Abstract
It has been hypothesized that one way in which lower socioeconomic status (SES) affects health is by increasing the rate of biological aging. A widely used marker of biological aging is telomere length. Telomeres are structures at the ends of chromosomes that erode with increasing cell proliferation and genetic damage. We aimed to identify, through systematic review and meta-analysis, whether lower SES (greater deprivation) is associated with shorter telomeres. Thirty-one articles, including 29 study populations, were identified. We conducted 3 meta-analyses to compare the telomere lengths of persons of high and low SES with regard to contemporaneous SES (12 study populations from 10 individual articles), education (15 study populations from 14 articles), and childhood SES (2 study populations from 2 articles). For education, there was a significant difference in telomere length between persons of high and low SES in a random-effects model (standardized mean difference (SMD) = 0.060, 95% confidence interval (CI): 0.002, 0.118; P = 0.042), although a range of sensitivity analyses weakened this association. There was no evidence for an association between telomere length and contemporaneous SES (SMD = 0.104, 95% CI: −0.027, 0.236; P = 0.119) or childhood SES (SMD = −0.037, 95% CI: −0.143, 0.069; P = 0.491). These results suggest weak evidence for an association between SES (as measured by education) and biological aging (as measured by telomere length), although there was a lack of consistent findings across the SES measures investigated here.
Keywords: biological aging; review, systematic; socioeconomic status; telomere length
INTRODUCTION
Social inequalities in health, with people experiencing progressively worse health with increasing socioeconomic deprivation, are present throughout the world (1–5). For example, the difference in life expectancy between the most deprived and least deprived persons in the United States has been found to be 4.5 years (6). Nevertheless, the pathways, particularly the underlying biological processes, between poorer socioeconomic status (SES) and ill health are less well understood.
One area of increasing focus is biological aging. This is the incremental, universal, and intrinsic degeneration of physical and cognitive functioning and the ability of the body to meet the physiologic demands that occur with increasing chronologic age. Telomeric DNA length has been proposed as a possible marker of biological aging (7). Telomeres are nucleoprotein structures present at the ends of chromosomes that help maintain chromosomal integrity. Telomeric DNA shortens in somatic cells with increasing rounds of cell division as a consequence of the “end-replication problem” (8). The progressive nature of telomere length shortening has made it an appealing, widely used measure of a person's biological age, in that it is hypothesized to act as a molecular clock (9) and has been shown to be associated with key age-related diseases (10–13) and death (14).
Even though chronologic age is also associated with a progressive decline in many biological functions, there is considerable variation in the incidence of degenerative diseases among persons of the same age. Different rates of biological aging could be a key reason for this, and it has been hypothesized that low SES might lead to accelerated aging, which in turn increases the risk of premature death and chronic diseases such as cardiovascular disease and most cancers (15, 16).
Lower SES exposes persons to damaging physical, mental, and behavioral insults and hence to greater risk of cellular and genomic damage and depleted repair and protection mechanisms (17). This in turn should be reflected in shorter telomere length (15). Therefore, it has been hypothesized that there will be an association between SES and telomere length, as a marker of biological aging (15).
Thus far, the evidence for an association between SES and telomere length has been mixed. Some studies have shown lower SES (greater disadvantage) to be associated with shorter telomere length (18, 19), whereas others have shown the opposite to be true (20). In addition, several studies have found nonsignificant associations between SES and telomere length (21–23). However, many of these studies have drawn on small and nonrepresentative samples and have used a wide range of SES markers, which makes comparisons difficult. Given these conflicting results, we aimed to systematically review and quantitatively assess (using meta-analysis) the evidence for an association between SES and telomere length in adulthood. To our knowledge, this is the first such systematic review with a meta-analysis in this field.
MATERIALS AND METHODS
The review protocol is available in the Web Appendix (posted at http://aje.oxfordjournals.org/), including the completed PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist (24). Our objectives, in terms of the PICOS (population, intervention, comparator, outcome, and study design) statement, were as follows:
Population: Adult men and women (age ≥18 years)
Intervention: Not applicable
Comparator: SES
Outcome: Telomere length differences between high- and low-SES groups
Study design: Longitudinal, cross-sectional, and repeat cross-sectional studies; population-/community-based studies; population-/community-based studies sampling from specific occupation, hospital admission, or disease state groups; case-control studies
We used 4 approaches to identify relevant articles. First, an electronic search was conducted, and second, an electronic Cited Reference Search was carried out on articles identified by the initial electronic search, both of which are described in detail below. Third, the reference sections of articles deemed suitable for review were scrutinized. Finally, experts in the field were contacted for relevant articles. This multidimensional approach was used to help identify articles not readily identified through traditional database searching alone (25).
Data sources and searches
Articles published before October 25, 2011, were identified by an information scientist (C. F.) searching ISI Web of Knowledge (http://wok.mimas.ac.uk; all years), Embase (www.embase.com; 1980–2011), and Medline (http://www.ncbi.nlm.nih.gov; 1948–2011). Searches were performed for articles that contained matches for both telomere length and SES (based on common measures of SES (26–28)). For telomere length, search terms included telomere, telomere binding proteins, cell aging, cell ageing, biological aging, biological ageing, cellular aging, cellular ageing, and nucleoprotein structure. For SES, terms included socio-economic, socioeconomic, education, income, area deprivation, neighbourhood, neighborhood, employment, housing, financial difficulties, car ownership, class, poverty, social status, and tenure. The Cited Reference Search was carried out using ISI Web of Knowledge. A full search statement for each database is included in the protocol.
Article selection and further searches
Two of the authors (T. R. and M. B.) independently reviewed the abstracts of the identified articles to assess whether they met the inclusion criteria for further review. To be included, articles had to have been published in English, have an abstract available, be a full report in a peer-reviewed journal (interviews, meeting summaries, and conference presentations/abstracts were excluded), have a human (not animal) study population, be community-based (not laboratory-based), and be an empirical article (reviews and cohort profiles were excluded). The full texts of articles were then scrutinized for evidence of SES-telomere length associations. Articles were excluded if the study contained no measures of telomere length, no measures of SES, or no descriptions of SES-telomere length analyses; if SES-telomere length analyses were described but no results were presented; or if they described children-only (age <18 years) samples.
Data extraction and quality assessment
Information on study participants (sample size, age range, sex, and study design), telomere length measure (technique and measurement units), SES measures, adjustments, and main results were extracted by T. R. and verified by M. B. These data were recorded in a standardized form and are shown in full in Web Table 1. Articles were assessed on their strengths and limitations according to 3 sets of criteria: sample (A), analysis (B), and presentation (C) (Table 1). This approach was based on a previously published scoring system (29), with some modifications regarding sample quality carried out to better fit the studies identified here. Full definitions for each criterion are available in the protocol (see Web Appendix). Scores were assigned for each criterion, with 2 points available for “A,” 3 points for “B,” and 1 point for “C.” Therefore, a maximum score of 6 was possible for each article. Scores were assigned independently by T. R. and M. B., differences were discussed, and a final score was determined. Each article's quality was then summarized as higher (a score of ≥4), intermediate (a score of 2 or 3), or lower (a score of ≤1).
Table 1.
Strengths and Limitations of the Criteria Used for the Review Quality Scorea
| Criteria Set | Strengths | Limitations |
|---|---|---|
| A | Community-/population-based study design (+1) | Other study design |
| A | Representative sample (+1) | Nonrepresentative sample |
| B | More than 1 SES dimension (+1) | Only 1 SES dimension |
| B | Hierarchical, graded SES categories (+1) | Binary SES variables |
| B | SES-telomere results adjusted for age or sex (where applicable) (+1) | No adjustment for age or sex in SES-telomere analysis (where applicable) |
| C | SES-telomere results presented in the form of β coefficients, mean values with standard deviation, standard error, confidence interval, and P value (+1) | Incomplete results presented for SES-telomere analysis |
Abbreviation: SES, socioeconomic status.
a Articles included in the review were assessed on their strengths and limitations according to 3 sets of criteria: sample (A), analysis (B), and presentation (C). More detailed definitions for each criterion can be found in the review protocol, which is presented in the Web Appendix (http://aje.oxfordjournals.org/).
Meta-analysis
In conducting the meta-analysis, we wished to minimize heterogeneity but maximize the number of studies included. Therefore, we performed 3 separate meta-analyses with the most frequently used SES measures across articles within the following groupings: SES measured contemporaneous with telomere length, childhood SES, and education. Within each group, we employed the most commonly used SES measure across the articles available for the analysis (29). For contemporaneous and childhood SES, social class (self/parent(s)) was the most prevalent measure. If social class was not available, income was used. Where income was not available, employment status was used. For education, where attainment was not available (the most common education measure used), years of full-time education were used.
To conduct the meta-analyses, specific results were required from each article to maintain consistency while allowing the maximum number of articles to be included. Each article had to meet the following criteria: 1) telomere length was analyzed as a continuous measure, and 2) SES measures were available as ordinal categories. The results presented needed to include mean telomere length, standard deviation, and the sample size for each SES category. If the full required results were not presented in the article, we contacted the authors and requested the missing information. Depending on the SES measures used, information might have been sought for SES measures across each of the 3 life-course SES groupings. All authors were contacted by e-mail for at least one of the aforementioned essential components needed for the meta-analysis. If there was no response to the initial e-mail, a second (and final) e-mail request was sent. Only 1 author failed to respond to our request, although 12 authors could not provide data for at least 1 of their SES measures.
Quality scores were recalculated for the meta-analysis on the basis of the complete data provided. If more than 1 article presented identical analysis, the earliest published article was used or sought. If telomere length was measured more than once, baseline telomere length was used or sought. Table 2 includes the reasons for exclusion from the meta-analyses for each article where appropriate. High-SES groups were compared with low-SES groups to maximize the number of studies that could be compared (29). If SES measures were binary, this comparison was straightforward. If SES was categorized into more than 2 groups, the highest and lowest categories were used. Standardized mean differences (SMDs) (plus standard errors) in telomere length between high- and low-SES groups were calculated for each article and were used as the outcome variable in the meta-analyses.
Table 2.
Studies Reporting on the Relation Between Socioeconomic Status and Telomere Length That Were Included in a Systematic Review
| First Author, Year (Reference No.) | Study or Sample | Country | Study Design | Telomere Sample Size (n) | Sex and Age Range, years | TL Measure | SES Measure | SES-TL Association (+, −, 0)a | Review Quality Scoreb and Rating | Reason for Exclusion From Meta-Analysisc |
|---|---|---|---|---|---|---|---|---|---|---|
| Adams, 2007 (21) | Newcastle Thousand Families Study | United Kingdom | PCB | 318 | Men and women aged 50 years | qPCR | Occupation (at age 25 years) | 0 | 6—Higher | Included |
| Occupation (at age 50 years) | 0 | |||||||||
| Occupation (parental at birth) | 0 | |||||||||
| Occupational mobility (birth vs. age 25 years) | 0 | |||||||||
| Occupational mobility (age 25 years vs. age 50 years) | 0 | |||||||||
| Occupational mobility (birth vs. age 50 years) | 0 | |||||||||
| Occupation (accumulated) | 0 | |||||||||
| Income (at age 50 years) | 0 | |||||||||
| Batty, 2009 (16) | West of Scotland Coronary Prevention Study | United Kingdom | Otherd | 1,542 | Men aged 45–64 years | qPCR | Employment | + | 4—Higher | 3 |
| Education | 0 | |||||||||
| Area deprivation | 0 | |||||||||
| Early-life social position (height as proxy) | 0 | |||||||||
| a) Chan, 2010 (35) | Elderly people living in Hong Kong | Hong Kong, China | PCB | a) 2,006 | Men and women aged ≥65 years | qPCR | a) Education | Men 0, women 0 | a) 3—Intermediate | a) Included |
| b) Woo, 2009 (20) | b) 1,936 | b) Social status ladder (self-rated) | Men –, women 0 | b) 5—Higher | b) Included | |||||
| a) Cherkas, 2006 (18) | St. Thomas’ Adult Twin Registry (Twins UK) | United Kingdom | PCB | a) 1,303 | a) Women aged 18–75 years | Southern blot | a) Occupation | a) + | a) 6—Higher | a) Included |
| Education | a) 0 | |||||||||
| Income | a) 0 | |||||||||
| b) Cherkas, 2008 (19) | b) 1,319 | b) Men and women aged 18–81 years | b) Occupation | b) + | b) 1—Lower | b) 4 | ||||
| Epel, 2006 (56) | Mothers of sick children | United States | Other | 62 | Women aged 20–50 years | qPCR | Education | 0 | 1—Lower | 1 |
| Epel, 2009 (37) | MacArthur Study of Successful Aging | United States | PCB | 235 | Men and women aged 70–79 years | qPCR | Education | 0 | 2—Intermediate | 3 |
| Fernandez-Egea, 2009 (57) | Psychotic patients | Spain | Other | 82 | Men and women aged 18–64 years | Southern blot | Occupation | 0 | 1—Lower | 3 |
| Geronimus, 2010 (38) | Study of Women's Health Across the Nation | United States | PCB | 215 | Women aged 49–55 years | qPCR | Poverty | 0 | 1—Lower | 2 |
| Harris, 2006 (39) | Lothian Birth Cohort 1921 | United Kingdom | PCB | 189 | Men and women aged 78–79 years | qPCR | Occupation | 0 | 5—Higher | Included |
| Harris, 2010 (22) | Lothian Birth Cohort 1936 | United Kingdom | PCB | 1,091 | Men and women aged 68–70 years | qPCR | Occupation | 0 | 6—Higher | Included |
| Education | 0 | |||||||||
| Honig, 2006 (40) | Washington Heights-Inwood Columbia Aging Project | United States | Other | 257 | Men and women aged 66–103 years | qPCR | Education | 0 | 1—Lower | Included |
| Hou, 2009 (41) | Gastric cancer patients | Poland | PCB | 716 (analysis 416 only) | Men and women aged 21–79 years | qPCR | Education | + | 5—Higher | Included |
| Houben, 2011 (42) | Zutphen Elderly Study | The Netherlands | PCB | 203 | Men aged 73–91 years | qPCR | Education | 0 | 2—Intermediate | Included |
| Kananen, 2010 (43) | Health 2000 Study | Finland | Other | 939 | Men and women aged 30–87 years | qPCR | Education | 0 | 4—Higher | Included (education) |
| Employment | 0 | 3 | ||||||||
| Childhood financial difficulties | 0 | 3 | ||||||||
| Unemployment (parental) | + | 3 | ||||||||
| Lee, 2011 (44) | Fels Longitudinal Study | United States | PCB | 345 (258 for SES analysis) | Men and women aged 8–90 years (18–90 years for SES analysis) | qPCR | Education | 0 | 2—Intermediate | Included |
| Mather, 2010 (45) | Personality and Total Health Through Life Project | Australia | PCB | 646 | Men and women aged 44–49 years and 64–70 years | qPCR | ≥40 cohort—Occupation | 0 | 4—Higher | Included |
| ≥60 cohort—Occupation | 0 | |||||||||
| Mirabello, 2009 (46) | Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial | United States | Other | 1,661 | Men aged 55–74 years | qPCR | Education | 0 | 2—Intermediate | Included |
| a) Nettleton, 2008 (47) | Multi-Ethnic Study of Atherosclerosis | United States | PCB | a) 840 | Men and women aged 45–84 years | qPCR | a) Education | − | a) 2—Intermediate | a) 1 |
| Income | 0 | |||||||||
| b) Diez Roux, 2009 (36) | b) 981 | b) Education | − | b) 5—Higher | b) 2 | |||||
| Income | 0 | |||||||||
| Nordfjall, 2008 (48) | Northern Sweden MONICA Study | Sweden | PCB | 440 | Men and women aged 25–74 years | qPCR | Education | 0 | 3—Intermediate | Included |
| O'Donovan, 2011 (49) | Persons with posttraumatic stress disorder | United States | Other | 90 | Men and women aged 21–49 years | qPCR | Education | 0 | 1—Lower | 3 |
| Parks, 2011 (50) | Sister Study | United States | Other | 647 | Women aged 35–74 years | qPCR | Employment | − | 2—Intermediate | Included |
| Risques, 2010 (23) | National Long-Term Care Survey | United States | PCB | 624 | Men and women aged 65–89 years | qPCR | Education | 0 | 2—Intermediate | Included |
| Shiels, 2011 (51) | Psychological, Social, and Biological Determinants of Ill Health Study | United Kingdom | PCB | 382 | Men and women aged 35–64 years | qPCR | Occupation | 0 | 3—Intermediate | Included |
| Education | 0 | |||||||||
| Income | 0 | |||||||||
| Housing tenure | 0 | |||||||||
| Steptoe, 2011 (52) | Whitehall II Study | United Kingdom | Other | 434 | Men and women aged 53–76 years | qPCR | Occupation | 0 | 5—Higher | Included |
| Education | + | |||||||||
| Income | 0 | |||||||||
| Surtees, 2011 (53) | EPIC–Norfolk population study | United Kingdom | PCB | 4,441 | Women aged 41–80 years | qPCR | Occupation | 0 | 4—Higher | 3 (contemporaneous) |
| Employment (parental) | 0 | 5 (childhood) | ||||||||
| Wolkowitz, 2011 (54) | Persons with major depressive disorder | United States | Other | 35 | Men and women aged 24–48 years | qPCR | Income | 0 | 2—Intermediate | 3 |
| Yaffe, 2011 (55) | Health, Aging and Body Composition Study | United States | PCB | 2,741 | Men and women aged 70–79 years | qPCR | Education | + | 1—Lower | 1 |
| Zheng, 2010 (34) | 2 studies— a) Roswell Park Cancer Institute sample |
a) United States |
a) Other |
a) 328 |
a) Women aged 43–69 years |
a) qPCR |
a) Income |
0 |
a) 0—Lower |
a) Included |
| b) Lombardi Comprehensive Cancer Center sample | b) United States | b) Other | b) 259 | b) Women aged 42–63 years | b) FISH | b) Income | 0 | b) 0—Lower | b) 3 |
Abbreviations: EPIC, European Prospective Investigation into Cancer and Nutrition; FISH, fluorescence in-situ hybridization; MONICA, Monitoring of Trends and Determinants in Cardiovascular Disease; PCB, population- or community-based; qPCR, quantitative polymerase chain reaction; SES, socioeconomic status; TL, telomere length.
a +, higher SES and longer TL; −, higher SES and shorter TL; 0, no SES-TL association.
b Out of a possible score of 6.
c 1 = TL treated as categorical; 2 = SES treated as continuous; 3 = summary statistics (mean value, standard deviation, sample size) not available; 4 = repeat of previous analysis.
d Case-control or population sample based on specific occupations, hospital admission, or disease state.
Comprehensive Meta-Analysis software (version 2.2.064; Biostat Inc., Englewood, New Jersey) was used for all analyses and for the production of forest plots/publication bias plots. Heterogeneity between articles was considered by fitting a random-effects model, with the inverse variation method used to weight articles' effect sizes (30). When heterogeneity was identified, its source was assessed with post hoc meta-regression. For the meta-regression, effect size was regressed on the quality score with the aim of accounting for the heterogeneity through differences in quality score. We also used the sensitivity analyses described below to ascertain whether the heterogeneity identified in any meta-analysis was linked to any subgroups or individual articles by calculating the same heterogeneity statistics for each sensitivity analysis.
Sensitivity analyses were carried out in 6 ways: 1) by applying a fixed-effects model (assumes equal effect size across all studies); 2) by limiting articles to those in which adjustments were made for only age, sex, and assay plate (i.e., excluding those with a range of possible mediators); 3) by removing articles that did not adjust for age or sex (if applicable); 4) by removing poorer-quality (lower- and intermediate-ranking) articles; 5) by repeating the meta-analyses with each article removed; and 6) by rerunning the meta-analyses with the ordinal measure of SES used as a continuous variable (to allow for more gradated associations between SES and telomere length). To do this, we fitted regression models for each article on the basis of the summary data (means, standard deviations, and sample sizes) using a modification of the method described by Larson (31). This involved calculating a relative index of inequality (RII) for each study variable and then regressing the RII score against telomere length. This allowed maximum utilization of data where multicategory measures had been reduced to binary for the main high-SES/low-SES comparison. Publication bias was considered with the Begg and Mazumdar rank correlation test, as well as through the use of a funnel plot in which the SMDs were plotted against the sample sizes (32, 33).
RESULTS
Articles identified
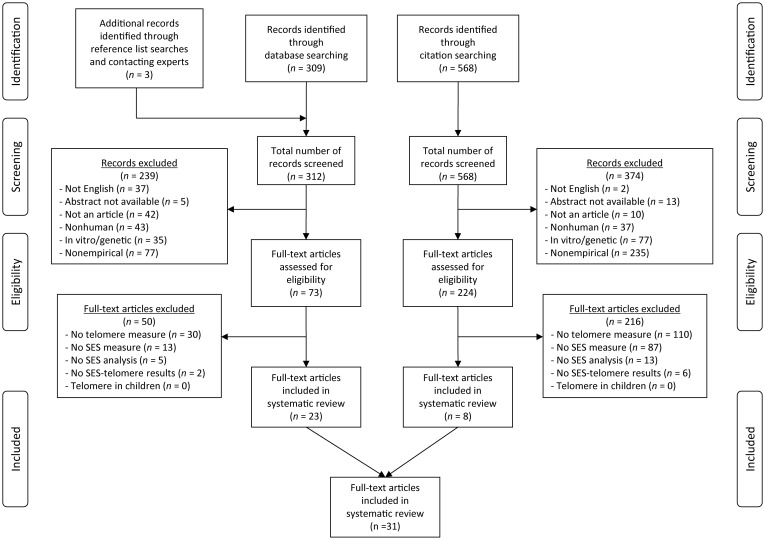
Herein, “article” (“articles”) refers to the published paper(s) or article(s), whereas “study” (“studies”) refers to the population, sample, cohort, or study (e.g., Whitehall II). Initial searches identified 309 unique articles for consideration (Figure 1). Of these, 70 satisfied the exclusion criteria, and after the full articles were reviewed, 20 met the full inclusion criteria. After reference lists were reviewed and experts in the field were contacted, 3 more unique articles were identified. The citation search of these 23 articles identified 568 unique references. Of these, 224 passed the exclusion criteria, and after the full articles were reviewed, 8 met the full inclusion criteria. No additional articles were identified by review of reference lists. Therefore, a total of 31 articles were identified for full review. Three pairs of articles used data from the same study (see Table 2), and 1 article contained 2 separate study populations (34); thus, 29 unique (nonoverlapping) study populations were included. The data extracted from the 31 articles/29 studies are summarized in Table 2 and are shown in fuller detail in Web Table 1.
Figure 1.
Selection and exclusion of publications for a systematic review of the association between socioeconomic status (SES) and telomere length.
Of the 29 studies, 17 were community-/population-based, whereas the rest were classed as “other” (including case-control studies and those that sampled population/community groups based on occupation, hospital admissions, or disease state). The majority of studies used respondents drawn from the United States (14) or the United Kingdom (8). The remaining studies were from Hong Kong (China), Spain, Poland, Finland, Australia, Sweden, and the Netherlands. Ages at study entry ranged from 8 years to 103 years, although only 6 studies sampled from similarly broad age ranges (18–90 years). Other studies focused on narrower ranges (10–40 years), typically encompassing midlife or older ages, although 5 also included younger respondents. Sample sizes ranged from 35 to 4,441, although only 7 studies had a sample size greater than 1,000. Of the 29 studies, 19 included both men and women, 7 sampled from women exclusively, and 3 included only male participants.
Measurement of telomere length
The most common technique used to measure telomere length (in 26 of the 29 studies) was quantitative polymerase chain reaction (16, 20–23, 34–56). Southern blot (18, 19, 57) and fluorescence in-situ hybridization (34) techniques were used in the remaining 3 studies. Telomere length typically was presented as either (kilo-) base pairs (16, 18–22, 34, 35, 37–39, 42, 49, 50, 54, 57) or relative T/S (telomere-to-single copy gene) ratios (23, 36, 40, 41, 43–48, 51, 52, 55, 56). Other units of measurement included fluorescent intensity units and delta Ct. Occasionally, telomere length was also log-transformed for normality.
Measurement of SES
SES is measured in a wide range of ways depending on the research question under consideration, geographic location, and the data available, but there is no “gold standard” (26–28). Although different theoretical hypotheses suggest why or how specific measures (e.g., occupation, education, income) might influence health, these measures generally are viewed as broad indicators of SES and typically are used interchangeably. The literature on SES and telomere length is no exception. However, in the broader social patterning literature, there is strong evidence of SES-health associations across all of these measures (58). The main ways in which SES was measured in the articles are shown in Web Table 1. These articles measured a wide array of SES constructs, including subjective and objective SES, and used registry and self-complete data. SES was assessed at different points in time in the identified articles: in adulthood contemporaneous with telomere length measurement; as persons moved from childhood to adulthood via educational measures; in childhood; prospectively (before telomere length was measured); and over time.
Contemporaneous measures included social class based on occupation (18, 19, 21, 22, 39, 45, 51–53, 57), income (18, 21, 34, 36, 47, 51, 52, 54), employment status (16, 43, 50), area deprivation (16), self-rated social status (20), and housing tenure (51). Education was assessed either as the total number of years spent in full-time education (22, 36, 37, 40, 46, 47, 49, 51, 56) or as educational attainment/qualifications (16, 19, 23, 35, 41–44, 48, 52, 55). Childhood SES was measured in several ways but typically focused on parental occupation or social class or on family financial circumstances (16, 21, 43, 53). Two articles measured prospective SES. In one of these studies, poverty (below or above a determined income threshold) was measured 7 years before telomere length ascertainment (38); in the other (a United Kingdom birth cohort), occupational social class was measured 25 years before telomere length (21). Measures of SES over time were used in 1 article (21), including measures of occupation-based social class mobility and accumulated social class. Ten articles used more than 1 SES measure (16, 18, 21, 22, 36, 43, 47, 51–53), and 21 articles included only 1 SES measure (18, 20, 23, 34, 35, 37–42, 44–46, 48, 50, 55–57).
Article quality
Twelve articles were judged to be of higher quality (16, 18, 20–22, 36, 39, 41, 43, 45, 53), with 3 scoring maximum points (18, 21, 22). Eleven articles were rated as being of intermediate quality (23, 35, 37, 42, 44, 46–48, 50, 51, 54), and 8 were rated as lower quality (19, 34, 38, 40, 49, 55–57). The article by Zheng et al. (34) contained 2 study populations that were scored separately (both rated as lower). See Table 2 and Web Table 1 for full scoring for each article.
Meta-analysis and narrative review
Contemporaneous SES
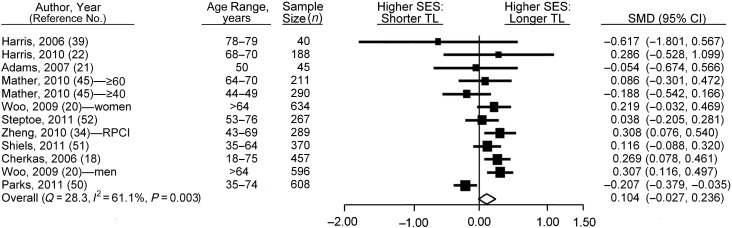
Of the 31 articles reviewed that met the criteria for inclusion in the systematic review, 17 articles examined the association between current SES and telomere length (16, 18–22, 34, 36, 39, 43, 45, 47, 50–54). Of these, it was possible to use data from only 10 in the meta-analysis because of a lack of comparable summary statistics in the remaining 7. Seven of the 10 authors provided additional data that were not available from the articles and therefore were not included in Web Table 1. The results of Woo et al. (20) were stratified by sex (combined analysis not available) and were included separately in the meta-analysis. The results of Mather et al. (45) were stratified by cohort and included separately. Therefore, 12 study populations were included in the meta-analysis (i.e., 12 individual effect sizes for higher SES vs. lower SES).
The random-effects meta-analysis, based on comparing high and low SES categories, found no association between telomere length and SES (SMD = 0.104, 95% confidence interval (CI): −0.027, 0.236; P = 0.119 (Figure 2)). There was also significant heterogeneity between the studies identified at the 95% level (Q = 28.313, I2 = 61.148, P = 0.003). Meta-regression on the quality score revealed no alteration in study heterogeneity (β = 0.052, 95% CI: −0.227, 0.330; P = 0.716).
Figure 2.
Results from random-effects meta-analysis for the standardized mean difference (SMD) (i.e., effect size) between low and high contemporaneous socioeconomic status (SES) categories in the relation of SES with telomere length (TL), ranked by weights applied in the analysis. Squares, SMDs for individual studies; diamond, overall SMD. Bars, 95% confidence interval (CI). (RPCI, Roswell Park Cancer Institute).
Application of a fixed-effects model produced minimal attenuation of the SES (high/low)-telomere length effect size, although this resulted in reduced error and a stronger association between higher SES and longer telomeres (SMD = 0.111, 95% CI: 0.037, 0.185; P = 0.003). Given the heterogeneity, these results need to be taken with caution. Rerunning the analysis after removing those studies that used multiple adjustments over and above age, sex, and assay plate (18, 52) (leaving 11 study populations included in the analysis) weakened the (random-effects) association further (SMD = 0.086, 95% CI: −0.075, 0.247; P = 0.298). All studies adjusted for age and sex. Removing studies rated as being of lower or intermediate quality (34, 50) significantly strengthened the association (SMD = 0.153, 95% CI: 0.052, 0.255; P = 0.003). Systematic removal of individual studies did little to alter the effect for the random model (see Web Figure 1 for full results). However, removal of the study by Parks et al. (50) resulted in significant strengthening of the SES-telomere association because this was the only included study which showed that higher SES was associated with shorter telomeres (after removal of the Parks et al. study, SMD = 0.173, 95% CI: 0.079, 0.268; P < 0.001). Removal of Parks et al. also removed the heterogeneity identified between articles (Q = 12.204, I2 = 18.062, P = 0.272). Use of continuous SES measures (RII analysis) showed no evidence for a gradational association between SES and telomere length (SMD = 0.027, 95% CI: −0.015, 0.068; P = 0.209). There was no evidence of a publication bias, with the strength of the SES-telomere length association not being related to the standard error (Kendall's tau (τ) = −0.258, P = 0.244 (Web Figure 2)).
Some articles had second or alternative measures of contemporary SES that were not included in the meta-analysis. For example, 6 articles had data on income (18, 21, 34, 36, 47, 51, 52, 54), although no associations were found between these measures and telomere length. Of the articles that could not be included in the meta-analysis, 2 examined the association between employment and telomere length. Batty et al. (16) found that employed persons had longer telomeres than those who classified themselves as unemployed. However, retired men or men unable to work because of ill health did not have shorter telomeres than men who were still employed. In contrast, Kananen et al. (43) found that employment status (unemployed vs. employed) was not associated with telomere length.
Education
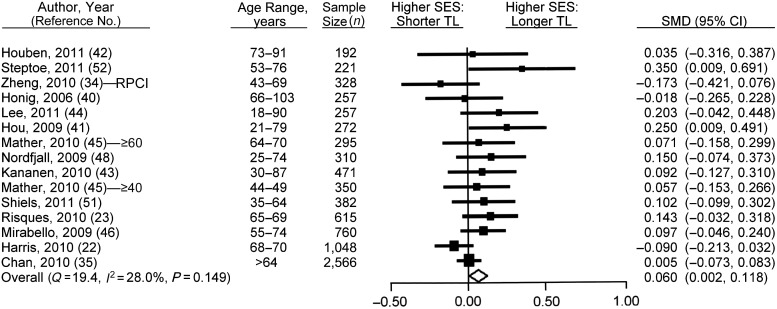
Twenty articles contained results on the association between education and telomere length (16, 18, 22, 23, 34–37, 40–49, 51–53, 55, 56). Of these, data were extracted successfully from 14 articles for use in the meta-analysis (again, exclusion was due to lack of sufficient summary statistics to make valid comparisons). Authors of 12 of the included articles provided additional data not available from the articles and therefore not included in Web Table 1. Within these articles, Mather et al. (45) also provided education data that were not reported in their article. As before, the results of Mather et al. were provided individually for 2 different cohorts and were included separately in the meta-analysis, giving us a total of 15 study populations.
The random-effects meta-analysis of high versus low education categories showed an association between higher SES groups and longer telomeres (SMD = 0.060, 95% CI: 0.002, 0.118; P = 0.042 (Figure 3)), with no heterogeneity identified (Q = 19.446, I2 = 28.006, P = 0.149). Meta-regression on the quality score revealed no alteration in study heterogeneity (β = 0.088, 95% CI: −0.060, 0.235; P = 0.245). Subgroup analysis of SES measurement type (years vs. attainment) also did not identify any significant subgroup heterogeneity.
Figure 3.
Results from random-effects meta-analysis for the standardized mean difference (SMD) (i.e., effect size) between low and high education categories in the relation of socioeconomic status (SES) with telomere length (TL), ranked by weights applied in the analysis. Squares, SMDs for individual studies; diamond, overall SMD. Bars, 95% confidence interval (CI). (RPCI, Roswell Park Cancer Institute).
The SES-telomere association was weakened by application of a fixed-effects model (SMD = 0.044, 95% CI: −0.001, 0.089; P = 0.053). Rerunning the analysis after exclusion of studies with multiple adjustments (52) weakened the random-effects association further (SMD = 0.049, 95% CI: −0.006, 0.104; P = 0.078). Removal of Risques et al. (23) for not adjusting for age or sex had a small attenuation effect (SMD = 0.054, 95% CI: −0.007, 0.114; P = 0.085). Removal of lower- and intermediate-quality studies (23, 34, 40, 46) weakened the association (SMD = 0.068, 95% CI: −0.002, 0.138; P = 0.056). Systematic removal of studies had little effect on the association, although the statistical significance both increased and decreased (see Web Figure 3 for full details). Use of continuous SES measures (RII analysis) revealed stronger evidence for an association between education and telomere length (SMD = 0.043, 95% CI: 0.011, 0.074; P = 0.008). There was no evidence of a publication bias, with the strength of the SES-telomere association not being strongly related to the standard error (Kendall's tau (τ) = 0.114, P = 0.553 (Web Figure 4)).
Education also was measured in 7 articles that were not included in the meta-analysis. Diez Roux et al. (36) found that a longer duration of education was actually associated with shorter telomeres, whereas Yaffe et al. (55) found that higher educational attainment was associated with longer telomere length. Epel et al. (37) were not able to replicate these findings, finding that education (based on years) was not associated with telomere length. Four other studies also observed no association between education and telomere length (16, 49, 51, 56).
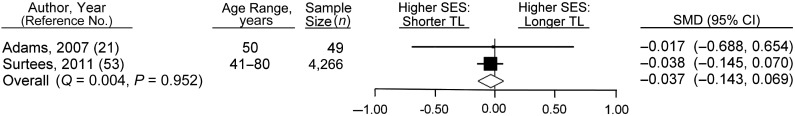
Childhood SES
Of the 31 articles reviewed, 4 contained results on the association between childhood SES measures and telomere length (16, 21, 43, 53). Of these, it was possible to include only 2 in the meta-analysis (because of a lack of suitable summary statistics) (21, 53). With such a small number of studies, the results must be treated with caution. The random-effects meta-analysis of high versus low childhood SES found there to be no significant difference in telomere lengths (SMD = −0.037, 95% CI: −0.143, 0.069; P = 0.491 (Figure 4)). There was no evidence of heterogeneity (Q = 0.004, P = 0.952).
Figure 4.
Results from random-effects meta-analysis for the standardized mean difference (SMD) (i.e., effect size) between low and high childhood socioeconomic status (SES) categories in the relation of SES with telomere length (TL), ranked by weights applied in the analysis. Squares, SMDs for individual studies; diamond, overall SMD. Bars, 95% confidence interval (CI).
Application of a fixed-effects model resulted in no change in association. Because of the low number of studies included, conducting sensitivity analyses (by rerunning the analyses with multiply adjusted studies removed, missing sex or age adjustments removed, lower- and intermediate-rated studies removed, or systematic removal) was not possible. Use of continuous measures of SES (RII analysis) had only a minimal impact on the association between SES and telomere length (SMD = −0.011, 95% CI: −0.041, 0.018; P = 0.440). Testing for publication bias was not possible because of the low number of studies.
Two articles with results on childhood SES measures were identified but not included in the meta-analysis. Childhood social position (with height as a proxy) was tested by Batty et al. (16) for its association with telomere length in middle-aged Scottish men at risk of heart disease, although no association was found. Kananen et al.'s (43) analysis of Finnish men and women in a case-control study of anxiety found that parental unemployment was linked with shorter telomere length but childhood financial difficulties were not.
SES over time
Only Adams et al. (21) considered SES over time, using respondents from the Newcastle Thousand Families Study. They found that when social class was assessed over time in the form of accumulated SES or as a mobility measure (birth vs. age 25 years, age 25 years vs. age 50 years, and birth vs. age 50 years), there were no associations between social class and telomere length.
Prospective SES
Geronimus et al. (38) measured poverty (income below a certain threshold) 7 years before telomere length, but no association was detected. As described above, Adams et al. (21) measured social class at birth and age 25 years but found no association with telomere length measured at age 50 years.
Age effects
t was not possible to easily group articles/studies by age in the meta-analyses to carry out subsample comparisons. For age, when only studies with narrow age cohorts were considered, visual inspection of each article's findings showed that there was no pattern in terms of effect direction or size between SES and telomere length in a comparison of middle-aged adults (ages 40–59 years) (16, 21, 38, 45), older adults (ages 60–69 years) (20, 35, 45), and the elderly (ages ≥70 years) (22, 37, 39, 42, 55). Populations with exclusively younger adults (ages <40 years) were not available.
Sex effects
As with age, quantitative comparisons between the sexes were not possible. Visual inspection of the data in studies with stratified analysis (20, 35) or only 1 sex included revealed no difference in the associations between telomere length and SES according to sex, with a mix of null, positive (higher SES–longer telomeres), and negative results in both male-only (16, 42, 46) and female-only (18, 34, 38, 50, 53, 56) samples.
DISCUSSION
To the best of our knowledge, this is the first systematic review and meta-analysis that has explored socioeconomic inequalities in telomere length. The review identified 31 journal articles containing analyses of the association between SES and telomere length in 29 studies, with a marked lack of consistent evidence. The meta-analyses confirmed this variability in results, in that high (compared with low) education was weakly associated with longer telomeres, whereas childhood SES and SES measured contemporaneously with telomere length were not.
Education has been hypothesized to be a potentially better marker for identifying associations with telomere length than adult SES. Steptoe et al. stated, “Education is an indicator of socioeconomic position at the onset of adult life that sets an individual's socioeconomic trajectory for the future. Effects of SES on telomeres may take many years to accumulate, so education may provide a more robust indicator of SES through early adult life and middle age than measures taken at the time of the study” (52, p. 1296). Our results do provide some evidence to support this, although the strength of the association is by no means certain. Removal of studies rated as lower quality, removal of studies with multiple adjustments, and application of a fixed-effects model (reducing the weighting for smaller studies) weakened the association. In addition, the analysis was sensitive to removal of individual studies, resulting in detection of both significant and nonsignificant associations between higher educational levels and longer telomeres.
More generally, it has been suggested that the potentially strongest driver of telomere length decline is long-term exposure to detrimental environments (15, 52). This would suggest that accumulated measures of SES would be most associated with telomere length or that the association would be most notable in persons with the longest exposure, namely the oldest persons. Unfortunately, there was only 1 study that included measures of SES over time (21), and no significant associations were identified. Across the literature, a wide range of ages were included in the studies, including narrow cohorts and 80-year age ranges. Given this, it was not possible to perform meta-regression or subsample analysis to identify whether the association varied with age. However, visual inspection of the data failed to identify any pattern of associations linked to the age structure of the study populations.
Quality of the review
There are several reasons why the accumulated evidence to date could have failed to find a consistent association between SES and telomere length. First, our review might not have captured all of the relevant articles. Second, the nature of the underlying studies and their measurement of the two factors of interest could have been problematic. Third, telomere length might not be an adequate marker of biological aging. Fourth, biological aging might not be a pathway between SES and health.
This was the first review and meta-analysis of the association between SES and telomere length. Despite this being a small and relatively new field, the systematic review identified several articles not previously cited in empirical articles examining telomere length differences according to SES. Different SES measures were included in the same analysis (social class, income, and employment status), which allowed each of the meta-analyses to be maximized in terms of size. A wide range of sensitivity analyses were conducted to investigate the effect of different aspects of study heterogeneity on the findings. These analyses both weakened and strengthened the results.
The results of any meta-analysis are subject to publication bias through the overrepresentation of positive, statistically significant results and availability. However, the systematic review suggests that positive results (higher SES–longer telomeres) are not the dominant finding. After publication of Cherkas et al.'s (18) evidence that higher social class was associated with longer telomeres, many researchers attempted to replicate those findings, but a mix of positive, null, and negative results were identified (16, 20, 21, 50–52). Perhaps reflecting this, the funnel plots and rank correlation results indicated that publication bias was not an issue in the contemporaneous SES and education meta-analyses.
Another form of publication bias is missing articles. However, a wide search was conducted, with the use of 3 bibliographic databases, as well as citation searches, reference list searches, and contact with experts. Although these searches focused on research from English-language journals and from developed countries, the cost and technical requirements for conducting telomere length analyses mean that the majority of studies will be from the developed world. In addition, there is the issue of the “file-drawer effect” (59), where null results are published less readily than statistically significant associations. However, given the number of studies identified that found null associations, this is unlikely to have been a major factor.
Quality of the underlying studies
The underlying studies included a wide range of study types, sample sizes, and populations, as well as concerns with the measurement of both SES and telomere length. For example, current techniques for measuring telomere length might not be sensitive enough to detect the small differences that could exist between persons of lower and higher SES (7, 9, 60, 61). Different techniques were used to measure telomere length, and there is some evidence of differences between measurement techniques. Increased variation in quantitative polymerase chain reaction techniques over Southern blot has been identified, which could reduce the chances of detecting small differences in telomere length (62, 63). Quantitative polymerase chain reaction was used in all but one of the studies included in the meta-analyses, so this could have been a factor. In all of these studies, telomere length was measured in mature leukocytes that circulate in the blood. However, these leukocytes are made up of a diverse mix of cell types of different ages, which could result in a range of telomere lengths (63, 64). Even within an individual cell type, there can be variability in the lengths of telomeres (61, 64), meaning that the average telomere length used in these studies might not have accurately represented the extent of telomere shortening. More importantly, we do not know whether telomere length at birth varies systematically between SES groups or whether the rate of decline could be a better indicator of accelerated biological aging than a measure taken at one point in time. Two studies have analyzed the change in telomere length over time against SES (37, 65). Both studies found no link between lower SES and greater decline in telomere length, although both were limited by a short time span between measurement events (2.5 years and 5 years, respectively).
The methods and time points for measuring SES varied greatly between and within studies. Given the variety of SES indicators available, and indeed used, in social and health research, this was not unexpected in the telomere length literature. However, a priori, it would be expected that accumulated disadvantage would result in greater reductions in telomere length through longer-term exposure to more detrimental and damaging environments. Taking all of these factors into account suggests that prospective studies that measure both SES and telomere length over relatively long periods of the life course and examine the associations at different life stages are theoretically more robust in their attempts to assess whether an association between SES and telomere length does exist.
Is telomere length an appropriate measure of biological aging?
The evidence from this systematic review and meta-analysis suggests that the evidence for an association between SES and biological aging is weak when telomere length is used as a marker of biological aging. There is an ongoing debate in the literature about the effectiveness of using telomere length as a marker of biological aging, and results are still equivocal despite telomere length being the strongest currently available candidate as a biomarker of aging (7, 9, 61, 64). We must note, though, that directly assessing the effectiveness of telomere length as a marker of biological aging was not the focus of the present study. In terms of finding a more suitable biomarker of aging in the future, it could be that a single biomarker alone is not a sufficient tool with which to accurately assess a person's biological age. Perhaps cumulative measures of several physiologic systems (such as allostatic load) are required to gain a better understanding of the concept of biological aging. However, we also need a better understanding of the fundamental mechanisms of health, disease, and the aging process, be they general pathways (biological aging) or specific pathogenic pathways (e.g., the etiology of cardiovascular disease) (15).
Conclusions
There are strong a priori reasons to expect that telomere length would be a good marker of biological aging and that this would be strongly socially patterned. However, this expectation is not borne out by the evidence synthesized here. Meta-analyses of high SES versus low SES (measured contemporaneously with telomere length, in childhood, and as education) have confirmed that there is mixed evidence for an association between higher SES and longer telomeres, dependent on how SES is measured. More studies focusing on the question of whether SES is associated with telomere length are needed, especially large, representative longitudinal studies. However, justification of these time-consuming and expensive studies could be difficult with such inconclusive results (15).
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: MRC/CSO Social and Public Health Sciences Unit, Glasgow, United Kingdom (Tony Robertson, Geoff Der, Candida Fenton, Michaela Benzeval); Institute of Cancer Sciences, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, United Kingdom (Paul G. Shiels); and Department of Epidemiology and Public Health, School of Life and Medical Sciences, University College London, London, United Kingdom (G. David Batty).
The work of Dr. Tony Robertson, Geoff Der, Candida Fenton, and Dr. Michaela Benzeval was funded by the Medical Research Council. Dr. G. David Batty is a Wellcome Trust Fellow.
We thank all of the authors who provided additional information and data for use in the study.
The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Dow WH, Rehkopf DH. Socioeconomic gradients in health in international and historical context. Ann N Y Acad Sci. 2010;1186(1):24–36. doi: 10.1111/j.1749-6632.2009.05384.x. [DOI] [PubMed] [Google Scholar]
- 2.Mackenbach JP, Bakker MJ. Tackling socioeconomic inequalities in health: analysis of European experiences. Lancet. 2003;362(9393):1409–1414. doi: 10.1016/S0140-6736(03)14639-9. [DOI] [PubMed] [Google Scholar]
- 3.Marmot MG. Fair Society, Healthy Lives. The Marmot Review. London, United Kingdom: University College London; 2010. (http://www.stituteofhealthequity.org/projects/fair-society-healthy-lives-the-marmot-review. ). (Accessed November 5, 2011) [Google Scholar]
- 4.Thomas B, Dorling D, Smith GD. Inequalities in premature mortality in Britain: observational study from 1921 to 2007. BMJ. 2010;341:c3639. doi: 10.1136/bmj.c3639. doi:10.1136/bmj.c3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frieden TR. Forward: CDC health disparities and inequalities report—United States, 2011. MMWR Surveill Summ. 2011;60(suppl):1–2. [PubMed] [Google Scholar]
- 6.Singh GK, Siahpush M. Widening socioeconomic inequalities in US life expectancy, 1980–2000. Int J Epidemiol. 2006;35(4):969–979. doi: 10.1093/ije/dyl083. [DOI] [PubMed] [Google Scholar]
- 7.von Zglinicki T, Martin-Ruiz CM. Telomeres as biomarkers for ageing and age-related diseases. Curr Mol Med. 2005;5(2):197–203. doi: 10.2174/1566524053586545. [DOI] [PubMed] [Google Scholar]
- 8.Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol. 1973;41(1):181–190. doi: 10.1016/0022-5193(73)90198-7. [DOI] [PubMed] [Google Scholar]
- 9.Shiels PG. Improving precision in investigating aging: why telomeres can cause problems. J Gerontol A Biol Sci Med Sci. 2010;65(8):789–791. doi: 10.1093/gerona/glq095. [DOI] [PubMed] [Google Scholar]
- 10.Blasco MA. Telomeres and human disease: ageing, cancer and beyond. Nat Rev Genet. 2005;6(8):611–622. doi: 10.1038/nrg1656. [DOI] [PubMed] [Google Scholar]
- 11.Carrero J, Stenvinkel P, Fellstrom B, et al. Telomere attrition is associated with inflammation, low fetuin-A levels and high mortality in prevalent haemodialysis patients. J Intern Med. 2008;263(3):302–312. doi: 10.1111/j.1365-2796.2007.01890.x. [DOI] [PubMed] [Google Scholar]
- 12.Maxwell F, McGlynn LM, Muir HC, et al. Telomere attrition and decreased fetuin-A levels indicate accelerated biological aging and are implicated in the pathogenesis of colorectal cancer. Clin Cancer Res. 2011;17(17):5573–5581. doi: 10.1158/1078-0432.CCR-10-3271. [DOI] [PubMed] [Google Scholar]
- 13.von Zglinicki T, Serra V, Lorenz M, et al. Short telomeres in patients with vascular dementia: an indicator of low antioxidative capacity and a possible risk factor? Lab Invest. 2000;80(11):1739–1747. doi: 10.1038/labinvest.3780184. [DOI] [PubMed] [Google Scholar]
- 14.Cawthon RM, Smith KR, O'Brien E, et al. Association between telomere length in blood and mortality in people aged 60 years or older. Lancet. 2003;361(9355):393–395. doi: 10.1016/S0140-6736(03)12384-7. [DOI] [PubMed] [Google Scholar]
- 15.Adams JM, White M. Biological ageing: a fundamental, biological link between socio-economic status and health? Eur J Public Health. 2004;14(3):331–334. doi: 10.1093/eurpub/14.3.331. [DOI] [PubMed] [Google Scholar]
- 16.Batty GD, Wang Y, Brouilette SW, et al. Socioeconomic status and telomere length: the West of Scotland Coronary Prevention Study. J Epidemiol Community Health. 2009;63(10):839–841. doi: 10.1136/jech.2009.088427. [DOI] [PubMed] [Google Scholar]
- 17.Adler NE, Stewart J. Health disparities across the lifespan: meaning, methods, and mechanisms. Ann N Y Acad Sci. 2010;1186(1):5–23. doi: 10.1111/j.1749-6632.2009.05337.x. [DOI] [PubMed] [Google Scholar]
- 18.Cherkas LF, Aviv A, Valdes AM, et al. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5(5):361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- 19.Cherkas LF, Hunkin JL, Kato BS, et al. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168(2):154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- 20.Woo J, Suen EWC, Leung JCS, et al. Older men with higher self-rated socioeconomic status have shorter telomeres. Age Ageing. 2009;38(5):553–558. doi: 10.1093/ageing/afp098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams J, Martin-Ruiz C, Pearce MS, et al. No association between socio-economic status and white blood cell telomere length. Aging Cell. 2007;6(1):125–128. doi: 10.1111/j.1474-9726.2006.00258.x. [DOI] [PubMed] [Google Scholar]
- 22.Harris SE, Martin-Ruiz C, von Zglinicki T, et al. Telomere length and aging biomarkers in 70-year-olds: the Lothian Birth Cohort 1936. Neurobiol Aging. 2012;33(7):1486.e3–1486.e8. doi: 10.1016/j.neurobiolaging.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 23.Risques RA, Arbeev KG, Yashin AI, et al. Leukocyte telomere length is associated with disability in older US population. J Am Geriatr Soc. 2010;58(7):1289–1298. doi: 10.1111/j.1532-5415.2010.02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. ( doi:10.1371/journal.pmed.1000097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogilvie D, Hamilton V, Egan M, et al. Systematic reviews of health effects of social interventions: 1. Finding the evidence: how far should you go? J Epidemiol Community Health. 2005;59(9):804–808. doi: 10.1136/jech.2005.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daly MC, Duncan GJ, McDonough P, et al. Optimal indicators of socioeconomic status for health research. Am J Public Health. 2002;92(7):1151–1157. doi: 10.2105/ajph.92.7.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Galobardes B, Shaw M, Lawlor DA, et al. Indicators of socioeconomic position (part 1) J Epidemiol Community Health. 2006;60(1):7–12. doi: 10.1136/jech.2004.023531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galobardes B, Shaw M, Lawlor DA, et al. Indicators of socioeconomic position (part 2) J Epidemiol Community Health. 2006;60(2):95–101. doi: 10.1136/jech.2004.028092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lorant V, Deliege D, Eaton W, et al. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157(2):98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- 30.Hunter JE, Schmidt FL. Fixed effects vs. random effects meta-analysis models: implications for cumulative research knowledge. Int J Select Assess. 2000;8(4):275–292. [Google Scholar]
- 31.Larson A. Analysis of variance with just summary statistics as input. Am Stat. 1992;46(1):151–152. [Google Scholar]
- 32.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 33.Sterne JAC, Becker BJ, Egger M. The funnel plot. In: Rothstein H, Sutton AJ, Borenstein M, editors. Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments. Chichester, United Kingdom: John Wiley & Sons Ltd; 2005. pp. 75–98. [Google Scholar]
- 34.Zheng YL, Ambrosone C, Byrne C, et al. Telomere length in blood cells and breast cancer risk: investigations in two case-control studies. Breast Cancer Res Treat. 2010;120(3):769–775. doi: 10.1007/s10549-009-0440-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan R, Woo J, Suen E, et al. Chinese tea consumption is associated with longer telomere length in elderly Chinese men. Br J Nutr. 2010;103(1):107–113. doi: 10.1017/S0007114509991383. [DOI] [PubMed] [Google Scholar]
- 36.Diez Roux AV, Ranjit N, Jenny NS, et al. Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell. 2009;8(3):251–257. doi: 10.1111/j.1474-9726.2009.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Epel ES, Merkin SS, Cawthon R, et al. The rate of leukocyte telomere shortening predicts mortality from cardiovascular disease in elderly men. Aging (Albany NY) 2009;1(1):81–88. doi: 10.18632/aging.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geronimus AT, Hicken MT, Pearson JA, et al. Do US black women experience stress-related accelerated biological aging? A novel theory and first population-based test of black-white differences in telomere length. Hum Nat. 2010;21(1):19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris SE, Deary IJ, MacIntyre A, et al. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci Lett. 2006;406(3):260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- 40.Honig LS, Schupf N, Lee JH, et al. Shorter telomeres are associated with mortality in those with APOE epsilon 4 and dementia. Ann Neurol. 2006;60(2):181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- 41.Hou LF, Savage SA, Blaser MJ, et al. Telomere length in peripheral leukocyte DNA and gastric cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(11):3103–3109. doi: 10.1158/1055-9965.EPI-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Houben JMJ, Giltay EJ, Rius-Ottenheim N, et al. Telomere length and mortality in elderly men: the Zutphen Elderly Study. J Gerontol A Biol Sci Med Sci. 2011;66(1):38–44. doi: 10.1093/gerona/glq164. [DOI] [PubMed] [Google Scholar]
- 43.Kananen L, Surakka I, Pirkola S, et al. Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One. 2010;5:e10826. doi: 10.1371/journal.pone.0010826. ( doi:10.1371/journal.pone.0010826) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee M, Martin H, Firpo MA, et al. Inverse association between adiposity and telomere length: the Fels Longitudinal Study. Am J Hum Biol. 2011;23(1):100–106. doi: 10.1002/ajhb.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mather KA, Jorm AF, Anstey KJ, et al. Cognitive performance and leukocyte telomere length in two narrow age-range cohorts: a population study. BMC Geriatr. 2010;10:62. doi: 10.1186/1471-2318-10-62. ( doi:10.1186/1471-2318-10-62) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirabello L, Huang WY, Wong JY, et al. The association between leukocyte telomere length and cigarette smoking, dietary and physical variables, and risk of prostate cancer. Aging Cell. 2009;8(4):405–413. doi: 10.1111/j.1474-9726.2009.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nettleton JA, Diez-Roux A, Jenny NS, et al. Dietary patterns, food groups, and telomere length in the Multi-Ethnic Study of Atherosclerosis (MESA) Am J Clin Nutr. 2008;88(5):1405–1412. doi: 10.3945/ajcn.2008.26429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nordfjall K, Eliasson M, Stegmayr B, et al. Increased abdominal obesity, adverse psychosocial factors and shorter telomere length in subjects reporting early ageing; the MONICA Northern Sweden Study. Scand J Public Health. 2008;36(7):744–752. doi: 10.1177/1403494808090634. [DOI] [PubMed] [Google Scholar]
- 49.O'Donovan A, Epel E, Lin J, et al. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol Psychiatry. 2011;70(5):465–471. doi: 10.1016/j.biopsych.2011.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Parks CG, DeRoo LA, Miller DB, et al. Employment and work schedule are related to telomere length in women. Occup Environ Med. 2011;68(8):582–589. doi: 10.1136/oem.2010.063214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shiels PG, McGlynn LM, MacIntyre A, et al. Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS One. 2011;6(7):e22521.. doi: 10.1371/journal.pone.0022521. ( doi:10.1371/journal.pone.0022521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steptoe A, Hamer M, Butcher L, et al. Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behav Immun. 2011;25(7):1292–1298. doi: 10.1016/j.bbi.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 53.Surtees PG, Wainwright NWJ, Pooley KA, et al. Life stress, emotional health, and mean telomere length in the European Prospective Investigation into Cancer (EPIC)-Norfolk Population Study. J Gerontol A Biol Sci Med Sci. 2011;66(11):1152–1162. doi: 10.1093/gerona/glr112. [DOI] [PubMed] [Google Scholar]
- 54.Wolkowitz OM, Mellon SH, Epel ES, et al. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress—preliminary findings. PLoS One. 2011;6(3):e17837. doi: 10.1371/journal.pone.0017837. ( doi:10.1371/journal.pone.0017837) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yaffe K, Lindquist K, Kluse M, et al. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol Aging. 2011;32(11):2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Epel ES, Lin J, Wilhelm FH, et al. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology. 2006;31(3):277–287. doi: 10.1016/j.psyneuen.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Fernandez-Egea E, Bernardo M, Heaphy CM, et al. Telomere length and pulse pressure in newly diagnosed, antipsychotic-naive patients with nonaffective psychosis. Schizophr Bull. 2009;35(2):437–442. doi: 10.1093/schbul/sbn169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Whitehead M, Dahlgren G. Concepts and Principles for Tackling Social Inequities in Health: Levelling Up Part 1. Copenhagen, Denmark: WHO Regional Office for Europe; 2007. (Studies on social and economic determinants of population health, no. 2) [Google Scholar]
- 59.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86(3):638–641. [Google Scholar]
- 60.Chen W, Kimura M, Kim S, et al. Longitudinal versus cross-sectional evaluations of leukocyte telomere length dynamics: age-dependent telomere shortening is the rule. J Gerontol A Biol Sci Med Sci. 2011;66(3):312–319. doi: 10.1093/gerona/glq223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mather KA, Jorm AF, Parslow RA, et al. Is telomere length a biomarker of aging? A review. J Gerontol A Biol Sci Med Sci. 2011;66(2):202–213. doi: 10.1093/gerona/glq180. [DOI] [PubMed] [Google Scholar]
- 62.Aviv A, Hunt SC, Lin J, et al. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 2011;139(20):e134. doi: 10.1093/nar/gkr634. ( doi:10.1093/nar/gkr634) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aviv A, Valdes AM, Spector TD. Human telomere biology: pitfalls of moving from the laboratory to epidemiology. Int J Epidemiol. 2006;35(6):1424–1429. doi: 10.1093/ije/dyl169. [DOI] [PubMed] [Google Scholar]
- 64.Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88(2):557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- 65.Farzaneh-Far R, Lin J, Epel E, et al. Telomere length trajectory and its determinants in persons with coronary artery disease: longitudinal findings from the Heart and Soul Study. PLoS One. 2010;5(1):e8612. doi: 10.1371/journal.pone.0008612. doi:10.1371/journal.pone.0008612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.