Abstract
Acrylonitrile(AN) is a neurotoxin both in animals and humans, but its effects on acetylcholinesterase (AChE) activity remain controversial. This study aimed to determine the dose-response effects of AN on AChE activity and the modulatory role of ethanol pre-treatment. A total of 144 Kunming mice were randomly divided into 18 groups: nine groups received 5% ethanol in their drinking water, and the remaining nine groups received regular tap water. One week later, both the ethanol and tap water only groups were given an intraperitoneal injection of AN at the following doses: 0 (control), 0.156, 0.3125, 0.625, 1.25, 2.5, 5, 10 or 20 mg AN/kg body weight. AChE activity was determined on whole blood and brain 24 h later. Blood AChE activity was higher in AN-injected mice than in controls at all doses. AChE activity in blood increased in a dose-dependent manner, peaking at 0.156 mg/kg, after which a gradual decrease ensued, displaying a β-typed dose-response relationship. In contrast, brain AChE activity, following a single AN injection, was consistently lower than in control mice, and continued to fall up to a dose of 0.313 mg/kg, and thereafter increased gradually with higher doses. Mice receiving a 20 mg/kg dose of AN exhibited AChE brain activity indistinguishable from that of control mice, demonstrating a typical U-typed dose-response relationship. The activity of AChE in the blood and brain of the AN + ethanol-treated groups displayed a shift to the right, and the magnitude of the decrease in AChE activity induced by AN was attenuated relative to the AN-only group. These results suggest that AN affects AChE activity in both mouse blood and brain in a hormetic manner. Pretreatment with ethanol modifies the effect of AN on AChE, indicating that parent AN has a more prominent role than its metabolites in modulating enzyme activity.
Keywords: acrylonitrile, acetylcholinesterase, biphasic effects, hormesis
INTRODUCTION
Acrylonitrile (AN) is a volatile, toxic liquid monomer that is widely used in the manufacture of synthetic rubber, styrene plastics, acrylic fiber, and adhesives. Globally, about 9 billion pounds of AN are produced annually (AN Group 2011). Workers can be exposed to high AN levels in industrial settings during the production and polymerization of the compound. For the general population, accidental explosion or leakage during transportation, and cigarette smoking, pose the greatest exposure risk (IARC 1999). Health care professionals, including surgeons, may also be exposed to low levels of AN during laparoscopic surgery (Wu et al. 1997; Chung et al. 2010).
AN is an acute neurotoxin in rodents and humans (Ahmed and Farooqui 1982; Ghanayem et al. 1991; Chen et al. 1999). In animals, AN-induced neurotoxicity presents in two distinct phases (Ahmed and Farooqui 1982; Ghanayem et al. 1991). The first phase occurs shortly after exposure, reaching a nadir 1 h later, and can be attenuated with atropine pretreatment. This initial phase is consistent with cholinergic overstimulation, with symptoms of excessive salivation, lacrimation, chromodacryorrhea, polyuria, miosis, vasodilatation of the face, ears and extremities, increased gastric secretion, and diarrhea. The second phase occurs 4 (or more) h later, and is characterized by signs of central nervous system (CNS) dysfunction, including tremor, ataxia, convulsions, lethargy, and respiratory failure (Ghanayem et al. 1991).
AN administration causes cholinergic toxicity (Fanini et al. 1985; Ghanayem et al. 1985; Ghanayem et al. 1991), resembling that caused by acetylcholine (ACh) mimetics or AChE inhibition (Ahmed and Farooqui 1982; Ghanayem et al. 1991; Zabrodskii et al. 2000a, b). These effects can be inhibited by atropine, a muscarinic receptor antagonist, suggesting the involvement of the cholinergic system in AN-induced neurotoxicity (Ghanayem et al. 1991). In humans, AN toxicity frequently presents as headaches, dizziness, convulsions, nausea, vomiting, abdominal pain, chest tightness, cough, and dyspnea (Chen et al. 1999). These symptoms appear to be due to disturbances of the CNS and the respiratory, digestive, and cardiovascular systems.
AN exposure also affects the peripheral nervous system (PNS). Acute exposure results in increased gastrointestinal (GI) motility and tracheal smooth muscle contraction, both of which appear to be due to cholinergic excitation (Satayavivad et al. 1991). The enhanced motility can be attenuated by pretreatment with cisapride, a drug that promotes ACh release from GI cholinergic plexus, which is part of the PNS. In addition, a high dose of AN (80 mg/kg) produces tremors in rats, similar to those seen after exposure to muscarinic agonists (Ghanayem et al. 1991). Furthermore, AN-treated animals display motor hyper-responsiveness to atropine (Satayavivad et al. 1998), suggesting that down-regulation of muscarinic receptors in the CNS may be involved in AN toxicity.
Despite accumulating research, the effects of AN on AChE activity in the brain and other tissues remain controversial and unclear. Xiao and Li reported that AN inhibits AChE activity in humans and mice, respectively (Li 1996; Xiao 1999). Our previous study also showed that AN can inhibit AChE activity in the cerebral cortex and hippocampus (Wang et al. 2004). However, Satayavivad et al. (1991, 1998) failed to observe an inhibitory effect of AN on AChE, and they suggest that the cholinomimetic effects of AN might be due to its ability to stimulate the release of ACh from presynaptic cholinergic fibers. Chantara and colleagues found that AN did not directly effect muscarinic receptors (Chantara et al. 2006). Furthermore, studies using primary cultures of neural cells, including neurons and glia, showed that, although AN can selectively damage cholinergic neurons, AChE activity was unaffected (Dorman et al. 1996).
In this study, we investigate the mechanisms responsible for AN-induced changes in AChE activity. We explore the effects of AN on AChE activity in both whole blood and brain of mice. We also examine the effects of AN on AChE activity following pretreatment with ethanol, an inducer of cytochrome P450 2E1, an enzyme that metabolizes AN (Sumner et al. 1999; Daiker et al. 2000; Wang et al. 2002). A major objective of this study was to determine the role of parent AN and the effects of its modified metabolism (with ethanol pretreatment) on cholinergic over-stimulation. Our results revealed that AN at different doses produces divergent AChE activity response. These results merit careful consideration in handling intoxicated patients with differential AN exposures, when anti-cholinergic antidotes are considered.
MATERIALS AND METHODS
Chemicals, animals, and treatments
AN (99% pure) was obtained from the Acrylonitrile Plant of Shanghai Petrochemical Company (Shanghai, China). Male Kunming mice, weighing 19–21 g, were obtained from the Laboratory Animal Center of Jiangsu University. Before and during testing, animals were housed in a controlled temperature (22 ± 2 °C) room with a 12 h light/dark cycle and were acclimated for at least 1 week prior to experimentation. Animal care was consistent with the guidelines set by the Laboratory Animal Committee of Jiangsu University. Animals received food and water ad libitum. Prior to experimentation, a total of 144 mice were individually weighed and randomly assigned into 18 groups. The first nine groups were administrated 0 (normal saline), 0.156, 0.3125, 0.625, 1.25, 2.5, 5.0, 10, or 20 mg/kg AN by intraperitoneal (i.p.) injection in the absence of anesthesia. The other nine groups were pretreated with 5% ethanol in their drinking water for one week prior to the administration of AN. Injections were administered between 8:00 and 10:00 AM.
Tissue samples
Animals were decapitated 24 h after AN injection. Blood was collected in heparin-coated tubes. The tubes were gently inverted several times to distribute the anticoagulant. Brains were quickly excised, rinsed with ice-cold normal saline, and frozen at −70°C for biochemical analysis.
Gross behavioral observations
Behavior was evaluated by three personnel blind to animal treatment. Immediately after AN or vehicle injection, each mouse was placed in an individual cage and symptoms such as salivation were observed by an individual blind to the various treatments.
Biochemical assays for AChE
Brain samples were homogenized (1:9, w/v) in cold buffer (pH 7.4, 10 mM tris-HCl, 0.1 mM EDTA-Na2, 0.8% NaCl, 10 mM sucrose) with a glass hand-homogenizer. AChE activity was determined as described by Ellman et al. (1961) in 2 mL assay solution containing 100 mM phosphate buffer, pH 8.0, and 1.0 mM 5,5′-dithiobis-2-nitrobenzoic acid (DTNB) at 25 °C. Fifty μL of diluted homogenate (30–50 μg protein) was added to the reaction mixture and incubated for 3 min. Hydrolysis was monitored by the formation of the thiolate dianion of DTNB at 412 nm for 2–3 min with a spectrophotometer (Model 722, Shanghai Xinmao Instrument Co. Ltd., Shanghai, China). All samples were analyzed in duplicates or triplicates. One unit (U) of enzyme activity was defined as 1 micromole of ACh hydrolyzed per min and per mg of brain homogenate or per ml of blood (pH 8.0, 25 °C).
Cytochrome P450 2E1 (CYP2E1) activity
Hepatic CYP2E1 activity was measured to confirm its induction by ethanol. CYP2E1 activity analysis was based on the rate of oxidation of p-nitrophenol (PNP) to p-nitrocatechol, initiated by NADPH according to the methods as reported in our previous studies (Guangwei et al. 2010; Suhua et al. 2010). Briefly, liver homogenate (10%) was prepared in chilled potassium phosphate (5 mM; pH 7.4) then centrifuged at 11,800 μg for 20 min at 4°C. The supernatant (1.5 ml) was added to ice-cold CaCl2 (final concentration: 8 mmol/L) and, 30 min later, was centrifuged at 11,800 μg for 20 min. The resulting pellets (microsomes) were resuspended in 50 mM sodium phosphate buffer (pH 7.4) and kept at −80°C for further study. The activity was determined in a final volume of 500 μl containing 100 μg liver microsomes, 100 mM potassium phosphate, pH 6.8, 0.1 mM PNP p-nitrophenol, 1 mM NADPH and 5 mM MgCl2 followed by incubation at 37°C for 30 min. The reaction was terminated by the addition of 100 μl of chilled trichloroacetic acid (20%, w/v) and centrifuged at 10,000 μg for 5 min. After vortexing and centrifugation, 100 μl of the supernatant was added to a 96-well plate with 50 μl of 10 M sodium hydroxide. The plate was read at 550 nm and quantified by reference to p-nitrocatechol standards. The reaction was performed with 100 μg of liver microsomal protein for 30 min at 37°C. The results are expressed as nmol products per minute per mg of microsomal protein.
Protein estimation
The protein concentration of the samples was determined with the BCA kit from Bio-Rad Laboratories Inc. (Hercules, CA, USA). Standards and blanks were assessed simultaneously.
Statistical analysis
The t-test was applied to determine the difference hepatic CYP2E1 activities between ethanol pretreatment and tap water controls. Analysis of variance (ANOVA) coupled with a post hoc Student-Newman-Keuls test was carried out to evaluate the effects of the various treatments on AChE activity. Statistical significance was set at p < 0.05.
RESULTS
Effects of ethanol pretreatment on hepatic CYP2E1 activity
As shown in Table 1, ethanol pretreatment significantly enhanced hepatic CYP2E1 activity to ∼150% of control values (t=8.02, degrees of freedom=8, p <0.05).
TABLE 1.
Effects of ethanol pretreatment on hepatic CYP2E1 activity in mice a
The CYP2E1 activity is expressed as nmol p-nitrocatecho/min/mg microsomal protein
Mice were pretreated with 5% ethanol in their drinking water for one week.
p<0.05 vs control group.
Gross behavioral changes
All AN-treated mice exhibited salivation and reddish ears, noses and paws. Treatment with AN doses greater than 2.5 mg/kg resulted in a generalized wet appearance, reflecting excessive sweating. AN-induced hyperactivity commenced within 5 min post injection; this was followed 30 min later by general hypoactivity. A single mouse in the 20 mg/kg single-AN injection group died, as did another mouse in the 20 mg/kg AN + ethanol group. No alteration in behavior was observed in mice drinking ethanol alone. In addition, no marked difference in behavior was observed between AN alone and AN + ethanol groups.
Effects of AN on blood AChE activity and its modulation by ethanol
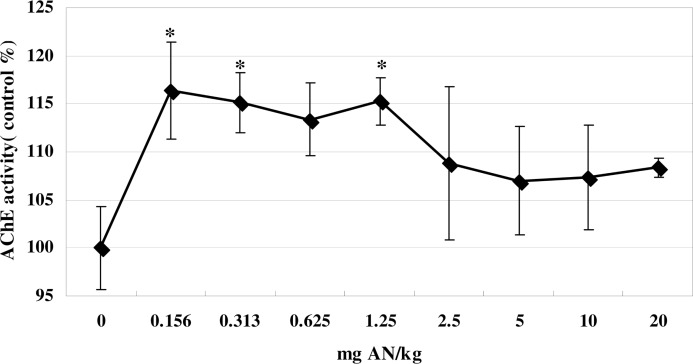
As shown in Figure 1, blood AChE activity significantly increased (14–16%) over control levels when animals were treated with a single injection of AN (0.156–1.25 mg/kg) (F (8, 53) = 9.6, p<0.05). At higher doses, AChE activity did not significantly exceed control levels, with only an 8% elevation at 20 mg/kg AN.
FIG. 1.
Effects of AN treatment on whole-blood AChE activity in mice. Values are means ± SD. * denotes significant difference compared with the control group, p<0.05. AN, acrylonitrile; AChE, acetylcholinesterase.
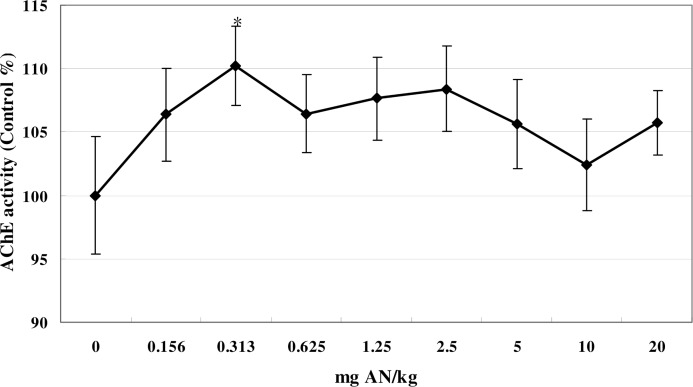
The modifying effect of ethanol pretreatment on blood AChE activity is shown in Figure 2. A comparable dose-response curve was seen when animals were allowed to drink ethanol ad libitum prior to AN treatment, and significant increase was observed at the dose of 0.313 mg/kg compared to controls (F (8, 53) = 6.84, p<0.05). There was no significant difference in the per-dose response of animals treated with AN and AN + ethanol (data not shown).
FIG 2.
Effects of AN treatment on whole-blood AChE activity in mice pretreated with 5% ethanol in their drinking water. * denotes a significant difference compared with the control group, p<0.05. Values are means ± SD. AN, acrylonitrile; AChE, acetylcholinesterase.
Effects of AN on brain AChE activity and its modulation by ethanol
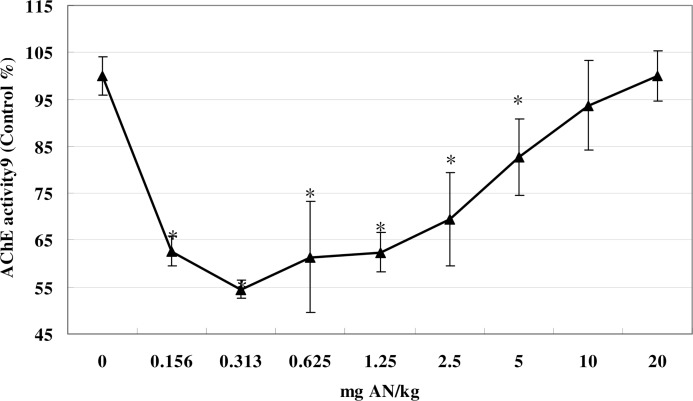
Systemic treatment with AN (0.156–5 mg/kg) markedly depressed brain AChE activity relative to that of control mice (F (8, 53) = 43.08, p<0.05) (Fig. 3). The lowest level of activity (45% of control) occurred at 0.313 mg/kg of AN. At higher doses, AChE activity decreased stepwise, and was inversely proportional to the dose, and at 20 mg/kg AN, brain AChE activity was indistinguishable from that of controls (Fig. 3).
FIG 3.
Effects of AN treatment on brain AChE activity in mice. Values are means ± SD. * denotes a significant difference compared with the control group, p < 0.05. AN, acrylonitrile; AChE, acetyl-cholinesterase.
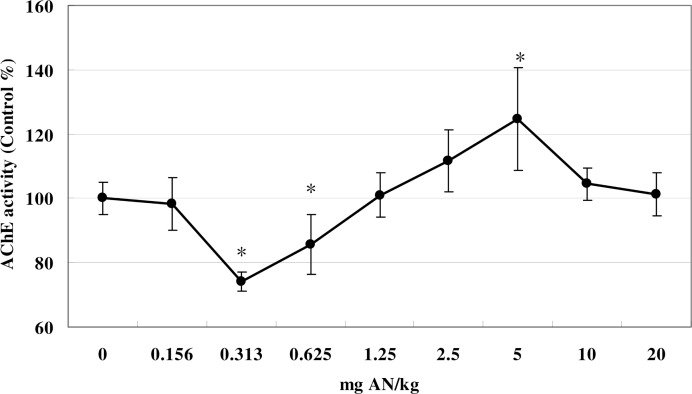
Brain AChE activity was also significantly affected by AN treatment in mice pretreated with ethanol (F (8, 53) = 20.56, p<0.05). The shape of the curve was broadly similar to that of mice not given an ethanol pretreatment, although the nadir was at 0.3125 mg AN/kg, when AChE activity was 75% that of control. Brain AChE activity was also depressed by 0.625 mg AN/kg. AChE activity was similar to controls at 1.25 mg. From 1.25 to 5.0 mg AN/kg AChE activity rose and exceeded control values. At doses greater than 5.0 mg/kg, brain AChE activity declined again and was indistinguishable from that of controls at 10 and 20 mg AN/kg (Fig. 4).
FIG 4.
Effects of AN treatment on brain AChE activity in mice pretreated with 5% ethanol in their drinking water. Values are means ± SD. * denotes a significant difference compared with the control group, p < 0.05. AN, acrylonitrile; AChE, acetylcholinesterase.
DISCUSSION
Although cholinergic overstimulation is observed in AN-intoxicated animals and humans (Ghanayem et al. 1991; Chen et al. 1999), the mechanisms by which AN affects the cholinergic system remain unclear. AN has been shown to affect both the central and peripheral cholinergic systems in vivo. AN inhibits AChE, enhances ACh release from presynaptic cholinergic fibers, and increases the agonist-binding affinity of the ACh receptor (Ghanayem and Ahmed 1986; Ghanayem et al. 1985, 1991; Satayavivad et al. 1991; Li 1996; Xiao 1999; Wang et al. 2004; Chantara et al. 2006). However, very little information is available on the role of AN and its metabolites in mediating the cholinergic effects.
The dosing regimen used in this study produced different patterns of change in AChE activity in blood and brain. AChE activity was differentially affected by a single systemic dose of AN. Specifically, while AN caused an increase in AChE activity at a low dose in blood, a decrease in AChE activity was observed in the brain with a similar AN treatment. While changes in AChE activity in blood were small (AN) or non-significant (AN + ethanol), those in the brain approached a 50% (AN) and a 30% (AN + ethanol) loss of enzyme activity. Satayavivad et al. (1991) have suggested that alterations in AChE activity following AN exposure are secondary to AN-stimulated ACh release. They also suggest that AN directly affects AChE activity by interacting with cysteine residues in the enzyme.
The explanation for the differential effect of AN on blood vs. brain AChE activity is unknown. In this study, the central cholinergic system appears to have been more severely affected by AN. This result is consistent with the observation that the two common forms of atropine have differential effects on cholinergic toxicity. Atropine methylbromide, which mainly blocks peripheral parasympathetic receptors, provides less protection against cholinergic excitotoxicity than atropine sulfate. The latter can block both central and peripheral parasympathetic receptors (Ghanayem et al. 1991).
The results from the AChE activity measurements demonstrate that the effects of AN are dose-dependent. It is known that low levels of toxins can elicit a compensatory response and that this process can lead to hormetic, biphasic dose-response relationships. Hormetic effects are often observed at low doses, below the no-observed-effect-level (NOEL; Calabrese and Baldwin 2001; Calabrese 2004). The classic pharmacological approach to examining dose-response relationships has been subject to criticism in an increasing number of studies demonstrating the importance of hormesis (Murado and Vasquez 2007). Because hormesis is generally characterized by low-dose stimulation and high-dose inhibition (Calabrese and Baldwin 2001), it is likely that short-term exposure to a low concentration of AN initiates a compensatory reaction that leads to a temporary increase in cholinesterase activity.
The biphasic alteration of AChE activity observed in the brain of mice after a single dose of AN is similar to earlier reports on AChE activity in the brains of rats exposed to aluminum (Gulya et al. 1990; Kumar 1998, 1999; Zatta et al. 1994). However, our study is the first to report AN-induced bidirectional changes in AChE activity in both blood and brain of mice. In our study, the increase in AChE activity at lower doses may be due to a compensatory or protective physiological response to toxin exposure. Continuous exposure to AN may exceed the cells’ ability to overcome the stress, thereby leading to a decrease in enzyme activity. Although the effect of low-level exposure to AN on AChE activity was minor, the imbalance may still affect peripheral and central cholinergic functions. Further investigation is necessary to clarify the effects of acute AN treatment on other members of the cholinergic system, including acetylcholine and its synthetic enzyme, choline acetyltransferase.
Our study suggests that AN targets blood and brain AChE differentially. Differences in binding characteristics (e.g., bimolecular binding rate constants) of each of the AChE enzymes may account for this effect, or it may reflect differences in the accessibility of AN to the enzyme (e.g., AN may have less access to AChE in blood compared to brain). However, understanding the mechanisms underlying these differences requires further investigation.
The rightward shift of the biphasic dose-response curves following ethanol pretreatment suggests that the parent chemical is primarily responsible for the observed cholinergic dysfunction. Ethanol pretreatment induces CYP 2E1 activity (Daiker et al. 2000), which has been shown to play a critical role in the bioconversion of AN, promoting degradation of AN and the release of cyanide ions (Sumner et al. 1999; Wang et al. 2002).
In conclusion, our results suggest that AN affects AChE activity in both blood and brain in a hormetic manner. Pretreatment with ethanol modifies the effects of AN on AChE activity. Whether the parent AN or its metabolites play a more important role in modulating cholinergic function has yet to be determined.
Acknowledgments
We are grateful to Dr. Peter Spencer at Oregon Health & Science University for his critical comments and English corrections. This work was supported in part by the Natural Science Foundation of China (No. 30872139, to Lu Rongzhu), by SCI-TECH (2008-018-02, to Xu Wenrong), by the Nutrition-Disease Team Fund of Jiangsu University (to Lu Rongzhu), and by the National Institute of Environmental Health Sciences (ES10563 and ES07331, to Michael Aschner).
REFERENCES
- Ahmed AE, Farooqui MY. Comparative toxicities of aliphatic nitriles. Toxicol Lett. 1982;12:157–163. doi: 10.1016/0378-4274(82)90179-5. [DOI] [PubMed] [Google Scholar]
- AN Group About acrylonitrile. 2011. Available at http://www.angroup.org/about.html.
- Calabrese EJ, Baldwin LA. Hormesis: U-shaped dose responses and their centrality in toxicology. Trends Pharmacol Sci. 2001;22:285–291. doi: 10.1016/s0165-6147(00)01719-3. [DOI] [PubMed] [Google Scholar]
- Calabrese EJ. Hormesis: from marginalization to mainstream A case for as the default dose-response model in risk assessment. Toxicol Appl Pharmacol. 2004;97:125–136. doi: 10.1016/j.taap.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Chantara W, Watcharasit P, Thiantanawat A, Satayavivad J. Acrylonitrile-induced extracellular signal-regulated kinase (ERK) activation via protein kinase C (PKC) in SK-N-SH neuroblastoma cells. J Appl Toxicol. 2006;26:517–523. doi: 10.1002/jat.1171. [DOI] [PubMed] [Google Scholar]
- Chen Y, Chen C, Jin S, Zhou L. The diagnosis and treatment of acute acrylonitrile poisoning: a clinical study of 144 cases. J Occup Health. 1999;41:172–176. [Google Scholar]
- Chung YJ, Lee SK, Han SH, Zhao C, Kim MK, Park SC, Park JK. Harmful gases including carcinogens produced during transurethral resection of the prostate and vaporization. Int J Urol. 2010;17:944–949. doi: 10.1111/j.1442-2042.2010.02636.x. [DOI] [PubMed] [Google Scholar]
- Daiker DH, Shipp BK, Schoenfeld HA, Klimpel GR, Witz G, Moslen MT, Ward JB., Jr Effect of CYP2E1 induction by ethanol on the immunotoxicity and genotoxicity of extended low-level benzene exposure. J Toxicol Environ Health A. 2000;59:181–196. doi: 10.1080/009841000156961. [DOI] [PubMed] [Google Scholar]
- Dorman DC, Struve MF, Ritzman TK, Owens JG, Morgan KT. Acrylonitrile-induced cytotoxicity in primary rat neural cell cultures. In Vitro Toxicol. 1996;9:361–371. [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Fanini D, Trieff NM, Sadagopa Ramanujam VM, Ahmed AE, Adams PM. Effect of acute acrylonitrile exposure on metrazol induced seizures in the rat. Neurotoxicology. 1985;6:29–34. [PubMed] [Google Scholar]
- Ghanayem BI, Ahmed AE. Prevention of acrylonitrile-induced gastrointestinal bleeding by sulfhydryl compounds, atropine and cimetidine. Res Commun Chem Pathol Pharmacol. 1986;53:141–144. [PubMed] [Google Scholar]
- Ghanayem BI, Boor PJ, Ahmed AE. Acrylonitrile-induced gastric mucosal necrosis: role of gastric glutathione. J Pharmacol Exp Ther. 1985;232:570–577. [PubMed] [Google Scholar]
- Ghanayem BI, Farooqui MY, Elshabrawy O, Mumtaz MM, Ahmed AE. Assessment of the acute acrylonitrile-induced neurotoxicity in rats. Neurotoxicol Teratol. 1991;13:499–502. doi: 10.1016/0892-0362(91)90056-3. [DOI] [PubMed] [Google Scholar]
- Guangwei X, Rongzhu L, Wenrong X, Suhua W, Xiaowu Z, Shizhong W, Ye Z, Aschner M, Kulkarni SK, Bishnoi M. Curcumin pretreatment protects against acute acrylonitrile-induced oxidative damage in rats. Toxicology. 2010;2010;267:140–146. doi: 10.1016/j.tox.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Gulya K, Rakonczay Z, Kasa P. Cholinotoxic effects of aluminium in rat brain. J Neurochem. 1990;54:1020–1026. doi: 10.1111/j.1471-4159.1990.tb02352.x. [DOI] [PubMed] [Google Scholar]
- IARC Re-evaluation of Some Organic Chemicals, Hydrazine, and Hydrogen Peroxide. International Agency for Research on Cancer, Lyon, France, IARC Monogr Eval Carcinog Risks Hum. 1999;71:43–108. [PMC free article] [PubMed] [Google Scholar]
- Kumar S. Biphasic effect of aluminum on cholinergic enzyme of rat brain. Neurosci Lett. 1998;248:121–123. doi: 10.1016/s0304-3940(98)00267-5. [DOI] [PubMed] [Google Scholar]
- Kumar S. Aluminum-induced biphasic effect. Med Hypotheses. 1999;52:557–559. doi: 10.1054/mehy.1997.0693. [DOI] [PubMed] [Google Scholar]
- Li ZL. Detection of four markers in mice blood treated with acrylonitrile. J Lanzhou Med Coll. 1996;22:43–44. (article in Chinese). [Google Scholar]
- Murado MA, Vázquez JA. The notion of hormesis and the dose-response theory: a unified approach. J Theor Biol. 2007;244:489–499. doi: 10.1016/j.jtbi.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Satayavivad J, Ruchirawat M, Mahidol C. Industrial chemicals and pesticides as modulators of autonomic functions. Proceeding of the First International Conference on Environmental and Industrial Toxicology: Research and Applications. Chulabhorn Research Institute; Bangkok, Thailand. 1991. pp. 203–215. [Google Scholar]
- Satayavivad J, Thiantanawat A, Tuntawiroon J, Watcharasit P, Ruchirawat M. Alterations of central muscarinic functions during subchronic exposure to acrylonitrile in rats. Res Commun Biol Psychol Psychi. 1998;23:29–42. [Google Scholar]
- Suhua W, Rongzhu L, Wenrong X, Guangwei X, Xiaowu Z, Shizhong W, Ye Z, Fangan H, Aschner M. Induction or inhibition of cytochrome P450 2E1 modifies the acute toxicity of acrylonitrile in rats: biochemical evidence. Arch Toxicol. 2010;84:461–469. doi: 10.1007/s00204-010-0519-7. [DOI] [PubMed] [Google Scholar]
- Sumner SC, Fennell TR, Moore TA, Chanas B, Gonzalez F, Ghanayem BI. Role of cytochrome P450 2E1 in the metabolism of acrylamide and acrylonitrile in mice. Chem Res Toxicol. 1999;12:1110–1116. doi: 10.1021/tx990040k. [DOI] [PubMed] [Google Scholar]
- Wang H, Chanas B, Ghanayem BI. Cytochrome P450 2E1 (CYP2E1) is essential for acrylonitrile metabolism to cyanide: comparative studies using CYP2E1-null and wild-type mice. Drug Metab Dispos. 2002;30:911–917. doi: 10.1124/dmd.30.8.911. [DOI] [PubMed] [Google Scholar]
- Wang SH, Lu R, Ren CL, Chen SX. Effects of acrylonitrile on activity of acetylcholinerase in rat brain. J Toxicol. 2004;18:25–26. (article in Chinese). [Google Scholar]
- Wu JS, Luttmann DR, Meininger TA, Soper NJ. Production and systemic absorption of toxic byproducts of tissue combustion during laparoscopic surgery. Surg Endos. 1997;11:1075–1079. doi: 10.1007/s004649900533. [DOI] [PubMed] [Google Scholar]
- Xiao W. Effect of acrylonitrile on blood cholinesterase activity. J Lanzhou Med Col. 1999;25:4–6. (article in Chinese). [Google Scholar]
- Zabrodskii PF, Kirichuk VF, Germanchuk VG, Belikov VG. Mechanisms of immunotoxic effects of acrylonitrile. Bull Exp Biol Med. 2000a;129:463–465. doi: 10.1007/BF02439803. [DOI] [PubMed] [Google Scholar]
- Zabrodskii PF, Kirichuk VF, Romashchenko SA, Germanchuk VG. Effect of the cholinesterase reactivator dipyroxime in various models of delayed hypersensitivity during acute intoxication by acrylonitrile. Eksp Klin Farmakol. 2000b;63:47–49. [PubMed] [Google Scholar]
- Zatta P, Benedetti PZ, Bruna V, Filipi B. Activation of acetylcholinesterase by aluminium (III): the relevance of the metal species. NeuroReport. 1994;5:1777–1780. doi: 10.1097/00001756-199409080-00023. [DOI] [PubMed] [Google Scholar]