Abstract
Background: In older adults, every 0.1-m/s slower gait speed is associated with a 12% higher mortality. However, little research has identified risk factors for gait-speed decline.
Objective: We assessed the association between several measures of body composition and age-related decline in gait speed.
Design: Data were from 2306 older adults who were participating in the Health, Aging, and Body Composition cohort and were followed for 4 y (50% women; 38% black). Usual walking speed (m/s) over 20 m was measured in years 2 through 6, and the baseline and changes in several measures of body composition were included in mixed-effects models.
Results: Gait speed declined by 0.06 ± 0.00 m/s over the 4-y period. Baseline thigh intermuscular fat predicted the annual gait-speed decline (±SE) in both men and women (−0.01 ± 0.00 and −0.02 ± 0.00 m/s per 0.57 cm2, respectively; P < 0.01). In men, but not in women, this relation was independent of total body adiposity. In longitudinal analyses, changes in thigh intermuscular fat and total thigh muscle were the only body-composition measures that predicted gait-speed decline in men and women combined. When modeled together, every 5.75-cm2 increase in thigh intermuscular fat was associated with a 0.01 ± 0.00-m/s decrease in gait speed, whereas every 16.92-cm2 decrease in thigh muscle was associated with a 0.01 ± 0.00-m/s decrease in gait speed.
Conclusions: High and increasing thigh intermuscular fat are important predictors of gait-speed decline, implying that fat infiltration into muscle contributes to a loss of mobility with age. Conversely, a decreasing thigh muscle area is also predictive of a decline in gait speed.
INTRODUCTION
Physical function declines with age (1, 2), and the loss of function consistently predicts higher rates of disability, institutionalization, and mortality (3–5). Thus for older adults, the preservation of physical function is critically important for prolonged and independent living. Because of the growing demographic of persons ≥65 y of age, the identification of modifiable predictors of functional decline is of considerable public health interest.
Gait speed is a simple, but important, indicator of functional status in older adults (6–8), with a 12% greater mortality associated with every 0.1-m/s slower gait speed (6). The act of walking requires considerable energy and coordination, and declines in gait speed signify reduced energy availability, potential damage to one or more body systems, and impairments in their integration. However, despite its importance, a paucity of literature has identified risk factors for age-related gait-speed decline (9).
Body composition, specifically excess adipose tissue and lower lean mass, is associated with worse physical function in older adults (10, 11). Previous longitudinal data reported an association between BMI, waist circumference, and body fat percentage and incident mobility limitation (12). Likewise, lower muscle mass is associated with increased risk of mobility loss in older men and women (13). Although provocative, these findings were based on self-reported mobility disability, and confirmation is warranted by using an objectively measured walking speed. Moreover, a delineation of the independent contributions of changes in lean- and fat-mass depots on gait-speed decline is needed because findings have the potential to optimize intervention strategies. Therefore, in this study, we aimed to assess the association between several dimensions of body composition at baseline and over time on the 4-y annualized change in gait speed in the Health, Aging, and Body Composition (Health ABC)5 cohort.
SUBJECTS AND METHODS
Study population
Participants were from the Health ABC study, which is a prospective cohort study of 3075 initially well-functioning white and black adults aged 70–79 y. Participants were recruited in 1997 and 1998 from a random sample of white and all-black Medicare-eligible beneficiaries residing in the areas surrounding Pittsburgh, PA, and Memphis, TN. Participants were excluded if they 1) reported difficulty walking one-quarter of a mile, walking up 10 steps, or performing basic activities of daily living; 2) had active cancer treatment in the past 3 y; or 3) had plans to move out of the area in the next 3 y.
Participants who attended the year 2 clinic visit (1998–1999), when their usual 20-m walking speeds were first measured, served as the baseline population for this analysis (n = 2998). Participants who lacked a baseline gait speed (n = 74), body-composition predictor variables (n = 604), and pertinent covariates (n = 14) were excluded, which yielded an analysis sample of 2306 participants. For analyses of the change in body composition, 1267 participants had follow-up data on all body-composition measures and were included. All participants provided written informed consent, which was approved by the institutional review boards (IRBs) of the clinical and coordinating sites (University of Tennessee IRB approval 95-05531-FB, University of Pittsburgh IRB approval 960212, and University of California, San Francisco IRB approval H5254-12688-15), and STrengthening the Reporting of OBservational studies in Epidemiology guidelines were followed.
Measurements
Gait speed
Gait speed was assessed by measuring the usual time to complete a 20-m walk annually in years 2 (baseline) through 6 of the study (14). Participants were instructed to walk at their usual pace for a distance of 20 m, while trained staff recorded time to the nearest 0.01 s.
Body composition
Body weight was measured to the nearest 0.1 kg with a standard balance-beam scale. Body height was measured to the nearest 0.1 cm by using a wall-mounted stadiometer. BMI (in kg/m2) was calculated by dividing body mass by the square of height in meters. Total body fat mass, percentage body fat, total body lean mass, appendicular lean mass, and leg lean mass were acquired from total body scans by using fan-beam dual-energy X-ray absorptiometry (Hologic QDR 4500A; Hologic) with dual-energy X-ray absorptiometry software (Hologic). Computed tomography scans of the abdomen and thigh were obtained in Memphis by using a Somatom Plus 4 scanner (Siemens) or a Picker PQ 2000S scanner (Marconi Medical Systems) and a 9800 Advantage scanner (General Electric) in Pittsburgh. Scans were obtained at 120 kVp, 200–250-mA seconds, at a slice thickness of 10 mm. Areas were calculated by multiplying the number of pixels of a given tissue type by the pixel area with Interactive Data Language development software (RSI Systems). Scans of the abdomen were taken at the level of the space between the fourth and fifth lumbar vertebrae (L4–L5). The scan at midthigh level was acquired halfway between the medial edge of the greater trochanter and the intercondyloid fossa. Visceral fat was manually distinguished from the abdominal subcutaneous fat area by tracing along the fascial plane that defines the internal abdominal wall. In the thigh, intermuscular fat tissue and visible intramuscular fat tissue were separated from subcutaneous adipose tissue by drawing a line along the deep fascial plane that surrounds the thigh muscles. Areas of left and right thighs were added. All body-composition variables were obtained at years 2 and 6 except computed tomography–acquired body-composition measures, which were obtained at years 1 and 6.
Covariates
Relevant covariates included sex, race, site, education, height, and smoking status at the year 1 visit and age, prevalent cardiovascular disease (CVD), chronic lung disease (CLD), diabetes, physical activity level, and chronic knee pain at the year 2 visit. Self-rated health was determined at both visits. Demographic characteristics (age, sex, race, and education), smoking status, and health status were ascertained by using an interviewer-administered questionnaire. Physical activity was based on the reported time spent walking for exercise or other walking (eg, for transportation) over the past 7 d. Knee pain on most days for ≥1 mo in the past 1 y was assessed by self-reports. The prevalence of diabetes, CVD (coronary artery disease or stroke), and CLD were determined by using algorithms that were based on self-reports and medication use.
Statistical analysis
All data were initially analyzed by using descriptive statistics. Baseline demographic data, including age, sex, race, site, educational level, height, usual walking speed, smoking status, prevalent disease (CVD, CLD, and diabetes), physical activity level, chronic knee pain, and self-rated health status, were used for population descriptive information as well as covariate analyses. Sample means and SDs were computed for continuous variables, and counts and proportions were calculated for discrete variables. To evaluate the association between baseline body-composition measures and gait-speed decline, mixed-effects models that included standardized measures of body composition were used. The basic model (model 1) estimated the association between the change in gait speed and each standardized body-composition measure individually, with adjustment for year, baseline (year 2) gait speed, age, race, study site, education, and height. Analyses were further adjusted (model 2) for smoking status, prevalent disease (CVD, CLD, and diabetes), physical activity, knee pain, and self-rated health status. Last, BMI was added to all models to determine the independence of individual body-composition measures from global adiposity. To minimize the heterogeneity of variance and best approximate the conditional normality assumption, baseline areas of abdominal subcutaneous fat, thigh muscle, thigh subcutaneous fat, and thigh intermuscular fat were log transformed. To model the association between changes in body-composition measures and gait-speed decline, mixed-effects models that included standardized measures of changes in body-composition variables (as well as the same covariates previously listed) were used, again by modeling the change in each standardized body-composition measure individually. Interactions between body-composition variables and sex were tested, with stratified results presented as appropriate. In all cases, the goodness of fit of a model was assessed by using the Akaike information criteria (AIC). All analyses were generated with SAS software (version 9.1; SAS Institute) with assumption of a type 1 error rate of 0.05 for all hypothesis tests.
RESULTS
Participant characteristics
The mean age of the study sample (n = 2306) was 74.6 ± 2.9 y and 50% of subjects were women. Excluded participants (n = 692) were more likely to be older, of female sex, black, have less than a high school education, currently smoke, and report worse overall health (P < 0.01). Excluded participants also had higher BMI and slower walking speed at baseline than the study sample did (P < 0.01). Characteristics of men and women at baseline are shown in Table 1. Compared with subjects included in analyses of the change in body composition (n = 1267), excluded individuals (n = 1039) had slower walking speed (−0.08 ± 0.01 m/s; P < 0.01) and more thigh intermuscular fat (1.14 ± 0.26 cm2; P < 0.01) at baseline.
TABLE 1.
Characteristics of men and women at baseline1
| Characteristics | Women (n = 1158) | Men (n = 1148) |
| Age (y) | 74.5 ± 2.8 | 74.7 ± 2.9 |
| Black race [n (%)] | 487 (42) | 399 (35) |
| Site (Memphis) [n (%)] | 584 (50) | 554 (48) |
| Years of education [n (%)] | ||
| Less than high school | 249 (21) | 287 (25) |
| High school graduate | 441 (38) | 292 (25) |
| Postsecondary | 468 (41) | 569 (50) |
| Smoking status [n (%)] | ||
| Never | 673 (58) | 349 (30) |
| Current | 96 (8) | 118 (10) |
| Former | 389 (34) | 681 (59) |
| Physical activity level [n (%)] | ||
| Low (<1000 kcal/wk) | 497 (43) | 396 (35) |
| Moderate (1000–2000 kcal/wk) | 383 (33) | 349 (30) |
| High (>2000 kcal/wk) | 278 (24) | 403 (35) |
| Prevalent disease [n (%)] | ||
| Cardiovascular disease | 260 (22) | 385 (34) |
| Chronic lung disease | 176 (15) | 133 (12) |
| Diabetes | 193 (17) | 254 (22) |
| Chronic knee pain [n (%)] | 331 (29) | 246 (21) |
| Excellent or good self-rated health status [n (%)] | 515 (45) | 578 (50) |
| Height (cm) | 159.6 ± 6.0 | 173.3 ± 6.4 |
| Weight (kg) | 69.1 ± 13.7 | 80.7 ± 13.0 |
| BMI (kg/m2) | 27.1 ± 5.1 | 26.9 ± 3.9 |
| DXA-acquired body-composition measures | ||
| Total fat mass (kg) | 28.3 ± 8.7 | 24.0 ± 7.2 |
| Percentage of body fat | 40.1 ± 5.7 | 29.3 ± 5.0 |
| Total lean mass (kg) | 40.9 ± 5.9 | 56.6 ± 7.2 |
| Appendicular lean mass (kg) | 17.4 ± 3.1 | 25.2 ± 3.7 |
| Leg lean mass (kg) | 13.1 ± 2.4 | 18.1 ± 2.7 |
| CT-acquired body-composition measures | ||
| Abdominal visceral fat (cm2) | 129.7 ± 58.7 | 153.9 ± 71.4 |
| Abdominal subcutaneous fat area (cm2) | 335.0 ± 122.4 | 226.3 ± 86.9 |
| Thigh intermuscular fat area (cm2) | 10.2 ± 5.9 | 9.8 ± 6.6 |
| Thigh subcutaneous fat area (cm2) | 104.4 ± 42.7 | 47.2 ± 20.1 |
| Total thigh muscle area (cm2) | 92.0 ± 16.7 | 131.8 ± 21.9 |
| Baseline usual 20-m walking speed (m/s) | 1.10 ± 0.21 | 1.19 ± 0.21 |
All values are means ± SDs for continuous variables and n (%) for categorical variables. All data were collected at the year 1 visit, except for weight, BMI, and DXA adiposity measures, which were obtained at the year 2 visit. CT, computed tomography; DXA, dual-energy X-ray absorptiometry.
Associations between gait-speed decline and baseline body composition
In the baseline study sample, the mean gait-speed decline was 0.06 ± 0.00 m/s over 4 y. The gait-speed decline was similar by sex; however, the baseline gait speed was 0.10 m/s slower in women, which resulted in consistently slower gait speeds across all time points.
Interactions between several baseline body-composition predictor variables and sex were significant (P > 0.05); therefore, relations between gait-speed decline and standardized baseline measures of body composition are presented for men and women separately (Tables 2 and 3, respectively). For men, after basic adjustment (model 1), baseline weight, BMI, and all adiposity-related variables, except abdominal subcutaneous fat area, were modestly and inversely associated with gait-speed decline (all P ≤ 0.05, except for thigh subcutaneous fat area, for which P = 0.08), whereas no association was shown between lean-mass measures and gait-speed decline. In the fully adjusted model, only thigh intermuscular fat was significantly associated with gait-speed decline in men (P < 0.01). The association between thigh intermuscular fat and gait-speed decline remained after additional adjustment for total-body fat mass. For women, all baseline measures of body composition, except the total thigh muscle area, were significantly and inversely associated with gait-speed decline in both the basic and fully adjusted model. AIC analysis revealed that the basic covariate model that contained thigh intermuscular fat had the best fit in men, whereas more-global measures of body composition (ie, body weight and BMI) had the best fit in women.
TABLE 2.
Baseline measures of body composition and gait-speed decline in men (n = 1148)1
| Standardized baseline predictor variable | Change in gait speed (m/s)2 | AIC | P |
| Weight (per 4.55 kg) | |||
| Model 1 | −0.009 ± 0.0047 | −4544.3 | 0.06 |
| Model 2 | −0.0052 ± 0.0048 | −4524.0 | 0.28 |
| BMI (per 14.57 kg/m2) | |||
| Model 1 | −0.0087 ± 0.0045 | −4544.4 | 0.05 |
| Model 2 | −0.0051 ± 0.0045 | −4524.0 | 0.26 |
| DXA-acquired body-composition measures | |||
| Total fat mass (per 83.07 g) | |||
| Model 1 | −0.0109 ± 0.0045 | −4546.5 | 0.02 |
| Model 2 | −0.0079 ± 0.0045 | −4525.7 | 0.08 |
| Percentage body fat (per 7.62%) | |||
| Model 1 | −0.0139 ± 0.0059 | −4546.7 | 0.02 |
| Model 2 | −0.011 ± 0.0059 | −4526.7 | 0.06 |
| Total lean mass (per 102.49 g) | |||
| Model 1 | −0.005 ± 0.0066 | −4542.0 | 0.45 |
| Model 2 | 0.0005 ± 0.0067 | −4523.5 | 0.94 |
| Appendicular lean mass (per 52.27 g) | |||
| Model 1 | 0.004 ± 0.0067 | −4541.8 | 0.55 |
| Model 2 | 0.0081 ± 0.0067 | −4524.9 | 0.22 |
| Leg lean mass (per 35.74 g) | |||
| Model 1 | 0.0029 ± 0.0062 | −4541.5 | 0.64 |
| Model 2 | 0.0065 ± 0.0063 | −4524.4 | 0.3 |
| CT-acquired body-composition measures | |||
| Abdominal visceral fat area (per 66.46 cm2) | |||
| Model 1 | −0.0083 ± 0.0037 | −4545.2 | 0.02 |
| Model 2 | −0.005 ± 0.0037 | −4524.1 | 0.18 |
| Abdominal subcutaneous fat area (per 0.46 cm2) | |||
| Model 1 | −0.0059 ± 0.0042 | −4542.5 | 0.16 |
| Model 2 | −0.0037 ± 0.0042 | −4523.3 | 0.37 |
| Thigh intermuscular fat area (per 0.57 cm2) | |||
| Model 1 | −0.0143 ± 0.0038 | −4554.4 | <0.01 |
| Model 2 | −0.0119 ± 0.0038 | −4531.9 | <0.01 |
| Thigh subcutaneous fat area (per 0.58 cm2) | |||
| Model 1 | −0.0091 ± 0.0053 | −4544.0 | 0.08 |
| Model 2 | −0.0084 ± 0.0052 | −4525.6 | 0.11 |
| Total thigh muscle area (per 0.25 cm2) | |||
| Model 1 | 0.0102 ± 0.0062 | −4544.0 | 0.1 |
| Model 2 | 0.0113 ± 0.0062 | −4526.7 | 0.07 |
Abdominal subcutaneous fat, thigh intermuscular fat, thigh subcutaneous fat, and total thigh muscle areas were log transformed. Model 1 was adjusted for year, baseline (year 2) gait speed, age, race, study site, education, and height. Model 2 was adjusted as for model 1 and for smoking status, prevalent disease (diabetes, cardiovascular disease, and chronic lung disease), physical activity, knee pain, and self-rated health status. P values were based on F tests for the significance of estimated mixed-model effects. All body-composition data were collected at the year 2 visit, except for CT measures, which were collected at year 1. AIC, Akaike information criteria; CT, computed tomography; DXA, dual-energy X-ray absorptiometry.
All values are normalized variable estimates (β) ± SEs.
TABLE 3.
Baseline measures of body composition and gait-speed decline in women (n = 1158)1
| Standardized baseline predictor variable | Change in gait speed (m/s)2 | AIC | P |
| Weight (per 4.55 kg) | |||
| Model 1 | −0.0249 ± 0.0045 | −5123.8 | <0.01 |
| Model 2 | −0.0214 ± 0.0046 | −5110.1 | <0.01 |
| BMI (per 14.57 kg/m2) | |||
| Model 1 | −0.0196 ± 0.0036 | −5122.5 | <0.01 |
| Model 2 | −0.0168 ± 0.0037 | −5109.0 | <0.01 |
| DXA-acquired body-composition measures | |||
| Total fat mass (per 83.07 g) | |||
| Model 1 | −0.0207 ± 0.0038 | −5123.0 | <0.01 |
| Model 2 | −0.0184 ± 0.0038 | −5111.2 | <0.01 |
| Percentage of body fat (per 7.62%) | |||
| Model 1 | −0.022 ± 0.0051 | −5112.5 | <0.01 |
| Model 2 | −0.0204 ± 0.0051 | −5104.6 | <0.01 |
| Total lean mass (per 102.49 g) | |||
| Model 1 | −0.0348 ± 0.0081 | −5113.4 | <0.01 |
| Model 2 | −0.0267 ± 0.0083 | −5100.3 | <0.01 |
| Appendicular lean mass (per 52.27 g) | |||
| Model 1 | −0.0333 ± 0.008 | −5112.3 | <0.01 |
| Model 2 | −0.0274 ± 0.0081 | −5101.3 | <0.01 |
| Leg lean mass (per 35.74 g) | |||
| Model 1 | −0.0305 ± 0.0071 | −5113.4 | <0.01 |
| Model 2 | −0.026 ± 0.0071 | −5103.0 | <0.01 |
| CT-acquired body-composition measures | |||
| Abdominal visceral fat area (per 66.46 cm2) | |||
| Model 1 | −0.019 ± 0.0043 | −5113.3 | <0.01 |
| Model 2 | −0.0142 ± 0.0044 | −5099.2 | <0.01 |
| Abdominal subcutaneous fat area (per 0.46 cm2) | |||
| Model 1 | −0.0143 ± 0.0045 | −5104.1 | <0.01 |
| Model 2 | −0.0127 ± 0.0045 | −5096.7 | <0.01 |
| Thigh intermuscular fat area (per 0.57 cm2) | |||
| Model 1 | −0.0188 ± 0.0041 | −5114.8 | <0.01 |
| Model 2 | −0.0168 ± 0.004 | −5105.6 | <0.01 |
| Thigh subcutaneous fat area (per 0.58 cm2) | |||
| Model 1 | −0.0215 ± 0.0053 | −5110.3 | <0.01 |
| Model 2 | −0.0215 ± 0.0053 | −5105.1 | <0.01 |
| Total thigh muscle area (per 0.25 cm2) | |||
| Model 1 | 0.0005 ± 0.0062 | −5094.6 | 0.94 |
| Model 2 | 0.0044 ± 0.0062 | −5089.9 | 0.47 |
Abdominal subcutaneous fat, thigh intermuscular fat, thigh subcutaneous fat, and total thigh muscle area were log transformed. Model 1 was adjusted for year, baseline (year 2) gait speed, age, race, study site, education, and height. Model 2 was adjusted as for model 1 and for smoking status, prevalent disease (diabetes, cardiovascular disease, and chronic lung disease), physical activity, knee pain, and self-rated health status. P values were based on F tests for the significance of estimated mixed-model effects. All body-composition data were collected at the year 2 visit, except for CT measures, which were collected at year 1. AIC, Akaike information criteria; CT, computed tomography; DXA, dual-energy X-ray absorptiometry.
All values are normalized variable estimates (β) ± SEs.
Associations between gait-speed decline and change in body composition
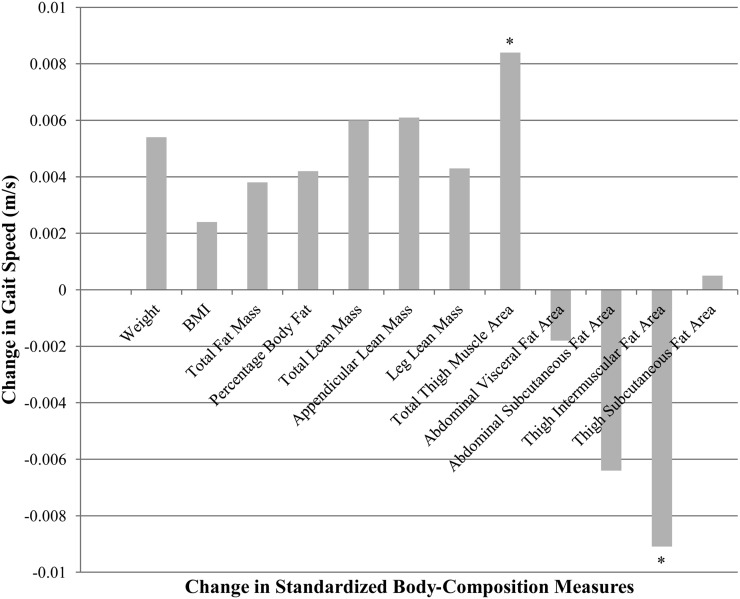
The average weight declined by 1.16 ± 4.51 kg over the 4-y period, and 49% and 66% of participants experienced a loss of fat and lean mass, respectively. No interactions of sex by change in body composition were shown to be significant (all P > 0.05); thus, gait-speed decline adjusted for changes in standardized measures of body composition in men and women combined is shown in Table 4. After full adjustment (model 2), only changes in thigh intermuscular fat area and total thigh muscle area were associated with declines in gait speed. Likewise, models that contained changes in total thigh muscle area and thigh intermuscular fat yielded the most negative AIC (−6448.8 and −6450.1, respectively) or best fit. As expected, the change in total thigh muscle area was directly associated with the change in gait speed, whereas the change in thigh intermuscular fat showed an inverse association. Findings remained after additional adjustment for total-body fat mass. A graphical depiction of variable estimates for each measure of the standardized change in body compartments is presented in Figure 1 and shown that the magnitude of the effect was greatest for the change in both thigh intermuscular fat and total thigh muscle area.
TABLE 4.
Changes in measures of body composition and gait-speed decline in men and women combined (n = 1267)1
| Standardized change in predictor variable | Change in gait speed (m/s)2 | AIC | P |
| Weight (per 4.51 kg) | |||
| Model 1 | 0.0049 ± 0.0033 | −6443.9 | 0.14 |
| Model 2 | 0.0054 ± 0.0032 | −6445.5 | 0.1 |
| BMI (per 4.32 kg/m2) | |||
| Model 1 | 0.0017 ± 0.0033 | −6442.0 | 0.61 |
| Model 2 | 0.0024 ± 0.0032 | −6443.3 | 0.45 |
| DXA-acquired body-composition measures | |||
| Total fat mass (per 32.52 g) | |||
| Model 1 | 0.0025 ± 0.0033 | −6442.3 | 0.46 |
| Model 2 | 0.0038 ± 0.0032 | −6444.1 | 0.24 |
| Percentage of body fat (per 2.62%) | |||
| Model 1 | 0.0026 ± 0.0034 | −6442.3 | 0.45 |
| Model 2 | 0.0042 ± 0.0033 | −6444.4 | 0.21 |
| Total lean mass (per 20.73 g) | |||
| Model 1 | 0.0072 ± 0.0035 | −6446.1 | 0.04 |
| Model 2 | 0.006 ± 0.0034 | −6446 | 0.08 |
| Appendicular lean mass (per 11.13 g) | |||
| Model 1 | 0.0077 ± 0.0034 | −6446.9 | 0.02 |
| Model 2 | 0.0061 ± 0.0033 | −6446.2 | 0.07 |
| Leg lean mass (per 8.97 kg) | |||
| Model 1 | 0.0055 ± 0.0034 | −6444.5 | 0.1 |
| Model 2 | 0.0043 ± 0.0033 | −6444.5 | 0.19 |
| CT-acquired body-composition measures | |||
| Abdominal visceral fat area (per 39.77 cm2) | |||
| Model 1 | −0.0023 ± 0.0034 | −6442.2 | 0.5 |
| Model 2 | −0.0018 ± 0.0033 | −6443.1 | 0.58 |
| Abdominal subcutaneous fat area (per 46.44 cm2) | |||
| Model 1 | −0.0052 ± 0.0034 | −6444.2 | 0.12 |
| Model 2 | −0.0064 ± 0.0033 | −6446.6 | 0.05 |
| Thigh intermuscular fat area (per 5.75 cm2) | |||
| Model 1 | −0.0099 ± 0.0034 | −6450.1 | <0.01 |
| Model 2 | −0.0091 ± 0.0034 | −6450.1 | <0.01 |
| Thigh subcutaneous fat area (per 26.30 cm2) | |||
| Model 1 | −0.001 ± 0.0033 | −6441.8 | 0.76 |
| Model 2 | 0.0005 ± 0.0033 | −6442.8 | 0.87 |
| Total thigh muscle area (per 16.92 cm2) | |||
| Model 1 | 0.0093 ± 0.0035 | −6448.8 | <0.01 |
| Model 2 | 0.0084 ± 0.0035 | −6448.8 | 0.02 |
Model 1 was adjusted for year, baseline (year 2) gait speed, age, race, sex, study site, education, and height. Model 2 was adjusted as for model 1 and for smoking status, prevalent disease (diabetes, cardiovascular disease, and chronic lung disease), physical activity, knee pain, and self-rated health status. P values were based on F tests for the significance of estimated mixed-model effects. All changes in adiposity are represented as the year 6 minus year 2 measurements, except for CT-acquired adiposity measures (year 6 minus year 1 measurements). AIC, Akaike information criteria; CT, computed tomography; DXA, dual-energy X-ray absorptiometry.
All values are normalized variable estimates (β) ± SEs.
FIGURE 1.
Change in gait speed per change in standardized body-composition measure. Variable estimates were adjusted for year, baseline (year 2) gait speed, age, race, study site, education, height, smoking status, prevalent disease (diabetes, cardiovascular disease, or chronic lung disease), physical activity, knee pain, and self-rated health status (n = 1267). *P < 0.05.
To assess whether these changes were independent of weight loss, we reran the longitudinal body-composition change model (Table 4; see Results) by using the change in weight as a covariate. Our findings remained mostly unchanged; both the change in thigh intermuscular fat area (P < 0.001) and the change in total thigh muscle area (P = 0.028) remained significant, whereas most other body-size and -composition predictors (eg, all except BMI) remained nonsignificant after adjustment for weight change. The lone exception was BMI, which became significant (P < 0.001) after adjustment for the change in weight; however, this finding was likely due to high multicollinearity between the BMI change and weight change at 6 y (r = 0.97).
Relative contributions of fat- and lean-mass measures as predictors of gait-speed decline
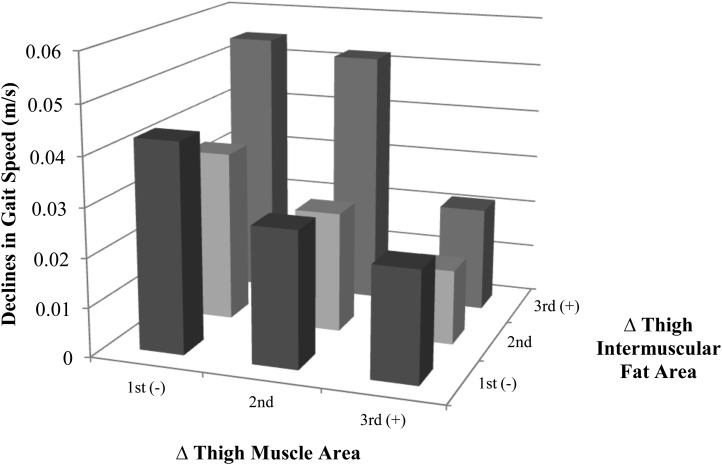
When thigh intermuscular fat and total thigh muscle area were included in the same model, both increasing thigh intermuscular fat and decreasing total thigh muscle area were significant predictors of gait-speed decline (both P < 0.01); however, a significant interaction between thigh intermuscular fat and total thigh muscle area was not observed (P = 0.79). Specifically, for every 5.75-cm2 increase in thigh intermuscular fat, the annual gait speed declined by 0.01 ± 0.00 m/s, and for every 16.92-cm2 decrease in thigh muscle area, the annual gait speed declined by 0.01 ± 0.00 m/s. As presented in Figure 2, the descriptive association between the change in gait speed by tertiles of change in thigh intermuscular fat (cutoffs: <2.86 and ≥6.95 cm2) and thigh muscle area (cutoffs: less than −15.29 and at least −1.84 cm2); where the first tertile represents the greatest relative decrease in area, and the third tertile represents the greatest relative increase in area. Results showed that participants who lost the most thigh muscle area and gained the most thigh intermuscular fat (ie, first and third tertiles of muscle and fat areas, respectively) had the greatest declines (ie, 0.05 m/s) in gait speed over the 4-y period.
FIGURE 2.
Association between the change in gait speed and changes in thigh intermuscular fat and thigh muscle area. Variable estimates were adjusted for year, baseline (year 2) gait speed, age, race, study site, education, and height (n = 1267). Tertile 1 represents the greatest relative decrease in body-composition area, and tertile 3 represents the greatest relative increase in body-composition area. Cutoffs for the change in thigh intermuscular fat were <2.86 and ≥6.95 cm2 and for the change in thigh muscle area were less than −15.29 and at least −1.84 cm2. Participants who lost the most thigh muscle area and gained the most thigh intermuscular fat saw the greatest declines (ie, 0.05 m/s) in gait speed over the 4-y period.
DISCUSSION
Usual gait speed declines with age, and in older adults, slower gait speeds predict higher rates of disability, institutionalization, and mortality (3–5). As the burden of disability becomes increasingly common and expensive, the identification of modifiable contributors to functional decline is emerging as a significant priority of public health research. Results from this study showed that a high and increasing thigh intermuscular fat area along with a decreasing total thigh muscle area are important predictors of gait-speed decline. Importantly, the 4-y decline in gait speed observed for individuals who lost the most thigh muscle area and gained the most intermuscular fat (ie, 0.05 m/s), although small, was clinically meaningful (15).
The cross-sectional association between a slower gait speed and elevated BMI is well established (10). A slow gait speed (<0.6 m/s) is more prevalent in obese men (21.3% compared with 14.5%) and women (31.7% compared with 14.6%) than in nonobese counterparts (16), with every SD increment of BMI associated with a 5% reduction in the usual walking speed (17). Likewise, several longitudinal studies suggested a link between elevated BMI and gait-speed decline (18–21). Although previous longitudinal data reported a direct association between the amount of fat mass (12) and an inverse (13) [or null (22, 23)] association between the amount of muscle mass and development of self-reported incident mobility limitation, to the best of our knowledge, this is the first article to address the independent association between changes in measures of body composition and gait speed. Interestingly, elevations of several baseline adiposity measures were associated with a decline in gait speed, especially in women; however, only the change in thigh intermuscular fat was a significant, independent predictor. Conversely, the baseline thigh muscle area was not associated with gait-speed decline; however, the change in thigh muscle area was directly associated with the change in gait speed. Collectively, these results suggest that the total adiposity and associated fat infiltration into muscle are important predictors of gait-speed decline, whereas gains in thigh muscle area may counter this process. Additional research is needed to clarify the cause of the concomitant age-related loss in muscle and gain in fat mass observed in this and other studies (24–26).
Little is known regarding the mechanisms of action that underlie the association between increased thigh intermuscular fat and gait-speed decline; however, part of this observation may be explained by the endocrine nature of adipose tissue. For instance, several cytokines are secreted from adipose tissue (27), and excessive fat accumulation can induce a proinflammatory state. Chronic inflammation is associated with lower muscle strength (28), and predicts disability in older adults (29, 30), potentially as a result of impaired muscle-fiber contractility (31). In addition, excessive adiposity can downregulate the anabolic actions of insulin (32), testosterone, (33) and growth hormone (34), all of which may contribute to a progressive loss of muscle mass and associated function, especially when they occur locally. The direct association observed between the change in thigh muscle area and gait speed is better understood; however, improvements in muscle-related factors other than mass, including strength and quality, may be more critical to the perseveration of physical function (13, 35).
Strengths of the current study included the large sample size, long follow-up duration, and objective measure of gait speed over several time points. In addition, the novel comparison of multiple, increasingly sophisticated measures of body composition was a strength.
However, certain limitations of the study design need to be considered. First, to allow for direct comparison of our statistical models, standardized measures of body composition were used. This technique requires symmetric data and necessitated the log transformation of certain body-composition measures, which diminished the clinical interpretability of our data. Second, a survivorship bias may have occurred that led to an underestimation of the association between changes in body-composition measures and gait-speed decline because those individuals who missed follow-up assessments (ie, due to health problems or death) likely had body-composition changes that were more unfavorable than those experienced by included participants. Furthermore, the modest reduction in sample sizes in models that associated a change in body composition with a change in gait speed may have limited the power to detect differential sex effects. Last, as with all observational studies, our ability to draw causal inferences from this data were limited; however, longitudinal evidence suggested that a localized decline in muscle mass and increase in fat infiltration are highly and independently predictive of declines in gait speed over time.
In conclusion, findings from this study suggest that both the preservation of thigh muscle mass and prevention of fat infiltration into muscle are important in the prevention of age-related declines in gait speed. Because gait speed represents a critically important and modifiable predictor of independent living in older adults, future research should test whether targeted reductions in thigh intermuscular fat, an augmentation of the thigh muscle area, or both yield an improved walking speed and whether such improvements ultimately translate into prolonged independence.
Acknowledgments
The authors’ responsibilities were as follows—TBH and ABN: designed the research; TFH: conducted the research; DPB and KMB: analyzed data; DKH, AK, ABN, EMS, SAS, BJN, and SBK: contributed to the writing of the manuscript; KMB: had primary responsibility for the final content of the manuscript; and all authors: read and approved the final manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: AIC, Akaike information criteria; CLD, chronic lung disease; CVD, cardiovascular disease; Health ABC, Health, Aging, and Body Composition; IRB, institutional review board.
REFERENCES
- 1.Onder G, Penninx BW, Lapuerta P, Fried LP, Ostir GV, Guralnik JM, Pahor MP. Change in physical performance over time in older women: the Women's Health and Aging Study. J Gerontol A Biol Sci Med Sci 2002;57:M289–93 [DOI] [PubMed] [Google Scholar]
- 2.Lindle RS, Metter EJ, Lynch NA, Fleg JL, Fozard JL, Tobin J, Roy TA, Hurley BF. Age and gender comparisons of muscle strength in 654 women and men aged 20-93 yr. J Appl Physiol 1997;83:1581–7 [DOI] [PubMed] [Google Scholar]
- 3.Vasunilashorn S, Coppin AK, Patel KV, Lauretani F, Ferrucci L, Bandilenni S, Guralnik JM. Use of the Short Physical Performance Battery Score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2009;64:223–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, Studenski S, Berkman LF, Wallace RB. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000;55:M221–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, et al. Gait speed and survival in older adults. JAMA 2011;305:50–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verghese J, Wang C, Lipton RB, Holtzer R, Xue X. Quantitative gait dysfunction and risk of cognitive decline and dementia. J Neurol Neurosurg Psychiatry 2007;78:929–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abellan van Kan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J Nutr Health Aging 2009;13:881–9 [DOI] [PubMed] [Google Scholar]
- 9.Shumway-Cook A, Guralnik JM, Phillips CL, Coppin AK, Ciol MA, Bandinelli S, Ferrucci L. Age-associated declines in complex walking task performance: the Walking InCHIANTI toolkit. J Am Geriatr Soc 2007;55:58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vincent HK, Vincent KR, Lamb KM. Obesity and mobility disability in the older adult. Obes Rev 2010;11:568–79 [DOI] [PubMed] [Google Scholar]
- 11.Rolland Y, Lauwers-Cances V, Cristini C, Abellan van Kan G, Janssen I, Morley JE, Vellas B. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l'OSteoporose) Study. Am J Clin Nutr 2009;89:1895–900 [DOI] [PubMed] [Google Scholar]
- 12.Koster A, Patel KV, Visser M, van Eijk JT, Kanaya AM, de Rekeneire N, Newman AB, Tylavsky FA, Kritchevsky SB, Harris TB. Joint effects of adiposity and physical activity on incident mobility limitation in older adults. J Am Geriatr Soc 2008;56:636–43 [DOI] [PubMed] [Google Scholar]
- 13.Visser M, Goodpaster BH, Kritchevsky SB, Newman AB, Nevitt M, Rubin SM, Simonsick EM, Harris TB. Muscle mass, muscle strength, and muscle fat infiltration as predictors of incident mobility limitations in well-functioning older persons. J Gerontol A Biol Sci Med Sci 2005;60:324–33 [DOI] [PubMed] [Google Scholar]
- 14.Simonsick EM, Montgomery PS, Newman AB, Bauer DC, Harris T. Measuring fitness in healthy older adults: the Health ABC Long Distance Corridor Walk. J Am Geriatr Soc 2001;49:1544–8 [DOI] [PubMed] [Google Scholar]
- 15.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc 2006;54:743–9 [DOI] [PubMed] [Google Scholar]
- 16.Angleman SB, Harris TB, Melzer D. The role of waist circumference in predicting disability in periretirement age adults. Int J Obes (Lond) 2006;30:364–73 [DOI] [PubMed] [Google Scholar]
- 17.Davis JW, Ross PD, Preston SD, Nevitt MC, Wasnich RD. Strength, physical activity, and body mass index: relationship to performance-based measures and activities of daily living among older Japanese women in Hawaii. J Am Geriatr Soc 1998;46:274–9 [DOI] [PubMed] [Google Scholar]
- 18.Atkinson HH, Rosano C, Simonsick EM, Williamson JD, Davis C, Ambrosius WT, Rapp SR, Cesari M, Newman AB, Harris TB, et al. Cognitive function, gait speed decline, and comorbidities: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2007;62:844–50 [DOI] [PubMed] [Google Scholar]
- 19.Ortega-Alonso A, Sipila S, Kujala UM, Kaprio J, Rantanen T. Genetic influences on adult body mass index followed over 29 years and their effects on late-life mobility: a study of twin sisters. J Epidemiol Community Health 2009;63:651–8 [DOI] [PubMed] [Google Scholar]
- 20.Zoico E, Di F, Di F, V, Mazzali G, Zivelonghi A, Volpato S, Bortolani A, Dioli A, Coin A, Bosello O, Zamboni M. High baseline values of fat mass, independently of appendicular skeletal mass, predict 2-year onset of disability in elderly subjects at the high end of the functional spectrum. Aging Clin Exp Res 2007;19:154–9 [DOI] [PubMed] [Google Scholar]
- 21.Stenholm S, Sainio P, Rantanen T, Koskinen S, Jula A, Heliovaara M, Aromaa A. High body mass index and physical impairments as predictors of walking limitation 22 years later in adult Finns. J Gerontol A Biol Sci Med Sci 2007;62:859–65 [DOI] [PubMed] [Google Scholar]
- 22.Visser M, Langlois J, Guralnik JM, Cauley JA, Kronmal RA, Robbins J, Williamson JD, Harris TB. High body fatness, but not low fat-free mass, predicts disability in older men and women: the Cardiovascular Health Study. Am J Clin Nutr 1998;68:584–90 [DOI] [PubMed] [Google Scholar]
- 23.Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, Wilson PW, Kiel DP. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci 1998;53:M214–21 [DOI] [PubMed] [Google Scholar]
- 24.Visser M, Pahor M, Tylavsky F, Kritchevsky SB, Cauley JA, Newman AB, Blunt BA, Harris TB. One- and two-year change in body composition as measured by DXA in a population-based cohort of older men and women. J Appl Physiol 2003;94:2368–74 [DOI] [PubMed] [Google Scholar]
- 25.Gallagher D, Ruts E, Visser M, Heshka S, Baumgartner RN, Wang J, Pierson RN, Pi-Sunyer TX, Heymsfield SB. Weight stability masks sarcopenia in elderly men and women. Am J Physiol Endocrinol Metab 2000;279:E366–75 [DOI] [PubMed] [Google Scholar]
- 26.Newman AB, Lee JS, Visser M, Goodpaster BH, Kritchevsky SB, Tylavsky TA, Nevitt M, Harris TB. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr 2005;82:872–8 [DOI] [PubMed] [Google Scholar]
- 27.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol 2005;115:911–9 [DOI] [PubMed] [Google Scholar]
- 28.Visser M, Pahor M, Taaffe DR, Goodpaster BH, Simonsick EM, Newman AB, Nevitt M, Harris TB. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci 2002;57:M326–32 [DOI] [PubMed] [Google Scholar]
- 29.Penninx BW, Kritchevsky SB, Newman AB, Nicklas BJ, Simonsick EM, Rubin S, Nevitt M, Visser M, Harris T, Pahor M. Inflammatory markers and incident mobility limitation in the elderly. J Am Geriatr Soc 2004;52:1105–13 [DOI] [PubMed] [Google Scholar]
- 30.Verghese J, Holtzer R, Oh-Park M, Derby CA, Lipton RB, Wang C. Inflammatory markers and gait speed decline in older adults. J Gerontol A Biol Sci Med Sci 2011;66:1083–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pahor M, Kritchevsky S. Research hypotheses on muscle wasting, aging, loss of function and disability. J Nutr Health Aging 1998;2:97–100 [PubMed] [Google Scholar]
- 32.Chevalier S, Gougeon R, Choong N, Lamarche M, Morais JA. Influence of adiposity in the blunted whole-body protein anabolic response to insulin with aging. J Gerontol A Biol Sci Med Sci 2006;61:156–64 [DOI] [PubMed] [Google Scholar]
- 33.Schaap LA, Pluijm SM, Smit JH, van Schoor NM, Visser M, Gooren LJ, Lips P. The association of sex hormone levels with poor mobility, low muscle strength and incidence of falls among older men and women. Clin Endocrinol (Oxf) 2005;63:152–60 [DOI] [PubMed] [Google Scholar]
- 34.Waters DL, Qualls CR, Dorin RI, Veldhuis JD, Baumgartner RN. Altered growth hormone, cortisol, and leptin secretion in healthy elderly persons with sarcopenia and mixed body composition phenotypes. J Gerontol A Biol Sci Med Sci 2008;63:536–41 [DOI] [PubMed] [Google Scholar]
- 35.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 2006;61:72–7 [DOI] [PubMed] [Google Scholar]