Abstract
The ADP-activated P2Y1 receptor is broadly expressed and plays a crucial role in ADP-promoted platelet aggregation. We previously synthesized 2-iodo-N6-methyl–(N)-methanocarba-2′-deoxyadenosine 3′,5′-bisphosphate (MRS2500), as a selective, high affinity, competitive antagonist of this receptor. Here we report utilization of a trimethylstannyl precursor molecule for the multistep radiochemical synthesis of a [125I]-labeled form of MRS2500. [125I]MRS2500 bound selectively to Sf9 insect cell membranes expressing the human P2Y1 receptor but did not specifically bind to membranes isolated from empty vector-infected cells. Binding of [125I]MRS2500 to P2Y1 receptors was saturable with a Kd of 1.2 nM. Known agonists and antagonists of the P2Y1 receptor inhibited [125I]MRS2500 binding to P2Y1 receptor-expressing membranes with potencies in agreement with those previously observed in functional assays of this receptor. A high-affinity binding site for [125I]MRS2500 also was observed on intact human platelets (Kd = 0.61 nM) and mouse platelets (Kd = 1.20 nM) that exhibited the pharmacological selectivity of the P2Y1 receptor. The densities of sites observed were 151 sites/platelet and 229 sites/platelet in human and mouse platelets, respectively. In contrast, specific binding was not observed in platelets isolated from P2Y1 receptor (−/−) mice. Taken together, these data illustrate the synthesis and characterization of a novel P2Y1 receptor radioligand and its utility for examining P2Y1 receptors natively expressed on human and mouse platelets.
Keywords: P2Y1 receptor, competitive antagonist, radioligand, MRS2500, platelet
1. Introduction
A broad range of physiological responses are promoted by extracellular nucleotides signaling through two classes of cell surface receptors [1]. The P2X receptors are ATP-activated ion channels, and the P2Y receptors are G protein-coupled receptors that are activated by ATP, UTP, ADP, UDP, or nucleotide sugars. The P2Y receptor family includes eight receptor subtypes [2]. The P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11 receptors activate Gαq/phospholipase C, and therefore, these receptors primarily signal through the inositol lipid signaling cascade. The P2Y12, P2Y13, and P2Y14 receptors activate Gi resulting in inhibition of adenylyl cyclase as well as G protein-dependent activation of ion channels.
The ADP-activated P2Y1 receptor is relatively broadly expressed in brain and peripheral tissues [3]. This signaling protein fulfills an obligatory role in ADP-promoted platelet aggregation by triggering shape change and an initial, reversible phase of aggregation. Study of the P2Y1 receptor on platelets and other tissues has been augmented by identification of selective antagonists and agonists for this receptor. Adenosine 3′,5′-bisphosphate was identified as the first selective P2Y1 receptor antagonist [4] and was utilized to resolve pharmacologically the relative actions of the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 receptor in platelet aggregation [5, 6]. Subsequent medicinal chemistry has focused on developing higher affinity non-nucleotide P2Y1 receptor antagonists [7, 8], and on generation of a high affinity P2Y1 receptor agonist that activates the P2Y1 receptor but not the ADP-activated P2Y12 or the ADP-activated P2Y13 receptor [9, 10] .
Mechanism(s) associated with both the function and the regulation of the P2Y1 receptor in platelets and other tissues are not fully understood. For example, the platelet P2Y1 receptor rapidly desensitizes [9, 11] and internalizes [12] but molecular understanding of these phenomena is lacking. Polymorphisms have been identified in the P2Y1 receptor gene, and several of these have been proposed to be associated with an increased risk of cardiovascular disease [13-17]. However, the molecular basis of such an association remains undefined.
An increase in molecular understanding of the P2Y1 receptor could be provided by application of a radioligand that allowed unambiguous quantification of the density, cellular distribution, and ligand binding properties of the receptor, and an important goal of our research is to identify such a molecule. The non-nucleotide antagonist, 2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′5′-bisphosphate (MRS2500) exhibits the highest apparent affinity (Ki ~ 1 nM) reported to date for an antagonist of the P2Y1 receptor [8], and we previously synthesized a [32P]-labeled form of this molecule and applied it for quantification of P2Y1 receptors in rat tissues [18]. However, the short half-life of 32P as well as inefficient and variable incorporation of 32P into the precursor molecule led us to consider synthesis of [125I]MRS2500 utilizing iododestannylation of an stannyl aryl intermediate as previously described for preparation of several other radioiodinated ligands [19, 20]. A direct one-step reaction was not suitable for production of MRS2500, and therefore, a synthetic route was modified to a 3-step radiochemical synthesis. We report here the binding properties of [125I]MRS2500, establishing it as an optimized radioligand for study of recombinant P2Y1 receptors and P2Y1 receptors natively expressed on human and mouse platelets.
2. Materials and methods
2.1. Chemical synthesis
2.1.1. Materials and instrumentation
Hexamethyltin, 1.0 M tetrabutylammonium dihydrogen phosphate, 32% peracetic acid, and other reagents were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO), except where noted. Sodium [125I]iodide (17.4 Ci/mg) in NaOH (1.0 × 10−5 M) was supplied by Perkin–Elmer Life and Analytical Science. 1H NMR spectra were obtained with a Varian Gemini 300 spectrometer using CDCl3 as solvent. Chemical shifts are expressed in δ values (ppm) with tetramethylsilane (δ 0.00). TLC analysis was carried out on aluminum sheets with silica gel F254 (0.2 mm) from Aldrich. HPLC mobile phases for the nonradioactive synthesis consisted of System A: linear gradient solvent system: CH3CN/triethylammonium acetate (10 mM) from 5/95 to 60/40 in 20 min, flow rate 1.0 ml/min; System B: linear gradient solvent system: CH3CN/tetrabutyl ammonium phosphate (5 mM) from 20/80 to 60/40 in 20 min, flow rate 1.0 ml/min. HPLC mobile phases for the radioactive synthesis consisted of System C: linear gradient solvent system: CH3CN/tetrabutylammonium phosphate (5 mM) from 0/100 to 10/90 in 0.1 min, 10/90 to 30/70 in 5 min, 30/70 to 100/0 in 20 min, flow rate 1.0 ml/min; system D: linear gradient solvent system: CH3OH/triethylammonium acetate (10 mM, pH 7) at 0/100 for 1-2 min and then 5/95 to 15/85 in 40 min, flow rate 1.0 ml/min. Column A was Luna 5 μ C18(2) HPLC column from Phenomenex, Inc., Torrence, CA. Column B was 5 μ, C18 HPLC column from MAC-MOD Analytical, Inc., Chadd’s Ford, PA.
2.1.2. Strategy for chemical synthesis
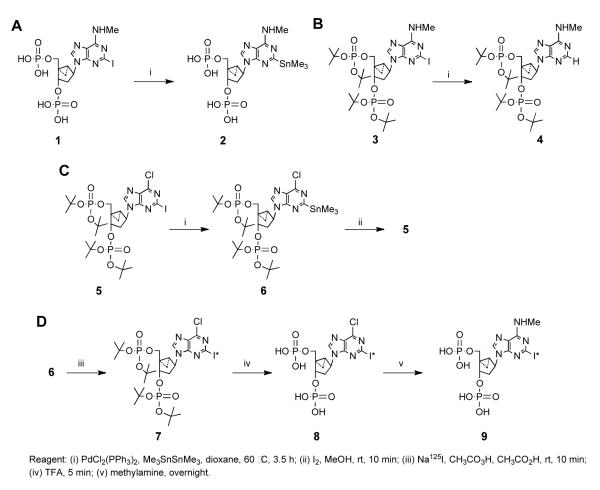
We previously reported that [32P]MRS2500 is a useful radioligand for quantification of P2Y1 receptors [18]. With the goal of generating a [125I]-labeled form of this molecule and after trying several abortive routes (Fig. 1A and B), we synthesized a trimethylstannyl analogue 6 (MRS2829) that potentially could be radio-iodinated as illustrated in Fig. 1C and D. The protected [125I]-labeled derivative 7 could be deprotected in TFA to yield 8 and then converted to [125I]MRS2500 9 via N6-methylation by nucleophilic attack with MeNH2.
Figure 1. Attempted approaches to the synthesis of [125I]MRS2500.
A, By direct stannylation of MRS2500. Intermediate 2 proved to be unstable. B, By direct stannylation of 3, a phosphate-protected form of MRS2500. Instead of the desired stannyl intermediate, only the 2-H compound 4 was obtained. C, By stannylation of 5, a phosphate-protected precursor of MRS2500, to which the 6-methylamino group is to be added later by substitution of a 6-chloroadenine group. The 2-trimethylstannyl intermediate 6 was synthesized and the feasibility of its re-conversion to an iodinated precursor of MRS2500 5 was demonstrated. D, The optimized, successful radiochemical synthesis, as described in Methods. [125I]MRS2500 9 was synthesized via [125I]-labeling of 6 to produce intermediate 7, deprotection of phosphates of 7 with TFA to produce the 6-chloro intermediate 8, and N6-methylation with methylamine to produce 9.
2.1.3. Synthesis of (1′R,2′S,4′S,5′S)-phosphoric acid di-tert-butyl ester 1-(di-tert-butoxy-phosphoryloxymethyl)-4-(6-chloro-2-trimethylstannylpurin-9-yl)bicyclo[3.1.0]hex-2-yl ester (6, MRS2829)
The iodo derivative 5 was prepared as previously described [21]. Me3SnSnMe3 (20 mg, 0.061 mmol) was added to a solution of 5 (2.7 mg, 0.0034 mmol) and PdCl2(PPh3)2 (3.5 mg, 0.0050 mmol) in dioxane (0.30 ml), and the mixture stirred at 60 °C for 3.5 h. The solvent was removed under vacuum and the residue was purified by TLC (AcOEt), which furnished the stannyl derivative 6 (0.6 mg, 23%): 1H NMR (CDCl3) 8.54 (s, 1H), 5.40-5.36 (m, 2H), 4.76 (m, 1H), 4.00 (m, 1H), 2.49 (m, 1H), 2.29 (m, 1H), 1.95 (m, 1H), 1.60 (s, 9H), 1.36 (s, 18H), 1.15 (m, 1H), 0.99 (m, 1H), 0.54 (s, 9H); MS (m/e) (positive-FAB): for C35H65ClN4O9P2Sn+ (M + tBuOH) 902.2937, found 902.2962. HPLC 3.71 min (90%) in solvent System A, 2.86 min (90%) in System B.
2.1.4. Small-scale conversion of compound 6 into compound 5
The formation of 5 from 6 (Fig. 1C) was piloted on a small scale with the goal of optimizing this reaction for production of 5 in a [125I]-labeled form. The trimethylstannyl intermediate 6 (0.1 mg) was reconverted to the iodo derivative 5 upon dissolving in MeOH (0.1 ml) followed by treatment with I2 (0.1 M in MeOH, 0.1 ml) for 10 min at room temperature. The structure 5 was confirmed by mass spectroscopy. MS (m/e) (positive-FAB): for C32H56ClIN4O9P2+ (M + tBuOH) 864.2256, found 864.2256.
2.1.5. Radiochemical synthesis of (1′R,2′S,4′S,5′S)-phosphoric acid di-tert-butyl ester 1-(di-tert-butoxy-phosphoryloxymethyl)-4-(6-chloro-2-[125I]iodo-9H-purin-9-yl)bicyclo[3.1.0]hex-2-yl ester (7)
A solution of the stannyl derivative MRS2829 6 in acetonitrile (25 μl, corresponding to 25 μg, 30 nmol) was added to ~80 μl Na125I solution (55 mCi, 25 nmol) in a glass container. A mixture of 32% peracetic acid/acetonitrile/glacial acetic acid was prepared in the ratio of 5/85/10 (v/v), and 2.5 μl of this peracetic acid solution (40 μg, 525 nmol) was added to the reaction mixture with brief mixing. After 10 min, the reaction mixture was diluted with 0.3 ml water, and the entire quantity was injected onto the HPLC (System C, Column B) to yield 8.8 mCi (32% yield) of 7 at 24 min. For confirmation of the identity of the radioactive product, a coinjected mixture of 5 and 7 eluted as a single peak
2.1.6. Radiochemical synthesis of [125I]MRS2500 (9)
The solution of [125I]7 was concentrated to dryness under vacuum, and the residue was treated with 200 μl trifluoroacetic acid with brief mixing followed by incubation at room temperature for 5 min. The reaction mixture was concentrated to dryness under vacuum. The residue was treated with 100 μl of 40% aqueous methylamine with brief mixing and incubation at room temperature for 5 min. Water (0.3 ml) was added, and this solution was injected onto the HPLC as above (System C, Column B). A peak containing 7.5 mCi was collected (85% and 26% radiochemical yield for the two steps). For confirmation of the identity of the radioactive product, coinjection of the unlabeled intermediate 8, unlabeled MRS2500, and labeled 9 resulted in elution of a chloride peak at 11 min without radioactivity and a radioactive MRS2500 peak at 14 min. The specific activity of the purified radioligand was 81 TBq (2200 Ci/mmol).
2.2. Pharmacological analyses
2.2.1. Materials
Apyrase was purified from potatoes as previously described [22]. ARC69931MX (Cangrelor) was provided by the Medicines Co. (Parsippany, NJ). MRS2179 and MRS2365 were from Tocris (Bristol, UK). ADP was from Boehringer (Mannheim, Germany). All other drugs were from Sigma-Aldrich Corporation (St. Louis, MO).
2.2.2. P2Y1 receptor expression in Sf9 insect cells
Membranes were prepared from Sf9 insect cells expressing the recombinant human P2Y1 receptor as described in detail previously [23]. Briefly, suspension cultures of Sf9 insect cells grown to a density of 1.4 × 106 cells/ml were infected with empty vector or recombinant baculovirus encoding the human P2Y1 receptor. Cells were harvested after two days by low speed centrifugation, and the cells were lysed using a Parr cavitation apparatus. A membrane fraction was isolated by high speed centrifugation, and the membranes were resuspended at a concentration of ~ 3.5 mg/ml in 20 mM Tris, pH 8.0, 250 mM sucrose, 2 mM 2-mercaptoethanol, 100 μg/ml phenylmethanesulphonylfluoride, 100 μM benzamidine, and 50 μg/ml tosylphenylalanyl chloromethyl ketone. Membranes were stored at −80°C and diluted approximately 300-fold for binding assays as described below.
2.2.3. Preparation of washed platelet suspension
Washed platelets from human or mouse blood were prepared by differential centrifugation as previously described [22]. Platelets were suspended in Tyrode’s buffer (137 mM NaCl, 2 mM KCl, 12 mM NaHCO3, 0.3 mM NaH2PO4, 1 mM MgCl2, 2 mM CaCl2, 5.5 mM glucose, 5 mM HEPES, pH 7.3) containing 0.35% human serum albumin and apyrase (0.02 U/ml), which degrades released ADP and prevents desensitization. Human platelets were maintained at a concentration of 300,000 platelets/μl at 37°C, and mouse platelets were maintained at a concentration of 150,000 platelets/μl at 37°C. Three different donors were utilized for isolation of three different preparations of platelets for each experiment.
2.2.4. Radioligand binding assay with recombinant P2Y1 receptors
Assays with membranes isolated from P2Y1 receptor-expressing Sf9 insect cells typically were with 0.2-0.5 nM [125I]MRS2500 and 100-300 ng of membranes in assay buffer (20 mM HEPES, 145 mM NaCl, 5 mM MgCl2, pH 7.5) in a total volume of 25 μl. All incubations were carried out in 12 × 75 mm conical tubes at 4° C and were terminated after 30 min by the addition of 3.5 ml of ice cold assay buffer followed by vacuum filtration over Whatman GF/A glass microfiber filters. The filters were washed with 7 ml of ice-cold assay buffer and radioactivity on each filter was quantified in a gamma counter. Specific binding was defined as total [125I]MRS2500 bound minus binding occurring in the presence of 30 μM 2MeSADP or 10 μM MRS2179.
2.2.5. Radioligand binding assay with human and mouse platelets
[125I]MRS2500 binding assays with intact washed platelets were performed at 20°C or 37°C. Assays with washed human platelets were initiated by addition of 900 μl of platelet suspension to a final volume of 1 ml of Tyrode’s buffer containing albumin. Assays with washed mouse platelets were initiated by addition of 400 μl of platelet suspension to a final volume of 0.5 ml of Tyrode’s buffer containing albumin. Steady state binding was attained within 5 min at 20°C or 37°C, and therefore, binding reactions were terminated after 5 min by vacuum filtration through GF/C glass fiber filters followed by five rapid washes with 3 ml of ice-cold Tyrode’s buffer. Radioactivity was quantified in a gamma counter. Specific binding was defined as total [125I]MRS2500 bound minus binding occurring in the presence of 10 μM MRS2179. Association, saturation, and competition binding experiments were performed using a single concentration of radiolabeled ligand (25 pM, 65,000 cpm/tube of 1 ml reaction mixture) in the presence of increasing concentrations of the appropriate unlabeled ligand.
2.2.6. Data Analysis
Data were analyzed using GraphPad Prism (GraphPad Software, San Diego, CA). The results are presented as the mean ± S.E.M. averaged from three or more experiments or in some cases as a data set from a single experiment that was typical of results from three or more experiments.
3. Results
3.1. Synthesis of [125I]MRS2500 and strategy for its application in a radioligand binding assay
We previously illustrated that iodo-substitution in a series of halo-substituted (N)-methanocarba bisphosphate analogues resulted in a competitive antagonist (MRS2500) that exhibited high affinity (Ki ~1 nM) and a high degree of binding selectivity for the P2Y1 receptor [8]. A monophosphate precursor of this high affinity antagonist was synthesized and conditions optimized for its [32P]phosphorylation using nucleotide kinase and [γ32P]ATP [18]. [32P]MRS2500 proved to be an excellent radioligand for quantification of both recombinant and natively expressed P2Y1 receptors. However, the short half-life of 32P as well as somewhat inefficient incorporation of 32P into the precursor molecule led us to consider alternative means of production of radiolabelled MRS2500.
We tried three different approaches for establishing a successful 3-step route from a stable stannylated intermediate 6. Two unsuccessful approaches to the synthesis of [125I]MRS2500 are shown in Figures 1A and B. The direct stannylation of MRS2500 1 gave an intermediate 2 that proved to be unstable. Although compound 2 was detected by HPLC, it was not sufficiently stable even when stored at low temperature (−80°C), and thus, it could not be purified. Alternatively, the attempted stannylation of 3, a phosphate-protected form of MRS2500, with trimethyltin under the same conditions gave only the undesired compound 4. This occurred upon replacement of the trimethylstannyl group by hydrogen readily available from acidic protons such as the 6-NHMe in the reaction [8]. Finally, a trimethylstanno derivative 6 was synthesized (Fig. 1C) from 5 by the method of Baranowska-Lortylewicz and coworkers with hexamethylditin in the presence of bis(triphenylphosphine)palladium(II) dichloride in dioxane for 3.5 h at 60 °C [24]. Intermediate 6 was intended for conversion to the iodo-form of 1 by a multi-step process to deprotect the phosphate groups and to exchange the functionality at the 6 position. Feasibility of the rapid re-conversion of 6 to an iodinated precursor 5 was demonstrated on small scale. We then took advantage of this procedure to produce a 125I-labeled precursor of MRS2500 7, which was purified by HPLC (Fig. 1D). The radiochemical yield for the conversion of 6 to 7 was 35%, including HPLC purification.
This radiolabeled molecule was deprotected to produce the intermediate 8, which in turn was converted to [125I]MRS2500 9 via N6-methylation by nucleophilic attack with methylamine. The final product 9 was purified by HPLC. It was also possible to combine the last two chemical steps successively in one pot (i.e., it was not necessary to purify 8). Using this procedure, 163 MBq (4.4 mCi) of [125I]MRS2500 was prepared. The overall radiochemical yield for the two steps from 7 to 9 was 23%, including HPLC purification.
3.2. Pharmacological analyses
3.2.1. Strategy
The approach utilized to examine [125I]MRS2500 as a potential radioligand for quantification of P2Y1 receptors included two different sources of P2Y1 receptors. First, we examined the binding properties of [125I]MRS2500 at human P2Y1 receptors in membranes prepared from Sf9 insect cells expressing recombinant receptor at high levels. Second, the P2Y1 receptor serves an obligatory physiological role in the effects of ADP on platelet shape change and aggregation [3]. Therefore, with the ultimate goal of applying an optimized radioligand for molecular study of the platelet P2Y1 receptor, we examined the properties of [125I]MRS2500 binding using human and mouse platelets.
3.2.2. Binding of [125I]MRS2500 to recombinant human P2Y1 receptors
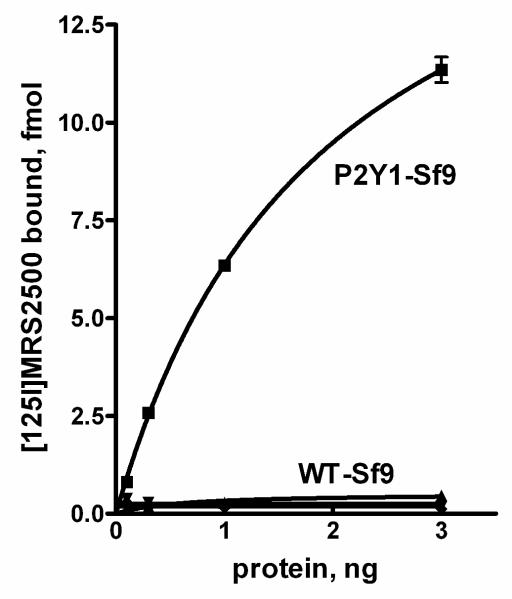
We previously generated a baculovirus expression vector that harbors the coding sequence of the human P2Y1 receptor. Infection of Sf9 insect cells with this baculovirus results in high level expression (~10 pmol/mg protein) of active P2Y1 receptor [23], and this system has been used as a source for purification of the human P2Y1 receptor to homogeneity [25]. As illustrated in Fig. 2, incubation of [125I]MRS2500 with membranes from wild-type insect cells resulted in no specific binding of radioligand over a wide range of membrane concentrations. In contrast, specific binding of [125I]MRS2500 was detected with as little 30 ng of membranes prepared from cells infected with P2Y1 receptor baculovirus. Binding of [125I]MRS2500 increased linearly with protein concentration up to 1 μg per assay at a concentration of radioligand of approximately 0.3 nM. Time course experiments (data not shown) revealed that steady state binding was obtained within 20 min in assays carried out with approximately 0.3 nM of [125I]MRS2500 and 300 ng of membranes, and a steady-state was maintained for at least 40 min.
Figure 2. Binding of [125I]MRS2500 to membranes from Sf9 insect cells expressing recombinant human P2Y1 receptors.
[125I]MRS2500 was incubated with the indicated amounts of membranes prepared from Sf9 insect cells infected with empty baculovirus (WT-Sf9) or human P2Y1 receptor baculovirus (P2Y1-Sf9) as described in Methods. Assays were in the absence (closed symbols) or presence (open symbols) of 30 μM 2MeSADP.
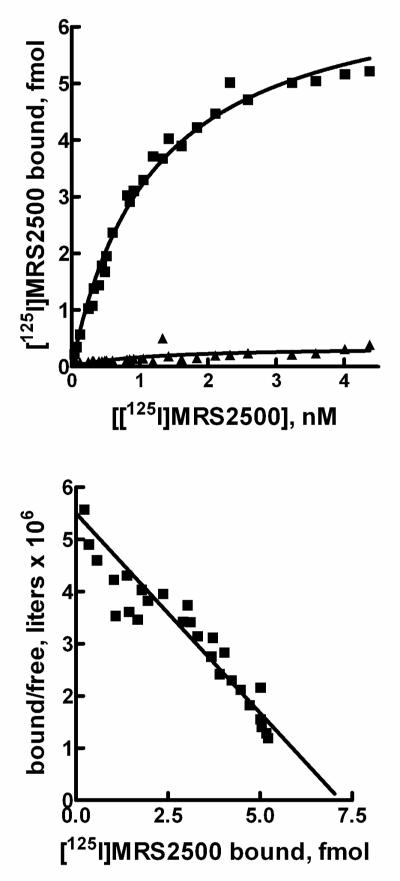
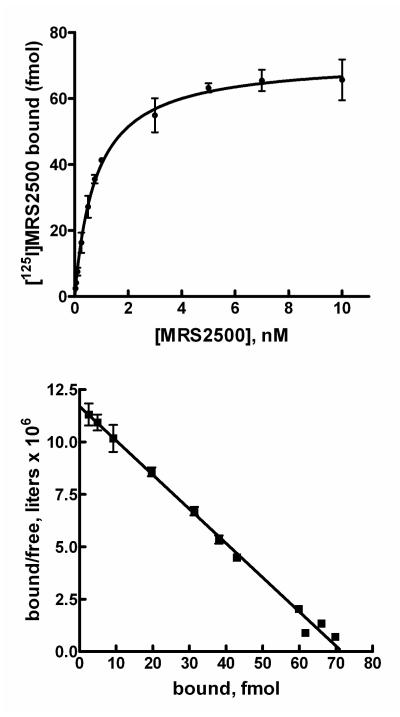
Saturation binding of [125I]MRS2500 was quantified over a wide range (0.04 nM to 5.15 nM) of concentrations. The resultant binding isotherm and transformation of these data using the Scatchard equation are presented in Fig. 3. Specific binding of [125I]MRS2500 exhibited one-site binding kinetics with a Kd of 1.1 ± 0.3 nM observed. The Bmax obtained for P2Y1 receptor expression in Sf9 cells was 13.3 ± 2.2 pmol/mg membrane protein.
Figure 3. Saturation binding of [125I]MRS2500 to membranes from Sf9 insect cells expressing recombinant human P2Y1 receptors.
Top panel, P2Y1 receptor-expressing membranes were incubated with the indicated concentrations of [125I]MRS2500 in the absence (■) or presence (▲) of 30 μM 2MeSADP as described in Methods. Bottom panel, The data presented in the top panel were plotted as the amount (fmol) of [125I]MRS2500 specifically bound on the X-axis versus the amount bound divided by the free concentration of [125I]MRS2500 on the Y-axis.
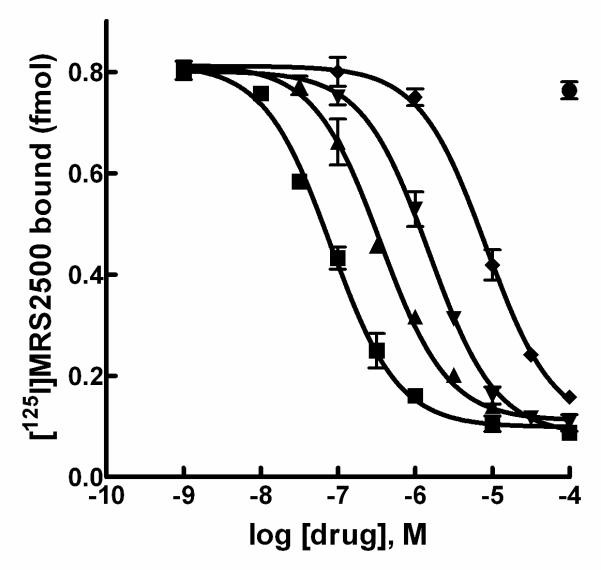
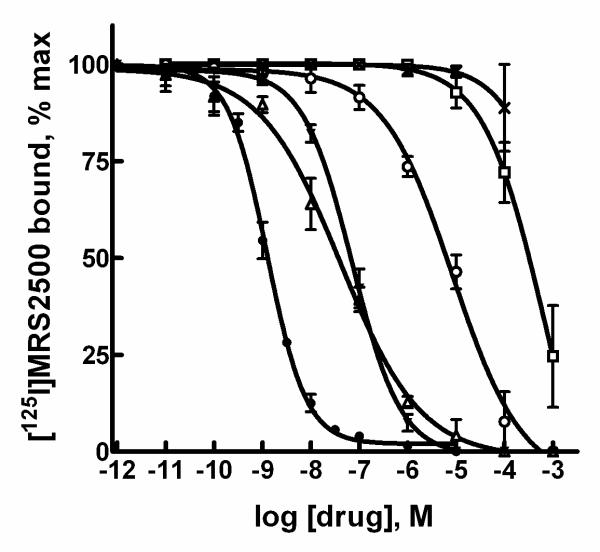
Competition curves were generated with a series of adenine nucleotides and analogues known to interact with the P2Y1 receptor (Fig. 4). The highest apparent affinity was observed with 2MeSADP (Ki = 70 ± 30 nM), followed in relative affinities by ADP (Ki = 625 ± 37 nM), ADP!S (Ki = 1750 ± 750 nM), and ATP (Ki = 16,250 ± 875 nM). UTP had no effect on [125I]MRS2500 binding at concentrations up to 30 μM.
Figure 4. Inhibition of [125I]MRS2500 binding to P2Y1 receptor-expressing membranes by P2Y1 receptor agonists.
Membranes prepared from Sf9 insect cells infected with human P2Y1 receptor baculovirus were incubated with [125I]MRS2500 and the indicated concentrations of 2MeSADP (■), ADP (▲), ADPβS (▼), ATP (◆), or UTP (●) as described in Methods.
3.2.3. Binding of [125I]MRS2500 to P2Y1 receptors on human platelets
We also examined the capacity of [125I]MRS2500 to interact with binding sites on human platelets. Incubation of [125I]MRS2500 with platelets over a range of concentrations up to 300,000 platelets/μl resulted in an essentially linear increase in the amount of radioligand bound (Fig. 5). In contrast, very little [125I]MRS2500 binding was observed in the presence of the selective P2Y1 receptor antagonist MRS2179 (10 μM). Time course experiments at 37°C revealed that binding reached apparent steady state within 60-90 sec, and [125I]MRS2500 binding was stable for at least 15 min (data not shown). All subsequent assays were carried out using 5 min incubations.
Figure 5. Binding of [125I]MRS2500 to human platelets.
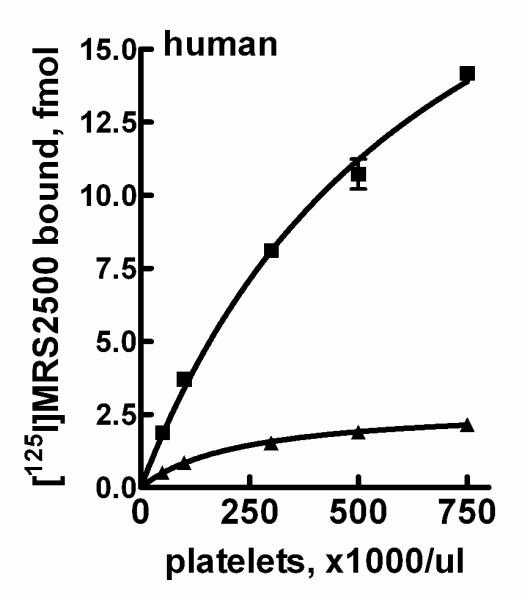
Platelet concentrations ranging from 50,000 platelets/μl to 750,000 platelets/μl were incubated with [125I]MRS2500 at 37°C in the absence (■) or presence (▲) of 10 μM MRS2179 as described in Methods.
The concentration of [125I]MRS2500 was varied from 0.025 nM to 10 nM as described in Methods, and specific binding was assessed as a function of radioligand concentration at 37°C. The resultant binding isotherm and transposition of these data using the Scatchard equation are presented in Fig. 6. Specific binding of [125I]MRS2500 to human platelets exhibited one-site binding kinetics with Kd = 0.61 ± 0.03 nM and a Bmax of 151 ± 1 sites/platelet. Saturation binding isotherms generated for human platelets at 20°C (data not shown) also were consistent with interaction with a single population of binding sites with Kd = 0.25 nM and 147 sites/platelet. Since all assays were carried out in the presence of apyrase, which degrades any released ADP, the binding values obtained were not influenced by events associated with agonist-induced desensitization.
Figure 6. Saturation binding of [125I]MRS2500 to human platelets.
Top panel, Human platelets were incubated with the indicated concentrations of [125I]MRS2500 at 37°C as is described in Methods. Specific binding (total binding minus binding in the presence of 10 μM MRS2179) is plotted. Bottom panel, The data presented in the top panel were plotted as the amount (fmol) of [125I]MRS2500 specifically bound on the X-axis versus the amount bound divided by the free concentration of [125I]MRS2500 on the Y-axis.
The capacity of agonists and antagonists of the P2Y1 receptor to inhibit [125I]MRS2500 binding to human platelets also was determined (Fig. 7). The antagonists MRS2179 and MRS 2500 inhibited radioligand binding in concentration dependent fashion, and the higher apparent affinity of MRS2500 (Ki = 1.20 ± 0.15 nM) compared to MRS2179 (Ki = 72.5 ± 17 nM) is consistent with the reported activities of these molecules. As also is predicted from previous studies, the synthetic agonist analogue 2MeSADP (Ki = 46.5 ± 1.7 nM) was more potent than the cognate agonist of the P2Y1 receptor, ADP (Ki = 15.4 ± 9 μM). Little effect of the P2Y12 receptor antagonist ARC-69931MX or P2X receptor agonist !!MeATP on [125I]MRS2500 binding was observed at concentrations of competing drugs up to 100 μM.
Figure 7. Inhibition of [125I]MRS2500 binding to human platelets by P2Y1 receptor ligands.
Platelets were incubated with [125I]MRS2500 and the indicated concentrations of MRS2500 (●), 2MeSADP (△), MRS2179 (▼), ADP (○), αβMeATP (□), or ARC-69931MX (x), as described in Methods.
3.2.4. Binding of [125I]MRS2500 to P2Y1 receptors on mouse platelets
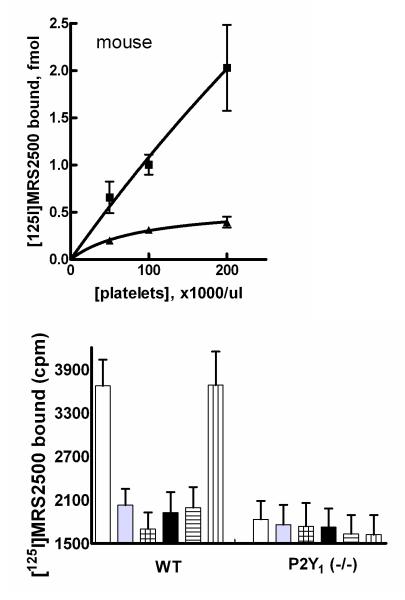
We also investigated [125I]MRS2500 as a potential radioligand for quantification of P2Y1 receptors on mouse platelets. As was observed with human platelets, [125I]MRS2500 binding increased linearly with increasing amounts of mouse platelets up to 200,000 platelets/μl (Fig. 8A). Whereas binding was inhibited by the P2Y1 receptor agonists ADP, 2MeSADP, and MRS2365 and the antagonist MRS2179, no effect was observed with the P2Y12 receptor antagonist ARC-69931MX (Fig 8B). Importantly, total binding of [125I]MRS2500 was much lower in platelets isolated from P2Y1(−/−) mice, and this low amount of binding was not inhibited by a high concentration of 2MeSDP. Thus, specific binding of [125I]MRS2500 was not observed in platelets isolated from P2Y1(−/−) mice, and both pharmacological and genetic data with mice platelets are consistent with the idea that [125I]MRS2500 specifically labels the P2Y1 receptor.
Figure 8. Binding of [125I]MRS2500 to mouse platelets.
Top panel, Platelet concentrations ranging from 50,000 platelets/μl to 200,000 platelets/μl were incubated with 50 fmol of [125I]MRS2500 at 37°C in the absence (■) or presence (▲) of 10 μM MRS2179 as described in Methods. Bottom panel, Platelets were prepared from wild-type or P2Y1 (−/−) mice and incubated with [125I]MRS2500 in the absence of additional drug (open bars) or with 100 μM ADP (gray bars), 10 μM 2MeSADP (squared bars), 10 μM MRS2179 (black bars), 10 μM MRS2365 (cross-hatched bars), or 10 μM ARC-69931MX (perpendicular-lined bars) as described in Methods.
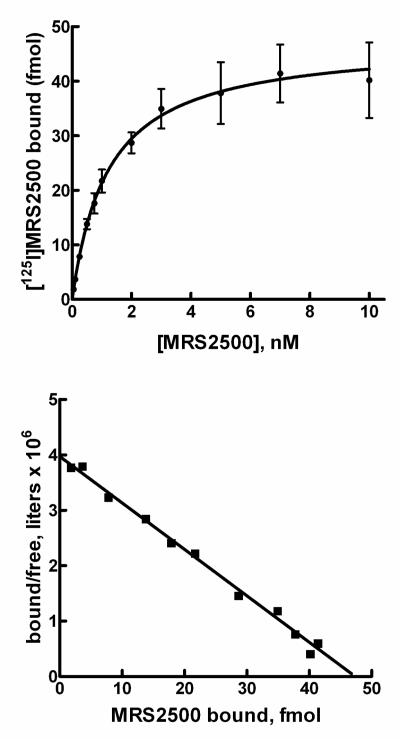
Saturation binding experiments also were carried out with platelets isolated from wild-type mice. The binding isotherm and transposition of these data using the Scatchard equation are presented in Fig. 9. Specific binding of [125I]MRS2500 to mouse platelets exhibited single site binding kinetics (Kd = 1.2 ± 0.1 nM) in saturation binding assays carried out at 37°C (Fig. 9), and a Bmax of 229 ± 23 sites/platelet was determined. Similar values (Kd = 0.5 nM and Bmax = 253 sites/platelet) were obtained in saturation binding assays carried out at 20°C (data not shown).
Figure 9. Saturation binding of [125I]MRS2500 to mouse platelets.
Top panel, Mouse platelets were incubated with the indicated concentrations of [125I]MRS2500 at 37°C as is described in Methods. Specific binding (total binding minus binding in the presence of 10 μM MRS2179) is plotted. Bottom panel, The data presented in the top panel were plotted as the amount (fmol) of [125I]MRS2500 specifically bound on the X-axis versus the amount bound divided by the free concentration of [125I]MRS2500 on the Y-axis.
4. Discussion
One of our research goals in drug development in recent years has been to develop an opitimized radioligand for direct quantification of the P2Y1 receptor. MRS2179 was one of the first synthetic antagonists to show high selectivity for the P2Y1 receptor [26], and although this molecule exhibits intermediate affinity (Kd ~100 nM) for this receptor, a [33P]-labeled form was used to detect P2Y1 receptors on human platelets [27]. MRS2279 was synthesized as a non-nucleotide P2Y1 receptor antagonist of higher affinity (Kd ~ 10 nM) that also exhibited high selectivity for the P2Y1 receptor over other P2Y receptors. [3H]2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate ([3H]MRS2279) provided the first widely applied radioligand for study of the P2Y1 receptor [23, 25]. We subsequently synthesized a non-nucleotide antagonist, 2-iodo-N6-methyl-(N)-methanocarba-2′deoxyadenosine-3′5′-bisphosphate (MRS2500), that inhibited [3H]MRS2279 binding to the P2Y1 receptor with a Ki value (~ 1 nM) ten times lower than that of MRS2279 [8]. More recently, a precursor of this molecule was synthesized that allowed facile, single-step kinase-catalyzed [32P]phosphorylation of a precursor molecule to yield [32P]MRS2500 [18]. This radioligand in turn was shown to bind selectively to the P2Y1 receptor versus other P2Y receptors and was utilized to quantify P2Y1 receptors natively expressed in rat brain and several other tissues. The binding characteristics of [32P]MRS2500 were excellent [7, 18], but the relatively low yield using enzymatic synthesis of the radioligand introduced limitations.
The chemistry described here takes advantage of a trimethylstannyl analogue 6 that allows facile synthesis of [125I]MRS2500. Only a few examples of direct iododestannylation on unprotected nucleotides exist, and there are no positions for straightforward incorporation of iodine into MRS2500. Our initial attempts to carry out iododestannylation in one or two steps failed. The successful synthetic route described here required three radiochemical steps, which otherwise would not be considered optimal because of the limitations of handling radioactive intermediates. However, each of these steps was carried out in a small volume and with high yield without excessive manipulation. Therefore, it constitutes a practical and efficient method, but one that requires nonaqueous reaction conditions for one step, i.e., the phosphate deprotection.
Given our previous success with application of [32P]MRS2500 in radioligand binding assays [18], it is not surprising that the [125I]-labeled form of this molecule also proves an outstanding radio-probe for quantification of P2Y1 receptors with a radioligand of relatively long radioactive half-life. The most well-established physiological function of this receptor involves its role with the P2Y12 receptor coordinating the shape change and aggregation response that occurs in platelets in response to ADP [3]. The data presented here establish radioligand binding assays with [125I]MRS2500 as a means to directly quantify and study native P2Y1 receptors on the cell surface of intact human and mouse platelets.
Methodology for quantification of P2Y1 receptor binding sites is crucial for advancing our understanding of a variety of conditions. For example, the platelet P2Y1 receptor rapidly desensitizes during agonist activation [9, 11, 12], but the molecular mechanisms that underpin this loss of responsiveness remain undefined. Similarly, polymorphisms of the P2Y1 receptor have been proposed to be associated with an increased risk of cardiovascular disease [13-17], but the relative densities and binding properties of the platelet P2Y1 receptor associated with these polymorphisms is not known. The studies reported here establish [125I]MRS2500 as a valuable new radioligand to investigate mechanistic aspects of the P2Y1 receptor in these and other conditions.
Acknowledgments
We thank Anthony Joseph (PerkinElmer) for technical assistance. This work was supported by National Institutes of Health grant GM38213 and by the NIDDK Intramural Research program. S. de Castro thanks Ministerio de Educación y Ciencia (Spain) for financial support.
ABBREVIATIONS
- AR-C69931MX
[dichloro-[[[(2R,3S,4R,5R)-3,4-dihydroxy-5-[6-(2-methylsulfanylethylamino)-2-(3,3,3-trifluoropropylsulfanyl)purin-9-yl]oxolan-2-yl]methoxy-hydroxyphosphoryl]oxy-hydroxyphosphoryl]methyl]phosphonic acid
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- 2MeSADP
2-methylthio-adenosine 5′-diphosphate
- MRS2179
2-chloro-N6-methyl-2′deoxyadenosine-3′,5′- bisphosphate
- MRS2279
2-chloro-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate
- MRS2365
(1′S,2′R,3′S,4′R,5′S)-4-[(6-amino-2-methylthio-9H-purin-9-yl)-1-diphosphoryloxymethyl]bicyclo[3.1.0]hexane-2,3-diol or 2-methylthio-(N)-methanocarba-adenosine-5′-diphosphate
- MRS2829
(1′R,2′S,4′S,5′S)-phosphoric acid di-tert-butyl ester 1-(di-tert-butoxy-phosphoryloxymethyl)-4-(6-chloro-2-trimethylstannylpurin-9-yl)bicyclo[3.1.0]hex-2-yl ester
- MRS2500
2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine 3′,5′-bisphosphate
- Tris
tris(hydroxymethyl)-aminomethane
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- 2.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gachet C. Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol. 2006;46:277–300. doi: 10.1146/annurev.pharmtox.46.120604.141207. [DOI] [PubMed] [Google Scholar]
- 4.Boyer JL, Romero-Avila T, Schachter JB, Harden TK. Identification of competitive antagonists of the P2Y1 receptor. Mol Pharmacol. 1996;50:1323–9. [PubMed] [Google Scholar]
- 5.Hechler B, Leon C, Vial C, Vigne P, Frelin C, Cazenave JP, et al. The P2Y1 receptor is necessary for adenosine 5′-diphosphate-induced platelet aggregation. Blood. 1998;92:152–9. [PubMed] [Google Scholar]
- 6.Jin J, Daniel JL, Kunapuli SP. Molecular basis for ADP-induced platelet activation. II. The P2Y1 receptor mediates ADP-induced intracellular calcium mobilization and shape change in platelets. J Biol Chem. 1998;273:2030–4. doi: 10.1074/jbc.273.4.2030. [DOI] [PubMed] [Google Scholar]
- 7.Houston D, Costanzi S, Jacobson KA, Harden TK. Development of selective high affinity antagonists, agonists, and radioligands for the P2Y1 receptor. Comb Chem High Throughput Screen. 2008;11:410–9. doi: 10.2174/138620708784911474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, et al. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a northern conformation: enhanced potency as P2Y1 receptor antagonists. J Med Chem. 2003;46:4974–87. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourdon DM, Mahanty SK, Jacobson KA, Boyer JL, Harden TK. (N)-methanocarba-2MeSADP (MRS2365) is a subtype-specific agonist that induces rapid desensitization of the P2Y1 receptor of human platelets. J Thromb Haemost. 2006;4:861–8. doi: 10.1111/j.1538-7836.2006.01866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chhatriwala M, Ravi RG, Patel RI, Boyer JL, Jacobson KA, Harden TK. Induction of novel agonist selectivity for the ADP-activated P2Y1 receptor versus the ADP-activated P2Y12 and P2Y13 receptors by conformational constraint of an ADP analog. J Pharmacol Exp Ther. 2004;311:1038–43. doi: 10.1124/jpet.104.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baurand A, Eckly A, Bari N, Leon C, Hechler B, Cazenave JP, Gachet C. Desensitization of the platelet aggregation response to ADP: differential down-regulation of the P2Y1 and P2cyc receptors. Thromb Haemost. 2000;84:484–91. [PubMed] [Google Scholar]
- 12.Baurand A, Eckly A, Hechler B, Kauffenstein G, Galzi JL, Cazenave JP, et al. Differential regulation and relocalization of the platelet P2Y receptors after activation: a way to avoid loss of hemostatic properties? Mol Pharmacol. 2005;67:721–33. doi: 10.1124/mol.104.004846. [DOI] [PubMed] [Google Scholar]
- 13.Li Q, Chen BL, Ozdemir V, Ji W, Mao YM, Wang LC, et al. Frequency of genetic polymorphisms of COX1, GPIIIa and P2Y1 in a Chinese population and association with attenuated response to aspirin. Pharmacogenomics. 2007;8:577–86. doi: 10.2217/14622416.8.6.577. [DOI] [PubMed] [Google Scholar]
- 14.Fontana P, Remones V, Reny JL, Aiach M, Gaussem P. P2Y1 gene polymorphism and ADP-induced platelet response. J Thromb Haemost. 2005;3:2349–50. doi: 10.1111/j.1538-7836.2005.01483.x. [DOI] [PubMed] [Google Scholar]
- 15.Hetherington SL, Singh RK, Lodwick D, Thompson JR, Goodall AH, Samani NJ. Dimorphism in the P2Y1 ADP receptor gene is associated with increased platelet activation response to ADP. Arterioscler Thromb Vasc Biol. 2005;25:252–7. doi: 10.1161/01.ATV.0000148708.44691.27. [DOI] [PubMed] [Google Scholar]
- 16.Lev EI, Patel RT, Guthikonda S, Lopez D, Bray PF, Kleiman NS. Genetic polymorphisms of the platelet receptors P2Y12, P2Y1 and GP IIIa and response to aspirin and clopidogrel. Thromb Res. 2007;119:355–60. doi: 10.1016/j.thromres.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Sibbing D, von Beckerath O, Schomig A, Kastrati A, von Beckerath N. P2Y1 gene A1622G dimorphism is not associated with adenosine diphosphate-induced platelet activation and aggregation after administration of a single high dose of clopidogrel. J Thromb Haemost. 2006;4:912–4. doi: 10.1111/j.1538-7836.2006.01869.x. [DOI] [PubMed] [Google Scholar]
- 18.Houston D, Ohno M, Nicholas RA, Jacobson KA, Harden TK. [32P]2-iodo-N6-methyl-(N)-methanocarba-2′-deoxyadenosine-3′,5′-bisphosphate ([32P]MRS2500), a novel radioligand for quantification of native P2Y1 receptors. Br J Pharmacol. 2006;147:459–67. doi: 10.1038/sj.bjp.0706453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kung MP, Kung HF. Peracetic acid as a superior oxidant for preparation of [iodine-123]IBZM: a potential dopamine D-2 receptor imaging agent. J. Labelled Compounds and Radiopharmaceuticals. 1989;27:691–700. [Google Scholar]
- 20.Owens J, Tebbutt AA, McGregor AL, Kodama K, Magar SS, Perlman ME, et al. Synthesis and binding characteristics of N-(1-naphthyl)-N′-(3-[125I]-iodophenyl)-N’-methylguanidine ([125I]-CNS 1261): a potential SPECT agent for imaging NMDA receptor activation. Nucl Med Biol. 2000;27:557–64. doi: 10.1016/s0969-8051(00)00102-5. [DOI] [PubMed] [Google Scholar]
- 21.Cattaneo M, Lecchi A, Ohno M, Joshi BV, Besada P, Tchilibon S, et al. Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem Pharmacol. 2004;68:1995–2002. doi: 10.1016/j.bcp.2004.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cazenave JP, Ohlmann P, Cassel D, Eckly A, Hechler B, Gachet C. Preparation of washed platelet suspensions from human and rodent blood. Methods Mol Biol. 2004;272:13–28. doi: 10.1385/1-59259-782-3:013. [DOI] [PubMed] [Google Scholar]
- 23.Waldo GL, Corbitt J, Boyer JL, Ravi G, Kim HS, Ji XD, et al. Quantitation of the P2Y1 receptor with a high affinity radiolabeled antagonist. Mol Pharmacol. 2002;62:1249–57. doi: 10.1124/mol.62.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baranowska-Lortylewicz J, Helseth LD, Lai J, Schneiderman MH, Schneiderman GS, Dalrymole GV. Radiolabeling kit/generator for 5-radiohalogenated uridines. J Labelled Compounds Radiopharm. 1994;34:513–521. [Google Scholar]
- 25.Waldo GL, Harden TK. Agonist binding and Gq-stimulating activities of the purified human P2Y1 receptor. Mol Pharmacol. 2004;65:426–36. doi: 10.1124/mol.65.2.426. [DOI] [PubMed] [Google Scholar]
- 26.Boyer JL, Mohanram A, Camaioni E, Jacobson KA, Harden TK. Competitive and selective antagonism of P2Y1 receptors by N6-methyl 2′-deoxyadenosine 3′,5′-bisphosphate. Br J Pharmacol. 1998;124:1–3. doi: 10.1038/sj.bjp.0701837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baurand A, Raboisson P, Freund M, Leon C, Cazenave JP, Bourguignon JJ, et al. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur J Pharmacol. 2001;412:213–21. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]