Summary
In pregnancy, trophoblast invasion and uterine spiral artery remodeling are important for lowering maternal vascular resistance and increasing uteroplacental blood flow. Impaired spiral artery remodeling has long been implicated in preeclampsia, a major complication of pregnancy, but the underlying mechanisms remain unclear1, 2. Corin is a cardiac protease that activates atrial natriuretic peptide (ANP), a cardiac hormone important in regulating blood pressure3. Unexpectedly, corin expression was detected in the pregnant uterus4. Here we identify a novel function of corin and ANP in promoting trophoblast invasion and spiral artery remodeling. We show that pregnant corin- or ANP-deficient mice developed high blood pressure and proteinuria, characteristics of preeclampsia. In these mice, trophoblast invasion and uterine spiral artery remodeling were markedly impaired. Consistently, we find that ANP potently stimulated human trophoblasts in invading Matrigels. In patients with preeclampsia, uterine corin mRNA and protein levels were significantly lower than that in normal pregnancies. Moreover, we have identified corin gene mutations in preeclamptic patients, which decreased corin activity in processing pro-ANP. These results indicate that corin and ANP are essential for physiological changes at the maternal-fetal interface, suggesting that defects in corin and ANP function may contribute to preeclampsia.
Pregnancy poses a serious challenge for maintaining normal blood pressure. Pregnancy-induced hypertension, a major cause of maternal and fetal deaths, occurs in ~10% of pregnancies5, 6. During pregnancy, the uterus undergoes profound morphological changes, including trophoblast invasion and spiral artery remodeling. In preeclampsia, impaired spiral artery remodeling is common, but the underlying mechanisms are unclear1, 2, 7-9. Studies indicate that vascular growth factor receptors, angiotensin and estradiol are involved in the disease10-14.
Corin is a cardiac protease that activates ANP, a cardiac hormone regulating blood pressure and sodium homeostasis15. In mice, lack of corin prevents ANP generation and causes hypertension16. In humans, corin variants are associated with hypertension17. Interestingly, corin expression was detected in the pregnant mouse4 (Fig. 1a) and human uterus (Supplementary Fig. 1). As a transmembrane protein, corin is expected to act at the expression sites, suggesting a possible function in the pregnant uterus.
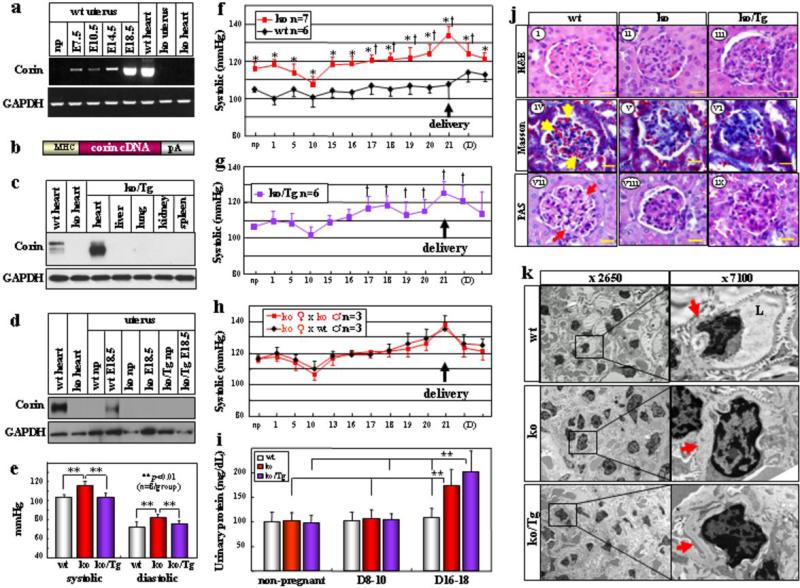
Figure 1. Hypertension, proteinuria and renal pathology in pregnant corin ko and ko/Tg mice.
a, Corin mRNA expression in mouse uteruses. b, Corin Tg construct. pA: poly A. c, d, Western analysis of corin protein in wt, corin ko and ko/Tg mice. e, Blood pressure (BP) (mean ± s.d.) in np females. BP increased in corin ko (f) and ko/Tg (g) mice in pregnancy. Data represent mean ± s.d. *P<0.05 vs. wt of the same time point. †P<0.05 vs. np level of the same genotype. h, Similar BP changes in corin ko females mated with ko or wt males. i, Late gestational proteinuria in corin ko and ko/Tg mice. Data represent mean ± s.d. **P<0.01, n=7-8 per group. j, Renal ischemia in pregnant corin ko and ko/Tg mice, shown in H&E (i-iii), Masson trichrome (iv-vi) or PAS (vii-ix) stained E18.5 sections. bar: 20 μm. Red blood cells (yellow arrows) and open capillaries (red arrows) in wt glumeruli are indicated. k, Narrow glomerular capillary lumen (L) and thick basement membranes (red arrows) in corin ko and ko/Tg mice at E18.5 shown by electron microscopy.
To understand the role of corin in pregnancy, we created a mouse model, in which a corin transgene was expressed under a cardiac promoter (Fig. 1b). The transgenic (Tg) and corin knockout (ko) mice were crossed to generate ko/Tg mice expressing corin only in the heart (Fig. 1c, d). In ko/Tg mice, transgenic corin expression restored pro-ANP processing in the heart (Supplementary Fig. 2) and normalized blood pressure (Fig. 1e), indicating that cardiac corin was sufficient to maintain normal blood pressure in non-pregnant (np) mice.
In pregnant corin ko mice, blood pressure increased at ~17 days post-coitus and rose further before returning to the np level after delivery (Fig. 1f), which resembled late gestational hypertension in preeclamptic women. In corin ko/Tg mice, which were normotensive, blood pressure increased similarly during pregnancy (Fig. 1g), indicating that cardiac corin expression did not prevent pregnancy-induced hypertension. The data also show that in these mice hypertension in pregnancy was not due to preexisting high blood pressure. In addition to in the uterus, corin mRNA was detected in the umbilical cord and placenta (Supplementary Fig. 3). To distinguish the role of maternal corin from that of placental or other fetal organs, corin ko females were mated with either wt or ko males. The resulting fetuses carried one or no copy of the functional corin gene. Normally, enzymes encoded by one gene copy are sufficient for their function. As shown in Fig. 1h, pregnant corin ko females, mated with either wt or ko males, had similarly increased blood pressure, indicating that lack of maternal, but not fetal, corin caused hypertension in pregnancy.
Proteinuria is a hallmark of preeclampsia. WT, corin ko and ko/Tg mice had similar urinary protein levels before pregnancy and at mid gestation. The levels, however, increased in corin ko and ko/Tg mice at late gestation (Fig. 1i), consistent with reported proteinuria in mouse models of preeclampsia18. Ischemic glomeruli, indicated by fewer red blood cells, were found in pregnant corin ko and ko/Tg mice (Fig. 1j, i-vi) but not in np mice (Supplementary Fig. 4). PAS staining revealed increased extracellular matrixes and collapsed glomerular capillaries in pregnant corin ko and ko/Tg mice (Fig. 1j, vii-ix). Electron microscopy showed narrow glomerular capillary lumens and thick basement membranes (Fig. 1k), suggesting endotheliosis and increased extracellular matrixes. Additional pathological features such as necrotic cells and calcium deposits in the placental labyrinth also existed in these mice (Supplementary Fig. 5), indicating insufficient uteroplacental perfusion. Consistently, corin ko and ko/Tg mice had smaller litters (7.1 ± 2.3 (n=28) and 6.8 ± 2.7 (n=28), respectively, vs. wt 9.1 ± 1.2 (n=21) pups/litter; p values <0.001).
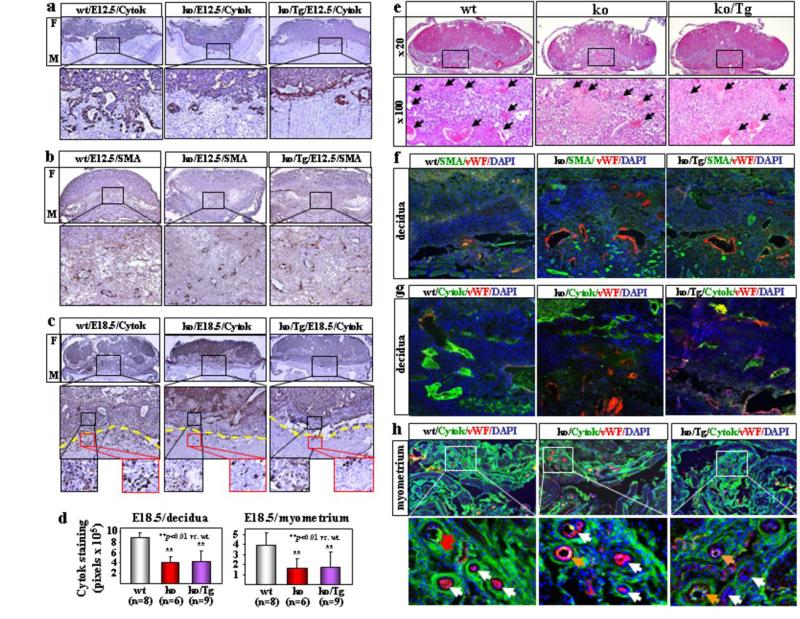
We examined embryos of E12.5 day, an early time point before blood pressure increase in corin ko and ko/Tg mice, and E18.5 day before delivery. WT E12.5 embryos exhibited obvious trophoblast invasion, shown by cytokeratin (cytok) staining (Fig. 2a), and large vessels mostly in the deep decidua, shown by smooth muscle actin (SMA) staining (Fig. 2b), indicating that smooth muscles in the superficial decidua were replaced by invading trophoblasts. In contrast, trophoblast invasion in corin ko and ko/Tg embryos was markedly reduced (Fig. 2a) and smaller arteries were found in both superficial and deep decidua (Fig. 2b). In E18.5 wt embryos, more abundant trophoblasts were found in the decidua and myometrium compared with those in corin ko and ko/Tg mice (Fig. 2c, d). By H&E staining, larger and more abundant decidual spiral arteries were observed in wt than corin ko or ko/Tg mice (Fig. 2e). Fig. 2f-h showed strong cytok (trophoblasts) staining but weak von Willebrand factor (vWF) (endothelial) and SMA (smooth muscles) staining in wt decidual and myometrial arteries. These data indicate that trophoblast invasion and spiral artery remodeling were impaired in corin ko and ko/Tg mice and that this defect occurred before blood pressure increased in these mice.
Figure 2. Impaired trophoblast invasion and spiral artery remodeling in corin ko and ko/Tg mice.
E12.5 embryo sections were stained for trophoblasts (a) or smooth muscles (b). Fetal (F) and maternal (M) sides are indicated. Boxed areas in top panels are shown at a higher magnification (x200). c, E18.5 embryo sections were stained for trophoblasts. In lower panels (x100), yellow lines indicate the decidua and myometrium boundary. d, Quantitative data (mean ± s.d.) of cytok staining. e, Fewer and smaller decidual spiral arteries (arrows) in H&E-stained E18.5 corin ko and ko/Tg embryos. f-h, Co-staining of SMA, vWF, cytok and nuclei in E18.5 embryos. Red arrows indicate cytok (green) signals, white arrows vWF (red) signals, and orange arrows mixed (yellow) signals.
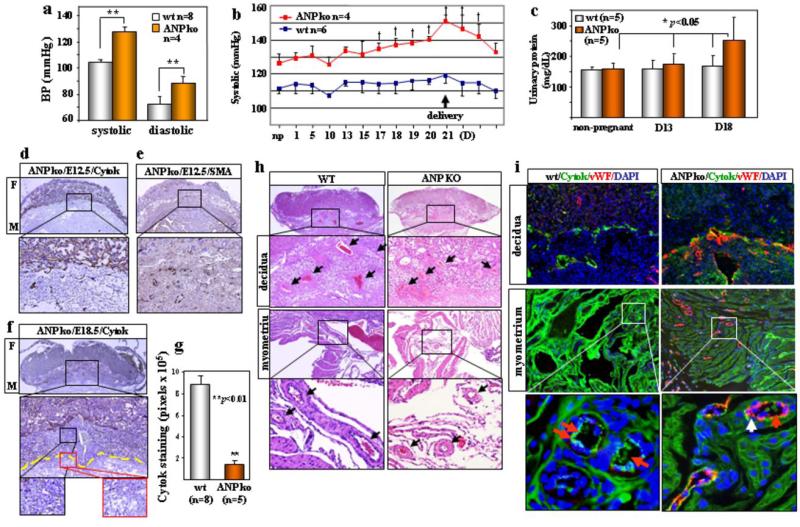
Corin activates ANP in the heart15. It was unknown if the corin function in pregnancy also was mediated by ANP. Pro-ANP is expressed in the np and pregnant uterus (Supplementary Fig. 6). If corin acts on pro-ANP to promote trophoblast invasion and spiral artery remodeling, thereby preventing hypertension in pregnancy, ANP and corin ko mice should have similar phenotypes. ANP ko mice are hypertensive (Fig. 3a) but their blood pressure was not monitored during pregnancy19. We found similarly increased blood pressure in pregnant ANP ko mice (Fig. 3b). The mice also had late gestational proteinuria (Fig. 3c) and smaller litters (4.4 ± 1.7 (n=25) vs. wt 9.1 ± 1.2 (n=21) pups/litter, p<0.001). By immunostaining, impaired trophoblast invasion and smaller spiral arteries were observed in E12.5 embryos (Fig. 3d, e). In E18.5 embryos, ANP ko mice had far fewer trophoblasts (Fig. 3f, g) and smaller arteries (Fig. 3h) in the decidua and myometrium than those in wt. Consistently, weak cytok-staining but strong vWF-staining were found in arteries in ANP ko mice (Fig. 3i). Thus, ANP and corin ko mice had very similar phenotypes, indicating that the role of corin in pregnancy is most likely mediated by ANP.
Figure 3. Hypertension, proteinuria and uteroplacental pathology in pregnant ANP ko mice.
a, BP (mean ± s.d.) in np females, **P<0.01. b, Elevated BP (mean ± s.d.) in pregnant ANP ko mice. †P<0.05 vs. np level. c, Gestational proteinuria in ANP ko mice. Data represent mean ± s.d. Impaired trophoblast invasion and smooch muscle remodeling in E12.5 embryos stained for cytok (d) or SMA (e). Boxed areas in top panels are shown at a higher magnification (x200). f, Impaired trophoblast invasion in E18.5 embryos stained for cytok. g, Quantitative data (mean ± s.d.) of cytok staining in E18.5 ANP ko embryos. h, Impaired decidual and myometrial artery remodeling (arrows) in H&E-stained E18.5 ANP ko embryos. i, Co-staining of cytok, vWF and nuclei in E18.5 ANP ko embryos. Red arrows indicate cytok (green) signals and white arrows vWF (red) signals.
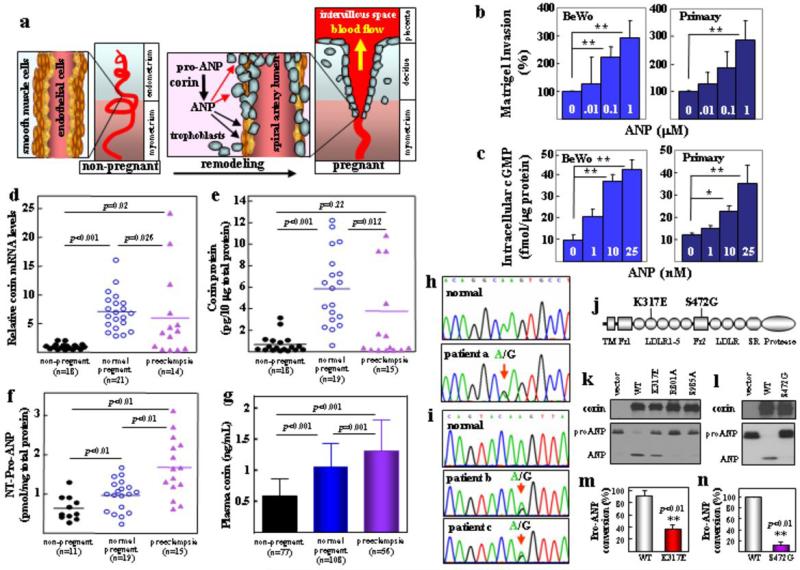
In the heart, corin produces ANP, which in turn regulates blood pressure by promoting natriuresis and vasodilation3. Here we found that lack of corin and ANP impaired trophoblast invasion and spiral artery remodeling, which was not rescued by cardiac corin expression in corin ko/Tg mice. ANP is known to relax vascular smooth muscles. Recently, ANP and its downstream cGMP-dependent protein kinase were shown to be important in angiogenic processes by promoting endothelial regeneration20, 21. Thus, ANP may function locally to remodel uterine arteries. Our results also suggest that ANP may directly promote trophoblast invasion (Fig. 4a). This hypothesis was tested. We found that ANP markedly stimulated human trophoblasts to invade Matrigels (Fig. 4b) (Supplementary Fig. 7a). In these cells, ANP receptor mRNA expression was confirmed (Supplementary Fig. 7b) and ANP-stimulated intracellular cGMP production was detected (Fig. 4c) (Supplementary Fig. 7c).
Figure 4. ANP-stimulated human trophoblast invasion, and impaired uterine corin expression and corin mutations in preeclamptic patients.
a, A model illustrating that corin-produced ANP in the pregnant uterus promotes trophoblast invasion (red arrows) and vascular wall remodeling (black arrows). ANP stimulated human BeWo and primary trophoblasts in Matrigel invasion (b) and intracellular cGMP production (c). Data represent mean ± s.d. *P<0.05; **P<0.01 vs. control. Corin mRNA (d) and protein (e) and N-terminal (NT) pro-ANP levels (f) in human uterus samples. Horizontal lines indicate mean values. g, Plasma soluble corin levels (mean ± s.d.) in preeclamptic patients and normal controls. h-j, CORIN gene mutations causing K317E (h) and S472G (i) changes in corin (j). TM, transmembrane; Fz, frizzled; LDLR, LDL receptor; SR, scavenger receptor. k,l, Expression of K317E and S472G mutants in HEK293 cells (top panels). Vector, wt corin and inactive corin R801A and S985A mutants were controls. K317E and S472G mutations reduced pro-ANP processing activity (bottom panels). m,n, Quantitative data (mean ± s.d.) from ≥3 experiments.
Our findings underscore the importance of locally produced ANP by corin, which acts on trophoblasts and vascular cells in the uterus. Because heart-derived ANP circulates inside the vessel, our model may explain why cardiac corin failed to promote trophoblast invasion and uterine artery remodeling, as shown in corin ko/Tg mice. To verify this hypothesis, we quantified corin mRNA and protein in human uteruses by RT-PCR and ELISA. The levels were low in np women but increased in pregnant women (Fig. 4d, e). In preeclamptic women, the levels were significantly lower than in normal pregnancies. Similar results were found by immunostaining (Supplementary Fig. 8). Consistently, pro-ANP levels in uterine tissues were significantly higher in preeclamptic women than normal pregnant women (Fig. 4f), indicating that reduced uterine corin expression impaired pro-ANP processing in these patients. Corin is a membrane-bound protein4, 15. Recent studies showed that corin can be shed from cardiomyocytes and that soluble corin was detected in human plasma22, 23. We found that plasma corin levels were higher in preeclamptic patients than np or normal pregnant women (Fig. 4g). Thus, plasma corin levels did not reflect that in tissues, indicating that plasma corin was likely derived from the heart, where corin expression was increased in response to high blood volume and pressure in pregnancy. These results further support a local corin function in the pregnant uterus.
We then sequenced the CORIN gene24 in preeclamptic patients and identified a mutation altering Lys to Glu at position 317 in LDL receptor repeat 2 in one woman (Fig. 4h, j) and another mutation altering Ser to Gly at position 472 in frizzled 2 domain in two women from the same family who had preeclampsia (Fig. 4i, j). In functional studies, K317E and S472G mutations did not affect corin expression in HEK293 cells but markedly reduced corin activity in processing pro-ANP (Fig. 4k-n). The data was consistent with previous findings that LDL receptor repeats and frizzled domains are critical for corin activity25, suggesting that the mutations may impair corin function in the patients, thereby contributing to preeclampsia. Interestingly, corin variants in frizzled 2 domain that impaired corin function have been reported in African Americans17, 26, a high risk population for preeclampisa.
Previously, high levels of plasma pro-ANP/ANP were detected in preeclamptic patients27, 28. As shown with our plasma soluble corin data, plasma protein levels may not reflect those in tissues. Together, we have identified a novel local function of corin and ANP in promoting trophoblast invasion and spiral artery remodeling to prevent hypertension in pregnancy. Our data suggest that impaired corin expression or function in the pregnant uterus may represent an important mechanism underlying preeclampsia. Studies to further understand impaired uterine corin expression in preeclamptic patients may help to develop new strategies to enhance the corin/ANP pathway to prevent or treat this life-threatening disease.
METHODS SUMMARY
Corin and ANP ko mice were described previously16, 19. Tg mice with cardiac corin expression were generated using a heart-specific promoter. Blood pressure was measured by radiotelemetry16. Tissue sections from np and pregnant mice were stained with H&E, Masson's trichrome, PAS or von Kossa or immunostained with antibodies against cytok, SMA, vWF or corin. Renal sections were also examined by electron microscopy. Transwell invasion assay was done with human primary villous trophoblasts (ScienCell) and trophoblastic JEG3, BeWo, JAR cell lines (ATCC) in Matrigel Invasion Chambers (BD Biosciences). ANP-stimulated cGMP production in trophoblasts was assayed in 96-well plates. Intracellular cGMP levels were determined using an EIA kit (Enzo Life Sciences). Corin levels in human blood and uterus tissue samples were measured by ELISA22. Pro-ANP levels in human uterus tissues were measured by ELISA (Alpco Diagnostics). CORIN gene exons24 from preeclamptic patients were PCR amplified and directly sequenced. Corin gene mutations identified were studied by expressing mutant corin proteins in HEK293 cells and testing their activities in pro-ANP processing assays, as described previously26.
SUPPLEMENTARY ONLINE METHODS
Knockout and transgenic mice
Corin ko mice were described previously16. ANP ko mice (B6.129P2-Nppa tm1Unc/J)19 were from the Jackson Lab. To make Tg mice expressing corin in the heart, the full-length mouse corin cDNA was inserted into a construct driven by the mouse α-myosin heavy chain (MHC) promoter. Pronuclear microinjection and production of Tg mice were done at the Case Western Reserve University Transgenic Core. Corin ko and Tg mice were crossed to generate ko/Tg mice. Littermates were used as controls. The animal study was conducted in accordance with the NIH guidelines and approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Blood pressure monitoring
Radiotelemetry was used for real-time blood pressure monitoring in conscious and unrestrained mice16. Female mice (8-12 weeks old) were chronically instrumented in the left carotid artery with a PA-C10 device (Data Sciences International) and rested for at least 7 days to recover from the surgery. The mice were mated and checked for vaginal plugs to establish gestation timing. The day when a plug was observed was defined as E0.5 day. The mating mice were homozygous except for the fetal testing experiment. Telemetry receivers (model RPC-1) were placed under individual cages for data acquisition using the Dataquest A.R.T. 4.0 Gold System (Data Sciences International). Data presented were from continuous recording of at least 6 h per day (10 a.m. to 4 p.m.) over the time periods indicated.
Urinary protein measurement
Urine samples were collected from non-pregnant mice and pregnant mice at mid (8-10 dpc) and late (16-18 dpc) gestational stages. Urinary protein levels were measured using a colorimetric assay based on a modified Bradford method (Bio-Rad).
RT-PCR, Western blotting and ELISA
Total RNAs were isolated from cultured cells or mouse and human tissues using TRIzol reagents (Invitrogen) or RNeasy kit (Qiagen) and used to synthesize first strand cDNAs. RT-PCR was done using oligonucleotide primers specific for the mouse or human corin, mouse ANP, or human ANP receptor genes. Quantitative RT-PCR for human corin mRNA expression in uterus tissues was done using the PRISM 7500 System (Applied Biosystems). β-Actin was used as an internal control. Quantitative RT-PCR for mouse ANP mRNA in uteruses was done using the iCycler system (Bio-Rad). For Western analysis of corin protein, membrane fractions from tissue homogenates were isolated by ultracentrifugation, as described previously29. Proteins were analyzed by SDS-PAGE and Western blotting using a polyclonal antibody (Berlex Biosciences). Western analysis of pro-ANP in heart samples was done using a polyclonal antibody (Santa Cruz). Processing pro-ANP by corin in transfected cells was analyzed by Western blotting, as described previously30. Pro-ANP in human uterus tissues was measured by an NT pro-ANP ELISA kit from Alpco Diagnostics. Human corin in uterus tissues or plasma was measured by ELISA, as described previously31.
Histology and immunohistochemistry
Tissues were fixed with 4% paraformaldehyde and embedded in paraffin. Sections were stained with H&E, Masson's trichrome, periodic acid-Schiff (PAS) or von Kossa. For immunohistochemical or immunofluorescent analysis, antibodies against smooth muscle α-actin (SMA) (Sigma-Aldrich), vWF (Sigma-Aldrich) and cytok (Dako) were used to label smooth muscle cells, endothelial cells, and trophoblasts, respectively. For human corin, an antibody from Berlex Biosciences was used. Secondary antibodies were conjugated with horseradish peroxidase or Alexa Fluor 488 (green) or 594 (red) (Invitrogen). Tissue sections were mounted with or without DAPI (blue) containing mounting medium (Dako). For ANP expression in mouse uterus tissues, a polyclonal antibody from Millipore was used. Control sections were treated similarly but without the primary antibodies. Photographs were taken with a light or fluorescent microscope equipped with a digital camera (Olympus). Data represented experiments from ≥5 mice per study group.
For immunohistochemical analysis of trophoblast invasion in mouse embryos, tissue samples from at least 5 mice per group and at least 2 implant sites per mouse were used. Serial sections (>50/embryo) of 5 μm in thickness were prepared. The position of maternal artery was used as a guide to orient section positions. At least 4-6 sections from the center of the placenta of each embryo were used for immunohistochemical analysis. Slides stained for cytok were examined by two individuals. The sections that showed the deepest trophoblast invasion were presented. These sections were also analyzed by ImagePro software to quantify cytok staining. For each section analyzed, the entire area of the decidua and myometrium was scanned by the software.
Electron microscopy
Kidneys from pregnant mice at E18.5 were fixed in 3% glutaraldehyde, treated with 1% osmium tetroxide, and embedded in an Araldite-Epon mixture. Semi-thin sections (0.6 μm) were prepared and examined with a transmission electron microscope (JEOL JEM-1210) at the Lerner Image Core of the Cleveland Clinic. Data represented experiments from at least three mice per study group.
Transwell invasion assay
Human trophoblastic JEG3, BeWo and JAR cells from ATCC were cultured in MEM (JEG3), RPMI1640 (JAR), and F-12K (BeWo) medium, respectively, with 10% FBS at 37°C. Primary human villous trophoblasts from ScienCell Research Labs (Carlsbad, CA) were cultured in the Trophoblast Medium (ScienCell) with 10% FBS. Transwell invasion assays were done using the BioCoat Growth Factor Reduced Matrigel Invasion Chambers (8-μm pore size) and control inserts (no Matrigel coating) (BD Biosciences) in 24-well plates. Culture medium containing human ANP (Calbiochem) was added to bottom wells, and cell suspension (5 × 104) was added to top wells and incubated at 37°C for 24 h. Non-invading cells were removed from the upper surface of the Matrigel layer by gentle scrubbing. The cells on the lower surface of the membrane were stained using Diff-Quick staining solutions. The membranes were excised and mounted onto glass slides. Invasion indices were determined by counting the number of stained cells on the membrane under a light microscope. The assay was done in duplicates in at least three independent experiments.
cGMP assay
ANP-stimulated intracellular cGMP production assay was performed with JEG3, JAR and BeWo cells and primary human trophoblasts using a method described previously32. The cells were grown in 96-well plates. Confluent cells were washed once with serum-free medium. Human ANP was added to serum-free medium and incubated with cells at 37°C for 30 min. In these experiments, ANP was more potent in stimulating intracellular cGMP production when serum-free medium was used (data not shown). The cells were lysed with 0.1M HCl. Intracellular cGMP levels in ANP-stimulated cells were determined using an EIA kit (Enzo Life Sciences). Each experimental condition was assayed in duplicate in at least three independent experiments.
Human blood and tissue samples
The study was approved by the local Ethics Committees and participants gave informed consent. Women of normal pregnancy or with preeclampsia and age-matched non-pregnant normal controls who underwent routine medical check-ups were recruited. All participants were ethnic Han Chinese. Hypertension was defined as diastolic pressure >90 mmHg and/or systolic pressure >140 mmHg on at least two occasions. Preeclampsia was defined as hypertension appeared after 20 weeks of gestation with proteinuria (>300 mg urinary protein/24 hours). Patients with chronic hypertension, chronic kidney disease, diabetes and heart disease were excluded. Uterus tissues were obtained during caesarean sections in pregnant women or operations for uterine leiomyoma in non-pregnancy women. Clinical characteristics of women who provided blood and uterus tissue samples were summarized in Tables S1 and S2, respectively.
CORIN gene sequences in patients
Blood samples from 56 patients with preeclampsia were collected into tubes containing EDTA as an anticoagulant. Genomic DNA was extracted from white blood cells using the QIAamp DNA Mini kit (Qiagen), and used in PCR to amplify exon sequences of the CORIN gene24. PCR products were used for direct DNA sequencing. Mutations identified were verified by independent PCR and DNA sequencing. Additional PCR and DNA sequencing were done with DNA samples from >100 normal controls to verify that mutations identified in patients did not exist in the normal population.
Expression and functional analysis of corin mutants
Plasmids expressing human wt corin and two inactive mutants R801A and S985A, in which the activation cleavage site and catalytic site residues were mutated, respectively, were described previously33. Plasmids expressing corin mutants K317E or S472G were constructed by PCR-based mutagenesis. Recombinant corin proteins expressed by these plasmids contained a C-terminal V5 tag to be detected by an anti-V5 antibody (Invitrogen)30. Plasmids were transfected into HEK293 cells using Lipofectamine 2000 (Invitrogen). Cells were lysed and proteins were analyzed by Western blotting using an anti-V5 antibody. To analyze the function of corin, recombinant human pro-ANP in conditioned medium was added to HEK293 cells expressing corin wt or mutants and incubated at 37°C for 2 h. Pro-ANP and ANP in the medium were immunoprecipitated and analyzed by Western blotting. Protein bands on x-ray films were scanned by densitometry. The percentage of pro-ANP to ANP conversion was calculated, as described previously30.
Statistical analysis
Results were presented as mean ± s.d. Differences between two groups were analyzed by Student's t-test. Data involving more than two groups were analyzed by ANOVA followed by the Tukey multiple comparison test. Comparisons for corin mRNA and protein and pro-ANP levels in human uterus samples were done by the Mann–Whitney– Wilcoxon test. A p value <0.05 was considered statistically significant.
ACKNOWLEDGMENTS
We thank J. Robbins for α-MHC promoter construct and L. Zhang for statistical analysis. This work was supported in part by grants from the Ralph Wilson Medical Foundation, the Bakken Heart-Brain Institute, and the National Institutes of Health (HL089298, HD064634), and by grants from the National Natural Science Foundation of China (31070716, 81170247) and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
AUTHOR CONTRIBUTIONS
Y.C., W.W., N.D., J.L., D.K.S., M.L., C.F., J.P., S.C., S.W., Z.L. and L.D. designed and performed experiments. N.D., W.C. and X.H. collected patient samples and analyzed clinical data. Q.W. conceived the study and designed experiments. Y.Z. and Q.W. wrote the manuscript. All authors analyzed and interpreted data, and critically read the manuscript.
Full Methods and any associated references are available in the online version of the paper at www.nature.com/nature.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Pijnenborg R, Vercruysse L, Hanssens M. The uterine spiral arteries in human pregnancy: facts and controversies. Placenta. 2006;27:939–58. doi: 10.1016/j.placenta.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Red-Horse K, et al. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114:744–54. doi: 10.1172/JCI22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Q, Xu-Cai YO, Chen S, Wang W. Corin: new insights into the natriuretic peptide system. Kidney Int. 2009;75:142–6. doi: 10.1038/ki.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yan W, Sheng N, Seto M, Morser J, Wu Q. Corin, a mosaic transmembrane serine protease encoded by a novel cDNA from human heart. J Biol Chem. 1999;274:14926–35. doi: 10.1074/jbc.274.21.14926. [DOI] [PubMed] [Google Scholar]
- 5.Lain KY, Roberts JM. Contemporary concepts of the pathogenesis and management of preeclampsia. Jama. 2002;287:3183–6. doi: 10.1001/jama.287.24.3183. [DOI] [PubMed] [Google Scholar]
- 6.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–99. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 7.Brosens IA, Robertson WB, Dixon HG. The role of the spiral arteries in the pathogenesis of preeclampsia. Obstet Gynecol Annu. 1972;1:177–91. [PubMed] [Google Scholar]
- 8.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69:1–7. doi: 10.1095/biolreprod.102.014977. [DOI] [PubMed] [Google Scholar]
- 9.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–8. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 10.Kanasaki K, et al. Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature. 2008;453:1117–21. doi: 10.1038/nature06951. [DOI] [PubMed] [Google Scholar]
- 11.Levine RJ, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83. doi: 10.1056/NEJMoa031884. [DOI] [PubMed] [Google Scholar]
- 12.Redman CW, Sargent IL. Latest advances in understanding preeclampsia. Science. 2005;308:1592–4. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 13.Venkatesha S, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9. doi: 10.1038/nm1429. [DOI] [PubMed] [Google Scholar]
- 14.Zhou CC, et al. Angiotensin receptor agonistic autoantibodies induce pre-eclampsia in pregnant mice. Nat Med. 2008;14:855–62. doi: 10.1038/nm.1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000;97:8525–9. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan JC, et al. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc Natl Acad Sci U S A. 2005;102:785–90. doi: 10.1073/pnas.0407234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dries DL, et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation. 2005;112:2403–10. doi: 10.1161/CIRCULATIONAHA.105.568881. [DOI] [PubMed] [Google Scholar]
- 18.Davisson RL, et al. Discovery of a spontaneous genetic mouse model of preeclampsia. Hypertension. 2002;39:337–42. doi: 10.1161/hy02t2.102904. [DOI] [PubMed] [Google Scholar]
- 19.John SW, et al. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science. 1995;267:679–81. doi: 10.1126/science.7839143. [DOI] [PubMed] [Google Scholar]
- 20.Kuhn M, et al. The natriuretic peptide/guanylyl cyclase--a system functions as a stress-responsive regulator of angiogenesis in mice. J Clin Invest. 2009;119:2019–30. doi: 10.1172/JCI37430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tokudome T, et al. Impaired recovery of blood flow after hind-limb ischemia in mice lacking guanylyl cyclase-A, a receptor for atrial and brain natriuretic peptides. Arterioscler Thromb Vasc Biol. 2009;29:1516–21. doi: 10.1161/ATVBAHA.109.187526. [DOI] [PubMed] [Google Scholar]
- 22.Dong N, et al. Plasma soluble corin in patients with heart failure. Circ Heart Fail. 2010;3:207–11. doi: 10.1161/CIRCHEARTFAILURE.109.903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang J, et al. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem. 2011;286:10066–72. doi: 10.1074/jbc.M110.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan J, et al. Genomic structures of the human and murine corin genes and functional GATA elements in their promoters. J Biol Chem. 2002;277:38390–8. doi: 10.1074/jbc.M205686200. [DOI] [PubMed] [Google Scholar]
- 25.Knappe S, Wu F, Madlansacay MR, Wu Q. Identification of domain structures in the propeptide of corin essential for the processing of proatrial natriuretic peptide. J Biol Chem. 2004;279:34464–71. doi: 10.1074/jbc.M405041200. [DOI] [PubMed] [Google Scholar]
- 26.Wang W, et al. Corin variant associated with hypertension and cardiac hypertrophy exhibits impaired zymogen activation and natriuretic peptide processing activity. Circ Res. 2008;103:502–8. doi: 10.1161/CIRCRESAHA.108.177352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Irons DW, Baylis PH, Butler TJ, Davison JM. Atrial natriuretic peptide in preeclampsia: metabolic clearance, sodium excretion and renal hemodynamics. Am J Physiol. 1997;273:F483–7. doi: 10.1152/ajprenal.1997.273.3.F483. [DOI] [PubMed] [Google Scholar]
- 28.Tihtonen KM, Koobi T, Vuolteenaho O, Huhtala HS, Uotila JT. Natriuretic peptides and hemodynamics in preeclampsia. Am J Obstet Gynecol. 2007;196:328, e1–7. doi: 10.1016/j.ajog.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, et al. Protease corin expression and activity in failing hearts. Am J Physiol Heart Circ Physiol. 2010;299:H1687–92. doi: 10.1152/ajpheart.00399.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao X, Wang W, Chen S, Wu Q. Role of glycosylation in corin zymogen activation. J Biol Chem. 2007;282:27728–35. doi: 10.1074/jbc.M703687200. [DOI] [PubMed] [Google Scholar]
- 31.Dong N, et al. Effects of anticoagulants on human plasma soluble corin levels measured by ELISA. Clin Chim Acta. 2010;411:1998–2003. doi: 10.1016/j.cca.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu F, Yan W, Pan J, Morser J, Wu Q. Processing of pro-atrial natriuretic peptide by corin in cardiac myocytes. J Biol Chem. 2002;277:16900–5. doi: 10.1074/jbc.M201503200. [DOI] [PubMed] [Google Scholar]
- 33.Qi X, Jiang J, Zhu M, Wu Q. Human corin isoforms with different cytoplasmic tails that alter cell surface targeting. J Biol Chem. 2011;286:20963–9. doi: 10.1074/jbc.M110.217570. [DOI] [PMC free article] [PubMed] [Google Scholar]