer, or placebo. All participants provided informed consent, and the University of Chicago Institutional Review Board approved all procedures, in accordance with the Declaration of Helsinki.
2.3. Procedure
Participants attended an orientation during which they were familiarized with procedures and practiced the EEfRT. They then completed five 5-hour individual study sessions. Here we will only report on the two sessions at which subjects received placebo and caffeine (200 mg). This caffeine dose is approximately equivalent to 2 cups of drip coffee (Barone and Roberts, 1996). In a previous study in a similar population, 150 mg caffeine was the minimum dose to produce significant subjective and cardiovascular effects, whereas a higher dose (450 mg dose) increased anxiety (Childs and de Wit, 2006). Participants consumed standardized breakfasts at 8 am in the morning of each study session. They arrived at the lab at 9:00 am, completed breath and urine tests and completed subjective and cardiovascular measures of drug effects every 30 min until noon. At 9:30 they ingested placebo capsules (part of the large study protocol not further described here), and at noon they ingested four opaque size 00 gelatin capsules containing either 200 mg caffeine or placebo. At 12:30 pm participants completed subjective and cardiovascular measures of drug effects, as well as cognitive tests not reported here. At 1:30 participants completed the EEfRT. At 1:50 pm they completed final measures of subjective and cardiovascular drug effects, and left the laboratory.
2.4. Measures
2.4.1. Subjective and cardiovascular drug effects
We measured subjective effects of caffeine using the Drug Effects Questionnaire (DEQ; Fischman and Foltin, 1991). The DEQ consists of a set of visual analog scales on which participants mark from 1 to 100 how much they like the drug's effects, dislike the drug's effects, feel the drug's effects, feel high, and want more of the drug. We focused on DEQ “Feel” scores. We also measured blood pressure using portable monitors (Life Source, A&D Company, Tokyo, Japan), with mean arterial pressure (MAP; [Systolic BP + 2* Diastolic BP] /3) as our measure of cardiovascular effects.
2.4.2. Effort expenditure for rewards task (“EEfRT”)
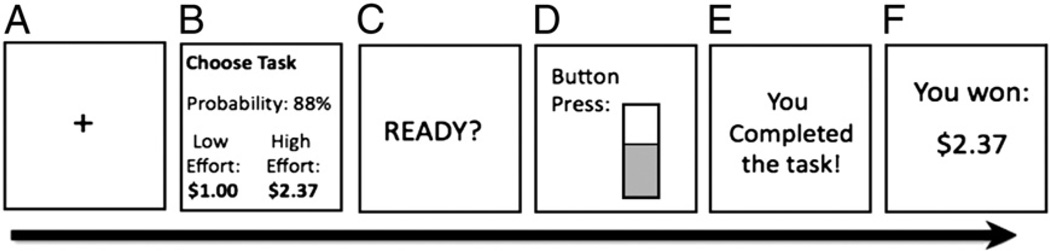
The EEfRT is amulti-trial game in which participants choose on each trial between an HC/HR and LC/LR option to obtain varying monetary rewards (Fig. 1). A detailed description has been published previously (Treadway et al., 2009). Briefly, each trial presents the subject with a choice between a ‘hard task’ (HC/HR option), requiring 100 button presses with the non-dominant pinky finger within 21 s, and an ‘easy task’ (LC/LR option), requiring 30 button presses with the dominant index finger within 7 s. For easy-task choices, subjects were eligible to win $1.00 for each successfully completed trial. For hard-task choices, subjects were eligible to win higher amounts that varied per trial within a range of $1.24–$4.30 (“reward magnitude”). Participants were not guaranteed to win the reward if they completed the task; some trials were “win” trials, in which the participant received the reward amount, while others were “no win” trials, in which the participant received no money. To help participants determine which trials were likely to be win trials, participants were provided with accurate probability cues during the choice period. Trials had three levels of probability: “high” 88% probability of a win trial, “medium” 50% and “low” 12%. Probability of a win applied to both the hard and easy task, and there were equal proportions of each probability level across the experiment. Probability and reward information, task progress, and feedback displays (as depicted in Fig. 1) were presented on a computer screen. Participants were informed that the task would last 20 min, regardless of their choices, which was long enough to complete at least 50 trials for most participants. The first 50 trials for each participant were analyzed.
Fig. 1.
Schematic diagram of a single effort expenditure for rewards task (‘EEfRT’) trial. A) 1 s fixation cue. B) 5 s choice period in which subjects are presented with reward magnitude of the hard task for that trial, and the probability of receiving reward for that trial. C) 1 s “ready” screen. D) Subjects make rapid button presses to complete the chosen task for 7 s (easy task) or 21 s (hard task). E) Feedback completion of the task. F) Feedback on whether they received any money for that trial.
2.5. Statistical analysis
Effects of caffeine on EEfRT choices were analyzed using generalized linear mixed effect (GLME) models. Button press rates on the EEfRT indexed general psychomotor speeding, and were analyzed using linear mixed effect (LME) models. Repeated measures of “feel drug” and MAP indexed typical caffeine effects and were also analyzed using LME models. All analyses were completed in the lme4 package (v 0.999375-42; Bates et al., 2011) of the R statistical computing environment (v. 2.14.0; R Development Core Team, 2011). (G)LME models offer significant advantages relative to traditional repeated-measures ANOVA in handling of missing data, relaxation of assumptions of normality and homogeneity of variance, and increased statistical power for smaller sample sizes (as they focus on the per-trial level rather than the per-subject level; Raudenbush and Bryk, 2002).1 EEfRT models (choice and press rate) included reward magnitude of the HC/HR option (RM), probability (P) and Expected value (EV; or the RM×P interaction) as independent (fixed) variables, and also included trial number and effect of session as covariates to control for possible fatigue over the task or over sessions. Subjective and cardiovascular drug effect models included the effect of time as an independent variable and effect of session as a covariate. The effect of drug in all models was a linear contrast between caffeine and placebo (all models included data from the other drug sessions to allow better estimation of order and session effects, but did not estimate effects of the other test substance on the DVs). All models also included a random effect for participant to account for non-independence of trials within participants. Effect sizes are reported as unstandardized coefficients (B) with standard errors (SE).
3. Results
3.1. Subjective/cardiovascular drug effects
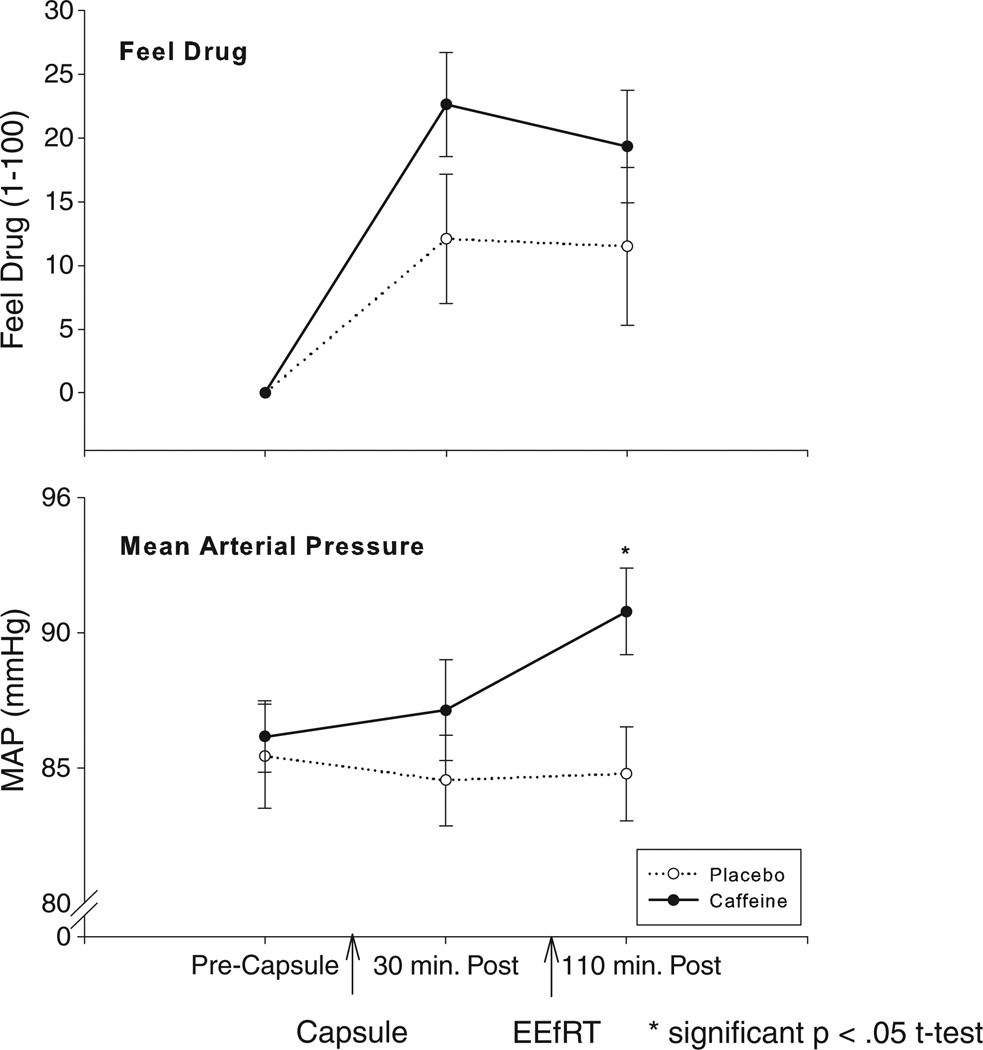
Caffeine slightly but significantly increased reports of feeling a drug effect; B = 4.65, SE = 1.65, t(22) = 2.83, p = 0.01, although neither the timepoint before or after the EEfRT demonstrated a significant difference from placebo in post-hoc paired t-tests (Fig. 2, top panel). Caffeine also significantly increased MAP; B = 1.86, SE = 0.59, t(22) = 3.15, p = 0.005, with the timepoint after the EEfRT differing significantly from placebo in post-hoc t-tests (Fig. 2, bottom panel).
Fig. 2.
Effects of caffeine on subjective “feel drug” scores and mean arterial pressure (MAP). Caffeine produced a significant overall increase in both feel drug and MAP, but only the MAP time point after the EEfRT was significantly different from placebo in post-hoc testing.
3.2. EEfRT
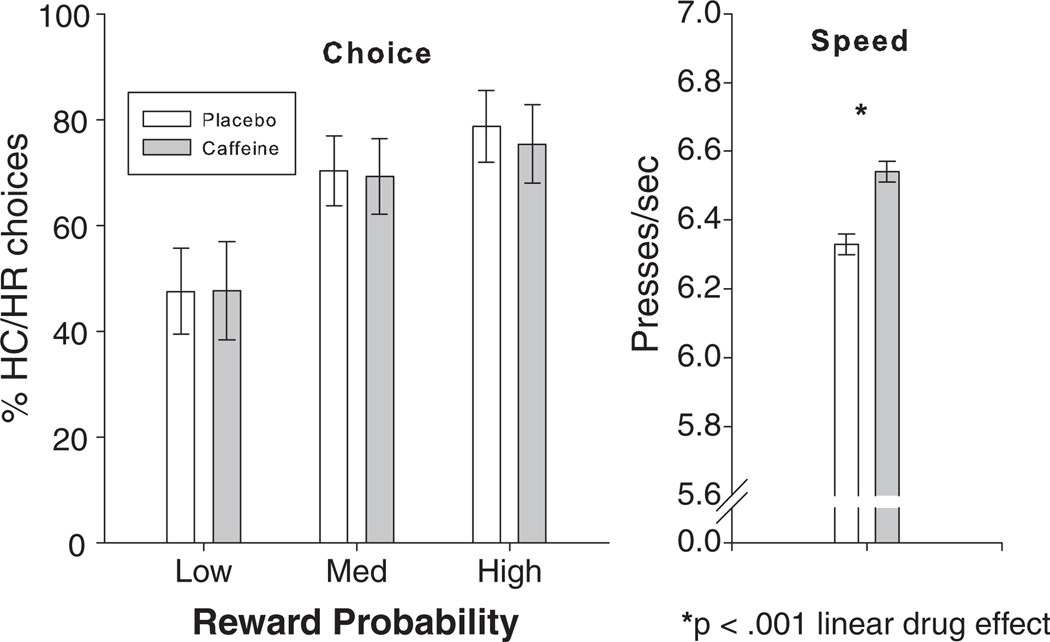
As shown in Table 1 and the left panel of Fig. 3, caffeine did not significantly affect choices on the EEfRT at any probability level. Congruent with previous findings, HC/HR choices increased with increasing probability of reward, reward amount and expected value (Table 1). As shown in Table 2 and the right panel of Fig. 3, consistent with caffeine's typical psychomotor effects, caffeine significantly increased press rate by about 0.2 presses/s. Press rate increased across sessions, and within sessions, but reward probability, reward value and expected value did not affect press rate (Table 2).
Table 1.
GLMER model of caffeine effects on EEfRT choice behavior (n = 18).
| Fixed effects | B (log odds of HC/HR choice) |
SE | z-value | p |
|---|---|---|---|---|
| Drug | −0.06 | 0.14 | 0.44 | 0.66 |
| Probability | ||||
| Linear | 2.14 | 0.11 | 19.53 | <0.001 |
| Quadratic | 0.39 | 0.09 | 4.28 | <0.001 |
| Reward value | 1.14 | 0.05 | 21.39 | <0.001 |
| Expected value | ||||
| (Probability×Reward value) | ||||
| Linear probability×Reward value | 0.12 | 0.12 | 0.99 | 0.32 |
| Quadratic probability×Reward value | 0.30 | 0.11 | 2.76 | 0.006 |
| Drug×Probability | ||||
| Drug×Linear probability | −0.34 | 0.33 | 1.05 | 0.29 |
| Drug×Quadratic probability | −0.01 | 0.29 | 0.04 | 0.97 |
| Drug×Reward value | −0.07 | 0.16 | 0.42 | 0.67 |
| Drug×Expected value | ||||
| Drug×Linear probability×Reward value |
0.18 | 0.38 | 0.47 | 0.64 |
| Drug×Quadratic probability×Reward value |
0.01 | 0.35 | 0.03 | 0.97 |
| Session | ||||
| Linear | 0.63 | 0.12 | 5.46 | <0.001 |
| Quadratic | −0.28 | 0.10 | 2.87 | 0.004 |
| Trial order | −0.008 | 0.003 | 2.89 | 0.004 |
Fig. 3.
Effects of caffeine on EEfRT. Left panel shows percent of high effort choices at low, medium and high probability of reward by drug condition as means +/− SE. Although effortful choice increased with increasing probability of reward, drug had no significant effect on choice. Right panel shows psychomotor speed in key presses per second, as estimated marginal means from LME model +/− SE of drug effect. Caffeine significantly increased key pressing speed.
Table 2.
LMER model of caffeine effects on press rate (n = 18).
| Fixed effects | B (taps/s) | SE | t(17) | p |
|---|---|---|---|---|
| Drug | 0.20 | 0.03 | 7.10 | <0.001 |
| Probability | ||||
| Linear | 0.007 | 0.02 | 0.32 | 0.75 |
| Quadratic | −0.02 | 0.02 | 0.99 | 0.34 |
| Reward value | 0.002 | 0.01 | 0.22 | 0.83 |
| Expected value (Probability×Reward value) | ||||
| Linear probability×Reward value | 0.02 | 0.02 | 0.96 | 0.35 |
| Quadratic probability×Reward value | −0.009 | 0.02 | 0.42 | 0.68 |
| Drug×Probability | ||||
| Drug×Linear probability | −0.09 | 0.07 | 1.37 | 0.19 |
| Drug×Quadratic probability | −0.005 | 0.06 | 0.08 | 0.94 |
| Drug×Reward value | −0.03 | 0.03 | 0.87 | 0.40 |
| Drug×Expected value | ||||
| Drug×Linear probability×Reward value | 0.10 | 0.08 | 1.36 | 0.19 |
| Drug×Quadratic probability×Reward value | −0.009 | 0.02 | 0.42 | 0.68 |
| Session | ||||
| Linear | 0.30 | 0.03 | 11.86 | <0.001 |
| Quadratic | 0.10 | 0.02 | 4.59 | <0.001 |
| Trial order | 0.004 | 0.0006 | 5.63 | <0.001 |
Because caffeine demonstrated comparatively mild effects on all measures, we further examined whether caffeine might have affected choices on the EEfRT for high caffeine responders (i.e. individuals who responded more strongly on feel drug or MAP) but not low caffeine responders, by conducting two further GLME models. The first included a mean-centered overall measure of MAP response and the second a mean-centered overall measure of feel drug response as additional independent (fixed) variables. We produced these overall response measures by first calculating change from baseline to the timepoint before and the timepoint after the EEfRT for each variable. We then averaged the change scores from each variable together, producing average MAP change and average feel drug change scores for the caffeine session and for the placebo session. We then subtracted the average scores for placebo from their respective average scores for caffeine to produce overall estimates of MAP response to caffeine and feel drug response to caffeine for each person. Caffeine did not affect choices differently for high feel drug responders vs. low feel drug responders, Feel response × Drug interaction; B = 0.005, SE = 0.005, z(18) = 1.04, p = 0.30. Caffeine did affect choices differently for high blood pressure responders vs. low blood pressure responders; MAP response×Drug interaction, B = −0.09, SE = 0.03, z(18) = 3.37, p < 0.001. To explore the nature of this interaction, we conducted a median split on MAP responses, and examined the effect of caffeine in the high responder and low responder groups separately. In high MAP responders, caffeine significantly decreased choice of the hard option, B = −0.53, SE = 0.20, z(10) = 2.64, p = 0.008, but in Low MAP responders, caffeine had no significant effect on choice B = .035, SE = 0.20, z(8) = 1.80, p = 0.07. Of note, there was not a corresponding significant interaction between MAP response and caffeine's effects on press rate; MAP response×Drug interaction, B = −0.003, SE = 0.004, t(17) = −0.72, p = 0.48.
4. Discussion
Contrary to our predictions, caffeine did not increase choice of the HC/HR option. In fact, when dividing the participants into high vs. low cardiovascular responders to caffeine, caffeine slightly decreased effortful choices in high cardiovascular responders. Although the dose of caffeine was relatively low, it did produce significant effects on the expected subjective and cardiovascular measures (‘feel drug’ and blood pressure), and, importantly, it significantly sped performance on the EEfRT. Thus, this behaviorally active dose of caffeine failed to increase willingness to exert effort, and may even have decreased it in individuals that responded particularly strongly to the drug. Moreover, it did not produce any effects on sensitivity to information about reward magnitude, probability or expected value when making effortful decisions, as evidenced by the lack of interactions between these variables and the caffeine condition.
These findings support the idea that choice behavior and psychomotor performance on the EEfRT are dissociable. In our previous study, amphetamine both increased psychomotor speed and increased choice of high effort options, raising the possibility these measures were related. In that study, when we statistically controlled for the increased motor performance, motor performance did not account for the increased high effort choice (Wardle et al., 2011). In the present study, caffeine increased motor speed without affecting effortful choice on the EEfRT, or even decreasing effortful choice slightly in high responders. This contributes further evidence for the validity of choice behavior on the EEfRT as a specific measure of effort-based decision-making.
The fact that caffeine did not increase effortful choices is slightly surprising, given the common use of caffeine to perform effortful tasks, and findings in the exercise psychology literature that caffeine reduces perceptions of effort (Doherty and Smith, 2005). However, this finding is in line with the animal literature, and underscores the complex nature of A2A–DA interactions relating tomotivation (Salamone et al., 2009). In rodents, adenosine A2A antagonists (unlike prototypic DA stimulants) do not increase preference for high effort options even though they reverse the effects of dopamine antagonism/depletion. In fact, in one article animals exhibited a trend towards fewer HC/HR choices after caffeine (40 mg/kg), a dose that ameliorated DA antagonism (Salamone et al., 2009). The current study suggests that in humans as well, A2A antagonists do not increase effort in the same way as dopaminergic stimulants. The reasons for the differences between caffeine and amphetamine on this measure are not known. However, interactions between adenosine and dopamine are complex, and caffeine may act on multiple receptor types (Ferré, 2008). The striatum is thought to be a primary site of adenosine's action on effort-related processes (Salamone et al., 2010), with both A1 adenosine receptors and A2A receptors present, co-localized with D1 and D2 receptors respectively (Ferré, 2008). Both D1 and D2 depletion/blockade affect exertion of effort, and A2A antagonists are able to reverse the effect of D2 antagonism on effort, while A1 antagonists are ineffective at ameliorating the effects of D1 or D2 antagonists (Nunes et al., 2010; Salamone et al., 2009). Additional preclinical work suggests that the arousal-related effects of caffeine are exclusively mediated by A2A receptor function, as A2A-knockout mice exhibit no benefits from caffeine on wakefulness, while A1 knockouts show normal effects of caffeine (Huang et al., 2005; Lazarus et al., 2011). Interestingly, A2A knockouts are also resistant to the normal effects of D2 receptor antagonism on effort-based decision-making (Pardo et al., 2012). Although caffeine is a non-selective adenosine antagonist that influences both A1 and A2A receptors, it may be that its A2A effects are not as evident in this human paradigm. One future direction for this research is to examine the effects of caffeine in humans in the presence of a dopamine D2 antagonist, such as haloperidol. This would test the possibility that adenosine antagonist effects on effort are only visible during D2 depletion/antagonism.
There are several limitations of this study that must be considered. First, only a single dose of caffeine (200 mg) was tested. This is approximately equivalent to 2 cups of drip coffee or one commercially available NoDoz® tablet (Barone and Roberts, 1996), and may have been either too low, or too high to produce effects on effort. This dose did produce significant psychomotor speeding; however, the magnitude of this speeding was smaller than amphetamine produced in our previous study (.20 more taps/s [SE 0.03] for 200 mg caffeine, compared to .37 more taps/s [SE 0.02] for 20 mg amphetamine), raising the possibility that the dose was too low to produce decisional effects. For comparison to preclinical studies, our volunteers received about 3 mg/kg which would scale to approximately 12 mg/kg in rats (acknowledging that difficulties in inter-species scaling make this a very approximate estimate; Bonati et al., 1984). This is within the range of caffeine doses that reversed the effects of haloperidol in rats (5.0 mg/kg to 40 mg/kg). However, the highest dose (40 mg/kg) in rats was the dose which, when tested alone, produced a trend towards decreased effort in animals, which also suggests that higher caffeine doses still might not effectively increase effort in humans. In addition, our own findings that higher cardiovascular responders to caffeine also decreased their effort suggest that a higher dose might not be effective. However, it may also have been that the dose was too high, and produced unpleasant effects in our sample of fairly light caffeine users (such as anxiety), which adversely impacted willingness to exert effort. Further research will thus be required to determine the dose–effect relationship of caffeine and effort in humans. Another limitation is the use of only one type of effort task. Although the bulk of the animal work on A2A antagonists and effort has utilized measures of physical effort like the one used here, recent preclinical findings suggest that tasks measuring cognitive effort may yield some what different effects of caffeine (Cocker et al., 2012).
There may also be individual differences moderating caffeine response that we were unable to detect due to our limited number and range of participants. Caffeine quickly produces tolerance, especially for A1 - mediated effects (Karcz-Kubicha et al., 2003), raising the possibility that habitual patterns of caffeine use, either abstinence or heavier use, might alter the acute effects of the drug on effort. Indeed, some researchers suggest that caffeine's effects on many measures are primarily attributable to withdrawal relief (James and Rogers, 2005). The participants we tested did not have a wide enough range of caffeine consumption to test these possibilities. There is also evidence from preclinical studies of cognitive effort (Cocker et al., 2012) indicating that caffeine had differential effects in animals who exhibited high effort at baseline (called “workers” by the authors), compared to low levels of effort at baseline (“slackers”). We could not examine this worker/slacker distinction in our data, as creating groups based on the placebo session would have confounded caffeine's effects with regression to the mean, but this issue could be addressed in future studies by adding a separate baseline session. Finally, responses to caffeine might be related to personality (introversion or extraversion) and time of day (Revelle et al., 1980). Interestingly, introverts and extroverts differ in DA (particularly D2) function (Netter, 2006), suggesting that biologically based individual differences related to D2 functioning might moderate the effect of caffeine on effort.
Disorders involving DA dysfunction, including Parkinson's disease, depression and drug addiction, are often also characterized by aberrant cost/benefit decision-making. Based on the pre-clinical evidence of adenosine/DA interactions, there has been increased interest in the adenosine system as a potential target for treatment of these disorders (Cunha et al., 2008; Filip et al., 2012). Thus, the current study represents an important step in translating pre-clinical research on adenosine/DA interactions into human decision-making behavior. Our findings suggest that a moderate dose of caffeine does not strongly influence motivational behavior in healthy normal adults, although further research will be required to establish whether this result applies at different caffeine doses, to different types of effort (cognitive vs. physical), during DA depletion, or in sub-populations with low baseline dopaminergic functioning. Translational extensions of pre-clinical work on interactions between DA and adenosine such as this one may eventually lead to development and evaluation of more effective treatments for neuropsychiatric disorders involving DA dysfunction.
Acknowledgments
The authors thank Lindsey Davis, Justin Birnholz, Zoya Hussein for their work on this study and Matthew Baggott for assistance with data processing. The research was supported by grants from the National Institute on Drug Abuse (R01 DA002812) to HdW. MCW is supported by a National Institute on Drug Abuse Training grant, T32 DA007255. The contribution of MTT to this work was supported by F31 MH1087015-02 from the National Institute of Mental Health.
Footnotes
p-values were calculated using the t-distribution with degrees of freedom (d.f.) = n − 1, except the binomial choice data, which uses a z-distribution. D.f. in mixed models are somewhat controversial (Baayen et al., 2008), with a proposed upper bound of the number of trials (over 1500 in our datasets) minus number of fixed effects. Our selected d.f. was considerably more conservative; however, p-values must be considered estimates.
References
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang. 2008;59:390–412. [Google Scholar]
- Bardgett ME, Depenbrock M, Downs N, Points M, Green L. Dopamine modulates effort-based decision making in rats. Behav Neurosci. 2009;123:242–251. doi: 10.1037/a0014625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone JJ, Roberts HR. Caffeine consumption. Food Chem Toxicol. 1996;34:119–129. doi: 10.1016/0278-6915(95)00093-3. [DOI] [PubMed] [Google Scholar]
- Bates DM, Meachler M, Bolker B. lme4: linear mixed-effects model using S4 classes. 2011 [Google Scholar]
- Bonati M, Latini R, Tognoni G, Young JF, Garattini S. Interspecies comparison of in vivo caffeine pharmacokinetics in man, monkey, rabbit, rat and mouse. Drug Metab Rev. 1984;15:1355–1383. doi: 10.3109/03602538409029964. [DOI] [PubMed] [Google Scholar]
- Chen JF, Beilstein M, Xu YH, Turner TJ, Moratalla R, Standaert DG, et al. Selective attenuation of psychostimulant-induced behavioral responses in mice lacking A2A adenosine receptors. Neuroscience. 2000;97:195–204. doi: 10.1016/s0306-4522(99)00604-1. [DOI] [PubMed] [Google Scholar]
- Childs E, de Wit H. Subjective, behavioral, and physiological effects of acute caffeine in light, nondependent caffeine users. Psychopharmacology (Berl) 2006;185:514–523. doi: 10.1007/s00213-006-0341-3. [DOI] [PubMed] [Google Scholar]
- Cocker PJ, Hosking JG, Benoit J, Winstanley CA. Sensitivity to cognitive effort mediates psychostimulant effects on a novel rodent cost/benefit decision-making task. Neuropsychopharmacology. 2012;37:1825–1837. doi: 10.1038/npp.2012.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA, Ferre S, Vaugeois J-M, Chen J-F. Potential therapeutic interest of adenosine A2A receptors in psychiatric disorders. Curr Pharm Des. 2008;14:1512–1524. doi: 10.2174/138161208784480090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M, Smith PM. Effects of caffeine ingestion on rating of perceived exertion during and after exercise: a meta-analysis. Scand J Med Sci Sports. 2005;15:69–78. doi: 10.1111/j.1600-0838.2005.00445.x. [DOI] [PubMed] [Google Scholar]
- Farrar A, Pereira M, Velasco F, Hockemeyer J, rgller C, Salamone J. Adenosine A2A receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat: implications for studies of psychomotor slowing. Psychopharmacology (Berl) 2007;191:579–586. doi: 10.1007/s00213-006-0554-5. [DOI] [PubMed] [Google Scholar]
- Ferré S. An update on the mechanisms of the psychostimulant effects of caffeine. J Neurochem. 2008;105:1067–1079. doi: 10.1111/j.1471-4159.2007.05196.x. [DOI] [PubMed] [Google Scholar]
- Filip M, Zaniewska M, Frankowska M, Wydra K, Fuxe K. The Importance of the adenosine A2A receptor-dopamine D2 receptor interaction in drug addiction. Curr Med Chem. 2012;19:317–355. doi: 10.2174/092986712803414231. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Strutured clinical interview for DSM-IV axis I disorders. New York: Biometrics Research Department; 1996. [Google Scholar]
- Fischman MW, Foltin RW. Utility of subjective-effects measurements in assessing abuse liability of drugs in humans. Br J Addict. 1991;86:1563–1570. doi: 10.1111/j.1360-0443.1991.tb01749.x. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Tse MT, Ghods-Sharifi S. Dopaminergic and glutamatergic regulation of effort- and delay-based decision making. Neuropsychopharmacology. 2008;33:1966–1979. doi: 10.1038/sj.npp.1301565. [DOI] [PubMed] [Google Scholar]
- Font L, Mingote S, Farrar A, Pereira M, Worden L, Stopper C, et al. Intra-accumbens injections of the adenosine A2A agonist CGS 21680 affect effort-related choice behavior in rats. Psychopharmacology (Berl) 2008;199:515–526. doi: 10.1007/s00213-008-1174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z-L, Qu W-M, Eguchi N, Chen J-F, Schwarzschild MA, Fredholm BB, et al. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat Neurosci. 2005;8:858–859. doi: 10.1038/nn1491. [DOI] [PubMed] [Google Scholar]
- James JE, Rogers PJ. Effects of caffeine on performance and mood: withdrawal reversal is the most plausible explanation. Psychopharmacology (Berl) 2005;182:1–8. doi: 10.1007/s00213-005-0084-6. [DOI] [PubMed] [Google Scholar]
- Karcz-Kubicha M, Antoniou K, Terasmaa A, Quarta D, Solinas M, Justinova Z, et al. Involvement of adenosine A1 and A2A receptors in the motor effects of caffeine after its acute and chronic administration. Neuropsychopharmacology. 2003;28:1281–1291. doi: 10.1038/sj.npp.1300167. [DOI] [PubMed] [Google Scholar]
- Lazarus M, Shen H-Y, Cherasse Y, Qu W-M, Huang Z-L, Bass CE, et al. Arousal effect of caffeine depends on adenosine A2A receptors in the shell of the nucleus accumbens. J Neurosci. 2011;31:10067–10075. doi: 10.1523/JNEUROSCI.6730-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liguori A, Hughes JR, Grass JA. Absorption and subjective effects of caffeine from coffee, cola and capsules. Pharmacol Biochem Behav. 1997;58:721–726. doi: 10.1016/s0091-3057(97)00003-8. [DOI] [PubMed] [Google Scholar]
- Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM, et al. Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neurosci. 2008;28:9037–9046. doi: 10.1523/JNEUROSCI.1525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford GK, Benowitz NL, Evans SM, Kaminski BJ, Preston KL, Sannerud CA, et al. Absorption rate of methylxanthines following capsules, cola and chocolate. Eur J Clin Pharmacol. 1996;51:319–325. doi: 10.1007/s002280050205. [DOI] [PubMed] [Google Scholar]
- Netter P. Dopamine challenge tests as an indicator of psychological traits. Hum Psychopharmacol Clin Exp. 2006;21:91–99. doi: 10.1002/hup.754. [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Santerre JL, Given AB, Sager TN, Correa M, et al. Differential effects of selective adenosine antagonists on the effort-related impairments induced by dopamine D1 and D2 antagonism. Neuroscience. 2010;170:268–280. doi: 10.1016/j.neuroscience.2010.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onge St, Jr, Chiu YC, Floresco SB. Differential effects of dopaminergic manipulations on risky choice. Psychopharmacology (Berl) 2010;211:209–221. doi: 10.1007/s00213-010-1883-y. [DOI] [PubMed] [Google Scholar]
- Pardo M, Lopez-Cruz L, Valverde O, Ledent C, Baqi Y, Müller CE, et al. Adenosine A2A receptor antagonism and genetic deletion attenuate the effects of dopamine D2 antagonism on effort-based decision making in mice. Neuropharmacology. 2012;62:2068–2077. doi: 10.1016/j.neuropharm.2011.12.033. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: applications and data analysis methods. Sage Publications Inc. 2002 [Google Scholar]
- Revelle W, Humphreys MS, Simon L, Gilliland K. The interactive effect of personality, time of day, caffeine: a test of the arousal model. J Exp Psychol Gen. 1980;109:1–31. [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, McCullough LD, Carriero DL, et al. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacol. Biochem. Behav. 1994;49:25–31. doi: 10.1016/0091-3057(94)90452-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Farrar AM, Font L, Patel V, Schlar DE, Nunes EJ, et al. Differential actions of adenosine A1 and A2A antagonists on the effort-related effects of dopamine D2 antagonism. Behav Brain Res. 2009;201:216–222. doi: 10.1016/j.bbr.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar AM, Nunes EJ, Collins LE. Role of dopamine/adenosine interactions in the brain circuitry regulating effort-related decision making: insights into pathological aspects of motivation. Futur Neurol. 2010;5:377–392. [Google Scholar]
- Simon NW, Montgomery KS, Beas BS, Mitchell MR, LaSarge CL, Mendez IA, et al. Dopaminergic modulation of risky decision-making. J Neurosci. 2011;31:17460–17470. doi: 10.1523/JNEUROSCI.3772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria G, Castane A, Ledent C, Parmentier M, Maldonado R, Valverde O. The lack of A2A adenosine receptors diminishes the reinforcing efficacy of cocaine. Neuropsychopharmacology. 2005;31:978–987. doi: 10.1038/sj.npp.1300876. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Schwartzman AN, Lambert WE, Zald DH. Worth the “EEfRT”? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS One. 2009:4. doi: 10.1371/journal.pone.0006598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: effects of d-amphetamine on human effort-based decision-making. J Neurosci. 2011;31:16597–16602. doi: 10.1523/JNEUROSCI.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]