Abstract
Background: Visceral adipose tissue (VAT) has been identified as a harmful fat depot, and sex and race differences in VAT have been reported in white and African Americans.
Objectives: We determined the clinical utility of VAT in the identification of individuals at elevated cardiometabolic risk in white and African American adults and compared the clinical utility with measures obtained by using dual-energy X-ray absorptiometry (DXA) and anthropometric measures.
Design: The sample included 429 white women, 311 African American women, 406 white men, and 100 African American men who were 18–74 y of age. VAT was measured by using computed tomography, fat mass (FM) and percentage of body fat were measured by using DXA, and waist circumference (WC) and BMI were assessed. Receiver operating characteristic curves were used to compare the utility of measures in the identification of participants in the upper quintile of a continuous score derived from principal components analysis of fasting glucose, HDL cholesterol, triglycerides, and blood pressure.
Results: The clinical utility of measures varied across sex-by-race groups. In the overall sample, the areas under the curve were significantly higher for VAT and WC in comparison with the other indicators. Identified VAT thresholds were higher in white men (140 cm2) and women (141 cm2) than in African American men (82 cm2) and women (97 cm2).
Conclusions: VAT and WC showed greater clinical utility than did other obesity measures. Because of the complexity of measuring VAT, the use of WC is recommended for the identification of adults with elevated cardiometabolic risk factors. The Pennington Center Longitudinal Study was registered at clinicaltrials.gov as NCT00959270.
INTRODUCTION
There is considerable scientific evidence that excess adiposity is associated with significant health risks, especially at high amounts (1). Abdominal obesity, in particular visceral adipose tissue (VAT)4, has been identified as a particularly harmful fat depot (2–4). Although the mechanisms are not yet fully understood, high amounts of VAT are predictive of insulin resistance and other metabolic abnormalities (3). Several studies have identified thresholds of VAT that are related to elevated cardiometabolic risk factors and metabolic syndrome (MetS) in white men and women (5–9). Limited studies have also identified optimal thresholds of VAT in other ethnic groups such as Indians (10), Chinese (11), Koreans (12, 13), Japanese (14, 15), and Japanese Americans (16).
Sex and race differences in VAT have been consistently shown in samples of white and African Americans. Men have higher amounts of VAT than women do, and white Americans have higher amounts of VAT than African Americans do (17–21). These ethnic differences in VAT persist even after statistical control for amounts of total body fat (19, 21). The relation between VAT and cardiometabolic risk factors has not been studied extensively across different ethnic groups; however, significant associations have been shown in both white and African American adults (22). One study has reported VAT thresholds in a combined sample of white and African American women (23); however, to our knowledge, differences in VAT thresholds among white and African Americans have not been studied. Thus, the purpose of this study was to determine the clinical utility of the use of VAT to identify individuals at elevated cardiometabolic risk in white and African American men and women and to compare the clinical utility to other common obesity measures obtained by using dual-energy X-ray absorptiometry (DXA) and anthropometric measures.
SUBJECTS AND METHODS
Sample
Participants were drawn from the baseline assessment for the Pennington Center Longitudinal Study (www.clinicaltrials.gov; NCT00959270), which is an ongoing investigation of the effects of obesity and lifestyle factors on the development of chronic diseases. The sample is composed of volunteers who have participated in a variety of clinical studies conducted at the Pennington Biomedical Research Center in Baton Rouge, LA, between 1992 and 2012. Participants were recruited from the greater Baton Rouge area through the local media and Web-based advertisements. The current cross-sectional study included 1246 participants (429 white women, 311 African American women, 406 white men, and 100 African American men) who were 18–74 y of age. Each participant provided their written informed consent, and all Pennington Center Longitudinal Study procedures were approved by the Pennington Biomedical Research Center Institutional Review Board.
VAT
Abdominal VAT cross-sectional areas (cm2) at the L4–L5 anatomic landmark were measured by using computed tomography on a scanner (General Electric) as previously described (21, 24). Commercially available software (Analyze; Analyze Direct) was used to electronically measure areas of adipose tissue by selecting regions of interest defined by using attenuation values (−30 to −190 Hounsfield units for adipose tissue).
Anthropometric measures and total body fat
Height and weight were measured by using a wall-mounted stadiometer and a digital scale, respectively, after the volunteer removed outer clothing, heavy pocket items, and shoes. BMI (in kg/m2) was calculated as weight divided by the square of height. Waist circumference (WC) was measured at the midpoint between the inferior border of the ribcage and the superior aspect of the iliac crest by using an inelastic measuring tape. Total-body fat mass (FM; in kg) and percentage of body fat were estimated by using DXA with a whole-body scanner (Hologic) as previously described (21).
Cardiometabolic risk factors
Resting blood pressure measurements were taken manually by using a stethoscope and standard sphygmomanometer or, in some cases, a validated automatic measuring device (Omron). Serum triglycerides, HDL cholesterol, and glucose were obtained from a 12-h fasting blood draw. Participants were asked to refrain from consuming alcohol or engaging in vigorous exercise ≥24 h before blood withdrawal.
Covariates
Participant age was computed from birth and observation dates. Smoking status was self-reported during the screening process, and participants were classified as nonsmokers, current smokers, or former smokers. Menopausal status (premenopausal compared with postmenopausal) was determined in women from their ages and responses to questions regarding their reproductive histories (21).
Statistical analysis
Abnormal levels of risk factors were defined according to current recommendations as follows: high blood pressure (≥140/90 mm Hg or reported hypertension) (25), high triglyceride concentrations (≥200 mg/dL) (26), high glucose concentrations (≥126 mg/dL or reported diabetes) (27), and low HDL-cholesterol concentrations (≤40 mg/dL) (26). A continuous MetS risk-factor score was derived by using a principal components analysis of MetS risk factors (ie, systolic and diastolic blood pressures, triglycerides, glucose, and HDL cholesterol) (28) with the exception of WC. Individuals at high risk were defined as those in the upper quintile (20%) of the risk factor score.
Logistic regression was used to determine the odds of having abnormal risk-factor levels and of being in the upper quintile of the continuous MetS score in each sex-by-ethnicity group. ORs are expressed per SD of the explanatory variable (VAT, BMI, WC, FM, and percentage of body fat). Age, smoking status, and menopausal status (in women) were included as covariates in logistic regression models. Receiver operating characteristic (ROC) curves were used to select thresholds that identified individuals in the upper quintile of the continuous MetS score in each sex-by-ethnicity group. Because the AUC is considered a measure of the utility of the predictor variable and represents the tradeoff between the correct identification of high-risk individuals (sensitivity) and the correct identification of low-risk individuals (specificity), the threshold was determined from the Youden index, which is the maximum value of J (16, 29, 30), whereby
Significant differences in AUCs in adiposity indicators were determined by using the nonparametric approach of DeLong et al (31). SAS software (version 9.3; SAS Institute Inc) was used for data management and preliminary analyses and MedCalc software (version 12.3) was used to perform ROC analyses. The level of significance was set at P ≤ 0.05.
RESULTS
Descriptive characteristics of the sample are presented in Table 1. The average age of the sample was 44.5 y (range: 18–74 y). Average BMI was 29.7, with a range from 17.3 to 48.7. The first principal component from the analysis of MetS risk factors was retained for additional analysis as a continuous MetS score. The component explained 37% of the variance in original risk-factor variables and was characterized by positive loadings for systolic blood pressure (0.62), diastolic blood pressure (0.64), glucose (0.50), and triglycerides (0.65) and a negative loading for HDL cholesterol (−0.62).
TABLE 1.
Descriptive characteristics of the analysis sample from the Pennington Center Longitudinal Study
| Women |
Men |
|||
| White | African American | White | African American | |
| Subjects (n) | 429 | 311 | 406 | 100 |
| Age (y) | 49.6 ± 11.51 | 40.2 ± 11.62 | 44.4 ± 13.5 | 36.5 ± 13.82 |
| Visceral adipose tissue (cm2) | 121.8 ± 61.2 | 91.0 ± 49.22 | 140.9 ± 70.8 | 90.3 ± 57.92 |
| BMI (kg/m2) | 29.0 ± 5.1 | 30.6 ± 5.42 | 30.0 ± 4.6 | 29.3 ± 5.2 |
| Waist circumference (cm) | 90.0 ± 13.0 | 91.2 ± 13.22 | 101.8 ± 13.4 | 95.5 ± 15.02 |
| Total fat mass (kg) | 30.2 ± 8.9 | 31.3 ± 9.4 | 25.9 ± 9.2 | 21.3 ± 9.92 |
| Percentage of body fat | 38.5 ± 5.5 | 37.6 ± 5.62 | 26.8 ± 6.2 | 22.0 ± 7.12 |
| Systolic blood pressure (mm Hg) | 120.0 ± 13.9 | 119.0 ± 13.7 | 121.6 ± 12.1 | 122.1 ± 11.1 |
| Diastolic blood pressure (mm Hg) | 74.9 ± 7.8 | 77.2 ± 8.82 | 78.0 ± 8.2 | 76.8 ± 9.1 |
| Triglycerides (mg/dL) | 135.8 ± 78.1 | 90.3 ± 48.82 | 155.3 ± 101.0 | 107.4 ± 68.62 |
| HDL cholesterol (mg/dL) | 59.7 ± 14.1 | 58.0 ± 13.9 | 46.2 ± 9.8 | 47.6 ± 9.9 |
| Glucose (mg/dL) | 98.4 ± 15.0 | 98.9 ± 16.9 | 104.8 ± 21.5 | 100.5 ± 15.72 |
| High blood pressure (≥140/90 mm Hg) (%)3 | 17.5 | 18.0 | 16.5 | 15.0 |
| High blood glucose (≥126 mg/dL) (%)4 | 9.6 | 11.9 | 11.3 | 10.0 |
| High triglycerides (≥200 mg/dL) | 16.6 | 4.82 | 23.7 | 7.02 |
| Low HDL cholesterol (<40 mg/dL) | 5.8 | 5.1 | 27.6 | 22.0 |
| Current smoking | 4.9 | 4.9 | 2.3 | 12.22 |
| Postmenopausal | 49.9 | 11.62 | — | — |
Mean ± SD (all such values).
Significant difference between white and African Americans, within sex, based on independent samples t test or chi-square test.
Or self-reported hypertension.
Or self-reported diabetes.
Partial correlations between adiposity variables and the risk factors, adjusted for age, are shown in Table 2. Correlations were consistently negative for HDL cholesterol and positive for the other risk factors. In addition, with few exceptions, correlations were significant.
TABLE 2.
Age-adjusted partial correlations in measures of adiposity and cardiometabolic risk factors1
| SBP | DBP | Glucose | Triglycerides | HDL cholesterol | Continuous MetS score | |
| White women | ||||||
| Visceral adipose tissue | 0.24 | 0.19 | 0.40 | 0.41 | −0.43 | 0.55 |
| BMI | 0.23 | 0.23 | 0.32 | 0.28 | −0.34 | 0.46 |
| Waist circumference | 0.20 | 0.19 | 0.36 | 0.26 | −0.41 | 0.49 |
| Fat mass | 0.21 | 0.20 | 0.26 | 0.26 | −0.30 | 0.40 |
| Percentage of body fat | 0.18 | 0.16 | 0.20 | 0.21 | −0.19 | 0.31 |
| African American women | ||||||
| Visceral adipose tissue | 0.062 | 0.112 | 0.38 | 0.35 | −0.29 | 0.36 |
| BMI | 0.15 | 0.18 | 0.23 | 0.26 | −0.28 | 0.37 |
| Waist circumference | 0.15 | 0.20 | 0.32 | 0.36 | −0.34 | 0.44 |
| Fat mass | 0.14 | 0.18 | 0.22 | 0.26 | −0.24 | 0.34 |
| Percentage of body fat | 0.092 | 0.14 | 0.18 | 0.18 | −0.19 | 0.25 |
| White men | ||||||
| Visceral adipose tissue | 0.17 | 0.21 | 0.24 | 0.36 | −0.27 | 0.43 |
| BMI | 0.21 | 0.25 | 0.18 | 0.26 | −0.29 | 0.37 |
| Waist circumference | 0.18 | 0.25 | 0.20 | 0.27 | −0.30 | 0.37 |
| Fat mass | 0.16 | 0.25 | 0.18 | 0.25 | −0.26 | 0.32 |
| Percentage of body fat | 0.13 | 0.24 | 0.14 | 0.24 | −0.23 | 0.28 |
| African American men | ||||||
| Visceral adipose tissue | 0.192 | 0.38 | 0.23 | 0.49 | −0.34 | 0.59 |
| BMI | 0.32 | 0.42 | 0.162 | 0.42 | −0.40 | 0.59 |
| Waist circumference | 0.28 | 0.42 | 0.182 | 0.40 | −0.43 | 0.60 |
| Fat mass | 0.29 | 0.42 | 0.172 | 0.38 | −0.37 | 0.56 |
| Percentage of body fat | 0.22 | 0.44 | 0.152 | 0.35 | −0.35 | 0.52 |
DBP, diastolic blood pressure; MetS Score, metabolic syndrome score derived from principal components analysis; SBP, systolic blood pressure.
All correlations were significant (P < 0.05) with the exception of those indicated.
The odds of having abnormal levels of risk factors and of being in the upper quintile of the continuous MetS score associated with each SD of obesity variables are presented in Table 3. With few exceptions, there are significantly higher odds of being in the upper quintile of the continuous MetS score associated with each SD of all obesity variables in all subgroups. ORs for all risk factors ranged from 1.7 to 6.2 for VAT, 1.5 to 4.5 for BMI, 1.6 to 5.0 for WC, 1.5 to 3.5 for FM, and 1.2 to 4.0 for percentage of body fat across the 4 sex-by-ethnicity groups.
TABLE 3.
Results of logistic regression analysis for visceral adipose tissue, BMI, waist circumference, fat mass, and percentage of body fat in the prediction of abnormal risk factor levels and the upper quintile (20%) of the continuous MetS score1
| Women |
Men |
|||
| White | African American | White | African American | |
| Visceral adipose tissue | ||||
| High blood pressure | 2.4 (1.8, 3.2) | 1.5 (1.1, 2.1) | 2.5 (1.8, 3.5) | 1.9 (1.0, 3.5) |
| High glucose | 4.4 (2.9, 6.8) | 4.2 (2.4, 7.1) | 2.4 (1.6, 3.5) | 1.8 (0.7, 4.6) |
| High triglycerides | 1.8 (1.4, 2.3) | 2.4 (1.4, 4.1) | 2.1 (1.6, 2.8) | 6.2 (2.0, 19.2) |
| Low HDL cholesterol | 3.4 (2.2, 5.3) | 1.9 (1.1, 3.5) | 1.8 (1.4, 2.3) | 1.7 (1.0, 3.1) |
| Continuous MetS score | 3.6 (2.6, 5.0) | 2.0 (1.4, 2.7) | 2.2 (2.7, 3.0) | 5.5 (2.3, 13.3) |
| BMI | ||||
| High blood pressure | 2.1 (1.5, 2.8) | 1.6 (1.2, 2.3) | 2.5 (1.8, 3.4) | 3.6 (1.6, 8.3) |
| High glucose | 3.9 (2.6, 6.0) | 3.2 (1.9, 5.2) | 2.3 (1.6, 3.3) | 3.0 (1.0, 8.7) |
| High triglycerides | 1.6 (1.2, 2.0) | 1.5 (0.9, 2.4) | 1.6 (1.2, 2.0) | 2.5 (1.1, 5.6) |
| Low HDL cholesterol | 3.0 (1.9, 4.9) | 1.6 (1.0, 2.7) | 1.7 (1.4, 2.2) | 1.7 (1.0, 2.8) |
| Continuous MetS score | 2.4 (1.8, 3.2) | 1.9 (1.4, 2.6) | 1.7 (1.4, 2.3) | 4.5 (2.1, 9.7) |
| Waist circumference | ||||
| High blood pressure | 2.1 (1.6, 2.8) | 1.7 (1.2, 2.4) | 2.1 (1.6, 3.0) | 4.3 (1.8, 10.4) |
| High glucose | 4.8 (3.0, 7.5) | 5.0 (2.7, 9.3) | 2.2 (1.5, 3.2) | 4.5 (1.1, 17.5) |
| High triglycerides | 1.9 (1.4, 2.4) | 2.1 (1.2, 3.6) | 1.6 (1.3, 2.1) | 2.8 (1.1, 6.6) |
| Low HDL cholesterol | 4.0 (2.4, 6.6) | 1.8 (1.0, 3.3) | 1.7 (1.4, 2.2) | 2.0 (1.2, 3.4) |
| Continuous MetS score | 3.2 (2.4, 4.3) | 2.3 (1.6, 3.2) | 1.8 (1.4, 2.4) | 2.3 (1.6, 3.2) |
| Fat mass | ||||
| High blood pressure | 1.7 (1.3, 2.2) | 1.5 (1.1, 2.1) | 2.2 (1.6, 3.1) | 3.4 (1.6, 7.5) |
| High glucose | 2.9 (2.0. 4.2) | 2.5 (1.6, 3.9) | 2.3 (1.6, 3.3) | 3.1 (0.9, 10.4) |
| High triglycerides | 1.5 (1.1, 1.9) | 1.5 (0.9, 2.5) | 1.5 (1.1, 1.9) | 2.6 (1.1, 6.1) |
| Low HDL cholesterol | 2.5 (1.6, 3.9) | 1.6 (1.0, 2.6) | 1.6 (1.3, 2.0) | 1.7 (1.0, 2.8) |
| Continuous MetS score | 2.1 (1.6, 2.8) | 1.8 (1.4, 2.4) | 1.7 (1.3, 2.1) | 3.5 (1.8, 6.9) |
| Percentage of body fat | ||||
| High blood pressure | 1.4 (1.1, 1.9) | 1.4 (1.0, 2.0) | 2.2 (1.6, 3.1) | 4.0 (1.6, 10.4) |
| High glucose | 2.4 (1.6, 3.7) | 2.5 (1.5, 4.2) | 2.2 (1.5, 3.4) | 2.2 (0.5, 8.8) |
| High triglycerides | 1.4 (1.0. 1.8) | 1.2 (0.7, 2.1) | 1.5 (1.1, 1.9) | 2.9 (1.0, 8.4) |
| Low HDL cholesterol | 1.6 (1.0, 2.6) | 1.3 (0.8, 2.2) | 1.5 (1.2, 1.9) | 1.7 (1.0, 2.9) |
| Continuous MetS score | 1.7 (1.3, 2.3) | 1.5 (1.1, 2.1) | 1.6 (1.2, 2.1) | 4.0 (1.8, 8.8) |
All values are ORs; 95% CIs in parentheses. ORs are expressed per SD of the explanatory variable from logistic regression analysis. All models included age, smoking, and menopausal status (women only) as covariates. Abnormal levels of risk factors were defined according to current recommendations as follows: high blood pressure: ≥140/90 mm Hg or self-reported hypertension; high glucose concentration: ≥126 mg/dL or self-reported diabetes; high triglycerides: ≥200 mg/dL; and low HDL-cholesterol concentration: <40 mg/dL. MetS score, metabolic syndrome score derived from principal components analysis.
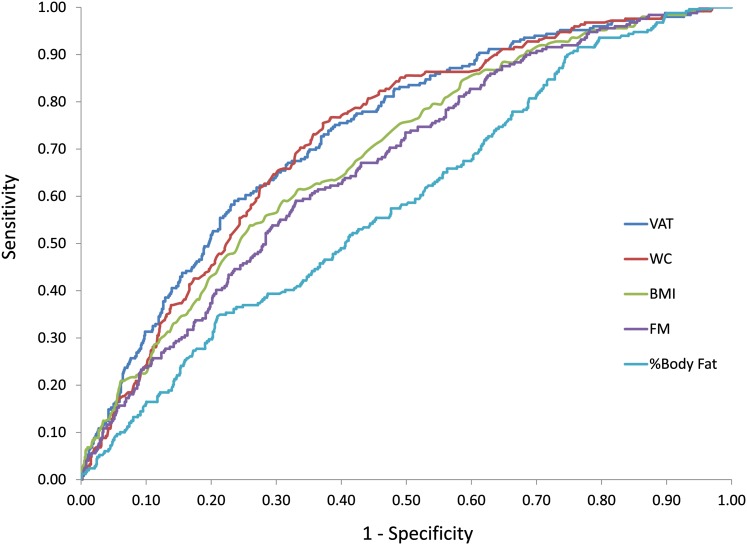
Identified thresholds of VAT, BMI, WC, and FM in this sample, along with the AUC and sensitivity and specificity of the optimal threshold at predicting individuals in the upper quintile of the continuous MetS score, are presented in Table 4. ROC curves for VAT, BMI, WC, FM, and percentage of body fat in the overall sample are presented in Figure 1. In the overall sample, the AUC was significantly higher for VAT and WC compared with the other indicators of obesity; whereas BMI, FM and percentage of body fat did not differ from one another. Optimal VAT thresholds were higher in white men (140 cm2) and women (141 cm2) than in African American men (82 cm2) and women (97 cm2). Optimal WC thresholds were higher in African American men (102 cm) than in white men (96 cm); however, thresholds were similar in African American (96 cm) and white (95 cm) women.
TABLE 4.
Results of receiver operating characteristic curve analyses for the utility of visceral adipose tissue (cm2), BMI (kg/m2), waist circumference (cm), total fat mass (kg), and percentage of body fat in the prediction of the upper quintile of the continuous metabolic syndrome score1
| AUC (95% CI) | Threshold | Sensitivity | Specificity | |
| White women | ||||
| Visceral adipose tissue | 0.785 (0.743, 0.823)a | 141.0 | 0.674 | 0.805 |
| BMI | 0.721 (0.676, 0.763)b | 31.1 | 0.616 | 0.732 |
| Waist circumference | 0.781 (0.739, 0.820)a | 95.1 | 0.663 | 0.776 |
| Total fat mass | 0.701 (0.655, 0.744)b | 34.3 | 0.593 | 0.752 |
| Percentage of body fat | 0.631 (0.583, 0.677)c | 40.3 | 0.605 | 0.624 |
| African American women | ||||
| Visceral adipose tissue | 0.696 (0.641, 0.746)a,b | 97.1 | 0.694 | 0.667 |
| BMI | 0.665 (0.609, 0.717)a | 30.2 | 0.742 | 0.526 |
| Waist circumference | 0.726 (0.673, 0.775)b | 95.6 | 0.710 | 0.715 |
| Total fat mass | 0.663 (0.607, 0.715)a | 31.1 | 0.726 | 0.558 |
| Percentage of body fat | 0.605 (0.548, 0.659)c | 40.2 | 0.532 | 0.691 |
| White men | ||||
| Visceral adipose tissue | 0.734 (0.689, 0.777)a | 140.2 | 0.778 | 0.609 |
| BMI | 0.646 (0.597, 0.693)b | 29.1 | 0.778 | 0.474 |
| Waist circumference | 0.680 (0.633, 0.726)b | 95.6 | 0.901 | 0.388 |
| Total fat mass | 0.644 (0.595, 0.691)c | 23.3 | 0.815 | 0.462 |
| Percentage of body fat | 0.616 (0.567, 0.663)d | 26.0 | 0.827 | 0.462 |
| African American men | ||||
| Visceral adipose tissue | 0.789 (0.696, 0.865)a | 81.9 | 0.900 | 0.575 |
| BMI | 0.814 (0.724, 0.885)a | 31.3 | 0.750 | 0.775 |
| Waist circumference | 0.799 (0.707, 0.872)a | 101.5 | 0.750 | 0.775 |
| Total fat mass | 0.791 (0.698, 0.866)a | 25.5 | 0.750 | 0.800 |
| Percentage of body fat | 0.788 (0.694, 0.863)a | 26.6 | 0.700 | 0.825 |
| Total sample | ||||
| Visceral adipose tissue | 0.734 (0.708, 0.758)a | 111.5 | 0.751 | 0.610 |
| BMI | 0.689 (0.663, 0.715)b | 31.1 | 0.614 | 0.666 |
| Waist circumference | 0.726 (0.700, 0.750)a | 95.1 | 0.767 | 0.616 |
| Total fat mass | 0.668 (0.641, 0.694)c | 31.1 | 0.590 | 0.670 |
| Percentage of body fat | 0.586 (0.558, 0.613)d | 26.6 | 0.900 | 0.253 |
Groups with different superscript letters were significantly different from each other within sex-by-race groups on the basis of pairwise comparisons by using the method of DeLong et al (31).
FIGURE 1.
Receiver operating characteristic curves for VAT, BMI, WC, FM, and %Body Fat for the prediction of the upper quintile of a continuous metabolic syndrome score in 1246 white and African American adults in the Pennington Center Longitudinal Study. FM, fat mass; VAT, visceral adipose tissue; WC, waist circumference; %Body Fat, percentage of body fat.
DISCUSSION
Results of this study indicated that VAT and WC have more utility as a marker of cardiometabolic risk than do BMI, FM, and percentage of body fat, although the results differed by ethnicity and sex. VAT thresholds were higher in white men and women than in African American women and men. Identified thresholds for VAT in white men (140 cm2) and women (141 cm2) in this study were somewhat higher but within the range of those identified in previous studies. For example, in a sample of white adults, Després et al (5) identified a threshold of 130 cm2 as indicative of metabolic disturbances. In addition, thresholds of 131 and 110 cm2 have been identified in samples of white men (6) and women (9), respectively, in the identification of elevated metabolic risk factors. von Eyben et al (8) reported a threshold of VAT of 144 cm2 (at L2–L3) in a small sample of Danish men and women to identify subjects with ≥2 cardiometabolic risk factors, and a recent study by Pickhardt et al (7) identified 125 and 70 cm2 (at the level of the umbilicus) as the best thresholds for the prediction of MetS in men and women, respectively. Differences in identified thresholds in these studies were likely due to differences in measurement protocols for VAT, outcomes used to assess metabolic risk, and analytic approaches used to determine the thresholds.
To our knowledge, the current study is the first to identify VAT thresholds associated with metabolic risk factors in African American adults; however, one previous study identified 106 and 163 cm2 as thresholds for elevated and significantly elevated cardiometabolic risk, respectively, in a combined sample of white and African American women (23). Ethnic differences in VAT thresholds observed in this study (58 cm2 in men and 44 cm2 in women) were greater than the ethnic differences in thresholds for the other obesity measures. VAT thresholds associated with risk were at or above the mean amount of VAT (Table 1) in all sex-by-race groups except African American men, where the identified threshold (81.9 cm2) was below the mean (90.3 cm2). Additional research is required to determine whether the ethnic differences in VAT thresholds in the current study are present in other samples and whether this risk is independent of risk associated with general adiposity. The degree to which differences in the VAT thresholds are due to true differences in risk associated with the visceral compartment compared with confounding as a result of the association between VAT and total adiposity has yet to be determined. This study addressed the clinical utility of absolute amounts of VAT; future studies should determine whether the use of a relative measure of VAT in relation to total adiposity might produce different results.
A recent study by Sumner et al (30) identified BMI thresholds of 30 and 32 in African American men and women, respectively, for the prediction of insulin resistance (30). These thresholds are similar to those obtained in the current study for African American men (31) and women (30) (Table 4). Corresponding WC thresholds in the study by Sumner et al (30) were 102 cm in men and 98 cm in women compared with 102 cm in men and 96 cm in women in the current study. The comparability of these results support the face validity of the results reported in the current study. More research is required to determine whether ethnic-specific BMI and WC thresholds are clinically more useful than single thresholds proposed by the NIH (32).
Body-composition estimates obtained by using DXA are becoming more common. For example, reference curves for body fat and bone mineral density have been produced from DXA data collected in the US NHANES (33). In the current study, FM and percentage of body fat had a somewhat lower utility than VAT, WC, and BMI (Table 4). A recent study in white and African Americans showed that DXA-derived estimates of FM were also inferior to WC in the prediction of MetS (34), which also supported earlier work that showed that the percentage of body fat from air-displacement plesthysmography was not superior to BMI and WC in the prediction of risk factors (35). Taken together, these results do not support the routine use of DXA in the assessment of obesity-related cardiometabolic risk when anthropometric measures such as BMI and WC are available; thus, the need for ethnic- and sex-specific thresholds of FM or percentage of body fat is not clear.
This study had several strengths and limitations. A marked strength was the large biethnic sample of men and women, with a wide range of age and BMI, and the availability of measured VAT, FM, percentage of body fat, and cardiometabolic risk factors. However, because the sample represented volunteers who have attended screening visits for clinical research studies, the results may not be generalizable to the wider population. Unfortunately, information on the use of lipid- or cholesterol-lowering medications was not available, and thus, we were unable to incorporate this information into the analysis. These results should be replicated in representative population samples as VAT data become more widely available. The current study uses a cross-sectional design, and as such, cause-and-effect conclusions could not be made. Future studies should focus on studying the association between VAT and cardiometabolic risk by using prospective research designs.
In conclusion, results of this study show that VAT is a useful clinical marker of cardiometabolic risk; however, its utility in this study was not any better than that of WC. Although VAT is currently measured most commonly by using computed tomography or magnetic resonance imaging, technological advances have allowed for more precise estimation by using DXA (36, 37), and it is likely that VAT will be assessed more readily in clinical settings in the near future. This study presents some preliminary thresholds for VAT that can be used clinically in white and African Americans until they can be verified or adapted by using data from representative population samples. In cases in which VAT cannot be directly assessed, it is recommended that WC should be measured.
Supplementary Material
Acknowledgments
We especially thank Emily Mire and Connie Murla for data management as well as the many clinical scientists and staff of the Pennington Biomedical Research Center who have contributed data to the development of the Pennington Center Longitudinal Study.
The authors’ responsibilities were as follows—PTK: designed and conducted the research, analyzed data, and had primary responsibility for the final content of the manuscript; and all authors: wrote the manuscript and read and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: DXA, dual-energy X-ray absorptiometry; FM, fat mass; MetS, metabolic syndrome; ROC, receiver operating characteristic; VAT, visceral adipose tissue; WC, waist circumference.
REFERENCES
- 1.NIH Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults–the evidence report. Obes Res 1998;6(suppl.2):51S–209S (Published erratum appears in Obes Res 1998;6:464.) [PubMed] [Google Scholar]
- 2.Cornier MA, Despres JP, Davis N, Grossniklaus DA, Klein S, Lamarche B, Lopez-Jimenez F, Rao G, St-Onge MP, Towfighi A, et al. Assessing adiposity: a scientific statement from the American Heart Association. Circulation 2011;124:1996–2019 [DOI] [PubMed] [Google Scholar]
- 3.Després JP, Lemieux I, Bergeron J, Pibarot P, Mathieu P, Larose E, Rodes-Cabau J, Bertrand OF, Poirier P. Abdominal obesity and the metabolic syndrome: contribution to global and cardiometabolic risk. Arterioscler Thromb Vasc Biol 2008;28:1039–49 [DOI] [PubMed] [Google Scholar]
- 4.Kuk JL, Katzmarzyk PT, Nichaman MZ, Church TS, Blair SN, Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity (Silver Spring) 2006;14:336–41 [DOI] [PubMed] [Google Scholar]
- 5.Després JP, Lamarche B. Effects of diet and physical activity on adiposity and body fat distribution: implications for the prevention of cardiovascular disease. Nutr Res Rev 1993;6:137–59 [DOI] [PubMed] [Google Scholar]
- 6.Hunter GR, Snyder SW, Kekes-Szabo T, Nicholson C, Berland L. Intra-abdominal adipose tissue values associated with risk of possessing elevated blood lipids and blood pressure. Obes Res 1994;2:563–8 [DOI] [PubMed] [Google Scholar]
- 7.Pickhardt PJ, Jee Y, O'Connor SD, Del Rio AM. Visceral adiposity and hepatic steatosis at abdominal CT: association with the metabolic syndrome. AJR Am J Roentgenol 2012;198:1100–7 [DOI] [PubMed] [Google Scholar]
- 8.von Eyben FE, Mouritsen E, Holm J, Dimcevski G, Montvilas P, Suciu G. Computed tomography scans of intra-abdominal fat, anthropometric measurements, and 3 nonobese metabolic risk factors. Metabolism 2006;55:1337–43 [DOI] [PubMed] [Google Scholar]
- 9.Williams MJ, Hunter GR, Kekes-Szabo T, Trueth MS, Snyder S, Berland L, Blaudeau T. Intra-abdominal adipose tissue cut-points related to elevated cardiovascular risk in women. Int J Obes Relat Metab Disord 1996;20:613–7 [PubMed] [Google Scholar]
- 10.Misra A, Wasir JS, Vikram NK, Pandey RM, Kumar P. Cutoffs of abdominal adipose tissue compartments as measured by magnetic resonance imaging for detection of cardiovascular risk factors in apparently healthy adult Asian Indians in North India. Metab Syndr Relat Disord 2010;8:243–7 [DOI] [PubMed] [Google Scholar]
- 11.Ye Y, Bao Y, Hou X, Pan X, Wu H, Li H, Wang C, Tang J, Lu H, Xiang K, et al. Identification of waist circumference cutoffs for abdominal obesity in the Chinese population: a 7.8-year follow-up study in the Shanghai urban area. Int J Obes (Lond) 2009;33:1058–62 [DOI] [PubMed] [Google Scholar]
- 12.Han JH, Park HS, Kim SM, Lee SY, Kim DJ, Choi WH. Visceral adipose tissue as a predictor for metabolic risk factors in the Korean population. Diabet Med 2008;25:106–10 [DOI] [PubMed] [Google Scholar]
- 13.Hyun YJ, Kim OY, Jang Y, Ha JW, Chae JS, Kim JY, Yeo HY, Paik JK, Lee JH. Evaluation of metabolic syndrome risk in Korean premenopausal women: not waist circumference but visceral fat. Circ J 2008;72:1308–15 [DOI] [PubMed] [Google Scholar]
- 14.Tanaka K, Okura T, Shigematsu R, Nakata Y, Lee DJ, Wee SW, Yamabuki K. Target value of intraabdominal fat area for improving coronary heart disease risk factors. Obes Res 2004;12:695–703 [DOI] [PubMed] [Google Scholar]
- 15.Oka R, Kobayashi J, Yagi K, Tanii H, Miyamoto S, Asano A, Hagishita T, Mori M, Moriuchi T, Kobayashi M, et al. Reassessment of the cutoff values of waist circumference and visceral fat area for identifying Japanese subjects at risk for the metabolic syndrome. Diabetes Res Clin Pract 2008;79:474–81 [DOI] [PubMed] [Google Scholar]
- 16.Hayashi T, Boyko EJ, McNeely MJ, Leonetti DL, Kahn SE, Fujimoto WY. Minimum waist and visceral fat values for identifying Japanese Americans at risk for the metabolic syndrome. Diabetes Care 2007;30:120–7 [DOI] [PubMed] [Google Scholar]
- 17.Demerath EW, Sun SS, Rogers N, Lee M, Reed D, Choh AC, Couch W, Czerwinski SA, Chumlea WC, Siervogel RM, et al. Anatomical patterning of visceral adipose tissue: race, sex, and age variation. Obesity (Silver Spring) 2007;15:2984–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Després J-P, Couillard C, Gagnon J, Bergeron J, Leon AS, Rao DC, Skinner JS, Wilmore JH, Bouchard C. Race, visceral adipose tissue, plasma lipids, and lipoprotein lipase activity in men and women: the Health, Risk Factors, Exercise Training, and Genetics (HERITAGE) family study. Arterioscler Thromb Vasc Biol 2000;20:1932–8 [DOI] [PubMed] [Google Scholar]
- 19.Hill JO, Sidney S, Lewis CE, Tolan K, Scherzinger AL, Stamm ER. Racial differences in amounts of visceral adipose tissue in young adults: the CARDIA (Coronary Artery Risk Development in Young Adults) study. Am J Clin Nutr 1999;69:381–7 [DOI] [PubMed] [Google Scholar]
- 20.Hoffman DJ, Wang Z, Gallagher D, Heymsfield SB. Comparison of visceral adipose tissue mass in adult African Americans and whites. Obes Res 2005;13:66–74 [DOI] [PubMed] [Google Scholar]
- 21.Katzmarzyk PT, Bray GA, Greenway FL, Johnson WD, Newton RL, Jr, Ravussin E, Ryan DH, Smith SR, Bouchard C. Racial differences in abdominal depot-specific adiposity in white and African American adults. Am J Clin Nutr 2010;91:7–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barreira TV, Staiano AE, Harrington DM, Heymsfield SB, Smith SR, Bouchard C, Katzmarzyk PT. Anthropometric correlates of total body fat, abdominal adiposity and cardiovascular disease risk factors in a biracial sample of men and women. Mayo Clin Proc 2012;87:452–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicklas BJ, Penninx BW, Ryan AS, Berman DM, Lynch NA, Dennis KE. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diabetes Care 2003;26:1413–20 [DOI] [PubMed] [Google Scholar]
- 24.Smith SR, Lovejoy JC, Greenway F, Ryan D, deJonge L, de la Bretonne J, Volafova J, Bray GA. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 2001;50:425–35 [DOI] [PubMed] [Google Scholar]
- 25.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jones DW, Materson BJ, Oparil S, Wright J, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 2003;289:2560–72 [DOI] [PubMed] [Google Scholar]
- 26.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97 [DOI] [PubMed] [Google Scholar]
- 27.American Diabetes Association Standards of medical care in diabetes—2012. Diabetes Care 2012;35(suppl 1):S11–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart JC, James WP, Loria CM, Smith SC., Jr Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5 [DOI] [PubMed] [Google Scholar]
- 29.Perkins NJ, Schisterman EF. The inconsistency of “optimal” cutpoints obtained using two criteria based on the receiver operating characteristic curve. Am J Epidemiol 2006;163:670–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sumner AE, Sen S, Ricks M, Frempong BA, Sebring NG, Kushner H. Determining the waist circumference in African Americans which best predicts insulin resistance. Obesity (Silver Spring) 2008;16:841–6 [DOI] [PubMed] [Google Scholar]
- 31.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 1988;44:837–45 [PubMed] [Google Scholar]
- 32.NIH The practical guide to the identification, evaluation and treatment of overweight and obesity in adults. Bethesda, MD: US NIH, 2000 [Google Scholar]
- 33.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS ONE 2009;4:e7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beydoun MA, Kuczmarski MT, Wang Y, Mason MA, Evans MK, Zonderman AB. Receiver-operating characteristics of adiposity for metabolic syndrome: the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. Public Health Nutr 2011;14:77–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bosy-Westphal A, Geisler C, Onur S, Korth O, Selberg O, Schrezenmeir J, Muller MJ. Value of body fat mass vs anthropometric obesity indices in the assessment of metabolic risk factors. Int J Obes (Lond) 2006;30:475–83 [DOI] [PubMed] [Google Scholar]
- 36.Kaul S, Rothney MP, Peters DM, Wacker WK, Davis CE, Shapiro MD, Ergun DL. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity (Silver Spring) 2012;20:1313–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Micklesfield LK, Goedecke JH, Punyanitya M, Wilson KE, Kelly TL. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity (Silver Spring) 2012;20:1109–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.