Abstract
BACKGROUND & AIMS
Hepatocyte injury occurs during liver fibrogenesis. MicroRNAs (miRNA) regulate some of these processes, and some are regulated by the farnesoid X receptor (FXR). We investigated the effect of repression of specific miRNAs by FXR in hepatocyte injury using fibrotic liver tissue from patients and hepatocytes.
METHODS
We used immunohistochemistry or real-time polymerase chain reaction to analyze proteins and miRNAs in human and mouse liver samples. HepG2 cells were transfected with pre-miRNA, antisense oligonucleotides, small interfering RNAs, the 3′-untranslated region of liver kinase B1 (LKB1) (STK11), or constructs for overexpression, and analyzed.
RESULTS
Liver tissue from patients with severe fibrosis had lower levels of FXR and greater amounts of hepatocyte death than samples from patients with mild disease. Levels of several miRNAs changed when FXR expression was disrupted in the liver; one of these, miR-199a-3p, was significantly up-regulated in patients with severe fibrosis. Activation of FXR by its ligand reduced the level of miR-199a-3p in HepG2 cells. LKB1 messenger RNA was identified as a target of miR-199a-3p, and its expression was reduced in human fibrotic liver tissue. Overexpression of FXR or incubation of cultured hepatocytes with the FXR ligand up-regulated LKB1; LKB1 was not induced in cells transfected with miR-199a-3p. Incubation of HepG2 cells with FXR ligand, or injection of the ligand into mice, protected hepatocytes from injury and increased levels of LKB1; levels of miR-199a-3p were reduced compared with cells that were not incubated with the FXR ligand. Activation of FXR reduced mitochondrial dysfunction and oxidative stress and increased hepatocyte survival.
CONCLUSIONS
In hepatocytes, FXR represses production of miR-199a-3p. In fibrotic livers of humans and mice, FXR expression is reduced, increasing levels of miR-199a-3p, which reduces levels of LKB1. FXR therefore protects hepatocytes from injury by repressing miR-199a-3p and thereby increasing levels of LKB1.
Keywords: Nuclear Receptor, Noncoding RNA, Mitochondrial Protection, Fibrotic Liver Injury
Liver fibrosis refers to the accumulation of fibrous scar tissue in the liver that is facilitated by orchestration of the complex events involving hepatocyte injury. When hepatocytes are injured because of hepatitis B virus (HBV) or hepatitis C virus (HCV) infection, or other pathologic conditions, the immune system is activated, and the repair process occurs.1 The injury or death of hepatocytes stimulates activation of hepatic stellate cells (HSCs) and production of extracellular matrix proteins. Until now, no drugs have been definitively shown to effectively reduce the development of liver fibrosis in humans. A limitation of the current antifibrotic approaches is that antifibrotic drugs are not efficiently taken up by activated HSCs and are often toxic to hepatic cells, producing unwanted adverse effects.2 Because the chronic hepatocyte death is a crucial factor for the initiation and progression of liver fibrosis and cirrhosis,3 the inhibition of hepatocyte injury or death may constitute an important strategy for the treatment of fibrosis.
Farnesoid X receptor (FXR) is highly expressed in major organs including the liver and serves as a ligand-mediated transcription factor that regulates the expression of various genes involved in liver homeostasis.4 FXR ligand treatments have anti-inflammatory and liver-regenerating effects.5,6 In patients with diabetes or nonalcoholic fatty liver disease, clinical trials of FXR agonists have recently been under-taken.7 Moreover, FXR activation has an antifibrotic potential. Although FXR is expressed in HSCs and negatively controls HSC activation,8 a recent study suggested that there is less FXR expression in human HSCs in liver fibrosis.9 This observation indicates that these cells may not be the direct target for FXR ligands. More importantly, the regulatory role of FXR on the viability of hepatocytes during the state of fibrotic disease remains undefined.
MicroRNAs (miRNAs) negatively regulate gene expression by base pairing with the 3′-untranslated region (UTR) of their target messenger RNAs (mRNA) and may modulate liver physiology.10 Thus, abnormal levels of miRNA could be one of the possible mechanisms responsible for the deregulation of protein expression during liver disease progression. However, specific miRNAs that induce hepatic cell injury responsible for the progression of liver disease and their molecular basis remained to be elucidated. Moreover, there is no information available on the regulatory role of FXR in the expression of miRNAs that affect hepatocyte viability during liver fibrosis.
In view of the lack of understanding on the miRNAs controlled by FXR, this study investigated the effect of specific miRNA repression by FXR against hepatocyte injury. We explored the miRNAs affected by FXR in the HBV patients with mild or severe liver fibrosis and elucidated the role of a specific miRNA in hepatocyte survival. In our finding, FXR and liver kinase B1 (LKB1) were both repressed in the livers of severe fibrotic patients with an increase in miR-199a-3p level. To address the complex regulation involving FXR and LKB1, we used several hepatic models to corroborate the role of FXR and miR-199a-3p in LKB1 expression and LKB1–AMP-activated protein kinase (AMPK)–dependent protection of mitochondria.
Materials and Methods
Materials
Information on the materials used in this study is given in the Supplementary Materials and Methods.
Patient Samples
Human liver tissues were obtained from patients who had been diagnosed with liver fibrosis or cirrhosis.11
Transient Transfection
The construct encoding for FXR was provided by Dr Bart Staels (Institut Pasteur de Lille, Lille, France).12
LKB1 3′UTR Luciferase Assay
The plasmid containing Luc-LKB1-3 UTR (product ID: HmiT017794-MT01; GeneCopoeia, Rockville, MD) was used in reporter assay.
Flow Cytometric Analysis of Mitochondrial Membrane Potential
Mitochondrial membrane potential (MMP) was measured with rhodamine123.13 Details for in vitro, in vivo, and human studies are given in the Supplementary Materials and Methods.
Results
FXR Repression and miR-199a-3p Induction in Patients With Severe Fibrosis
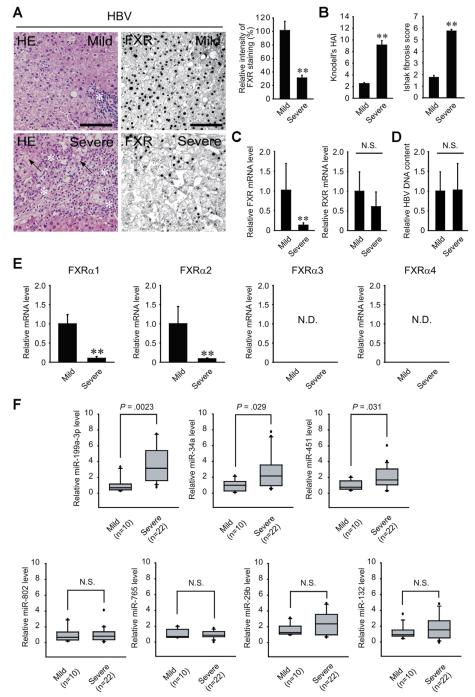
In an effort to find the biologic relevance of FXR function in a clinical situation of liver injury, the level of FXR expression was compared in a group of HBV patients with mild (Ishak fibrosis score of 3 or less) or severe (Ishak fibrosis score of 5 or 6) fibrosis. Histopathologic and immunohistochemical analyses of the liver sections revealed that the levels of FXR were lower in the patients with severe fibrosis than those with mild fibrosis (Figure 1A). As expected, the degrees of hepatocyte death and inflammation were much higher in the patients with severe fibrotic livers, as indicated by mean increases in modified Knodell histologic activity index (Figure 1B). Consistently, FXR mRNA levels were significantly decreased as the disease progressed to the severe fibrotic stage, whereas those of retinoid X receptor (RXR) mRNA were not (Figure 1C). Hepatic HBV DNA contents were not different (Figure 1D). Among the human FXR isoforms, the levels of FXRα1 and FXRα2 transcripts, most abundant in the liver, were much lower in HBV patients with severe fibrosis (Figure 1E). FXRα3 and FXRα4 mRNAs were undetectable. Similarly, FXR levels were also lower in the severe fibrotic livers of HCV patients (Supplementary Figure 1). Thus, there exists a correlation between FXR repression and hepatocyte death in the fibrotic liver of viral patients.
Figure 1.
Hepatic FXR and microRNA levels in HBV patients with fibrosis. (A) HE staining and FXR immunohistochemistry in the livers of HBV patients with mild or severe fibrosis. Arrows indicate eosinophilic necrosis of hepatocytes, and asterisks indicate infiltration of inflammatory cells (scale bar, 100 μm). The staining intensity of FXR was quantified in the liver sections (right). (B) The degrees of hepatocyte death, inflammation, and fibrosis in the human liver samples. Knodell histologic activity index and Ishak fibrosis scores were measured in the patient samples. (C) The levels of FXR and RXR mRNA. mRNA levels were assessed using real-time PCR assays. (D) The relative HBV DNA contents in the liver. HBV DNA was determined using real-time PCR assays. (E) The levels of FXR isoform mRNAs. ND, not detectible. For panels A–E, values represent the mean ± standard error of mean (significantly different as compared with mild fibrosis, **P < .01) (n = 10/group). (F) The levels of microRNAs in the livers of HBV patients with fibrosis. In the box plots, the lower and upper ends of each box represent the 25th and 75th percentiles, the horizontal lines inside the boxes display the median value, and the whiskers indicate 1.5 times the interquartile range. Dots denote outlier values. NS, not significant.
Aberrant expression of miRNAs is a crucial cause of various diseases.10 In a mouse model, FXR gene knockout affected the expression levels of certain miRNAs in the liver.14 Using the database, the miRNAs whose levels were increased by a deficiency in FXR at the threshold of 1.5-fold or higher were chosen, and their expression levels were compared in the human liver samples. Among the miRNAs, the levels of miR-199a-3p, miR-34a, and miR-451 were significantly up-regulated in the livers of HBV patients with severe fibrosis compared with those with mild fibrosis (Figure 1F). In particular, the level of miR-199a-3p was most significantly increased, suggesting that FXR may regulate the miRNA expression in association with hepatocyte death.
miR-199a-3p Down-regulation by FXR Activation
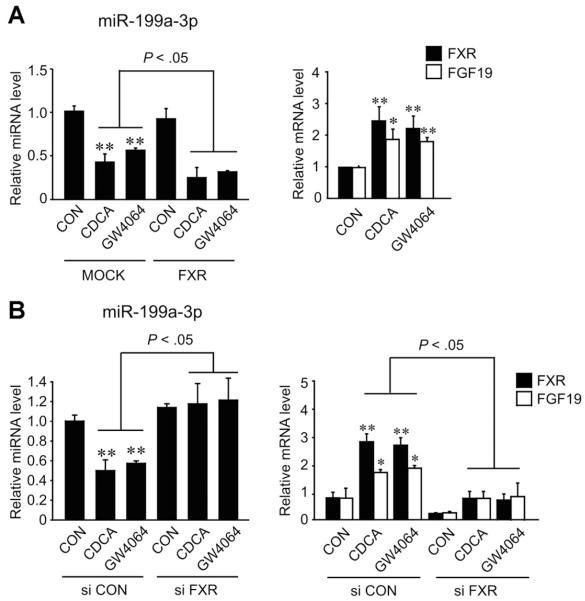
Given the link between FXR and miR-199a-3p, we studied the regulatory role of FXR in the expression of the miRNA in HepG2 cell model. The activation of FXR by its ligand treatments caused a decrease in miR-199a-3p level (Figure 2A). In addition, overexpression of FXR enhanced the ability of FXR ligand to decrease miR-199a-3p level. Moreover, a deficiency in FXR by small interfering RNA knockdown reversed the effect of FXR ligand treatment on miR-199a-3p expression (Figure 2B), corroborating the inhibitory role of FXR in the expression of the miRNA. FXR ligand treatment increased not only the levels of FXR mRNA but also those of FGF19 mRNA (the transcription target of FXR) (Figure 2A, right), which was abolished by FXR knockdown (Figure 2B, right). Our results showed that the activation of FXR inhibited the expression of miR-199a-3p.
Figure 2.
microRNA-199a-3p repression by FXR activation. (A) miR-199a-3p repression by FXR ligand treatment with or without FXR overexpression. The levels of miR-199a-3p, FXR, and FGF19 transcripts were measured using real-time PCR assays in HepG2 cells transfected with MOCK or the plasmid encoding for FXR. HepG2 cells were treated with either CDCA (100 μmol/L) or GW4064 (2 μmol/L) for 3 hours. (B) The effect of FXR knockdown on the repression of miR-199a-3p by FXR. After small interfering RNA transfection, the cells were incubated with FXR ligand for 3 hours. For panels A and B, values represent the mean ± standard error of mean of 3 replicates (significantly different as compared with MOCK or vehicle-treated control, *P < .05, **P < .01).
miR-199a-3p as a Repressor of LKB1 Translation
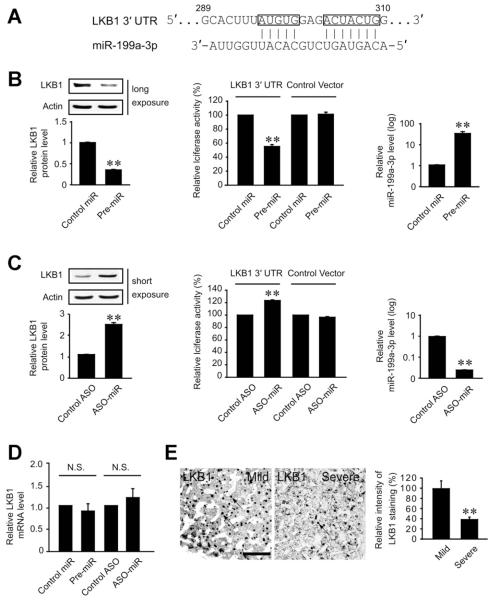
Having identified the repression of miR-199a-3p by FXR, we next explored the functional role of miR-199a-3p in the expression of protein necessary for cell survival. To search for the target of miR-199a-3p, we focused on the candidate target genes responsible for cell survival. Bioinformatic analyses using miRanda (MICROCOSM; EMBL-EBI, Cambridgeshire, United Kingdom) and TargetScan (Whitehead Institute for Biological Research, Cambridge, MA) algorithms enabled us to select the possible targets regulated by miR-199a-3p. In this approach, we focused on 7 candidate genes that have a potential to promote cell survival (Supplementary Table 1). Among them, LKB1, a protein known to protect mitochondria from oxidative stress, was expected to be the most appropriate molecule. The pairing between LKB1 3′UTR region and miR-199a-3p sequence was nearly perfect (Figure 3A), which suggested that LKB1 mRNA might be a target of miR-199a-3p.
Figure 3.
LKB1 mRNA as a target of miR-199a-3p. (A) Prediction of miR-199a-3p binding to the 3′UTR of LKB1 mRNA. (B) LKB1 repression by miR-199a-3p. Immunoblottings were performed on the lysates of HepG2 cells transfected with control miR or pre-miR-199a-3p (left). Luciferase assays of the 3′UTR of LKB1 were conducted in cells transfected with control miR or pre-miR-199a-3p in combination with a reporter comprising a luciferase cDNA fused to the 3′UTR of LKB1 (pEZX-LKB1 3′UTR) or control vector (pEZX-Control) (middle). Real-time PCR assay verified the transfection of miR-199a-3p (right). (C) LKB1 induction by the antisense oligonucleotide (ASO) directed against miR-199a-3p. The assays were performed as described in panel B after ASO-miR-199a-3p transfection. For panels B and C, data represent the mean ± standard error of mean of 3 replicates (significantly different from transfection control, **P < .01). (D) The effect of pre-miR-199a-3p or ASO-miR-199a-3p on LKB1 mRNA level. (E) Immunohistochemistry of LKB1 in the livers of HBV patients with fibrosis. Immunohistochemistry was performed on the liver sections (left). Arrows indicate LKB1 staining in liver tissue (scale bar, 100 μm). Values represent the mean ± standard error of mean (significantly different as compared with mild fibrosis, **P < .01) (n = 10/group).
To precisely define the inhibitory role of miR-199a-3p for LKB1, in vitro functional assays were performed by enhancing or silencing miR-199a-3p. As expected, transfection with pre-miR-199a-3p significantly decreased the level of LKB1 protein (Figure 3B, left). By the same token, pre-miR-199a-3p transfection decreased luciferase activity from p-EZX-LKB1 luciferase vector comprising the LKB1 3′UTR region, whereas scrambled control miRNA had no effect (Figure 3B, middle). In addition, transfection with the antisense oligonucleotide (ASO) directed against miR-199a-3p not only induced LKB1 but promoted luciferase expression from the LKB1 3 UTR reporter construct, confirming the repressing effect of miR-199a-3p on LKB1 translation (Figure 3C, left and middle). Either pre-miR-199a-3p or ASO-miR-199a-3p had no effect on the LKB1 mRNA content (Figure 3D). In view of the inhibitory role of miR-199a-3p on LKB1 mRNA translation, LKB1 levels were compared in the groups of HBV patients with mild or severe fibrosis. As expected, LKB1 expression was significantly lower in the livers of severe fibrotic patients (who had higher miR-199a-3p content in Figure 1F) than those with mild fibrosis (Figure 3E). Our finding regarding the repression of LKB1 in the patients with severe fibrosis corroborates the regulatory effect of miR-199a-3p on the key molecule necessary for hepatocyte viability.
LKB1 Induction by FXR Activation
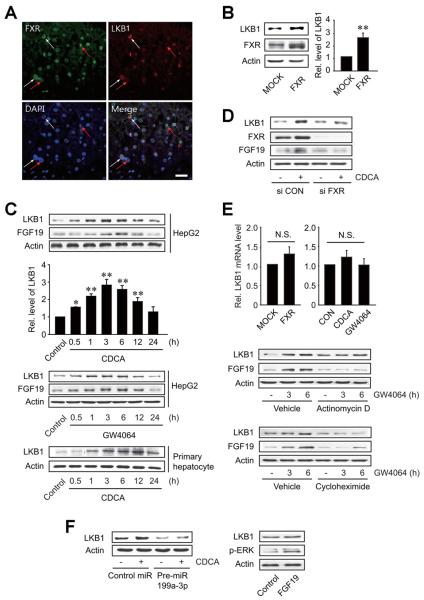
To directly link FXR and LKB1, we examined their localization using immunohistochemisty (Figure 4A). In the human liver samples, FXR was simultaneously stained with LKB1 (Figure 4A), being consistent with the concept that FXR regulates the expression of LKB1. In another effort to understand the association between FXR and LKB1, we explored the effect of FXR overexpression on LKB1 level. As expected, FXR overexpression notably induced LKB1 in HepG2 cells (Figure 4B). Moreover, treatment of the cells with FXR ligand resulted in a time-dependent increase in LKB1 with a maximal increase being noted at 3 hours (Figure 4C). The induction of LKB1 by FXR ligand treatment was confirmed in primary rat hepatocytes. In addition, FXR knockdown prevented the induction of LKB1 by chenodeoxycholic acid (CDCA) treatment (Figure 4D), verifying the role of FXR in the induction of LKB1. Fibroblast growth factor 19 (FGF19) expression was confirmed as a known target of FXR activation (Figure 4C and D). No changes in the LKB1 mRNA after FXR overexpression or ligand treatment supported the post-transcriptional regulatory mechanism (Figure 4E). Actinomycin D (an inhibitor of RNA polymerase II) did not inhibit LKB1 induction by GW4064 (an FXR ligand) but did that of FGF19. However, cycloheximide (an inhibitor of translational elongation) blocked both LKB1 and FGF19 induction (Figure 4E). Given our finding that the miR-199a-3p targets the 3′UTR of LKB1 mRNA, we further determined whether it intervenes with LKB1 induction by FXR ligand. As expected, pre-miR-199a-3p transfection completely inhibited the ability of CDCA to induce LKB1 (Figure 4F, left). The lack of LKB1 induction by recombinant FGF19 treatment excludes the possibility that FXR induction of LKB1 is mediated with FGF19 (Figure 4F, right).
Figure 4.
The induction of LKB1 by FXR. (A) Immunohistochemistry of FXR or LKB1 in the livers of HBV patients with fibrosis. Red arrows indicate colocalization of FXR and LKB1, whereas white arrows indicate background of staining. Tissue sections were mounted with vectashield containing 4′-6-diamidino-2-phenylindole (blue) for nuclear staining. Pictures are representative results from at least 8 experiments. Scale bar, 50 μm. (B) LKB1 induction by FXR overexpression. Immunoblottings were performed on the lysates of HepG2 cells transfected with MOCK or FXR. (C) LKB1 induction by FXR ligand treatment. Hepatocytes were treated with 100 μmol/L chenodeoxycholic acid (CDCA) or 2 μmol/L GW4064 for the indicated times. For panels B and C, data represent the mean ± standard error of mean of 3 replicates (treatment mean significantly different from MOCK or vehicle-treated control, *P < .05, **P < .01). (D) The effect of FXR knockdown on LKB1 induction by CDCA. (E) Post-transcriptional regulation of LKB1 by FXR. HepG2 cells were transfected with the construct encoding FXR or treated with either CDCA or GW4064 for 3 hours (upper). LKB1 and FGF19 were immunoblotted on HepG2 cells treated with GW4064 after actinomycin D or cycloheximide treatment for 5 hours (lower). (F) The effect of miR-199a-3p transfection on LKB1 induction by CDCA. HepG2 cells were treated with CDCA for 3 hours after pre-miR-199a-3p transfection. LKB1 and p-ERK were immunoblotted on cells treated with 250 ng/mL recombinant FGF19 for 1 hour.
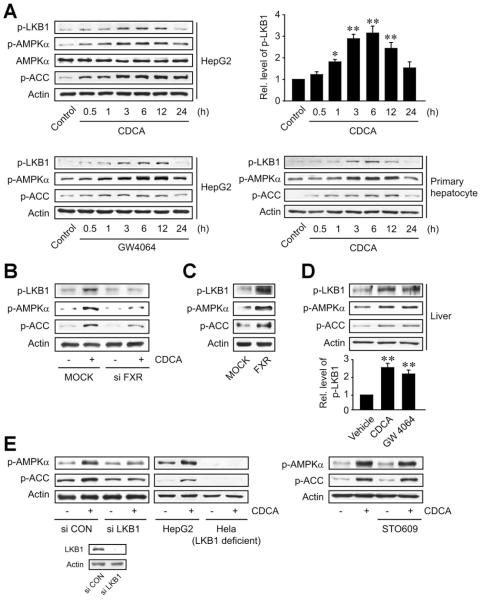
FXR Activation of LKB1
Because FXR induces LKB1, the activity of LKB1 may increase after FXR activation. Treatment of HepG2 cells with FXR ligand enhanced the activating phosphorylation of LKB1 in a time-dependent manner with a maximal increase observed at 6 hours after treatment (Figure 5A). Prior to activation, LKB1 translocates to the cytoplasm when it binds to STE20-related adaptor (STRAD) and/or calcium binding protein 39 (CAB39).15 We found that CDCA treatment facilitated complex formation of LKB1 with STRAD and CAB39 maximally at 3 hours post-treatment (Supplementary Figure 2). Because LKB1 is an upstream kinase of AMPK, FXR ligand treatment activated AMPK in hepatocytes, as shown in part previously.16 Abrogation of CDCA-induced LKB1 phosphorylation by FXR knockdown supported the regulatory role of FXR in this event (Figure 5B). Consistently, FXR overexpression facilitated LKB1 phosphorylation (Figure 5C). Furthermore, mice treated with either CDCA or GW4064 displayed increases in the phosphorylation of LKB1 in the liver (Figure 5D), verifying the activation of LKB1 by FXR in vivo. FXR activation of AMPK was also confirmed in vivo.
Figure 5.
LKB1 activation by FXR ligand treatment. (A) LKB1 activation by FXR ligand treatment. Immunoblottings were performed on the lysates of HepG2 cells or primary rat hepatocytes treated as described in Figure 4C. (B) FXR-dependent activation of LKB1. Proteins were immunoblotted on the lysates of HepG2 cells treated with chenodeoxycholic acid (CDCA) (100 μmol/L, for 6 hours) after small interfering RNA (siRNA) transfection. (C) The activation of LKB1 by FXR overexpression. Immunoblottings were done on the lysates of HepG2 cells transfected with FXR. (D) LKB1 activation in the liver of mice treated with FXR ligand. Mice were treated with a single dose of CDCA (30 mg/kg) or GW4064 (30 mg/kg) for 6 hours. For panels A and D, data represent the mean ± standard error of mean of 3 replicates (treatment mean significantly different from vehicle-treated control; *P < .05, **P < .01). (E) LKB1-dependent activation of AMPK. HepG2 cells were treated with CDCA for 6 hours after siRNA transfection (left). Hela cells deficient in LKB1 were also treated with CDCA for 6 hours (middle). After treatment with 1 μg/mL STO-609 for 30 minutes, HepG2 cells were incubated with CDCA for 6 hours (right).
The failure of CDCA to increase AMPKα phosphorylation in HepG2 cells transfected with LKB1 siRNA or Hela cells deficient in LKB1 supported the role of LKB1 in this effect (Figure 5E, left). Calcium/calmodulin-dependent kinase kinase (CaMKK)-β is another kinase that may activate AMPK. STO-609, an inhibitor of CaMKK-β, had no effect on the activation of AMPK by CDCA (Figure 5E, right). Our results demonstrate that LKB1 induction by FXR led to LKB1 activation, which promotes AMPK activation.
Hepatocyte Protection by FXR In Vivo and in a Cell Injury Model
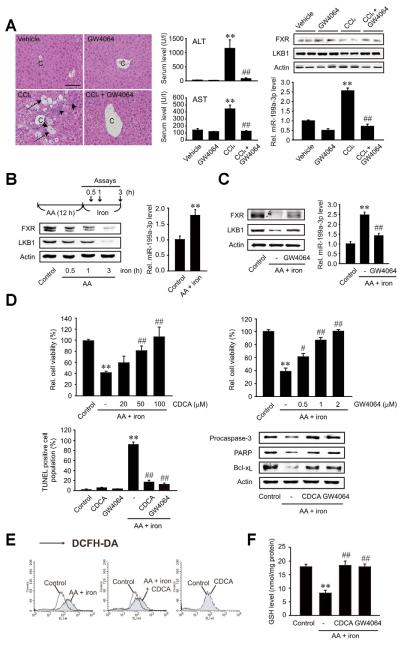
To link the LKB1 activation by FXR to functional improvements, the effect of FXR on hepatocyte survival was assessed in animal and cell models. First, we examined the effect of FXR ligand treatment on the fibrotic injury in mice treated with CCl4 (Figure 6A). GW4064 treatment prevented increases in serum alanine aminotransferase and aspartate aminotransferase activities. The levels of FXR and LKB1 were also restored. Consistently, GW4064 treatment abrogated the increase in miR-199a-3p level by CCl4.
Figure 6.
The role of FXR in hepatocyte viability on in vivo and in vitro hepatocyte injury models. (A) The effect of GW4064 treatment in mice treated with CCl4 (0.6 mL/kg, intraperitoneally, twice a week for 5 weeks). Arrows indicate eosinophilic necrosis of hepatocytes, and arrowheads indicate infiltration of inflammatory cells (scale bar, 100 μm). Data represent the mean ± standard error of mean of 4 replicates (treatment mean significantly different from vehicle-treated control, **P < .01; or CCl4, ##P < .01). (B) The levels of FXR, LKB1, and miR-199a-3p in an in vitro cell injury model. HepG2 cells were incubated with 10 μmol/L AA for 12 hours and continuously exposed to 5 μmol/L iron for 0.5–3 hours. (C) The effect of GW4064 on miR-199a-3p-mediated repression of LKB1 in an in vitro model. HepG2 cells were treated with AA for 12 hours following GW4064 treatment for 1 hour and continuously incubated with iron for 3 hours. (D) The effect of FXR ligand treatment on cell viability. Viability was assessed using MTT or terminal transferase-mediated dUTP nick-end labeling assays. Apoptotic marker proteins were immunoblotted on the lysates. (E) Hydrogen peroxide production. DCF fluorescence was monitored in HepG2 cells similarly treated as described in panel C (iron exposure for 1 hour). Results were confirmed by 2 repeated experiments. (F) The cellular GSH content. For panels B, C, D, and F, data represent the mean ± standard error of mean of 3 replicates (treatment mean significantly different from vehicle-treated control, **P < .01; or AA+iron, #P <.05, ##P < .01).
A combinatorial treatment of arachidonic acid (AA) and iron induces cell death through oxidative stress.13 Treatment of HepG2 cells with AA and iron elicited FXR repression as the incubation time with iron was prolonged (Figure 6B). In parallel, LKB1 expression was suppressed. Moreover, exposure of the cells to AA + iron significantly increased the miR-199a-3p content. Of note, FXR ligand treatment prevented the repression of both FXR and LKB1 in the hepatocyte injury model. The increase in miR-199a-3p was also abolished by the treatment (Figure 6C), strengthening the ability of FXR to regulate LKB1 and the role of miR-199a-3p in this event.
To assess the causal effect of activated FXR on cell viability, we determined the effects of FXR ligands on the viability of hepatocytes (Figure 6D). Either CDCA or GW4064 treatment protected HepG2 cells from death elicited by AA + iron. The ability of FXR to protect cells from the oxidative stress was confirmed by alterations in the levels of proteins associated with apoptosis. Incubation of HepG2 cells with AA and iron stimulates H2O2 production and causes glutathione (GSH) depletion. Ligand activation of FXR diminished the increase in cellular H2O2 and prevented GSH depletion (Figure 6E and F). Overall, ligand activation of FXR enhances LKB1 expression by down-regulating miR-199a-3p, which may contribute to protecting hepatocytes from injury and enhancing antioxidant capacity in hepatocytes.
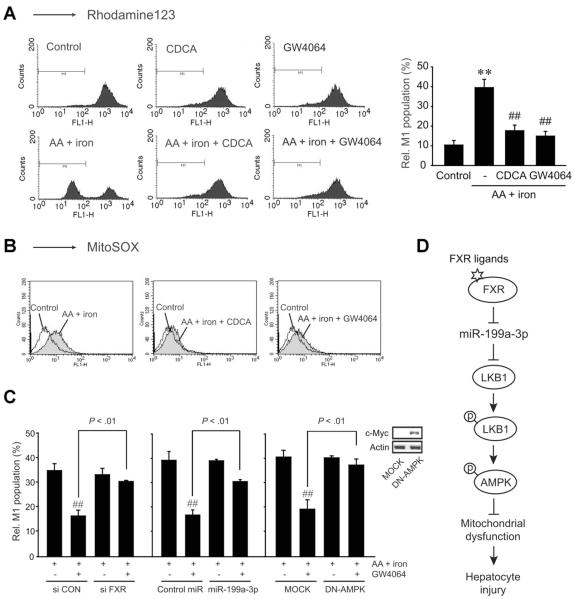
Mitochondrial Protection by FXR
The mitochondrion is the main source of reactive oxygen species (ROS). Thus, impairment of mitochondrial function may cause oxidative stress and apoptosis.17 As a continuing effort to study the functional role of LKB1 for the protection of mitochondria in association with miR-199a-3p down-regulation by FXR, we measured the MMP using fluorescence-activated cell sorter analysis. Exposure of HepG2 cells to AA + iron resulted in an increase in the subpopulation of M1 fraction. This MMP change was completely abrogated by either CDCA or GW4064 treatment (Figure 7A). Consequently, FXR activation leads to protect mitochondria from oxidative injury. In addition, FXR ligand treatment diminished mitochondrial superoxide production in the cell model, as monitored by an assay using MitoSOX (Molecular Probes, Eugene, OR) (Figure 7B). To confirm the bona fide regulatory role of FXR in the protection of mitochondria, the effect of FXR ligand treatment on M1 population was determined after FXR knockdown. As expected, the ability of GW4064 to maintain the proportion of M1 population under the condition of severe oxidative stress was antagonized by FXR knockdown (Figure 7C, left). Transfection of the cells with either pre-miR-199a-3p or DN-AMPK exerted similar effects (Figure 7C, middle and right). Overall, FXR activation indeed contributes to protecting mitochondria from oxidative injury, which may depend on LKB1-AMPK activation via LKB1 induction as a consequence of miR-199a-3p down-regulation (Figure 7D).
Figure 7.
FXR protection of mitochondria from oxidative injury. (A) Mitochondrial membrane potential. The population of M1 fraction (low rhodamine123 fluorescence) was measured in HepG2 cells treated as described in Figure 6C. (B) Superoxide production in mitochondria. (C) Relative M1 populations. After transfection with FXR siRNA, miR-199a-3p, or dominant negative-AMPK (DN-AMPK), HepG2 cells were treated as described in Figure 6C. For panels A and C, data represent the mean ± standard error of mean of 3 and 4 replicates, respectively (treatment mean significantly different from vehicle-treated control, **P < .01; or iron+AA, ##P < .01). (D) A proposed pathway by which FXR activation induces LKB1 for the protection of hepatocytes from oxidative injury via miR-199a-3p suppression.
Discussion
Chronic infection with HBV or HCV leads to long-term liver damage and fibrosis.1 Hepatocyte death initiates and stimulates matrix deposition as an adaptive repair response. Our work highlights the novel role of FXR in hepatocyte survival in the HBV patients with fibrosis. The present study revealed that FXR expression, especially FXRα1 and FXRα2, became lower in the liver of HBV patients with fibrosis as the disease progressed to a severe grade. Similar results were additionally obtained in HCV patients with mild or severe fibrosis (Supplementary Figure 1). Other etiologies such as steatosis or cholestasis-induced liver injury also showed that FXR was decreased in parallel with disease progression.18,19 Our result is also in line with the findings that the FXR gene knockout enhanced liver damage elicited by various experimental conditions.20–22
The bioinformatic analysis of the miRNA array data obtained by a knockout of the FXR gene and experimental approaches allowed us to extract certain miRNAs potentially involved in the process of cell viability.14 Among them, miR-199a-3p was the most significantly up-regulated in human fibrotic liver samples, which was accompanied by the repression of FXR. In an in vitro cell model, our result supported the functional role of FXR in the inhibition of miR-199a-3p expression. The level of miR-199a-3p primary transcript was not changed in different fibrosis stages but increased those of the precursor and mature forms in the severe fibrotic liver sample (Supplementary Figure 3 and Figure 1F), suggesting that FXR negatively regulates miR-199a-3p at the post-transcriptional step. Several miRNAs may become mature by altered expression of miRNA-processing factors (eg, p53 and SAM- and MAD-related protein) in the absence of changes in primary precursors.23,24 Thus, the up-regulation of miR-199a-3p might be attributable to the processing steps but not the genomic and epigenetic alterations.
The role of miR-199a-3p in liver carcinogenesis and progression had been investigated; it might inhibit hepatocellular carcinoma cell cycle progression.25 Here, we report the identification of miR-199a-3p as a regulator of hepatocyte survival in liver fibrosis and its elevation in the liver of HBV patients with severe fibrosis. Therefore, our result showing the role of FXR in the repression of miR-199a-3p may provide a key strategy for antifibrotic therapy. In other studies, the levels of miR-21 and miR-122 were changed in the liver of patients with HCV,26 whereas those of miRNA-29 family members were decreased in rat hepatic stellate cells.27 Hence, a set of miRNAs may work together for the progression of liver fibrosis. In other organs, fibrosis may be affected by other miRNAs.28 Because hepatocyte death is primarily controlled by miR-199a-3p and repetitive injury of hepatocytes facilitates the progression of liver fibrosis, one could predict the development of effective and safe miRNA inhibitors and mimics as a strategy for the treatment of liver fibrosis.
Having identified the role of miR-199a-3p in hepatocyte survival, we extracted 9 potential target molecules including STK11/LKB1, FIGF, PAWR, PRDX1, 2 mitogen-activated protein kinases, CYB5R4, PAK4, and mTOR (Supplementary Table 1). Among them, LKB1 is induced upon starvation and acts as a critical regulator of energy homeostasis in the liver.29 Thus, LKB1 plays a role in the protection of hepatocytes from injury.13,30 We revealed the miR-199a-3p as a direct suppressor of LKB1 induction, as indicated by the ability of the miRNA to inhibit LKB1 translation and decrease the expression of pEZX-LKB1 3′UTR reporter. The role of miR-199a-3p in the regulation of LKB1 was strengthened by the reversal of the effect using the ASO. Moreover, exogenous miR-199a-3p prevented the induction of LKB1 by FXR. FXR ligand treatment unchanged LKB1 mRNA content, excluding the possibility of FXR as a transcription factor of this gene. This concept is in line with the finding that actinomycin D did not prevent LKB1 induction by GW4064, whereas cycloheximide did so. These observations represent the first evidence that miR-199a-3p is down-regulated by FXR, which promotes LKB1 induction and the consequent activation of LKB1.
Another important finding of this study is the observation that LKB1 expression was lower in patients with severe fibrosis. LKB1 was colocalized with FXR in the fibrotic liver samples. To strengthen our observation, we confirmed the relationship between FXR and LKB1 in animal models; administration of FXR ligand to mice protected them against fibrotic injury and increased levels of LKB1 with miR-199a-3p repression, compared with mice that were not given FXR ligand. In addition, we used FXR knockout mice to reveal the direct effect of FXR on LKB1 expression. Levels of LKB1 were lower in FXR knockout mice than in wild-type mice (Supplementary Figure 4). CCl4 treatment further decreased LKB1 levels in the knockout mice; LKB1 levels matched with changes in liver histopathology.17 Moreover, overexpression of FXR or its ligand treatment promoted the induction and activation of LKB1 in HepG2 cells. Although protein kinase C ξ is one of upstream kinase of LKB1,31 it was not affected by CDCA treatment (data not shown). Our finding that FXR lowered the miRNA levels in hepatocytes supports the functional role of FXR in the induction of LKB1, being consistent with the elevation in the miRNA responsible for LKB1 repression in the fibrotic patients. Also, our finding of the increases in other miRNAs (miR-34a and miR-451) by severe fibrosis in conjunction with their known inhibitory effects on Sirt1 and CAB39 suggests that the miRNAs may negatively affect the activity of LKB1.14,32
AMPK serves as a crucial regulator of determining hepatocyte survival or death in response to pathologic stress.13,30 Because LKB1 is a primary upstream kinase of AMPK, the activation of LKB1-AMPK axis plays a role in maintaining cell viability, so the loss of LKB1 causes disorganization of cell signaling and viability under energetically unfavorable conditions. In the present study, the activation of FXR exerted an antioxidant, mitochondria-protective and cytoprotective effect at least in part through AMPK activation as mediated by LKB1. The role of AMPK in the protection of hepatocytes from injury was corroborated by increases in cell viability with the treatment of AMPK activator, which helps to protect mitochondria and inhibit ROS production in our hepatocyte injury model.13 Similarly, FXR ligand treatment protected hepatocytes from oxidative injury by inhibiting mitochondrial impairment and ROS production. The importance of FXR-mediated LKB1 activation was strengthened by the reversal of these effects by FXR knockdown, pre-miR-199a-3p transfection, or DN-AMPK overexpression.
Oxidative stress is critical for hepatocyte death. Experimental data from animal models and clinical data from patients with chronic infection of HBV or HCV suggest that an excess of ROS produced by inflammatory mediators facilitates the progression of liver fibrosis.1 In our observation, the HBV patients with severe fibrosis had high Knodell score, implying that the severe fibrotic liver might have an increased level of ROS. In fact, extensive oxidative damage had been observed in HBV-positive hepatocytes and HBV-infected humans.33 The oxidative stress in the pathogenesis of hepatitis virus might be caused by a combination of chronic liver damage, inflammation, and iron overload; therefore, the hepatocyte injury model used in the present study is likely to reflect the clinical situations (ie, ROS production, GSH depletion, repression of FXR and LKB1, and increase of miR-199a-3p).
Collectively, our results demonstrate that activated FXR protects hepatocytes from injury in fibrosis through LKB1 activation, which may result from the induction of LKB1. Moreover, our findings that the induction of LKB1 by FXR relies on the down-regulation of miR-199a-3p and that this miRNA targets LKB1 mRNA 3′ UTR provide key information not only in understanding the pharmacology and action mechanism of FXR but in designing therapeutic strategy and the use of FXR ligands in the treatment of acute or chronic liver diseases.
Supplementary Material
Supplementary Figure 1. FXR and LKB1 expression in the liver of HCV patients with mild or severe fibrosis. (A) H&E and immunohistochemical stainings for FXR and LKB1. Arrows indicate eosinophilic necrosis of hepatocytes (scale bar, 100 μm). (B) The degrees of hepatocyte death, inflammation, and fibrosis in the human liver samples. Knodell histologic activity index and Ishak fibrosis scores were measured in the patient samples (n = 3). (C) The staining intensities of FXR and LKB1 were quantified in the liver sections. For panels B and C, values represent the mean ± standard error of mean (significantly different as compared with mild fibrosis, **P < .01).
Supplementary Figure 2. LKB1 binding with CAB39 and STRAD by FXR ligand treatment. Either CAB39 or STRAD was immunoblotted on the LKB1 immunoprecipitates from HepG2 cells treated with 100 μmol/L CDCA for the indicated times.
Supplementary Figure 3. The levels of pri-miR-199a or pre-miR-199a in HBV patients with fibrosis. The levels of pri-miR-199a or premiR-199a were measured in the livers of HBV patients with mild or severe fibrosis. Values represent the mean ± standard error of mean. NS, not significant.
Supplementary Figure 4. LKB1 expression in the liver of wild-type or FXR knockout (KO) mice treated with CCl4. Immunohistochemistry for LKB1 was done on the liver sections of wild-type or FXR KO mice treated with a single dose of CCl4 (0.75 mL/kg, intraperitoneally, for 48 hours). The same paraffin blocks had been used for a different study.6 The relative LKB1 intensities were quantified (n = 3 animals/each group). Data represent the mean ± standard error of mean of 3 replicates (treatment mean significanty different from vehicle-treated control, **P < .01).
Supplementary Table 1. The Candidate Target Genes of miR-199a-3p That Are Associated With Cell Survival
Funding
Supported by the National Research Foundation of Korea grant (MEST No. 2011-0001204) funded by the government of Korea; and in part by the World Class University project (R32-2011-000-10098-0) and National Institutes of Health (NCI-R01-139158-02) (to W.H.).
Abbreviations used in this paper
- AA
arachidonic acid
- AMPK
AMP-activated protein kinase
- ASO
antisense oligonucleotide
- CAB39
calcium binding protein 39
- CDCA
chenodeoxycholic acid
- DCFH-DA
2′,7′-dichlorofluorescein diacetate
- FGF19
fibroblast growth factor 19
- FXR
farnesoid X receptor
- GSH
glutathione
- LKB1
liver kinase B1
- mRNA
messenger RNA
- miRNA
microRNA
- MMP
mitochondrial membrane potential
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide
- ROS
reactive oxygen species
- STRAD
STE20-related adaptor
- UTR
untranslated region
Footnotes
Supplementary Material Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at doi:10.1053/j.gastro.2012.01.007.
Conflicts of interest The authors disclose no conflicts.
References
- 1.Nakamoto Y, Kaneko S. Mechanisms of viral hepatitis induced liver injury. Curr Mol Med. 2003;3:537–544. doi: 10.2174/1566524033479591. [DOI] [PubMed] [Google Scholar]
- 2.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takehara T, Tatsumi T, Suzuki T, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–1197. doi: 10.1053/j.gastro.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Lefebvre P, Cariou B, Lien F, et al. Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev. 2009;89:147–191. doi: 10.1152/physrev.00010.2008. [DOI] [PubMed] [Google Scholar]
- 5.Wagner M, Zollner G, Trauner M. Nuclear bile acid receptor farnesoid X receptor meets nuclear factor-κB: new insights into hepatic inflammation. Hepatology. 2008;48:1383–1386. doi: 10.1002/hep.22668. [DOI] [PubMed] [Google Scholar]
- 6.Huang W, Ma K, Zhang J, et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312:233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 7.Fallowfield JA. Therapeutic targets in liver fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011;300:G709–G715. doi: 10.1152/ajpgi.00451.2010. [DOI] [PubMed] [Google Scholar]
- 8.Fiorucci S, Antonelli E, Rizzo G, et al. The nuclear receptor SHP mediates inhibition of hepatic stellate cells by FXR and protects against liver fibrosis. Gastroenterology. 2004;127:1497–1512. doi: 10.1053/j.gastro.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Fickert P, Fuchsbichler A, Moustafa T, et al. Farnesoid X receptor critically determines the fibrotic response in mice but is expressed to a low extent in human hepatic stellate cells and periductal myofibroblasts. Am J Pathol. 2009;175:2392–2405. doi: 10.2353/ajpath.2009.090114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Hara SP, Mott JL, Splinter PL, et al. MicroRNAs: key modulators of posttranscriptional gene expression. Gastroenterology. 2009;136:17–25. doi: 10.1053/j.gastro.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SG, Kim YM, Choi YH, et al. Pharmacokinetics of oltipraz its major metabolite in patients with liver fibrosis or cirrhosis: relationship with suppression of circulating TGF-β1. Clin Pharmacol Ther. 2011;31:861–869. doi: 10.1038/clpt.2010.89. [DOI] [PubMed] [Google Scholar]
- 12.Claudel T, Sturm E, Duez H, et al. Bile acid-activated nuclear receptor FXR suppresses apolipoprotein A-I transcription via negative FXR response element. J Clin Invest. 2002;109:961–971. doi: 10.1172/JCI14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3β inhibition downstream poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol. 2009;76:884–895. doi: 10.1124/mol.109.058479. [DOI] [PubMed] [Google Scholar]
- 14.Lee J, Padhye A, Sharma A, et al. A pathway involving farnesoid receptor and small heterodimer partner positively regulates patic sirtuin 1 levels via microRNA-34a inhibition. J Biol Chem. 2010;285:12604–12611. doi: 10.1074/jbc.M109.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boudeau J, Baas AF, Deak M, et al. MO25α/β interact STRADα/β enhancing their ability to bind, activate and localize LKB1 in the cytoplasm. EMBO J. 2003;22:5102–5114. doi: 10.1093/emboj/cdg490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noh K, Kim YM, Kim YW, et al. Farnesoid X receptor activation chenodeoxycholic acid induces detoxifying enzymes through activated protein kinase and extracellular signal-regulated kinase 1/2-mediated phosphorylation of CCAAT/enhancer binding tein β. Drug Metab Dispos. 2011;39:1451–1459. doi: 10.1124/dmd.111.038414. [DOI] [PubMed] [Google Scholar]
- 17.Kroemer G, Dallaporta B, Resche-Rigon M. The mitochondrial death/life regulator in apoptosis and necrosis. Ann Rev Physiol. 1998;60:619–642. doi: 10.1146/annurev.physiol.60.1.619. [DOI] [PubMed] [Google Scholar]
- 18.Chen F, Ananthanarayanan M, Emre S, et al. Progressive familial intrahepatic cholestasis, type 1, is associated with decreased farnesoid receptor activity. Gastroenterology. 2004;126:756–764. doi: 10.1053/j.gastro.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int. 2010;4:741–748. doi: 10.1007/s12072-010-9202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng Z, Wang Y, Wang L, et al. FXR regulates liver repair CCl4-induced toxic injury. Mol Endocrinol. 2010;24:886–897. doi: 10.1210/me.2009-0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner M, Fickert P, Zollner G, et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver in bile duct-ligated mice. Gastroenterology. 2003;125:825–838. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- 22.Sinal CJ, Tohkin M, Miyata M, et al. Targeted disruption of nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki HI, Yamagata K, Sugimoto K, et al. Modulation of croRNA processing by p53. Nature. 2009;460:529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 24.Davis BN, Hilyard AC, Nguyen PH, et al. Smad proteins bind conserved RNA sequence to promote microRNA maturation Drosha. Mol Cell. 2010;39:373–384. doi: 10.1016/j.molcel.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou J, Lin L, Zhou W, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell. 2011;19:232–243. doi: 10.1016/j.ccr.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Marquez RT, Bandyopadhyay S, Wendlandt EB, et al. Correlation between microRNA expression levels and clinical parameters associated with chronic hepatitis C viral infection in humans. Lab Invest. 2010;90:1727–1736. doi: 10.1038/labinvest.2010.126. [DOI] [PubMed] [Google Scholar]
- 27.Roderburg C, Urban GW, Bettermann K, et al. Micro-RNA profiling reveals a role for miR-29 in human and murine liver fibrosis. Hepatology. 2011;53:209–218. doi: 10.1002/hep.23922. [DOI] [PubMed] [Google Scholar]
- 28.Jiang X, Tsitsiou E, Herrick SE, et al. MicroRNAs and the regulation of fibrosis. FEBS J. 2010;277:2015–2021. doi: 10.1111/j.1742-4658.2010.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw RJ, Lamia KA, Vasquez D, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alexander A, Walker CL. The role of LKB1 and AMPK in cellular responses to stress and damage. FEBS Lett. 2011;585:952–957. doi: 10.1016/j.febslet.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Xie Z, Dong Y, Scholz R, et al. Phosphorylation of LKB1 at serine 428 by protein kinase C-zeta is required for metformin-enhanced activation of the AMP-activated protein kinase in endothelial cells. Circulation. 2008;117:952–962. doi: 10.1161/CIRCULATIONAHA.107.744490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godlewski J, Nowicki MO, Bronisz A, et al. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell. 2010;37:620–632. doi: 10.1016/j.molcel.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kitada T, Seki S, Iwai S, et al. In situ detection of oxidative DNA damage, 8-hydroxydeoxyguanosine, in chronic human liver disease. J Hepatol. 2001;35:613–618. doi: 10.1016/s0168-8278(01)00171-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. FXR and LKB1 expression in the liver of HCV patients with mild or severe fibrosis. (A) H&E and immunohistochemical stainings for FXR and LKB1. Arrows indicate eosinophilic necrosis of hepatocytes (scale bar, 100 μm). (B) The degrees of hepatocyte death, inflammation, and fibrosis in the human liver samples. Knodell histologic activity index and Ishak fibrosis scores were measured in the patient samples (n = 3). (C) The staining intensities of FXR and LKB1 were quantified in the liver sections. For panels B and C, values represent the mean ± standard error of mean (significantly different as compared with mild fibrosis, **P < .01).
Supplementary Figure 2. LKB1 binding with CAB39 and STRAD by FXR ligand treatment. Either CAB39 or STRAD was immunoblotted on the LKB1 immunoprecipitates from HepG2 cells treated with 100 μmol/L CDCA for the indicated times.
Supplementary Figure 3. The levels of pri-miR-199a or pre-miR-199a in HBV patients with fibrosis. The levels of pri-miR-199a or premiR-199a were measured in the livers of HBV patients with mild or severe fibrosis. Values represent the mean ± standard error of mean. NS, not significant.
Supplementary Figure 4. LKB1 expression in the liver of wild-type or FXR knockout (KO) mice treated with CCl4. Immunohistochemistry for LKB1 was done on the liver sections of wild-type or FXR KO mice treated with a single dose of CCl4 (0.75 mL/kg, intraperitoneally, for 48 hours). The same paraffin blocks had been used for a different study.6 The relative LKB1 intensities were quantified (n = 3 animals/each group). Data represent the mean ± standard error of mean of 3 replicates (treatment mean significanty different from vehicle-treated control, **P < .01).
Supplementary Table 1. The Candidate Target Genes of miR-199a-3p That Are Associated With Cell Survival