Abstract
MTLn3 cells derived from mouse mammary epithelium are known to be highly malignant and are resistant to both radio- and chemo-therapy. We exposed MTLn3 cells to various doses of inorganic Arsenic trioxide (As2O3) in combination with ionizing radiation. Cells were treated with a series of As2O3 concentrations ranging from 20 μM to 1.22 nM for 8 hour, 24 hour and 48 hour periods. Post-treated cell proliferation was quantified by measuring mitochondrial activity and DNA analysis. Cells exposed to radiation and As2O3 at concentration greater than 1.25 μM showed apoptosis and radiations alone treated cells were statistically not different from the control. Hormesis was observed for As2O3 concentrations in the range of 0.078 μM to 0.625 μM while the combined chemo and radiation treatments of the cells did not affect the hormetic effect. We have demonstrated that As2O3 (in the presence and absence of ionizing radiation) in specific low concentrations induced apoptosis in the otherwise chemoresistant cancer cells. This low concentration-mediated cell death is immediately followed by a surge in cell survival. Low dosing dosimetry is highly desirable in metronomic therapy however, it has a narrow window since necrosis, hormesis, apoptosis and other dose-dependent biological processes take place in this region. Further quantifiable dosimetry is highly desired for routine clinical practice.
Keywords: Cancer cells, Arsenic trioxide, Ionizing radiation, Apoptosis, Hormesis
1. INTRODUCTION
Many patients treated for chronic diseases develop drug related injuries. In addition to these side effects, the rising burden of chronic diseases that in most cases transition into malignancies, which is one of the major challenges faced by the health organizations throughout the world. The chronic diseases and malignancies are routinely treated with drugs based on the maximum tolerable dose (MTD) protocol. One of the main issues with MTD is tissue necrosis, which results in the continuous release of inflammatory factors into the body. Inflammatory onslaughts wreak havoc on the living system and over time can cause chronic diseases. The drug induced diseases are becoming more prominent among a broad range of patients. The cancer survival rate in patients is improved due to advancements in therapeutic techniques, but these instances of therapy related survival are eclipsed by the dose related side effects. Drug related side effects in cardiovascular diseases are well documented (Senkus and Jassem 2010). High dose treatment of breast cancer is criticized by Zander (Zander and Kroger 2005) who argued that such treatment is based on the acceptance of several nonrandom studies. Chemotherapy-induced long term effects such as cardiac toxicity, secondary leukemia, neuronal disruption, cognitive problems and sexual dysfunctions are of great concern among physicians as well as patients and some researchers questioned if MTD chemotherapy is an adjuvant therapy (Azim et al. 2011). Results of breast cancers in patients treated with chemo and radio-therapy are significant which is why we need dose optimization to achieve effective treatment while avoiding such severe effects (Cutuli et al. 2011). Physicians are exploring the low dose therapy to offset such side effects. Lower doses of chemotherapy and tightly blocked radiation fields may not only decrease the risk of secondary cancers, but also provide environmental, ecological and economic benefits.
Metronomic therapy has been suggested for older patients to avoid severe dose related side effects (Fontana et al. 2010) but there is no low dose standardization. On the other hand, the so called ‘low dose’ is not low enough to avoid secondary cancers among children (O’Brien et al. 2010) and in specific cases patients treated with low doses of ionizing radiation require re-irradiation (Mendenhall et al. 2008). The simple reason is the lack of any metric which defines “low dose” per se in clinical practice.
There is also a growing trend to use smaller doses of drugs to treat advanced cancers (Rajdev et al. 2011; Satti 2009). This technique, known as low dose metronomic (LDM) therapy, claims equal or superior clinical results while keeping the side effects to minimum levels. Although this methodology is attractive, it is not without its own shortcomings. Some studies have reported that although tolerated with minimum toxicity, side effects such as lymphopenia have been seen in some patients (Lord et al. 2007). Problems with so called LDM have been reported with some suggestions (Emmenegger et al. 2010). Tumors are heterogeneous in nature and consisting of cells with various metastatic potential and treatment with certain drugs may eventually develop resistance in certain population of cells. This is due to the existence of narrow dose regions that create apoptosis and hormesis in cells depending on the dose. Apoptosis and hormesis are two opposing phenomenon adjacent to each other in the dose response index, both with useful applications in medicine.
Although maximum tolerable dose and low dose metronomic both produce cellular resistance (Gonzalez-Angulo et al. 2007; Massaccesi et al. 2010; Thoenes et al. 2010), the mechanism by which the cancer cells become resistant to LDM is entirely different from that to MTD. Studies show that even tumors that acquire LDM resistance are still sensitive to MTD (Emmenegger et al. 2011). A score of biological processes including necrosis, apoptosis, hormesis, autophagy and bystander effect occur in the narrow window of low doses. Some of these mutually exclusive biological processes such as stimulation and inhibition may happen in the overlapping concentrations of certain drugs, making it challenging to quantify the dose for each process. A broad spectrum for drug quantification is required to avoid unwanted biological processes.
In our study, we chose As2O3, a popular chemotherapeutic agent in a variety of cancer treatments. As2O3 has been investigated as a potential sensitizer of cancer stem cells for possible modality to treat Glioblastoma Multiforme (GBM) (Tomuleasa et al. 2010). The methodology exploits the effects of radiation and chemotherapy to treat inoperable GBM. Scientists have reported an increase cure rate when As2O3 and radiation therapy were used concurrently (Ning and Knox 2004). Enhanced effects of As2O3 such as therapeutic efficiency with radiation have also been reported in oral squamous carcinoma (Kumar et al. 2008). Other researchers reported an increased killing rate of human fibrosarcoma cells with As2O3 in both in vitro and in vivo settings (Chiu et al. 2010). One advantage of As2O3 is its capability to penetrate into the subcellular compartments of living cells (Bacquart et al. 2010).
In our research, a wide dilution range of As2O3 was tested on highly malignant breast cancer cells (Goswami et al. 2004; Raja et al. 2010; Raof et al. 2011; Xue et al. 2006) (MTLn3). The cells were treated with lower concentrations of As2O3 in combination with ionizing radiation. The inhibition in cell growth and stimulation in cell proliferation was observed in various dose ranges of As2O3. Quantification of the drug amount to produce different dose responses will open new opportunities in clinical practice. The conventional clinical practice is heavily based on the MTD model. The MTD methodology later induces severe side effects due to necrotic processes. That is why clinicians are now exploring alternative methods to MTD.
2. MATERIALS AND METHODS
2.1. Cell line and culture
MTLn3 rat mammary adenocarcinoma cells (from Dr. John Condeelis) were cultured in alpha minimum essential medium (αMEM) medium (Invitrogen Corporation, Carlsbad, CA). The medium was supplemented with 5% fetal bovine serum (FBS) (Gemini Bio-Products, West Sacramento, CA), 0.2% Sodium Bicarbonate (Sigma-Aldrich, St. Louis, MO), and penicillin / streptomycin (Gibco / Invitrogen Corporation Carlsbad, CA). The cells were kept at 37°C under humid conditions with 5% carbon dioxide. In all experiments, the cells were used between passages 5 and 12.
2.2. Arsenic trioxide preparation and dilutions
As2O3 99.0% pure, powder (Sigma-Aldrich, St. Louis, MO) was dissolved in boiling deionized water (diH2O) for 40 minutes. The solution was then cooled to room temperature and filtered with a 0.22 μm filter. The starting weight of As2O3 was subtracted from the un-dissolved As2O3 and final volume of diH2O was measured to determine the bulk concentration of As2O3 in water (i.e. 50mM). As2O3 solution was then diluted in cell culture media by the serial dilution technique. The concentrations of As2O3 used on MTLn3 cells are discussed in the cell treatment section of materials and methods.
2.3. Ionizing radiation and experimental setup
MTLn3 cells were irradiated with various doses of ionizing radiation using a 6 Mega electron Volt (MeV) beam. The cells were cultured in 96-well plates and placed at dmax (depth of maximum dose) and the dose was delivered with the following settings; cone size 6x10 cm2, Source to Surface Distance (SSD) = 100 cm and cell at dmax of 1.13 cm MTLn3 cells treated with various concentrations of As2O3 were exposed to a standard a therapeutic radiation dose of 200 centi Gray (cGy), while untreated cells were exposed to 65 cGy, 120 cGy, 265 cGy and 330 cGy one dose per day for successive days, respectively.
2.4. Cell treatment
MTLn3 Cells were seeded at a density of approximately 2500 cells per well in transparent 96 well plates (Corning, Inc. Corning, NY) overnight and then treated with ionizing radiation and As2O3. The serial dilutions of As2O3 (20 μM to 1.22 nM) were administered as As2O3 alone and As2O3 with ionizing radiation together, and a group treated with ionizing radiation alone. The doses of ionizing radiation (mentioned above) were delivered to specific dishes at 8, 24 and 48 hour time points (one dose per day). Each condition and concentration of As2O3 was replicated 5 times per experiment and repeated 3 times.
2.5. Cell proliferation assay
Cellular responses to various treatments were quantified by a cell viability assay, in which the mitochondrial activity of live cells was measured using premixed WST-1 cell proliferation assay (Clontec Inc. Mountain View, CA) following manufacturer’s protocol. Briefly, tetrazolium salt was reduced to formazan by mitochondria succinate-tetrazolium reductase present in living cells. As cells keep proliferating, the amount of these enzymes would increase, reflective of the number of metabolically active cells in culture. The plates treated under different conditions were incubated with tetrazolium salt for 1 hour and absorption was measured at 440 nm with background absorption at 650 nm using microplate reader (BioTek, Winooski, VT). The data from all the wells were normalized with the control (untreated cells).
2.6. Apoptosis assay
Cellular apoptosis was measured using a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay from R&D system (Minneapolis, MN) to detect the DNA fragments in apoptotic cells. The TUNEL assay used in a 96 well format according to the manufacturer’s protocol. Briefly, cells were washed and fixed in formaldehyde solution then treated with Cytonin™ solution. After the removal of Cytonin™ and wash steps, MTLn3 cells were treated with hydrogen peroxide solution for approximately 5 minutes and then washed in diH2O. Terminal deoxynucleotidyl transferase (TdT) solution was added to each well followed by centrifugation. Then, the supernatant was discarded and labeling reaction mix was added. The reaction was halted with TdT stop buffer followed by two rinse steps and then, the cells were treated with biotin/streptavidin-horseradish peroxidase. Finally, the substrate solution was added in each well after rinsing steps and the reaction kinetics was measured at 630 nm. The reaction was stopped using 0.2 N HCl solution per well and the absorption was measured at 450 nm.
2.7. Cell death assay
MTLn3 cell death was measured by quantifying the presence of lactate dehydrogenase in the medium containing dead cells. The cells were cultured overnight then treated with different conditions as mentioned above. On the day of measurement, the treated cells were centrifuged in 96 well plates and supernatants were carefully transferred to new 96 well plates, and LDH cytotoxicity detection reagents (Clontec Inc. Mountain View, CA) were added to each well per manufacturer’s instructions. Measured values from each well were normalized with the control (untreated cells) and plotted.
3. RESULTS
3.1. Effects of hormesis induced by arsenic trioxide
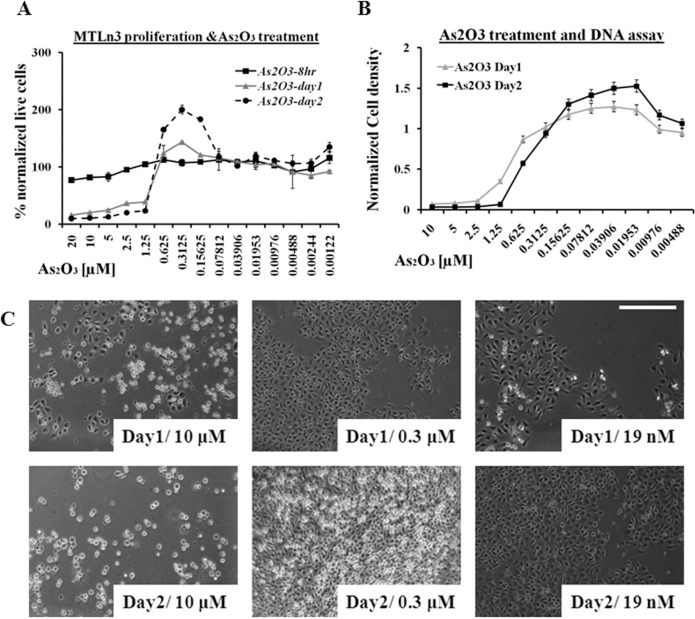
MTLn3 cells have shown resistance to available chemotherapy drugs (Goswami et al. 2004). The over expression of epidermal growth factor (EGF) receptor make them highly sensitive to chemoattractant (i.e. EGF) signals in their surroundings (Xue et al. 2006), which makes them more sensitive to more subtle chemical gradients resulting in high metastatic ability. When these cells were treated with various concentrations of inorganic arsenic trioxide, apoptosis and hormesis effects were observed. In the first 8hrs of arsenic trioxide (from 20 μM to 1.25 μM) treatment, a slight decline in cell proliferation was observed due to the cytotoxic effect of the drug, which was more pronounced in 24 and 48 hour time periods, as shown in Figure 1A. The rapid proliferation of MTLn3 cells or hormesis was observed in the dose range from 0.625 μM to 78.1 nM, which was more obvious at 48 hrs time period as compared to 24 hrs while in early events (4 to 12 hrs), hormesis effect was not that evident. Figure 1A shows that the MTLn3 cells, when treated with low concentration of arsenic trioxide, showed proliferation rates similar to untreated cells. The cell proliferation assay was based on the mitochondrial activities of the cells and a DNA based assay was used as an alternative technique to verify these results (shown in Figure 1B). These results confirmed that arsenic trioxide was not only increasing the proliferation rate of these cells in the hormesis region (1.25μM – 78nM) by increasing the total number of cells (shown in Figure 1B, C) but stimulating the mitochondrial activity as well as shown in Figure 1A. Figure 1C are the representative images collected from these cells at different time points, verifying the increase in number of cells at low concentration of arsenic trioxide.
FIGURE 1.
A) Cancer cell proliferation (mitochondrial activity based assay) treated with various dilution of arsenic trioxide for 2 days. B) DNA bases cell proliferation assay treated with arsenic trioxide dilutions for 2 days. C) Phase contrast images of MTLn3 cancer cells at different time points (24 hr and 48 hr) after treated with arsenic trioxide. (Error bars represent mean data ± standard deviation).
3.2. An extended effect of hormesis induced by a combination of arsenic trioxide and ionizing radiation
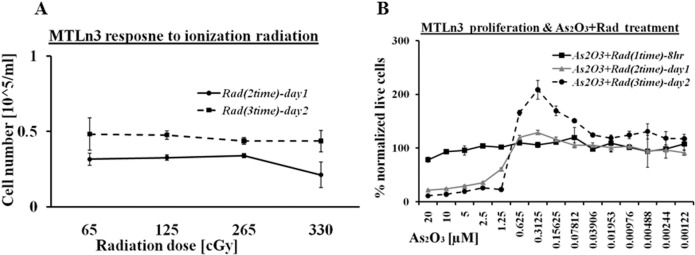
Ionizing radiation (i.e. electron beam with an energy of 6 MeV) is one of the most common radiotherapy techniques used for cancer patients. MTLn3 cells were exposed to various doses of 6 MeV electron beam i.e. from 65 cGy to 330 cGy one dose per day for two days at 24 hour time intervals. The maximum exposure of ionization radiation was performed in two day periods, which showed little or no effect on these cells. The proliferation profile of MTLn3 exposed to ionizing radiation is shown in Figure 2A which indicates the cells are growing normally after exposure to various doses of radiation. These results led us to combine the ionizing radiation with arsenic trioxide treatment. It was expected that the arsenic trioxide would disrupt the DNA repair process caused by the ionization radiation. Figure 2B shows the proliferation activity of MTLn3 cells treated with both arsenic trioxide and ionization radiation (200cGy). Both cellular death and stimulation in growth were observed in these experiments similar to arsenic trioxide treated cells (Figure 1). Cellular growth was arrested in the range from 20 μM to 1.25 μM, while an increase in cellular proliferation was observed in concentration of arsenic trioxide lower than 1.25 μM. The hormesis signal in this treatment was as high as in the cells treated with arsenic trioxide alone. Interestingly on day two the cells treated with low dilution of arsenic trioxide and ionization were proliferating more than normal. It is possible that low chemical treatment with cytotoxic agents stressed the cells and when combined with another stressing element (i.e. ionizing radiation), it inhibited cell death, increased survival and stimulated cell proliferation.
FIGURE 2.
A) MTLn3 cancer cells response to 6 MeV electron beam ionization radiation at 24 hr and 48 hr without arsenic trioxide treatment. B) Proliferation of cancer cell treated both with arsenic trioxide and electron beam ionization radiation. (Error bars represent mean data ± standard deviation).
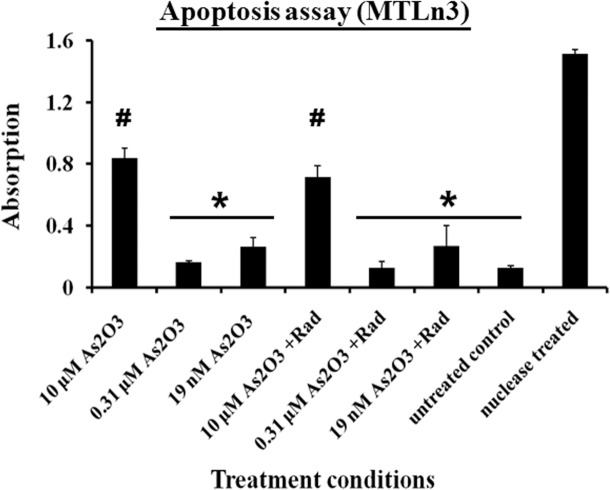
Under conditions of arsenic trioxide treatment or a combination of arsenic trioxide and ionization radiation treatment, an arrest and stimulation in cell proliferation was observed at various levels of drug treatment. These behaviors were verified by both the mitochondrial activities and DNA analysis of the cells. To further quantify the level of apoptosis in these cells, fragments of the DNA were measured using the TUNEL assay. Apoptosis was measured after 48 hr of treatment in both conditions. The cells treated with 10 μM of arsenic trioxide alone and in combination with ionizing radiation showed the highest levels of apoptosis as seen in Figure 3 while nuclease treatment was a positive control. In the hormesis region (0.31 μM) a negligible level of apoptosis was observed in those cells as compared to the untreated cells (Figure 3). The cells treated with low concentration of arsenic trioxide such as 19 nM showed a slight increase in the mean value of the apoptosis signal relative to untreated cells as indicated in Figure 3. These results suggest that the chemical treatment alone has dual effects on MTLn3 cells but the cells treated with combination of ionizing radiation and the chemical showed no significant difference on the cells proliferation behavior relative to chemical treatment alone.
FIGURE 3.
Apoptosis measurements from MTLn3 cancer cells treated under different conditions for 48 hours with negative control (untreated cells) and positive control (nuclease treated cells). * and # belong to different groups, on the bases of 95% confidence interval using ANOVA, (Error bars represent mean data ± standard deviation).
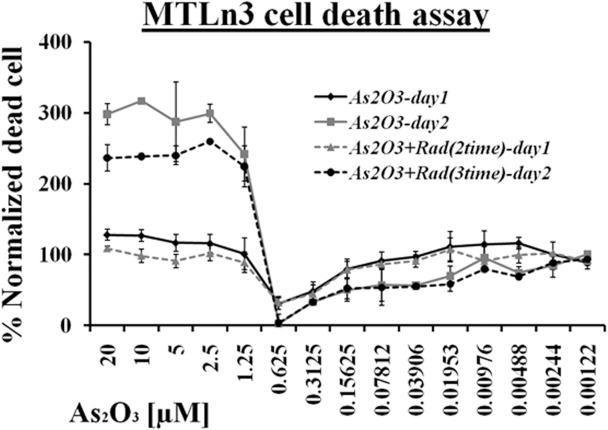
These results were also confirmed by measuring cell death in the whole range of arsenic trioxide treatment with and without ionization radiation. Figure 4 showed that at high concentrations of arsenic trioxide treatment (i.e. 20 μM – 1.25 μM), most of the cells died. However, at low concentrations (below 78 nM), cellular death was observed and the results were consistent with the TUNEL assay. No significant effect was observed between ionization treated and untreated cells at lower concentrations of arsenic trioxide. The minimum level of cell death was recorded in the hormesis region and at 0.31 μM, almost no cell death was recorded as shown in Figure 4.
FIGURE 4.
LDH based cell death measurement response of MTLn3 cancer cells treated with various dilutions of arsenic trioxide alone and in combination with ionizing radiation for 24 hours and 48 hours. (Error bars represent mean data ± standard deviation).
4. DISCUSSION
Conventional dosimetry protocols based on maximum tolerable dose (MTD) are constantly challenged by the fact that surviving patients are developing secondary cancers, among other problems. A certain dose may be stimulatory to immune cells, an effect that is highly undesirable in malignant cells. Likewise, an apoptosis-inducing dose is required to treat unwanted cells, but the same effects on normal cells would be disastrous. Quantification of dosing is therefore necessary to establish the delicate thresholds of separating different competing phenomena in the low dose region. This quantification is needed for different drugs in different cell lines to draw some standards.
One would argue that one size does not fit all as a given dose may induce apoptosis in a robust individual while induce necrosis in a compromised one. However, a dose response will form a fundamental index in clinical dosimetry, and the dose-response character of the cell biology is a strong index to consider in any clinical setting. Since inhibition and stimulation processes can occur in neighboring regions of narrow windows of different concentrations, quantitative measurements are necessary to specify these dosimetric parameters. In the case of As2O3, its dual and opposing effects have been established. These can be due to genetic, epigenetic factors or metabolism of the drug (Cui et al. 2008; Platanias 2009). Hormesis is a phenomenon of stimulatory effects whereas apoptosis is the altruistic suicide or self sacrifice by a cell. Both are useful therapeutic modalities based on their intended biological targets; however, one may be desirable in one patient while at the same time the induction of the other may prove disastrous or even fatal. Hence, there is a need to further quantify these phenomena.
Various authors have investigated the specific dose for hormesis. Hormesis is a useful biological phenomenon. For example, different cardiac chemo-preventative effects of resveratrol have been reported due to hormesis (Juhasz et al. 2010). Hormesis is a universal phenomenon found across all living species. The radiation-induced cellular stress-resistance mechanisms have been found with different genetic background in Drosophila of both sexes (Moskalev et al. 2011). Hence, hormesis is a genetic as well as sex dependent phenomenon. The hormetic effects of individual chemicals as well as of mixtures have been found in different experiments (Belz et al. 2008; Ge et al. 2010). However, the phenomenon of chemical hormesis that exists in the presence of ionizing radiation in the same dose window is reported here for the first time. This shows that hormesis is a universal phenomenon that exists under different sets of conditions or combined onslaughts of different modalities, such as radiation and chemicals together. Radiation-induced hormesis is not inhibited by chemicals or vice versa. It stands out in all forms of stresses.
Scientists are focused on firmly establishing dose standards in therapeutic drugs, among others. Although radiation alone does not produce effects it enhances cell death in the presence of As2O3 in lower concentrations, verifying the presence of hormesis. The same drug at various concentrations can produce different effects. Here, we documented that inhibition and stimulation can be achieved by adjusting the dose ranges. We found that the effects produce by certain concentrations of drugs can exist even if the biological system is simultaneously exposed to an alternative treatment. We particularly observed that chemo and radiation resistant cancer cells that respond to certain chemicals (e.g. As2O3) has conserved responses (hormesis and apoptosis) even when exposed to ionizing radiation. Cellular death that was observed between 20 μM and 2 μM of As2O3 concentration, while a higher concentration (>20 μM) can also produce cell death, but may have other side effects in vivo. The present study is focused to cell response in in vitro setting. However, it is possible that the lower dose can be tested and quantified by the same methodology of Kim (Kim et al. 2008), which assumes the same range of drug (i.e. drug/kg, for example 10 μmol/kg) will produce the same effect (apoptosis) as seen in vitro.
5. CONCLUSIONS
Arsenic trioxide is an emerging chemotherapeutic reagent for solid tumors and is already in use (clinically) for other types of cancers. We evaluate arsenic trioxide on a highly metastatic, multidrug resist breast cancer cell line from solid tumors. Our results showed that arsenic trioxide was able to generate apoptosis in these cells; however, in lower doses treatment with arsenic trioxide causes rapid cell proliferation. The hormesis peak was recorded when cells were challenged with approximately 0.3 μM of arsenic trioxide. This effect disappeared at lower concentrations of arsenic trioxide. Although ionizing radiation alone had no effect on cell proliferation, when combined with the low concentration treatment of arsenic trioxide which alone had no effect, cellular proliferation was stimulated. Cellular death was observed on both sides of the hormesis peak.
Further research is warranted to benchmark the dose amount (dosimetry) for apoptosis and hormesis processes. The apoptotic process is highly desirable in malignant diseases. This technique can be used to eradicate pathogens or viral infected mutated cells with minimal side effects. On the other hand, a dose sufficient to produce hormesis may be suitable to control the autoimmune diseases such as arthritis, allergies, diabetes, lupus, etc. Since these phenomena are complementary, a wide spectrum of diseases can be addressed with such dosimetry methods.
Acknowledgments
The authors would like to acknowledge Dr. Yubing Xie at the College of Nanoscale Science and Engineering for valuable discussions and access to her lab and Dr. John Condeelis at the Albert Einstein College of Medicine for providing the cells.
REFERENCES
- Azim HA, Jr, de Azambuja E, Colozza M, Bines J, Piccart MJ. Long-term toxic effects of adjuvant chemotherapy in breast cancer. Ann Oncol. 2011 doi: 10.1093/annonc/mdq683. [DOI] [PubMed] [Google Scholar]
- Bacquart T, Deves G, Ortega R. Direct speciation analysis of arsenic in sub-cellular compartments using micro-X-ray absorption spectroscopy. Environ Res. 2010;110(5):413–416. doi: 10.1016/j.envres.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Belz RG, Cedergreen N, Sorensen H. Hormesis in mixtures — can it be predicted? Sci Total Environ. 2008;404(1):77–87. doi: 10.1016/j.scitotenv.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Chiu HW, Lin JH, Chen YA, Ho SY, Wang YJ. Combination treatment with arsenic trioxide and irradiation enhances cell-killing effects in human fibrosarcoma cells in vitro and in vivo through induction of both autophagy and apoptosis. Autophagy. 2010;6(3):353–365. doi: 10.4161/auto.6.3.11229. [DOI] [PubMed] [Google Scholar]
- Cui X, Kobayashi Y, Akashi M, Okayasu R. Metabolism and the paradoxical effects of arsenic: carcinogenesis and anticancer. Curr Med Chem. 2008;15(22):2293–2304. doi: 10.2174/092986708785747526. [DOI] [PubMed] [Google Scholar]
- Cutuli B, Kanoun S, Tunon De Lara C, Baron M, Livi L, Levy C, Cohen-Solal-Lenir C, Lesur A, Kerbrat P, Provencio M, Gonzague-Casabianca L, Mege A, Lemanski C, Delva C, Lancrenon S, Velten M. Breast cancer occurred after Hodgkin’s disease: Clinico-pathological features, treatments and outcome: Analysis of 214 cases. Crit Rev Oncol Hematol. 2011 doi: 10.1016/j.critrevonc.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Emmenegger U, Francia G, Chow A, Shaked Y, Kouri A, Man S, Kerbel RS. Tumors that acquire resistance to low-dose metronomic cyclophosphamide retain sensitivity to maximum tolerated dose cyclophosphamide. Neoplasia. 2011;13(1):40–48. doi: 10.1593/neo.101174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmenegger U, Francia G, Shaked Y, Kerbel RS. Metronomic chemotherapy: principles and lessons learned from applications in the treatment of metastatic prostate cancer. Recent Results Cancer Res. 2010;180:165–183. doi: 10.1007/978-3-540-78281-0_10. [DOI] [PubMed] [Google Scholar]
- Fontana A, Falcone A, Derosa L, Di Desidero T, Danesi R, Bocci G. Metronomic chemotherapy for metastatic prostate cancer: a ‘young’ concept for old patients? Drugs Aging. 2010;27(9):689–696. doi: 10.2165/11537480-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Ge HL, Liu SS, Zhu XW, Liu HL, Wang LJ. Predicting Hormetic Effects of Ionic Liquid Mixtures on Luciferase Activity Using the Concentration Addition Model. Environ Sci Technol. 2010 doi: 10.1021/es1018948. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Angulo AM, Morales-Vasquez F, Hortobagyi GN. Overview of resistance to systemic therapy in patients with breast cancer. Adv Exp Med Biol. 2007;608:1–22. doi: 10.1007/978-0-387-74039-3_1. [DOI] [PubMed] [Google Scholar]
- Goswami S, Wang W, Wyckoff JB, Condeelis JS. Breast cancer cells isolated by chemotaxis from primary tumors show increased survival and resistance to chemotherapy. Cancer Res. 2004;64(21):7664–7667. doi: 10.1158/0008-5472.CAN-04-2027. [DOI] [PubMed] [Google Scholar]
- Juhasz B, Mukherjee S, Das DK. Hormetic response of resveratrol against cardioprotection. Exp Clin Cardiol. 2010;15(4):e134–138. [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, Chung HY, Mattson MP, Lee J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;283(21):14497–14505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Gao Q, Ning Y, Wang Z, Krebsbach PH, Polverini PJ. Arsenic trioxide enhances the therapeutic efficacy of radiation treatment of oral squamous carcinoma while protecting bone. Mol Cancer Ther. 2008;7(7):2060–2069. doi: 10.1158/1535-7163.MCT-08-0287. [DOI] [PubMed] [Google Scholar]
- Lord R, Nair S, Schache A, Spicer J, Somaihah N, Khoo V, Pandha H. Low dose metronomic oral cyclophosphamide for hormone resistant prostate cancer: a phase II study. J Urol. 2007;177(6):2136–2140. doi: 10.1016/j.juro.2007.01.143. discussion 2140. [DOI] [PubMed] [Google Scholar]
- Massaccesi M, Forni F, Spagnuolo P, Macchia G, Mignogna S, De Ninno M, Cellini N, Giardina B, Carbone A, Morganti AG. Multiple tumor marker elevation in androgen ablation-refractory prostate cancer with long-term response to metronomic chemotherapy: a case report. Int J Biol Markers. 2010;25(4):243–247. [PubMed] [Google Scholar]
- Mendenhall WM, Mendenhall CM, Malyapa RS, Palta JR, Mendenhall NP. Re-irradiation of head and neck carcinoma. Am J Clin Oncol. 2008;31(4):393–398. doi: 10.1097/COC.0b013e3181637398. [DOI] [PubMed] [Google Scholar]
- Moskalev AA, Plyusnina EN, Shaposhnikov MV. Radiation hormesis and radioadaptive response in Drosophila melanogaster flies with different genetic backgrounds: the role of cellular stress-resistance mechanisms. Biogerontology. 2011 doi: 10.1007/s10522-011-9320-0. [DOI] [PubMed] [Google Scholar]
- Ning S, Knox SJ. Increased cure rate of glioblastoma using concurrent therapy with radiotherapy and arsenic trioxide. Int J Radiat Oncol Biol Phys. 2004;60(1):197–203. doi: 10.1016/j.ijrobp.2004.02.013. [DOI] [PubMed] [Google Scholar]
- O’Brien MM, Donaldson SS, Balise RR, Whittemore AS, Link MP. Second malignant neoplasms in survivors of pediatric Hodgkin’s lymphoma treated with low-dose radiation and chemotherapy. J Clin Oncol. 2010;28(7):1232–1239. doi: 10.1200/JCO.2009.24.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platanias LC. Biological responses to arsenic compounds. J Biol Chem. 2009;284(28):18583–18587. doi: 10.1074/jbc.R900003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raja WK, Gligorijevic B, Wyckoff J, Condeelis JS, Castracane J. A new chemotaxis device for cell migration studies. Integr Biol (Camb) 2010;2(11–12):696–706. doi: 10.1039/c0ib00044b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajdev L, Negassa A, Dai Q, Goldberg G, Miller K, Sparano JA. Phase I trial of metronomic oral vinorelbine in patients with advanced cancer. Cancer Chemother Pharmacol. 2011 doi: 10.1007/s00280-011-1580-5. [DOI] [PubMed] [Google Scholar]
- Raof NA, Raja WK, Castracane J, Xie Y. Bioengineering embryonic stem cell microenvironments for exploring inhibitory effects on metastatic breast cancer cells. Biomaterials. 2011;32(17):4130–4139. doi: 10.1016/j.biomaterials.2011.02.035. [DOI] [PubMed] [Google Scholar]
- Satti J. The emerging low-dose therapy for advanced cancers. Dose Response. 2009;7(3):208–220. doi: 10.2203/dose-response.08-010.Satti. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkus E, Jassem J. Cardiovascular effects of systemic cancer treatment. Cancer Treat Rev. 2010;37(4):300–311. doi: 10.1016/j.ctrv.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Thoenes L, Hoehn M, Kashirin R, Ogris M, Arnold GJ, Wagner E, Guenther M. In vivo chemoresistance of prostate cancer in metronomic cyclophosphamide therapy. J Proteomics. 2010;73(7):1342–1354. doi: 10.1016/j.jprot.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Tomuleasa C, Soritau O, Kacso G, Fischer-Fodor E, Cocis A, Ioani H, Timis T, Petrescu M, Cernea D, Virag P, Irimie A, Florian IS. Arsenic trioxide sensitizes cancer stem cells to chemoradiotherapy. A new approach in the treatment of inoperable glioblastoma multiforme. J BUON. 2010;15(4):758–762. [PubMed] [Google Scholar]
- Xue C, Wyckoff J, Liang F, Sidani M, Violini S, Tsai KL, Zhang ZY, Sahai E, Condeelis J, Segall JE. Epidermal growth factor receptor overexpression results in increased tumor cell motility in vivo coordinately with enhanced intravasation and metastasis. Cancer Res. 2006;66(1):192–197. doi: 10.1158/0008-5472.CAN-05-1242. [DOI] [PubMed] [Google Scholar]
- Zander AR, Kroger N. High-dose therapy for breast cancer - a case of suspended animation. Acta Haematol. 2005;114(4):248–254. doi: 10.1159/000088585. [DOI] [PubMed] [Google Scholar]