Abstract
Background: Weight loss (WL) is associated with a decrease in calcium absorption and may be one mechanism that induces bone loss with weight reduction.
Objective: Because vitamin D supplementation has been shown to increase true fractional calcium absorption (TFCA), the goal of this study was to examine the effect of vitamin D during WL or weight maintenance (WM).
Design: A randomized, placebo-controlled, double-blind 6-wk study was conducted in 82 postmenopausal women [BMI (in kg/m2; ±SD): 30.2 ± 3.7] with 25-hydroxyvitamin D [25(OH)D] concentrations <70 nmol/L during either WL or WM. All women were given 10 μg vitamin D3/d and 1.2 g Ca/d and either weekly vitamin D3 (375 μg) or a placebo equivalent to 63 μg (2500 IU)/d and 10 μg (400 IU)/d, respectively. We measured TFCA with the use of dual-stable isotopes, 25(OH)D, parathyroid hormone, estradiol, calcitriol, and urinary calcium at baseline and 6 wk in weight loss and vitamin D3–supplementation (WL-D; n = 19), weight maintenance and vitamin D3–supplementation (WM-D; n = 20), weight loss and placebo (n = 22), and weight maintenance and placebo (n = 21) groups.
Results: WL groups lost 3.8 ± 1.1% of weight with no difference between vitamin D3 supplementation and the placebo. The rise in serum 25(OH)D was greatest in the WL-D group (19.8 ± 14.5 nmol/L) compared with in WM-D (9.1 ± 10.3 nmol/L) and placebo groups (1.5 ± 10.9 nmol/L). TFCA increased with vitamin D3 supplementation compared with placebo treatment (P < 0.01) and decreased during WL compared with WM. Serum 25(OH)D or 1,25-dihyroxyvitamin D did not correlate with TFCA.
Conclusion: These data show that vitamin D supplementation increases TFCA and that WL decreases TFCA and suggest that, when calcium intake is 1.2 g/d, either 10 or 63 μg vitamin D/d is sufficient to maintain the calcium balance. This trial was registered at clinicaltrials.gov as NCT00473031.
INTRODUCTION
Vitamin D regulates calcium absorption and skeletal homeostasis. The active form 1,25-dihydroxyvitamin D [1,25(OH)2D3]4 facilitates the intestinal absorption of calcium by mediating active calcium transport across the intestinal mucosal brush border to the basolateral side of the cell (1). Previous studies have shown that serum concentrations of 1,25(OH)2D3 are associated with calcium absorption and are sometimes, but not always, associated with serum 25-hydroxyvitamin D [25(OH)D] (2–4). The proportion of ingested calcium that is absorbed is best estimated by true fractional calcium absorption (TFCA) that is in the range from 0.10 to 0.50 across all age groups (including women >50 y of age) and decreases with aging or in conditions of vitamin D deficiency. Low TFCA will ultimately lead to bone loss in the absence of adequate calcium intake.
Body weight has a positive effect on bone mineral density (BMD) and has been attributed to many factors including a greater efficiency of calcium absorption at a higher body weight (5) and in obese women (6). Weight reduction is recommended to reduce comorbidities associated with obesity, but it is also associated with loss of bone. Caloric restriction (CR) reduces TFCA efficiency by ∼10% (7), which may be one mechanism whereby weight reduction induces bone loss. Several endocrine changes occur during CR and may contribute to the decrease in calcium absorption including a decrease in serum estrogen concentrations (8, 9) or the presence of higher cortisol (7, 10, 11). Significantly, these hormonal changes that are due to CR are more pronounced in older (7) than younger women (12).
Nutrient interventions such as increasing protein intake positively influence TFCA (13) during weight maintenance (WM). Similarly, some, but not all, studies have shown that vitamin D supplementation increases calcium absorption (4, 14–19). Differences in these findings may be due to several factors such as age, dose of vitamin D, form of vitamin D (vitamin D2 compared with vitamin D3), the baseline 25(OH)D concentration, calcium intake, adiposity, and the presence of other physiologic conditions (20–22). CR is associated with a decrease in TFCA in postmenopausal women. The primary aim in this study was to determine whether supplementation with vitamin D would increase TFCA during CR in a randomized, double-blind trial, and determine whether hormones could explain changes in TFCA. We hypothesized that a high vitamin D intake would attenuate the decline in TFCA during CR compared with a rise in weight-stable women.
SUBJECTS AND METHODS
Subjects
Postmenopausal women who reported no menstruation for ≥2 y before the study were recruited. Recruitment and the intervention were done during the winter months in an effort to minimize seasonal effects on serum concentration of 25(OH)D. Advertisements in local newspapers and university online bulletins were done (December through February) over a 2-y period. All participants were aged between 50 and 70 y and free from any disease states or medications known to influence bone metabolism. Subjects were recruited for weight loss (WL) or WM for the 6-wk period. Before the initiation of any study procedures including screening, subjects signed an informed consent approved by the Institutional Review Board at Rutgers University and an external data safety-monitoring advisory board. Volunteers were monitored for adverse side effects during the trial. This trial was registered at clinicaltrials.gov as NCT0047303. The protocol met the ethical standards in accordance with the Helsinki Declaration.
All participants had to pass a 3-step screening process that included telephone, laboratory, and physical screenings. The telephone screening included questions about current diseases and medications and participation in previous WL programs in the past few months. Participants had a comprehensive metabolic panel test and physical examination and were screened for serum 25(OH)D concentrations before the study. Individuals with 25(OH)D concentrations >75 nmol/L were excluded. In addition, subjects were excluded if they took medications known to influence bone metabolism or had evidence of metabolic bone disease, immune disease, anemia, diabetes, or renal disease, or had a history of heart attack, stroke, kidney stones, or active cancer or cancer therapy in the past 12 mo. For participants who were taking thyroid medications, a stable dose for ≥2 y was required for inclusion. Subjects between BMI (in kg/m2) of 25 and 40 were included who had not recently lost or gained >2% of body weight in the past 6 mo. Subjects who passed all parts of the screening were considered eligible for the study.
Study design
Study participants underwent a month of stabilization during which they were instructed to consume a multivitamin/mineral and calcium supplement to total 1.2 g Ca/d and asked to maintain body weight. All participants completed a calcium and vitamin D food-frequency questionnaire under the guidance of the research staff. A standard multivitamin/mineral supplement (NatureMade Multi 50+) for women >50 y of age was also provided throughout the study to standardize nutritional status. The supplement contained 200 mg Ca, 5 μg (400 IU) vitamin D3, 10 μg vitamin K, 100 mg Mg, 48 mg P, and other standard nutrients. Recommendations were made to adjust intake to 1.2 g Ca/d in some subjects by using a calcium citrate supplement without added vitamin D (200 mg Ca/tablet; Citracal; Bayer Health care LLC). The extra calcium supplement was only given if calcium intake was <1.2 g from the habitual dietary intake and the daily multivitamin/mineral supplement. At the end of the stabilization period, participants brought back their empty pill containers, and baseline TFCA measurements were performed before random assignment into intervention arms.
In this randomized, double-blind study, participants received either vitamin D3 supplements or matching placebos (Bio Tech Pharmacal). Women were asked to consume 3 tablets of vitamin D3 or placebo once weekly for 5 wk with final measurements at week 6. Subjects in the vitamin D group were given a dose equivalent to 62.5 μg vitamin D (2500 IU)/d (375 μg/wk from a vitamin D3 supplement + 10 μg/d from a multivitamin), and subjects in the placebo group received 10 μg vitamin D3 (400 IU)/d (placebo + 10 μg/d from a multivitamin). To enhance compliance, vitamin D3, multivitamin/mineral, and calcium tablets were distributed biweekly, and the first week of pills were distributed in a weekly pill dispenser with instructions to use the dispenser in the subsequent weeks. All participants were asked to take these supplements with the largest meal of the day.
Women were recruited for WM and WL protocols separately by using different advertisements. WM volunteers were asked to maintain their weight for 6 wk; afterward, they offered a couple of free WL counseling sessions with the dietitian as an incentive to improve adherence during the maintenance period. For the WL group, a standard nutrition-education and behavior-modification weight-reduction program was provided, whereas weekly morning instruction was given under the supervision of a dietitian (n = ∼10/group) to reduce energy by 500–600 kcal/d while maintaining habitual exercise levels.
Food records
All participants were instructed to maintain food diaries for 1 wk during the stabilization period to reflect the baseline intake and again for ≥3 d/wk (2 weekdays and 1 weekend day) during the intervention. Dietary intakes were analyzed with Food Works software (version 10).
Weight, height, and body composition
At baseline, weight and height were measured with a balance-beam scale and stadiometer, respectively (Detecto). During the intervention, women were weighed weekly in the morning, and this was recorded without shoes and with minimal clothing. Dual-energy X-ray absorptiometry scans (Lunar Prodigy Advanced; GE-Lunar) (CV <1% for all sites were performed by using enCORE software (version 8.10.027; GE Lunar) to assess total hip, lumbar spine, and total-body BMD as well as total fat and lean mass.
TFCA
42Ca (96% enrichment) and 43Ca (90% enrichment) spikes (Trace Sciences International) were prepared for human use by the Pharmacy Department at St Peters University Hospital (New Brunswick, NJ). All isotopes were tested for sterility and pyrogenicity before use. Briefly, on the day of the calcium-absorption test protocol, women were admitted between 0700 and 0900 after an overnight fast. The dose was individualized according to body weight (0.012 mg 42Ca/kg and 0.017 mg 43Ca/kg ) by using stock solutions of 42Ca and 43Ca (eg, 0.70 and 0.48 mg/mL, respectively). The administered volume was calculated on the basis of body weight before the test. After collection of a fasting blood and urine sample, subjects were served a standard breakfast that consisted of milk, toast, fruit, and orange juice. The total calcium load of this meal was 200 mg and included 43Ca that had been mixed in one-half cup of skim milk (153 mg Ca) that had been equilibrated overnight (∼12 h). The milk was consumed in its entirety under supervision, and the cup was rinsed with deionized water 3 times. All of the rinsed water was also consumed by patients. Immediately after breakfast, an intravenous injection of 42Ca was administered over ∼3 min. Syringes that contained the isotopes (that were mixed with the milk or infused intravenously) were weighed before and after administration on a precision balance scale. The women were given instructions on how to collect a pooled 24-h urine sample, monitored during the day, and asked to remain overnight at the University Inn proximal to the clinical facility. On completion of the 24-h urine collection, women returned their samples to the researchers the next morning.
Laboratory methods
TFCA measurement
After administration of oral and intravenous enriched calcium isotopes, 24-h spot pooled urine specimens were collected before random assignment and after 6 weeks of treatment. In addition, an 8-h sample was collected for methods development. At baseline and before administration of isotope calcium spikes, a fasting urine sample was collected to determine the individual's natural calcium-isotope abundances (see Equation 1). Calcium was precipitated as the oxalate salt and ashed to examine the mineral content. The ratio of each isotope to 44Ca (the natural internal standard) was determined by using high-resolution, inductively coupled plasma mass spectrometry (Element-1; Finnigan MAT) (23). The precision and accuracy of the inductively coupled plasma mass spectrometry instrument were ∼1% (SD), and the day-to-day CV for 6 women measured twice was 1.2% (SD). Briefly, TFCA was calculated as the relative recovery in the 24-h pooled urine of the oral isotopes (43Ca) divided by the intravenous isotope (42Ca) as follows:
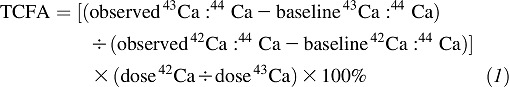 |
Net calcium absorption was calculated as
Blood and urine analysis
Fasting blood and urine samples were collected at baseline and week 6 and were analyzed in batch analysis. 25(OH)D and serum 1,25(OH)2D3 (DiaSorin) (interassay and intraassay CVs <12.5% and <15%, respectively) were measured by radioimmunoassay using internal and external standards. Our laboratory also participates and met certification in a vitamin D external quality-assessment scheme that monitors the performance of the 25(OH)D assay. In addition, 25(OH)D was measured in a subset (n = 80) twice at baseline (1–5 d apart) with a CV of 5.7%. To measure estradiol, we used an ultrasensitive estradiol (E2) 125I radioimmunoassay (Diagnostic Systems Laboratory, DSL) (interassay and intraassay CVs of 12.2% and 8.9%, respectively). Intact parathyroid hormone (PTH) was determined by immunoradioassay (Scantibodies) (interassy and intraassay CVs <6.8%). Urinary calcium was measured in a pooled 24-h sample and serum by using the o-cresolphthalein complex method with the use of a spectrophotometer and were also checked by atomic absorption spectrometry (Solaar Series; Thermo Electron Corp).
Statistical analysis
The study aimed to determine whether vitamin D supplementation and WL influences the primary outcome variable TFCA. The hormones PTH, 25(OH)D, 1,25(OH)2D3, and estradiol were analyzed to determine whether they could explain changes in the primary outcome variable between groups. This study was designed to achieve a serum 25(OH)D concentration >75 nmol/L with a weekly dose of 375 μg vitamin D3 (equivalent to 63 μg/d) supplementation in overweight/obese persons during WL. In a report that was available when this study was designed, calcium absorption at 2 doses of vitamin D intake (0 and 800 IU every other day) showed a fractional calcium-absorption difference of 0.14, with an SD of 0.12 (4). A power analysis was performed with α set at 0.05 and β set at 0.10 and showed that 16 subjects were required for a 90% probability to detect significant differences between groups (4). To control for one covariate, as had been deemed necessary in a previous study (7), and to allow for anticipated dropouts, additional subjects per group were recruited. We used single-block randomization for vitamin D and placebo assignments. To assess baseline differences between groups, 1-factor ANOVA was used. Variables that differed between groups at baseline were used as covariates during additional analysis. The influence of treatment (vitamin D3 supplementation or placebo) and weight (WL or WM) on TFCA and hormones was measured by using 2-way ANCOVA. Tukey's post hoc analysis was performed when the interaction was significant. Stepwise regression analysis was performed with changes in hormones as independent variables and the change in TFCA as the dependent variable performed, wherein predictors of TFCA were added one at a time to the model as long as the F statistic P value was below the specified α of 0.05. Pearson's correlation coefficients were used to evaluate associations between changes in different variables measured. Analyses were conducted with SAS Statistical Package (version 9.2; SAS Institute). Values are expressed as means ± SDs. P < 0.05 was considered significant.
RESULTS
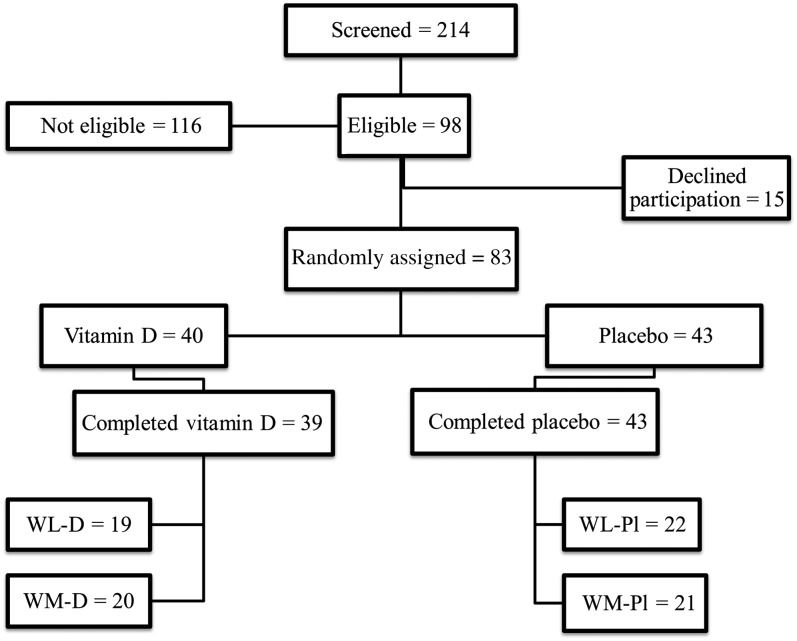
A total of 214 women were screened, and 98 women were shown to be eligible and passed all parts of the screening (Figure 1). Eighty-three women were randomly assigned to vitamin D (n = 40) and placebo (n = 43) groups, of which 82 women completed the study in weight loss and vitamin D3 supplementation (WL-D; n = 19), weight maintenance and vitamin D3 supplementation (WM-D; n = 20), weight loss and placebo supplementation (WL-Pl; n = 22), and weight maintenance and placebo supplementation (WM-Pl; n = 21). Of these subjects, 67 women were white, 10 women were African American, 2 women were Hispanic, 2 women were Asian, and one woman was of mixed ethnic background. The women had a mean age of 58 ± 6 y and BMI of 30.2 ± 3.7 with no differences between the 4 groups at baseline. At screening, the serum 25(OH)D concentration for the eligible women was 55.3 ± 17.8 nmol/L. After 1 mo of stabilization with the multivitamin/mineral pill and calcium supplementation, women brought back empty containers, and 25(OH)D concentration rose slightly to 62.3 ± 14.3 nmol/L at baseline. Thirteen percent of these women had osteopenia (T score less than −1.0 and greater than −2.5) at the total hip and lumbar spine, and only one woman had a T-score less than −2.5 for the lumbar spine. Fat, lean mass, and BMD at the total hip and lumbar spine and TFCA at baseline are presented in Table 1. Variables were not different between the 4 groups except for hip BMD, which was lower in the WM-Pl than in the WL-D group at baseline (Table 1).
FIGURE 1.
Flow diagram of study participants. Vitamin D, vitamin D supplementation; WL-D, weight loss and vitamin D3 supplementation; WL-Pl, weight loss and placebo; WM-D, weight maintenance and vitamin D3 supplementation; WM-Pl, weight maintenance and placebo.
TABLE 1.
Baseline characteristics of participants who completed the study1
| Variables | WL-D (n = 19) | WM-D (n = 20) | WL-Pl (n = 22) | WM-Pl (n = 21) | P |
| Age (y) | 58.1 ± 5.7 | 58.3 ± 6.8 | 56.3 ± 5.4 | 58.3 ± 5.1 | 0.625 |
| YSM | 6.9 ± 4.7 | 9.2 ± 8.0 | 7.7 ± 9.9 | 8.4 ± 6.9 | 0.793 |
| BMI (kg/m2) | 30.1 ± 3.2 | 30.4 ± 3.7 | 30.9 ± 4.5 | 29.2 ± 3.2 | 0.504 |
| Fat mass (kg) | 35.1 ± 8.2 | 35.6 ± 7.0 | 37.3 ± 8.3 | 30.9 ± 9.3 | 0.080 |
| Lean mass (kg) | 40.5 ± 5.0 | 41.8 ± 5.3 | 42.4 ± 7.0 | 38.4 ± 4.4 | 0.108 |
| Total-body BMC (g) | 2455 ± 372 | 2416 ± 238 | 2489 ± 358 | 2273 ± 326 | 0.161 |
| Total-body BMD (g/cm2) | 1.176 ± 0.097 | 1.161 ± 0.078 | 1.171 ± 0.086 | 1.117 ± 0.078 | 0.112 |
| Total hip BMD (g/cm2) | 1.011 ± 0.120a | 0.976 ± 0.108a,b | 1.000 ± 0.130a,b | 0.914 ± 0.095b | 0.038 |
| Lumbar spine L2–L4 (g/cm2) | 1.235 ± 0.19 | 1.196 ± 0.14 | 1.238 ± 0.18 | 1.134 ± 0.13 | 0.139 |
| TFCA (fraction) | 0.24 ± 0.09 | 0.22 ± 0.06 | 0.25 ± 0.06 | 0.20 ± 0.05 | 0.225 |
All values are means ± SDs. Eleven percent of women were taking thyroid medications [WL-D (n = 1), WM-D (n = 2), WL-Pl (n = 4), and WM-Pl (n = 1)]. P values represent results of 1-factor ANOVA for the 4 groups. Values in the same row with different superscript letters represent significant differences in group means, P < 0.05. Tukey's post hoc analysis was performed when a P value was significant. BMC, bone mineral content; BMD, bone mineral density; TFCA, true fractional calcium absorption; WL-D, weight loss and vitamin D3 supplementation; WL-Pl, weight loss and placebo; WM-D, weight maintenance and vitamin D3 supplementation; WM-Pl, weight maintenance and placebo; YSM, years since menopause.
Weight, hormones, and urinary calcium at baseline and during CR
Baseline values for serum hormones [25(OH)D, 1,25(OH)2D3, PTH, and estradiol] and urinary calcium did not differ between groups at baseline (Table 2). Because of significant differences in weight between groups at baseline, baseline values were used as covariates for examining the change in other variables because of the intervention. In addition, although hip BMD was also different between groups, it was not added as an additional covariate because it is both a component and correlates with total weight (r = 0.49, P < 0.001). The mean WL in WL-D and WL-Pl groups was −3.8 ± 1.1% and did not differ between the groups. Seventy-nine percent of women had serum concentrations >50 nmol/L and 21% of women (n = 17) had serum 25(OH)D concentrations between 29 and <50 nmol/L and were distributed between all 4 groups (9 women in placebo groups and 8 women in vitamin D–supplemented groups). Serum 25(OH)D concentrations increased with 63 μg/d vitamin D supplementation by 19.8 ± 14.5 nmol/L in the WL-D group and by 9.1 ± 10.3 nmol/L in the WM-D group (P < 0.05). There was no significant change in 25(OH)D in placebo groups. PTH was influenced by treatment (P = 0.023) with a greater decrease in groups supplemented with vitamin D3 compared with no decrease in placebo groups, whereas estradiol and 1,25(OH)2D3 was not influenced by WL or vitamin D supplementation. The change in 24-h urinary calcium excretion was not different between the 4 groups (Table 2). None of the subjects reported a change in any medications when questioned during the intervention.
TABLE 2.
Baseline characteristics and changes in body weight, serum hormones, net calcium absorption, and urinary excretion after 6 wk of weight loss or weight maintenance with vitamin D supplementation or placebo1
| WL-D (n = 19) |
WM-D (n = 20) |
WL-Pl (n = 22) |
WM-Pl (n = 21) |
Vitamin D | Δ Weight | Weight × vitamin D3 supplementation | |||||
| Variables | Baseline | Change | Baseline | Change | Baseline | Change | Baseline | Change | P-change | P-change | P-change |
| Weight (kg) | 79.1 ± 12.7 | −3.0 ± 1.2 | 80.1 ± 10.1 | −0.2 ± 1.4 | 84.3 ± 14.2 | −3.3 ± 1.2 | 73.3 ± 10.4 | −0.1 ± 1.1 | 0.646 | <0.001 | 0.929 |
| 25(OH)D (nmol/L) | 64.5 ± 14.5 | 19.8 ± 14.5a | 61.8 ± 14.0 | 9.1 ± 10.3b | 61.8 ± 17.3 | 1.3 ± 11.8 b | 63.0 ± 14.0 | 1.8 ± 10.5 b | <0.001 | 0.118 | 0.013 |
| PTH (pmol/L) | 4.2 ± 2.0 | −0.4 ± 1.0 | 4.8 ± 3.0 | −0.6 ± 1.6 | 4.2 ± 1.5 | 0.01 ± 1.7 | 4.2 ± 1.3 | 0.5 ± 1.2 | 0.023 | 0.661 | 0.218 |
| Estradiol (pmol/L) | 46.9 ± 12.5 | −4.0 ± 16.5 | 41.1 ± 13.9 | −2.6 ± 13.6 | 42.2 ± 17.3 | 1.8 ± 10.6 | 49.5 ± 16.9 | −0.4 ± 18.7 | 0.235 | 0.714 | 0.391 |
| 1,25(OH)2D3 (pmol/L) | 127.0 ± 46.8 | 2.6 ± 37.3 | 137.0 ± 44.9 | 12.2 ± 32.9 | 147.1 ± 37.2 | −4.8 ± 34.1 | 130.0 ± 39.8 | −4.1 ± 42.5 | 0.114 | 0.402 | 0.544 |
| Net calcium absorption (mg/d) | 271 ± 96 | −6 ± 46 | 267 ± 69 | 45 ± 90 | 274 ± 73 | −32 ± 78 | 238 ± 57 | −8 ± 46 | 0.012 | 0.018 | 0.359 |
| Urinary calcium (mg/d) | 144.2 ± 94.5 | −24.3 ± 57.6 | 139.5 ± 62.9 | 1.9 ± 48.3 | 149.4 ± 71.1 | −2.2 ± 49.7 | 150.8 ± 92.9 | −16.7 ± 45.9 | 0.857 | 0.528 | 0.111 |
All values are means ± SDs. Baseline blood samples were taken between January and February, and all endpoint blood samples were taken between March and the first week of April. Two-way ANCOVA for weight × vitamin D3 supplementation (controlled for baseline weight). Weight factor included weight-loss and weight-maintenance groups. Vitamin D3 included 2 intakes (375 μg/wk from a vitamin D3 supplement plus 10 μg/d from a multivitamin; placebo groups received a placebo once weekly plus 10 μg vitamin D/d from a multivitamin). Values in the same row with different superscript letters represent significant differences in group means, P < 0.05. Tukey's post hoc analysis was performed when the interaction was significant. At baseline, no variables were significantly different between groups except for weight (WL-Pl differed from WM-Pl; P < 0.05). PTH, parathyroid hormone; WL-D, weight loss and vitamin D3 supplementation; WL-Pl, weight loss and placebo; WM-D, weight maintenance and vitamin D3 supplementation; WM-Pl, weight maintenance and placebo; 1,25(OH)2D3, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
Changes in TFCA during the intervention
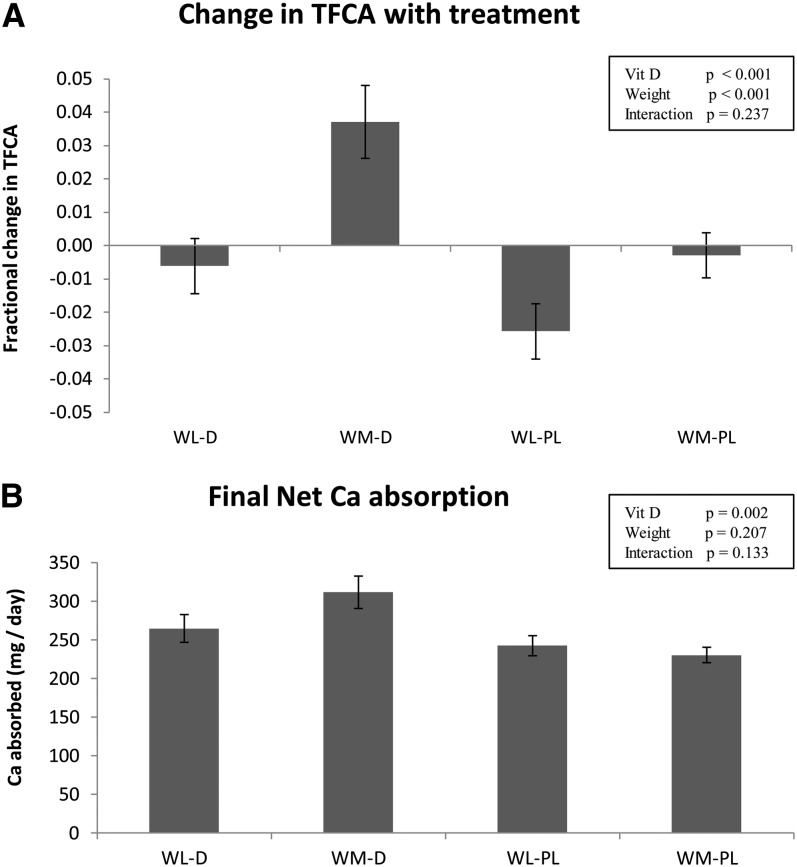
TFCA increased because of vitamin D treatment (P < 0.001) and, as expected, decreased during WL (P < 0.001), and there was no weight × treatment effect (Figure 2A). The fractional changes in TFCA from baseline during the intervention in the 4 groups were −0.006 ± 0.037 (WL-D), +0.037 ± 0.049 (WM-D), −0.026 ± 0.037 (WL-Pl), and −0.003 ± 0.030 (WM-Pl) (Figure 2A). The final net calcium absorption was higher as a result of vitamin D supplementation (Figure 2B), and the change (mg/d) from baseline (Table 2) showed that both the vitamin D treatment and WL influenced the net calcium absorption. Serum concentrations of 25(OH)D and 1,25(OH)2D3 did not correlate with TFCA before or after the intervention. Stepwise regression analysis showed that none of the hormones [25(OH)D, 1,25(OH)2D3, PTH, or estradiol) explained changes in TFCA for the entire population or individual groups.
FIGURE 2.
Mean (±SEM) changes in TFCA (A) and final net calcium absorption (B) by using baseline weight as a covariate with vitamin D3 supplementation (375 μg/wk plus 10 μg/d from a multivitamin supplement) compared with placebo (placebo once weekly plus 10 μg vitamin D/d from multivitamin) in weight-loss and weight-maintenance groups of postmenopausal women. The 4 groups are WL-D (n = 19), WM-D (n = 21), WL-Pl (n = 22), and WM-Pl (n = 21). P values are shown for 2-way ANCOVA for vitamin D (vitamin D or placebo) and weight (weight loss or weight maintenance). TFCA, true fractional calcium absorption; Vit D, vitamin D; WL-D, weight loss and vitamin D3 supplementation; WL-Pl, weight loss and placebo; WM-D, weight maintenance and vitamin D3 supplementation; WM-Pl, weight maintenance and placebo.
Nutrient intake during the intervention
Mean daily intake of macronutrients and micronutrients at baseline and during the intervention are presented in Table 3. The mean calcium intake from diet and multivitamin and calcium supplements in the 4 groups during the intervention was 1184 ± 138 mg/d compared with 687 ± 272 mg/d from diet alone. There was no evidence of noncompliance to vitamin D or other supplements over the 6 wk based on pill counts at weekly visits. Other macronutrient and micronutrient intakes did not differ between the 4 groups at baseline or final measurements.
TABLE 3.
Nutrient intakes at baseline and after 6 wk of weight loss or weight maintenance in postmenopausal women randomly assigned to receive vitamin D supplementation or placebo during caloric restriction1
| WL-D (n = 19) |
WM-D (n = 20) |
WL-Pl (n = 22) |
WM-Pl (n = 21) |
|||||
| Baseline | 6 wk | Baseline | 6 wk | Baseline | 6 wk | Baseline | 6 wk | |
| Energy (kcal) | 1432 ± 246 | 1289 ± 281 | 1428 ± 350 | 1394 ± 279 | 1499 ± 269 | 1343 ± 339 | 1358 ± 287 | 1308 ± 304 |
| Protein (g) | 62.2 ± 13.3 | 58.6 ± 11.6 | 67.1 ± 14.8 | 67.0 ± 16.6 | 72.4 ± 9.6 | 66.6 ± 11.6 | 66.0 ± 15.1 | 60.9 ± 12.0 |
| Fat (g) | 57.1 ± 11.4 | 54.7 ± 20.1 | 46.3 ± 15.2 | 49.7 ± 13.5 | 63.7 ± 28.4 | 47.5 ± 21.4 | 54.3 ± 20.0 | 46.5 ± 14.5 |
| Carbohydrates (g) | 174.4 ± 43.7 | 139.2 ± 33.0 | 188.3 ± 47.8 | 170.0 ± 41.6 | 161.9 ± 32.3 | 168.5 ± 46.0 | 153.8 ± 57.5 | 168.0 ± 62.9 |
| Calcium (mg)2 | 1169 ± 83 | 1170 ± 90 | 1246 ± 207 | 1221 ± 143 | 1176 ± 150 | 1168 ± 204 | 1201 ± 77 | 1179 ± 65 |
| Vitamin D (μg)23 | 13.2 ± 1.9 | 66.2 ± 1.8 | 13.7 ± 2.0 | 66.2 ± 2.1 | 13.0 ± 1.1 | 13.1 ± 1.3 | 13.8 ± 2.7 | 13.8 ± 2.8 |
| Phosphorus (mg)2 | 946 ± 244 | 929 ± 185 | 1053 ± 210 | 1009 ± 200 | 1121 ± 237 | 1092 ± 277 | 993 ± 229 | 889 ± 244 |
| Magnesium (mg)2 | 320 ± 102 | 298 ± 92 | 340 ± 59 | 333 ± 80 | 326 ± 112 | 343 ± 105 | 317 ± 71 | 315 ± 68 |
| Sodium (mg) | 2239 ± 627 | 1879 ± 396 | 2716 ± 820 | 2746 ± 887 | 2473 ± 603 | 2448 ± 923 | 2018 ± 710 | 2120 ± 510 |
| Vitamin K (μg)2 | 102 ± 120 | 98 ± 69 | 121 ± 208 | 102 ± 117 | 133 ± 142 | 95 ± 70 | 125 ± 167 | 136 ± 167 |
All values are means ± SDs. MV, multivitamin-mineral supplement; WL-D, weight loss and vitamin D3 supplementation; WL-Pl, weight loss and placebo; WM-D, weight maintenance and vitamin D3 supplementation; WM-Pl, weight maintenance and placebo.
Intake was from the diet, vitamin D supplement (or placebo), and MV supplement (48 mg P, 10 μg vitamin D, 100 mg Mg, 10 μg vitamin K, and other standard micronutrients). Subjects were stabilized for 1 mo before baseline measurements to receive calcium and an MV. If intake was <1200 mg from the diet and MV, a calcium supplement was provided.
Two-way ANOVA showed no significant differences for any nutrient at baseline or during the diet between the 4 groups, except for higher vitamin D than placebo because of the intervention (P < 0.05).
Safety
Mean urinary and serum calcium concentrations did not differ significantly between groups at any time during the study. No individual in either group had evidence of hypercalciuria (24-h urinary calcium >300 mg) or hypercalcemia (>10.5 mg/dL) at 6 wk. There were no serious adverse events in participants during this study. Nonserious adverse events were recorded at baseline and after 6 wk to determine the safety of vitamin D supplementation. These events included pain in legs, swelling in legs, pain or heaviness in chest, headaches, dizziness, nausea, fatigue, muscle weakness, muscle aches, abnormal urinary frequency, and abdominal pain. The cumulative frequency of nonserious adverse events for these 11 symptoms was 13 ± 7% (n = 59) and 13 ± 9% (n = 61) after 1 mo of stabilization (baseline) in women in vitamin D3–supplementation and placebo groups, respectively. After 6 wk, the cumulative frequency of events was 9 ± 8% (n = 41) and 7 ± 4% (n = 31) for vitamin D3–supplementation and placebo groups, respectively, and did not differ significantly between groups. For individual symptoms, the number of mild headaches differed between groups from baseline to wk 6 (P < 0.05). There were 8–9 mild headaches at baseline, and after 6 wk, there were 11 mild headaches in the vitamin D3–supplementation group, and 4 mild headaches in the placebo group. Besides mild headaches, none of the following events changed significantly over time for vitamin D3–supplementation and placebo groups: pain in legs (−2 and −2, respectively), swelling in legs (−2 and −1, respectively), pain or heaviness in chest (0 and 0, respectively), headaches (+3 and −5, respectively), dizziness (−2 and 0, respectively), nausea (−2 and 0, respectively), fatigue (−4 and −8, respectively), muscle weakness (−2 and −3, respectively) muscle aches (−5 and −5, respectively), abnormal urinary frequency (−1 and −4, respectively), and abdominal pain (−1 and −2, respectively). In a separate analysis, after 6 wk, there was no difference in cumulative or individual events in WL compared WM groups.
DISCUSSION
The vitamin D endocrine system is an important regulator of intestinal calcium absorption. In this 6-wk study, postmenopausal women were supplemented with 2 doses of vitamin D3 to understand the combined effect of supplementation and WL on TFCA. CR reduced TFCA by 10%, as we have shown previously (7), and may be one mechanism that regulates the loss of bone mass during weight reduction. In this study, we showed that vitamin D supplementation with 375 μg vitamin D3/wk and 10 μg vitamin D3/d during WL increased the serum 25(OH)D concentration by 20 nmol/L, but TFCA did not increase significantly compared with baseline. In contrast, in WM women, there was only a 9-nmol/L increase in the 25(OH)D concentration ,and the rise in TFCA was 0.037. The disparity in the larger rise in 25(OH)D but smaller increase in TFCA in the vitamin D–supplemented WL compared with WM groups suggested that other factors are important in the regulation of calcium absorption during CR.
Overweight/obese women in this study had a serum 25(OH)D concentration of 62 nmol/L and, 20% of women had concentration <50 nmol/L (2% of women were deficient at <30 nmol/L). These concentrations are similar to those seen in the general population (24). The attenuated rise in serum 25(OH)D because of vitamin D supplementation (∼0.2 nmol/μg vitamin D intake) in weight-stable women may have been due to their relatively higher baseline concentrations than in other studies and their excess adiposity (20–22, 25). In addition, a rise in serum 25(OH)D as a result of WL has been shown previously (11, 12, 26, 27), and although not significant here, it has been attributed to its release from adipose tissue.
The absence of a significant effect of vitamin D on TFCA in the WL group, despite a greater rise in serum 25(OH)D, suggested that factors other than the concentration of serum 25(OH)D act together or alone to suppress a rise in TFCA. For example, there is an increase in serum cortisol that is due to CR that may attenuate the ability of supplemental vitamin D to increase TFCA (7). In addition, a decrease in serum estradiol because of a loss of adipose tissue and reduced peripheral androdiestrone synthesis could decrease TFCA (28). Both estradiol and cortisol are important regulators of calcium absorption during CR (7, 9). Previously, we showed that serum PTH is the major predictor of TFCA during CR in women who received 10 μg vitamin D/d (7). The suppression of PTH with a higher intake of vitamin D in the current study may have attenuated the ability of PTH to explain the TFCA variance. It is well established that a high or low calcium intake will decrease and increase calcium absorption, respectively. However, we controlled for calcium and other micronutrients, and thus, this should not have been a factor in the attenuation of TFCA during CR. However, it is possible that a reduction in dietary protein (13) or fat (6, 29) intake that positively affect calcium absorption contributed to the reduction in TFCA with CR.
Vitamin D supplementation had a positive effect on TFCA in the current trial. There was a decrease in serum PTH with vitamin D supplementation, as expected (30, 31), with a rise in 1,25(OH)2D3. Stepwise regression analysis showed that none of the hormones could explain why TFCA differed because of vitamin D intake. The hypothesis that serum concentrations of 25(OH)D or 1,25(OH)2D3 can predict TFCA (32) is not consistent with our findings, which showed no relation. This inconsistency was possibly due to the relatively high baseline serum 25(OH)D in the current study, although the absence of this relation has also been reported in other cohorts (6, 33). Therefore, mechanisms that regulate the rise in TFCA because of vitamin D supplementation are not clear.
Several studies have shown that vitamin D supplementation has either a positive or no effect on TFCA. In a study that examined calcium absorption by using the AUC, calcium absorption was estimated to increase by 65% at serum concentration of 25(OH)D of 86 compared with 50 nmol/L (4). However, the method (AUC) (4) only estimates calcium absorption from a rise in serum calcium, whereas the dual stable-isotope method used in this study is the most precise method because it calculates calcium absorption from the relative enrichment of 2 isotopes. In another study (14), TFCA was examined after supplementation with 1250 μg vitamin D2/d (50,000 IU/d) for 15 d in vitamin D–insufficient women. These postmenopausal women increased serum 25(OH)D concentrations from 55 to 160nmol/L and increased TFCA from 0.24 to 0.27 (14), which was similar to the 0.037 rise in the current study. In a recent dose-response study (with a range from 400 to 4800 IU/d) that used radioactive isotopes, there was a positive correlation between the vitamin D3 dose and calcium absorption after 1 y that correlated to a greater extent with the final serum 25(OH)D concentrations than the vitamin D intake (19). Postmenopausal women who were given 12.5 μg vitamin D2/d for 3 mo and 25 μg vitamin D2/d for another 3 mo increased 25(OH)D concentrations from 36 to 61 nmol/L but did not increase calcium absorption (18). In another study (16), 120 postmenopausal women who received 1200 mg Ca/d plus 25μg vitamin D2/d compared with calcium only for 1 y showed no increase in calcium absorption. These negative findings were consistent with another study in 302 postmenopausal women who received 25μg vitamin D2/d or a matching placebo (17). It is possible that the shorter half-life of vitamin D2 compared with vitamin D3 may have contributed to negative findings in some of these studies. In contrast, only 70 μg vitamin D3/wk (∼10 μg/d) plus alendronate in postmenopausal women increased fractional calcium absorption by 0.07, but the large rise was attributed to the alendronate effect on serum PTH and 1,25(OH)2D3 (34). In adolescent girls and young children with rickets, supplementation with vitamin D3 showed no increase in calcium absorption (15, 35).
Several factors may have contributed to discrepancies in the findings between studies, such as baseline 25(OH)D and PTH concentrations, the calcium-absorption method, calcium intake, impaired renal function, or variable doses of vitamin D used in these studies. However, the studies have consistently shown that the marked positive association between calcium absorption and serum 25(OH)D at low concentrations <20 nmol/L (36) is not present when serum concentrations are higher, but there is a continued small increase in TFCA of ∼0.03 because of vitamin D supplementation (14). This finding is important because dietary calcium may need to be increased in some individuals, especially in patients with compromised calcium absorption.
It has been estimated that ≥200 mg absorbed Ca/d is required to offset obligatory calcium loss (32). In the current study, the net absorption of calcium ranged between 227 and 310 mg/d in all groups, irrespective of vitamin D supplementation or WL. These data suggest that when calcium intake is at the recommended intake of 1200 mg Ca/d, the use of higher doses of vitamin D than the recommended intake of 600 IU/d is not needed to avoid a negative calcium balance. However, the average calcium intake from dietary sources was only 650 mg/d in these women and, without additional calcium supplements, would have led to a net absorption of ∼140 mg/d, with assumption of 22% TFCA during CR. If 200 mg Ca/d is required to maintain a balance, this 60-mg/d deficit would be expected to result in an additional 0.9% bone loss/y.
There were several strengths and limitations to this study. The dual-isotope method is considered a gold standard to estimate absorption and was used in this study. Also, the double-blind, placebo-controlled design and weekly pill distribution and counts were strengths to increase compliance in this study. Our laboratory participates in the vitamin D external quality-assessment scheme, which is an international quality assurance group that monitors the performance of the 25(OH)D assay. To our knowledge, this was the first study to address the influence of vitamin D supplementation during CR. These studies were carried out in the winter and early spring to avoid the influence of sun on serum 25(OH)D measures. Some limitations of this study included the potential generalizability of the data because women who were enrolled in the study were primarily white, and thus, results may not apply to other ethnic groups. Second, women in our studies were younger postmenopausal women, and it is possible that there is a differential response of vitamin D on TFCA in older individuals because of a decline in renal function (conversion to calcitriol) or other factors. In addition, although there was compliance to the vitamin D and placebo intake on the basis of pill counts, we did not watch subjects consume the pills, and thus, it is possible that compliance was lower than reported. Finally, it is possible that a higher vitamin D dose or longer duration >6 wk could have increased the TFCA response to CR; however, we achieved a serum 25(OH)D concentration >80 nmol/L.
In conclusion, CR reduced calcium absorption in postmenopausal women as expected. Vitamin D supplementation equivalent to 63 μg/d (2500 IU/d) compared with 10 μg/d (400 IU/d) did not increase absorption during CR, despite a 20-nmol/L rise in serum 25(OH)D. The mechanism that regulates the decline in calcium absorption during CR is not clear; however, lowered calcium absorption might contribute to bone loss associated with weight reduction. Because bone loss and a higher fracture risk can be expected after WL (37), understanding its cause remains important. These findings suggest that, if both vitamin D and calcium intake are at recommended intakes (24), a calcium balance would be expected even during CR.
Acknowledgments
We thank the laboratory and clinical staff for their invaluable technical and clinical assistance. The effort of the following members of the external data safety monitoring board was appreciated: Lester Katzel, Robert Recker, and Bruce Barton. We also thank R Zurfluh, G Regis-Andrews, B Dobrzynski, and the volunteers in this study.
The authors’ responsibilities were as follows—SAS: was responsible for conception of the study and had primary responsibility for final content of the manuscript; DS and SAS: contributed to the execution of the study, laboratory analysis, and statistical tests and were primary writers of the manuscript; YS: contributed to the study design and had primary responsibility for random assignment and statistical interpretation; SHS: was the study physician, interpreted laboratory findings, and contributed to safety reports; RMS, MPF, and HA-S: contributed to the study design and laboratory analysis; and all authors: contributed to the interpretation of the results and the data analysis and reviewed and contributed to the writing of the manuscript. None of the authors had a conflict of interest.
Footnotes
Abbreviations used: BMD, bone mineral density; CR, caloric restriction; PTH, parathyroid hormone; TFCA, true fractional calcium absorption; WL, weight loss; WL-D, weight loss and vitamin D3 supplementation; WL-Pl, weight loss and placebo; WM, weight maintenance; WM-D, weight maintenance and vitamin D3 supplementation; WM-Pl, weight maintenance and placebo; 1,25(OH)2D3, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D.
REFERENCES
- 1.Norman AW. Intestinal calcium absorption: a vitamin D-hormone-mediated adaptive response. Am J Clin Nutr 1990;51:290–300 [DOI] [PubMed] [Google Scholar]
- 2.Aloia JF, Chen DG, Yeh JK, Chen H. Serum vitamin D metabolites and intestinal calcium absorption efficiency in women. Am J Clin Nutr 2010;92:835–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seamans KM, Cashman KD. Existing and potentially novel functional markers of vitamin D status: a systematic review. Am J Clin Nutr 2009;89:1997S–2008S [DOI] [PubMed] [Google Scholar]
- 4.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 2003;22:142–6 [DOI] [PubMed] [Google Scholar]
- 5.Barger-Lux MJ, Heaney RP. Calcium absorptive efficiency is positively related to body size. J Clin Endocrinol Metab 2005;90:5118–20 [DOI] [PubMed] [Google Scholar]
- 6.Shapses SA, Sukumar D, Schneider SH, Schlussel Y, Brolin RE, Taich L. Hormonal and dietary influences on true fractional calcium absorption in women: role of obesity. Osteoporos Int 2012;23:2607–14.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cifuentes M, Riedt CS, Brolin RE, Field MP, Sherrell RM, Shapses SA. Weight loss and calcium intake influence calcium absorption in overweight postmenopausal women. Am J Clin Nutr 2004;80:123–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O'Dea JP, Wieland RG, Hallberg MC, Llerena LA, Zorn EM, Genuth SM. Effect of dietery weight loss on sex steroid binding sex steroids, and gonadotropins in obese postmenopausal women. J Lab Clin Med 1979;93:1004–8 [PubMed] [Google Scholar]
- 9.Cifuentes M, Advis JP, Shapses SA. Estrogen prevents the reduction in fractional calcium absorption due to energy restriction in mature rats. J Nutr 2004;134:1929–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergendahl M, Iranmanesh A, Mulligan T, Veldhuis JD. Impact of age on cortisol secretory dynamics basally and as driven by nutrient-withdrawal stress. J Clin Endocrinol Metab 2000;85:2203–14 [DOI] [PubMed] [Google Scholar]
- 11.Riedt CS, Cifuentes M, Stahl T, Chowdhury HA, Schlussel Y, Shapses SA. Overweight postmenopausal women lose bone with moderate weight reduction and 1 g/day calcium intake. J Bone Miner Res 2005;20:455–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riedt CS, Schlussel Y, von Thun N, Ambia-Sobhan H, Stahl T, Field MP, Sherrell RM, Shapses SA. Premenopausal overweight women do not lose bone during moderate weight loss with adequate or higher calcium intake. Am J Clin Nutr 2007;85:972–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kerstetter JE, O'Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab 2005;90:26–31 [DOI] [PubMed] [Google Scholar]
- 14.Hansen KE, Jones AN, Lindstrom MJ, Davis LA, Engelke JA, Shafer MM. Vitamin D insufficiency: disease or no disease? J Bone Miner Res 2008;23:1052–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park CY, Hill KM, Elble AE, Martin BR, DiMeglio LA, Peacock M, McCabe GP, Weaver CM. Daily supplementation with 25 mug cholecalciferol does not increase calcium absorption or skeletal retention in adolescent girls with low serum 25-hydroxyvitamin D. J Nutr 2010;140:2139–44 [DOI] [PubMed] [Google Scholar]
- 16.Zhu K, Bruce D, Austin N, Devine A, Ebeling PR, Prince RL. Randomized controlled trial of the effects of calcium with or without vitamin D on bone structure and bone-related chemistry in elderly women with vitamin D insufficiency. J Bone Miner Res 2008;23:1343–8 [DOI] [PubMed] [Google Scholar]
- 17.Zhu K, Devine A, Dick IM, Wilson SG, Prince RL. Effects of calcium and vitamin D supplementation on hip bone mineral density and calcium-related analytes in elderly ambulatory Australian women: a five-year randomized controlled trial. J Clin Endocrinol Metab 2008;93:743–9 [DOI] [PubMed] [Google Scholar]
- 18.Francis RM, Boyle IT, Moniz C, Sutcliffe AM, Davis BS, Beastall GH, Cowan RA, Downes N. A comparison of the effects of alfacalcidol treatment and vitamin D2 supplementation on calcium absorption in elderly women with vertebral fractures. Osteoporos Int 1996;6:284–90 [DOI] [PubMed] [Google Scholar]
- 19.Gallagher JC, Yalamanchili V, Smith LM. The effect of vitamin D on calcium absorption in older women. J Clin Endocrinol Metab 2012;97:3550–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690–3 [DOI] [PubMed] [Google Scholar]
- 21.Zittermann A, Frisch S, Berthold HK, Gotting C, Kuhn J, Kleesiek K, Stehle P, Koertke H, Koerfer R. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. Am J Clin Nutr 2009;89:1321–7 [DOI] [PubMed] [Google Scholar]
- 22.Gallagher JC, Sai A, Templin T, Smith L. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med 2012;156:425–37 [DOI] [PubMed] [Google Scholar]
- 23.Field MP, Shapses S, Cifuentes M, Sherrell RM. Precise and accurate determination of calcium isotope ratios in urine using HR-ICP-SFMS. J Anal At Spectrom 2003;18:727–33 [Google Scholar]
- 24.IOM (Institute of Medicine) Dietary Reference Intakes for calcium and vitamin D. Washington, DC: The National Academies Press, 2011 [PubMed] [Google Scholar]
- 25.Blum M, Dallal GE, Dawson-Hughes B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J Am Coll Nutr 2008;27:274–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason C, Xiao L, Imayama I, Duggan CR, Bain C, Foster-Schubert KE, Kong A, Campbell KL, Wang CY, Neuhouser ML, et al. Effects of weight loss on serum vitamin D in postmenopausal women. Am J Clin Nutr 2011;94:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012;20:1444–8 [DOI] [PubMed] [Google Scholar]
- 28.Van Cromphaut SJ, Rummens K, Stockmans I, Van HE, Dijcks FA, Ederveen AG, Carmeliet P, Verhaeghe J, Bouillon R, Carmeliet G. Intestinal calcium transporter genes are upregulated by estrogens and the reproductive cycle through vitamin D receptor-independent mechanisms. J Bone Miner Res 2003;18:1725–36 [DOI] [PubMed] [Google Scholar]
- 29.Wolf RL, Cauley JA, Baker CE, Ferrell RE, Charron M, Caggiula AW, Salamone LM, Heaney RP, Kuller LH. Factors associated with calcium absorption efficiency in pre- and perimenopausal women. Am J Clin Nutr 2000;72:466–71 [DOI] [PubMed] [Google Scholar]
- 30.Lips P, Wiersinga A, van Ginkel FC, Jongen MJ, Netelenbos JC, Hackeng WH, Delmas PD, van der Vijgh WJ. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J Clin Endocrinol Metab 1988;67:644–50 [DOI] [PubMed] [Google Scholar]
- 31.Björkman M, Sorva A, Tilvis R. Responses of parathyroid hormone to vitamin D supplementation: a systematic review of clinical trials. Arch Gerontol Geriatr 2009;48:160–6 [DOI] [PubMed] [Google Scholar]
- 32.Heaney RP. Vitamin D and calcium interactions: functional outcomes. Am J Clin Nutr 2008;88:541S–4S [DOI] [PubMed] [Google Scholar]
- 33.Sai AJ, Walters RW, Fang X, Gallagher JC. Relationship between vitamin D, parathyroid hormone, and bone health. J Clin Endocrinol Metab 2011;96:E436–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shapses SA, Kendler DL, Robson R, Hansen KE, Sherrell RM, Field MP, Woolf E, Berd Y, Mantz AM, Santora AC. Effect of alendronate and vitamin D(3) on fractional calcium absorption in a double-blind, randomized, placebo-controlled trial in postmenopausal osteoporotic women. J Bone Miner Res 2011;26:1836–44 [DOI] [PubMed] [Google Scholar]
- 35.Thacher TD, Obadofin MO, O'Brien KO, Abrams SA. The effect of vitamin D2 and vitamin D3 on intestinal calcium absorption in Nigerian children with rickets. J Clin Endocrinol Metab 2009;94:3314–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Need AG, O'Loughlin PD, Morris HA, Coates PS, Horowitz M, Nordin BE. Vitamin D metabolites and calcium absorption in severe vitamin D deficiency. J Bone Miner Res 2008;23:1859–63 [DOI] [PubMed] [Google Scholar]
- 37.Ensrud KE, Ewing SK, Stone KL, Cauley JA, Bowman PJ, Cummings SR. Intentional and unintentional weight loss increase bone loss and hip fracture risk in older women. J Am Geriatr Soc 2003;51:1740–7 [DOI] [PubMed] [Google Scholar]