Abstract
Thoracic outlet syndrome (TOS) is a well‐described condition resulting from compression of the brachial plexus, subclavian artery and/or vein. Though symptoms of pain, numbness, tingling and signs of muscular weakness associated with this condition usually begin insidiously, on rare occasions the presentation is of acute onset and may represent an acute vascular compression. An unusual form of “effort” thrombosis of the subclavian vein may require emergency care in order to ensure controlled clot lysis and thrombus dissolution. Confirmation of subclavian thrombus is obtained by venography and makes use of real time videography to assess for venous flow impairment. Definitive treatment for the underlying cause of this form of TOS is first rib resection and scalenectomy. This case report presents a competitive swimmer who developed an acute onset of limb cyanosis and turgidity during swim training. Awareness of the possibility of acute thrombosis obstructing venous return and producing such signs and symptoms should lead the astute clinician to consider recommending contrast venography to assess the lesion and lead to appropriate medical intervention.
INTRODUCTION
Shoulder pain from impingement syndrome is the most common musculoskeletal complaint among competitive swimmers. Thoracic outlet syndrome (TOS), though rare, has been reported on occasion among upper extremity athletes.1‐3 This disorder is characterized by compression of the subclavian artery, vein, or brachial plexus and may present as neurogenic TOS (brachial plexus involvement) or vascular TOS (venous or arterial compression). Signs and symptoms of TOS include any combination of pain, numbness and tingling, weakness or evidence of vascular compromise in the upper extremity.1‐3 Venous TOS is more common than the arterial variety and is characterized by swelling and cyanosis of the extremity, pain, a heavy feeling and venous distension in the upper arm and shoulder region. Thrombosis of the subclavian vein which may be caused by TOS is referred to as Paget‐Schroetter Syndrome4 and represents a medical emergency when it occurs. The following case describes a subclavian thrombosis in an elite collegiate swimmer that necessitated initial intensive medical care followed by surgical excision of the first rib and simultaneous scalenectomy to achieve ultimate resolution.
CLINICAL PRESENTATION AND INITIAL IMAGING
The patient was a 21 year‐old male collegiate swimmer who had experienced a 15‐20 pound weight gain over a two‐year period with a weight lifting program in support of his swim training. He first noticed acute onset swelling and pain in the left non‐dominant upper extremity during an intense preseason swimming practice. The athlete was examined by the on‐site athletic trainer and advised to discontinue practicing and put ice on the shoulder. Symptoms and signs subsided over a 24‐hour period and the athlete was allowed to return to a modified practice regimen with emphasis on lower extremity training in order to avoid over‐using the upper extremities. Ten days following initial onset of symptoms, an identical episode occurred during swim practice and the swimmer was instructed by a physical therapist to go to the local hospital emergency room where a Doppler venous ultrasound of the upper extremity could be performed. This study failed to identify any impairment in blood flow in the left arm (Figure 1). Color Doppler sonography has the benefit of being non‐invasive and is reported to be 70–100% sensitive with a specificity of 93%.4‐6 A complication in completing sonography at this location is the surrounding bony anatomy that often makes direct compression of the subclavian vein during the procedure difficult or impossible.7 The best use of sonography is perhaps as a screening tool and not for consideration regarding surgical intervention.8 Despite the negative sonographic results, clinical suspicion persisted based on examination findings, which included virtual obliteration of the radial pulse with Wright's hyperabduction test (Figure 2), arm distension and limb cyanosis. Particular suspicion was warranted given the combination of such vascular symptoms known to common with thoracic outlet syndromes1‐3,8 and the athlete's presence in a high‐risk group for such conditions. Further examination indicated the patient to have normal skin sensation in C4T1 dermatomes, normal deep tendon reflexes for the biceps, brachioradialis, and triceps and no evidence of radicular symptoms arising from the cervical spine. Muscle testing demonstrated general weakness (4/5) of all muscles in the left upper extremity during the period in which the arm was cyanotic and distended. All peri‐scapular muscles were graded at 5/5 by manual muscle testing at the time of this clinical examination. The weakness demonstrated in the left upper extremity reverted to normal over a 24‐hour period as the signs of venous distention subsided. A vascular surgeon at the hospital, upon having the athlete's case described to him, strongly recommended a venogram be obtained in order to rule out vascular compromise. A contrast venogram from the level of the proximal forearm to the superior vena caval confluence was performed 12 days following the initial onset of acute symptoms. This test revealed a major blockage in the subclavian vein, venous stenosis, and concomitant thrombosis (Figure 3). Venogram is considered the “gold standard” for diagnosis of venous vascular TOS, but requires the challenge of cannulation distally of the edematous limb.8 The venogram and clinical exam findings necessitated admission to the Intensive Care Unit and immediate administration of heparin and tissue plasminogen activator (tPA) over a three day period in order to achieve thrombolysis. A repeat contrast venogram following the three‐day course of medical intervention revealed 70% patency of the subclavian vein with apparent permanent stenosis accounting for the failure to achieve 100% patency. The patient was begun on a therapeutic dose of Coumadin® and began daily abdominal self‐injections of Lovenox® as a blood thinning regimen. Emergent care in a case such as the one described herein is based on the avoidance of potential catastrophic consequences that may ensue should a thrombo‐embolus be dislodged from the subclavian vein. In the case of such an event, severe regional tissue anoxia and consequent necrosis may occur in the heart, lungs or brain.7
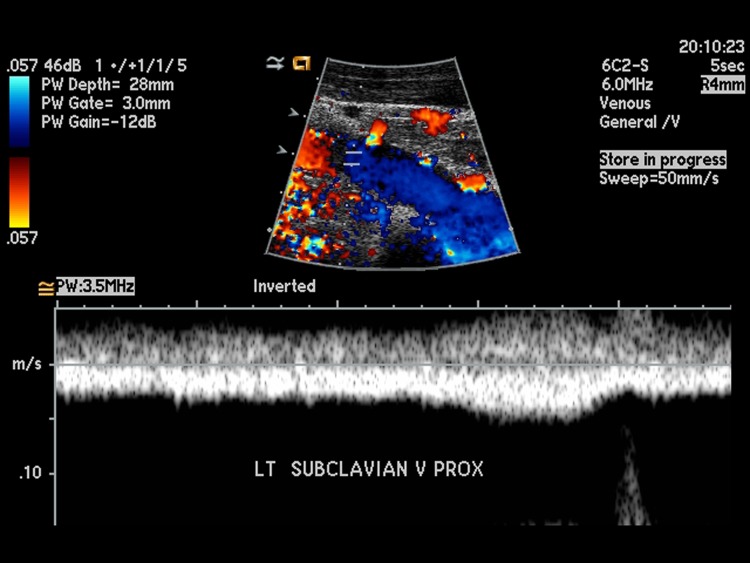
Figure 1.
Color Doppler Ultrasound indicating no acute deep venous thrombosis. The color, indicating the direction and velocity of blood flow, shows the red of the subclavian artery and blue of the subclavian vein as adjacent structures.
Figure 2.

Hyperabduction Test. In this test, the examiner first palpates a radial pulse. The positioning of the upper extremity is then achieved by the subject hyperabducting the shoulder and flexing the elbow to 90 degrees. The head is then rotated toward the non-test side. This has also been described with bilateral upper extremity positioning. The test has not been well studied for clinical value.
Figure 3.
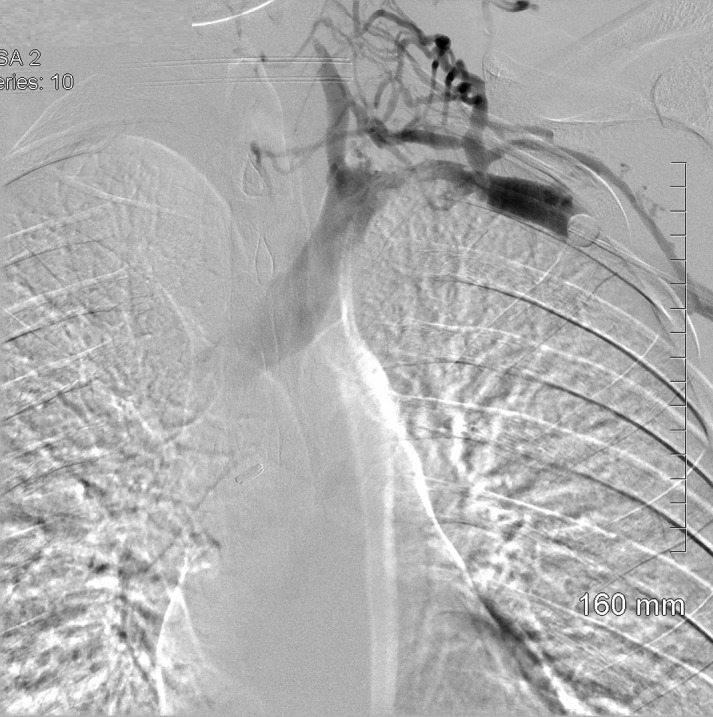
Contrast venogram indicating occlusion of the left subclavian vein. Note the intensity of the injected contrast distally compared to the proximal portion of the vessel.
In order to examine the possibility of a concomitant neurogenic TOS a standard electromyogram (EMG) and nerve conduction study (NCS) was performed four weeks after the date of original presentation. This electrical study emphasized nerve conduction across Erb's point (brachial plexus stimulation site) and needle EMG of muscles commonly involved in neurogenic TOS (those supplied by C8T1 nerve roots and the lower trunk of the brachial plexus). Nerve conduction across Erb's point was completely normal at 70 meters per second, well above the standard lower limit of normal of 50 meters per second. The Median nerve F‐wave for this 74 inch tall individual was 31.0 milliseconds and the Median nerve H‐reflex to the flexor carpi radialis muscle was 17.88 – all values within the normal range. The F‐wave and H‐reflex values represent action potentials that provide evidence of neural conductivity in proximal segments of the nerve. For instance, significant proximal nerve compression from a brachial plexopathy or nerve root compression would produce a slowed F‐wave or H‐reflex, alerting the examining clinician to focus additional attention in this area. Furthermore, the needle EMG failed to reveal any evidence of muscle membrane instability associated with denervation (no positive sharp waves or fibrillations present), nor were any motor unit recruitment abnormalities identified in any of the muscles studied. Muscles examined by EMG included the following: Upper trapezius, serratus anterior, rhomboids, deltoid, biceps, triceps, pronator teres, flexor carpi radialis, flexor digitorum superficialis, extensor digitorum communis, extensor pollicis longus, first dorsal interosseous, abductor digiti minimi, abductor pollicis brevis and opponens pollicis.
SURGICAL INTERVENTION
The student‐athlete returned to a modified swim training schedule and was able to complete the season, competing in the conference swim meet approximately three months following initial onset of signs and symptoms at which he won the 100 and 200 yard breaststroke conference championships. Though having qualified for national competition the swimmer declined to participate further, electing to proceed with definitive surgical correction. The patient underwent transaxillary first rib resection in the manner described by Atasoy.9 The surgical procedure involves sectioning the first rib into two pieces to permit removal. Surgical resection of the attached middle and anterior scalene muscles is also accomplished with this procedure, due to their origins on the first rib. Post‐surgically, the patient was again begun on a one week course of Lovenox® injections, followed by a three month regimen of oral Coumadin®.
Post‐operative Complication
Five days following the surgery the patient complained of an unusual feeling in the posterior aspect of the shoulder. Careful examination of the shoulder revealed a winging scapula produced by impairment of the serratus anterior muscle (Figure 4). The long thoracic nerve, supplying the serratus anterior muscle, lies in close proximity to the site of rib resection and the surgeon acknowledged that stretch of this neural structure could have occurred during the procedure. Three weeks following surgery clinical examination revealed continued scapular winging and weakness of the serratus anterior muscle, but all other muscles tested manually in the left upper extremity were graded at 5/5. Another EMG was undertaken at this juncture in order to objectively document the nature of the neuromuscular impairment noted following surgery. The deltoid, biceps, supraspinatus, infraspinatus, upper trapeziues, pectoralis major, and serratus anterior muscles were examined by EMG, using an 28 gauge Teflon®‐coated EMG needle. All muscles except the serratus anterior were normal with needle insertion, at rest and during recruitment. By contrast, examination of the serratus anterior muscle demonstrated increased insertional activity, 2+ positive sharp waves and fibrillations potentials and 50% reduction in motor unit recruitment (all electrical signs of muscle membrane instability usually associated with denervation). The presence of 50% reduction of voluntary motor unit recruitment, though an indicator of neurologic impairment, was, nevertheless an encouraging sign of likely recovery from this state of partial denervation. No significant increase in small amplitude polyphasic motors (usually interpreted as a sign of initial re‐innervation) was identified in this early post‐operative electrical study.
Figure 4.
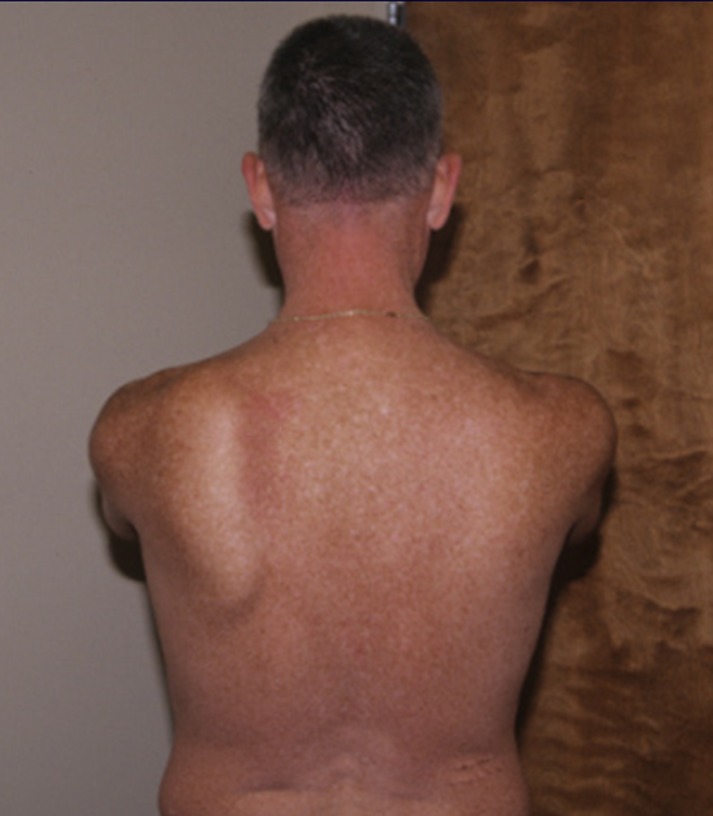
An example of the presentation of a winging scapula. Note: This is not the subject of this case.
PHYSICAL THERAPY MANAGEMENT
In light of the patient's neuromuscular condition post‐operatively, a generalized rehabilitation regimen was instituted. Specifically, the patient was instructed to be aware of scapular position to minimize stress on the denervated serratus anterior muscle and other soft tissues restraining the scapula. It is believed that minimizing the extent of winging by limiting the demands on the surrounding musculature provides an optimal environment for re‐innervation and eventual full recovery. Emphasis was placed on interscapular stabilization exercises and closed chain movement patterns that minimized scapular winging events. No electrical stimulation was applied during the post‐operative recovery as evidence is inconclusive and conflicting as to value of this intervention toward promoting re‐innervation and functional recovery.10‐12 The patient responded favorably to this exercise‐based protocol and improvement was noted in upper extremity function while minimal scapular dysfunction was experienced. Full strength (5/5 by manual muscle testing) was achieved in all muscles except the serratus anterior within three weeks of surgical excision of the first rib. The patient was placed on a home program in which he continued the upper extremity strength training, worked on soft tissue stretching of the shortened pectoralis major muscle and began a protocol of scar mobilization to limit the tendency to develop adhesions in and about the surgical scar.
Though scapular winging persisted for approximately five months, a repeat EMG examination of the serrratus anterior at three months post‐operatively revealed numerous small amplitude polyphasic motor units (so called “nascent motor units”) in the serratus anterior muscle, generally indicative of motor unit recovery. Complete resolution of scapular winging occurred at the five months post‐op with 5/5 muscle strength in the serrratus anterior muscle noted by clinical examination. The patient experienced no further sequalae and has been able to participate in all desired activities with no restrictions since the time of complete resolution.
CONCLUSION AND RECOMMENDATIONS
This case report demonstrates the multifaceted nature of assessment for patients with unusual clinical presentation. Effort‐induced limb cyanosis, swelling, and limb weakness strongly suggested a vascular cause for the findings. Definitive identification of subclavian venous stenosis and thrombus was obtained by venography following standard clinical examination. Ultimate resolution of the anatomical abnormality in this case required surgical resection of the first rib and scalenectomy. Post‐operative complication of long thoracic nerve injury was confirmed by standard electrophysiological assessment using EMG examination and nerve conduction studies. Scapular winging persisted for several months following surgery and eventually resolved at five months post‐operatively. Clinicians working with upper extremity athletes are cautioned to be especially alert for signs of vascular compromise, as noted in this case, as the condition may require specialized imaging studies for confirmation (venography) and emergent medical intervention.
REFERENCES
- 1.Braun RM. Thoracic outlet syndrome: a primer on objective methods of diagnosis. J Hand Surg-Am. Sep 2010;35(9):1539–1541; quiz 1541. [DOI] [PubMed] [Google Scholar]
- 2.Brandao LR, Williams S, Kahr WH, Ryan C, Temple M, Chan AK. Exercise-induced deep vein thrombosis of the upper extremity. 1. Literature review. Acta Haematol. 2006;115(3‐4):214–220 [DOI] [PubMed] [Google Scholar]
- 3.Chang K, Graf E, Davis K, Demos J, Roethle T, Freischlag JA. Spectrum of thoracic outlet syndrome presentation in adolescents. Arch Surg. Dec 2011;146(12):1383–1387 [DOI] [PubMed] [Google Scholar]
- 4.Melby SJ, Vedantham S, Narra VR, et al. Comprehensive surgical management of the competitive athlete with effort thrombosis of the subclavian vein (Paget-Schroetter syndrome). J Vasc Surg. Apr 2008;47(4):809–820; discussion 821 [DOI] [PubMed] [Google Scholar]
- 5.Prandoni P, Polistena P, Bernardi E, et al. Upper-extremity deep vein thrombosis. Risk factors, diagnosis, and complications. Arch Intern Med. Jan 13 1997;157(1):57–62 [PubMed] [Google Scholar]
- 6.Mustafa BO, Rathbun SW, Whitsett TL, Raskob GE. Sensitivity and specificity of ultrasonography in the diagnosis of upper extremity deep vein thrombosis: a systematic review. Arch Intern Med. Feb 25 2002;162(4):401–404 [DOI] [PubMed] [Google Scholar]
- 7.Smith RA, Dimitri SK. Diagnosis and management of subclavian vein thrombosis: three case reports and review of literature. Angiology. Feb-Mar 2008;59(1):100–106 [DOI] [PubMed] [Google Scholar]
- 8.Ferrante MA. The thoracic outlet syndromes. Muscle & nerve. Jun 2012;45(6):780–795 [DOI] [PubMed] [Google Scholar]
- 9.Atasoy E. Thoracic outlet compression syndrome. Orthop Clin N Am. Apr 1996;27(2):265–303 [PubMed] [Google Scholar]
- 10.Salvini TF, Durigan JL, Peviani SM, Russo TL. Effects of electrical stimulation and stretching on the adaptation of denervated skeletal muscle: implications for physical therapy. Rev Bras Fisioter. Jun 2012;16(3):175–183 [DOI] [PubMed] [Google Scholar]
- 11.Gordon T, Amirjani N, Edwards DC, Chan KM. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp Neurol. May 2010;223(1):192–202 [DOI] [PubMed] [Google Scholar]
- 12.Gordon T, Udina E, Verge VM, de Chaves EI. Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Motor Control. Oct 2009;13(4):412–441 [DOI] [PubMed] [Google Scholar]