Abstract
Many so-called “alternative medicine” techniques such as Reiki and acupuncture produce very good outcomes for intractable pain and other chronic illnesses but the efficacy is often dismissed as being psychosomatic. However a plausible mechanism does exist i.e. that the treatments alter the electromagnetic fields in living organisms and thereby prevent or reduce activity of neurons which lead to the pain. Low doses of ionising radiation have similar effects on electromagnetic fields and are known to induce signaling cascades in tissues due to ion gradients. To test this hypothesis cell cultures were exposed to Reiki – like and to acupuncture – like treatments, both performed by qualified practitioners. The cells were exposed either before or after the treatment to x-rays and were monitored for production of direct damage or bystander signals. The data suggest that the alternative techniques altered the response of cells to direct irradiation and altered bystander signal mechanisms. We conclude that alternative medicine techniques involving electromagnetic perturbations may modify the response of cells to ionizing radiation. In addition to the obvious implications for mechanistic studies of low dose effects, this could provide a novel target to exploit in radiation protection and in optimizing therapeutic gain during radiotherapy.
Keywords: radiation-induced bystander effects, complementary and alternative medicine, Reiki, acupuncture, non-linear dose response, bioenergy
INTRODUCTION
Alternative medicine techniques have been practiced for thousands of years and have achieved good results in animals as well as humans. The techniques have yet to gain credibility in mainstream Western medicine mainly because the energy which is balanced in living things as a result of many of these treatments, cannot be measured scientifically. Two methods where bioenergy is thought to be relevant are acupuncture and Reiki (Rubik, 2002). Reiki is a biofield therapy. The biofield is an extremely weak electromagnetic field of the organism thought to involve electromagnetic bioinformation important for regulating homeodynamics (Rubik 2002). Acupuncture involves inserting steel needles with coiled tops into the skin at well defined points along meridians (Lo 2002). These meridians are thought to be energy flow paths and to consist of polarized molecules. Balancing energy flow is critical to achieve therapeutic improvement. Critical to both therapies is the concept of “Qi “ or universal energy which organisms share with their environment. Living systems constantly exchange energy with information at multiple levels of organization with their environment in order to maintain themselves (Rubik 2002).
The field of radiation biology has undergone a paradigm shift in the last 10 years which has changed the way low doses of radiation are viewed. The twin pillars of this shift are the discovery of genomic instability and lethal mutations (delayed reproductive death) in the 1980’s and early 1990’s. (Seymour et al. 1986; Alper et al. 1988; Seymour and Mothersill, 1989; Chang and Little, 1991; Seymour and Mothersill, 1992; Chang and Little 1992; Kadhim et al. 1992; Kadhim et al. 1994; Marder and Morgan, 1993; Brown and Trott, 1994) and the discovery of bystander effects in the early 1990’s (Nagazawa and Little, 1992). As with any paradigm shift, there were data that did not fit the prevailing view going back to 1915 (Murphy and Norton, 1915). The old literature is reviewed by Mothersill and Seymour (2001) but the seminal papers which began the move away from a DNA centric view of radiation biology and a linear non-threshold (LNT) relationship between dose and effect, were the discovery of the non-clonal appearance of lethal mutations in apparently healthy distant progeny of irradiated cells in 1986 (Seymour et al. 1986), the discovery of non-clonal appearance of chromosomal aberrations in distant progeny of alpha particle irradiated hematopoietic stem cells in 1992 (Kadhim et al. 1992). Both reports showed high yields of damage in distant progeny which could not be explained by any conventional mechanism. Implicit in the 1992 paper was the realization that cells which did not get an alpha track could show damage associated with receiving such a track. This was formally demonstrated later in 1992 (Nagazawa and Little, 1992) when it was shown that cells exposed to low fluences of alpha particles such that not all cells received an energy deposition, more cells than could have been hit showed chromosomal aberrations. This effect was termed the radiation-induced bystander effect (RIBE). Since 1986 the field has grown exponentially and these low dose phenomena are regarded as interrelated non-targeted effects of radiation with a consensus that genomic instability is being driven by bystander associated mechanisms including the production of reactive oxygen and reactive nitrogen species (ROS/NOS), calcium fluxes and activation of ion gated channels (Clutton et al. 1996; Lyng et al. 2001; Mothersill et al, 2005, Lyng et al. 2006; Mothersill and Seymour, 2006, Shao et al. 2006; Shao et al. 2008; Mothersill et al. 2010; Lyng et al. 2011). However the nature of the released factors which travel from cell to cell are still a mystery and there are suggestions that the factors may have a physical component (Mosse et al. 2006; Mothersill et al. 2007; Dr Olga Senyiuk, Chernobyl Research Centre, Chernobyl Village, Ukraine, p.comm 2011, cited with permission).
Consideration of the possibility of a physical component to the factor prompted us to review the field of electromagnetic frequency effects on cells and organisms. There is considerable evidence that bioelectric or biomagnetic fields can affect cells (Gandhi, 2002; Brodsky et al. 2003; Swanson and Kheifets, 2006; Yamaguchi et al. 2006, Wiegant and Van Wijk, 2010), mainly by influencing membranes resulting in altered permeability to ions as a result of effects on the potential difference across the membrane (Jasti et al. 2001; Lohmann et al. 2003; Dini and Abbro, 2005). Aaron’s group has also reported growth factor production associated with bioelectric field exposure (Aaron et al. 2004). Fractures which will not heal can often be helped by bioelectric or magnetic treatment and the mechanism is reported to involve TGF_ production consequent on electromagnetic (EM) stimulation, (Bodamyali et al. 1998; Aaron et al. 2002). There is also a whole industry built around the ability of plastics to conduct EM waves and optical signals (Werneck and Barrientos, 1994, http://www.lumigen.com). Fibre optic technology relies on this and in the microplate reader industry, many precautions have to be taken to prevent optical conductance in microwell plates. A branch of medical imaging depends on electrical impedance of tissues (Boone et al, 1997, Eyübog_lu, 2006, Eyuboglu and Degirmenci, 2011) which is another mainstream example. Thus at one level the results suggesting that bystander effects may be transmitted via biomagnetic or bioelectric mechanisms are not that surprising. We know that ion channels are important in the initiation of bystander responses (Lyng et al. 2006) and also know that drugs which transduce electrochemical signaling via receptors associated with ion-gated membrane channels are involved in triggering bystander signal production (Poon et al. 2007, Saroya et al. 2009).
Given the possible link between bystander signal production and bioelectromagnetic phenomena, we decided to explore two modalities where the beneficial health effect of the treatment was attributed to bioenergy balancing. Our hypothesis was that application of these treatments to cells before or after radiation exposure would reduce or otherwise modulate bystander stress signaling between cells by modifying energy fields.
METHODS
Reiki-like and acupuncture-like technique
The authors include a professional radiotherapist (Wong) who is also a qualified acupuncturist, and a Reiki level 3 master (Seymour) who is also a radiobiologist. The treatment of cells with acupuncture involved opening the flask in a level 2 biosafety cabinet and placing a standard steel acupuncture needle in the flask of cells making sure the cell layer was in contact with the needle tip for 20 mins. Sham controls included opening the flask for the same length of time with no needles or exposure using plastic toothpicks instead of acupuncture needles. Treatment was given before irradiation to donor flasks or after irradiation to donor or recipient flasks.
Reiki was practiced by bioenergising the cells for 20 minutes either before or after irradiation. This is done by the practioner holding his hands over the flasks without touching them, while concentrating on transmitting energy to the living cells. In this case the sham control flasks were removed from the incubator for the same time interval but were left on the bench while Reiki-like treatment was given to the test flasks.
Acute X-ray treatment and bystander effect induction
X-ray doses were administered using a portable X-ray machine (Faxitron X-ray Corporation cabinet X-ray system; Wheeling, Illinois, USA), delivering 100mGy per min, exactly as previously described (Mothersill et al. 2006). Flasks were kept in the dark (in polystyrene boxes) during irradiation and while being transported to and from the irradiator to prevent UV light exposure being a confounding variable and also to minimize temperature fluctuations as the irradiation was in a separate building from the cell culture laboratory. Flasks were returned to the incubator immediately after irradiation.
Cell culture techniques
The HPV-G cell line was originally given to us by Dr J DiPaolo, National Institutes of Health (NIH), Bethesda, Maryland, USA (Pirisi et al. 1988), it was obtained at an early passage and expanded and frozen in our laboratory. The cell line has a well-characterized and stable bystander response, showing a reduction in cloning efficiency of ∼40% over a wide range of radiation doses, and well-characterized calcium fluxes and mitochondrial effects (Lyng et al. 2002, Maguire et al. 2007). This makes it ideal as a reporter system. All cell culture procedures were performed in a class II biosafety cabinet. The cells were grown in RPMI medium containing 60 ml pre-screened foetal bovine serum (FBS), 5 ml penicillin-streptomycin, 5 ml L-glutamine, 15 mM Hepes buffer, and 1 mg/ml hydrocortisone. All reagents were manufactured by Gibco, Biocult and obtained from VWR, Burlington, Ontario, Canada. The serum was pre-screened to ensure it supported a bystander effect when tested using a positive control x-ray exposure of HPV-G cells.
Clonogenic Assay Technique and Bystander Protocol
Cell cultures that were 85–90% confluent and that had received a medium change the previous day were selected. Cells were removed from the flasks using 0.25% w/v trypsin/1 mM Ethylenediaminetetraacetic acid (EDTA) solution (1:1) obtained from VWR, Burlington, Ontario, Canada. When the cells had detached, they were resuspended in medium, and an aliquot was counted using a Z2 Coulter particle count and size analyzer (Beckman Coulter Electronics, Mississauga, Ontario, Canada). Appropriate cell numbers (∼500) were plated for the recipient or bystander flasks to optimize the ratio of signal molecules to cell number. 48hrs after set up of the explants, medium was poured off from the flasks containing the explants. The medium was filtered through a 0.22-mm filter to remove any debris from the explant culture medium and then added to the cells in the reporter flasks from which the original medium had been removed. Ten days later, colonies of reporter cells were stained with Carbol Fuchsin (Ziehl Nielsson, Sigma) and colonies were counted to determine reporter cell survival.
Statistical analysis
All data are expressed as mean ± standard deviation (n = 3 in all cases) and compared by Analysis of Variance (ANOVA) followed by Least Square Difference (LSD), using the “Statistix” statistical analysis software (Analytical Software, Tallahassee, FL, USA); in all cases a p <0.05 was considered statistically significant. On each figure the letters, annotated above the bars, indicate those data which are statistically similar on the bar graph and those which are statistically different. The asterisk indicates those data which show a statistically significant difference to the completely untreated control cells.
RESULTS
Acupuncture experiments
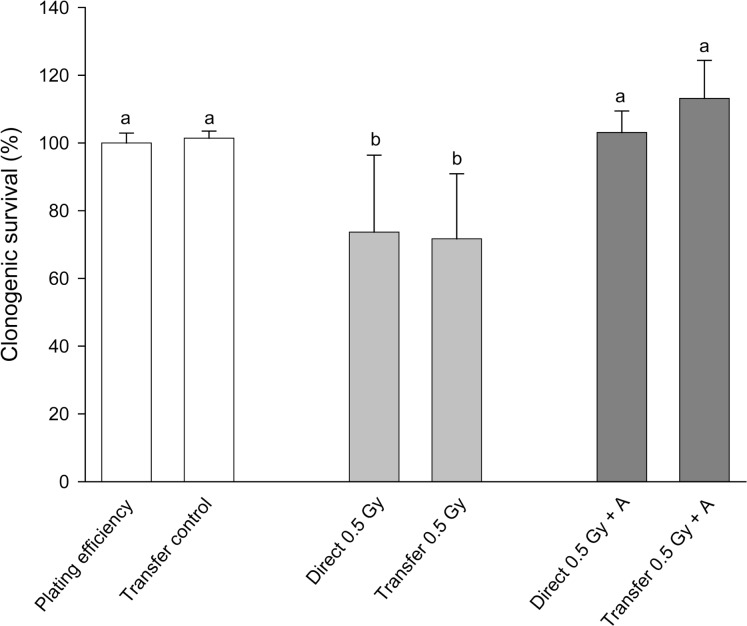
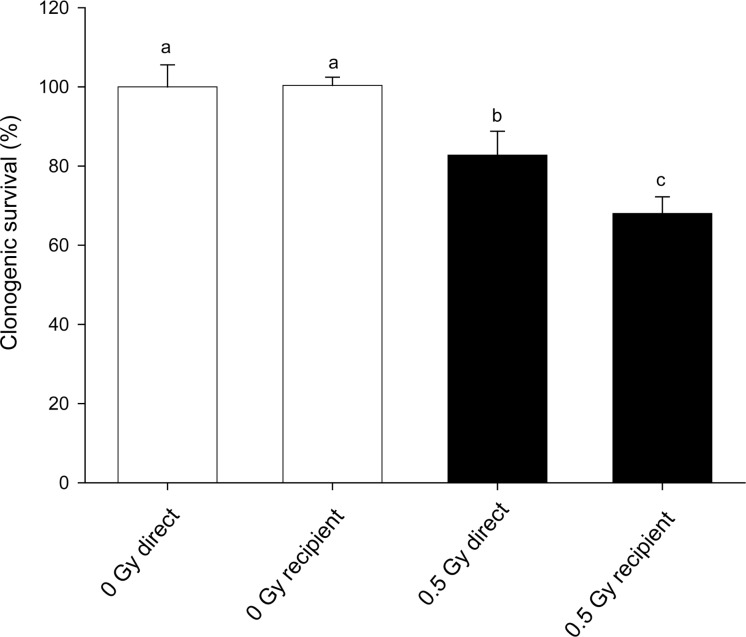
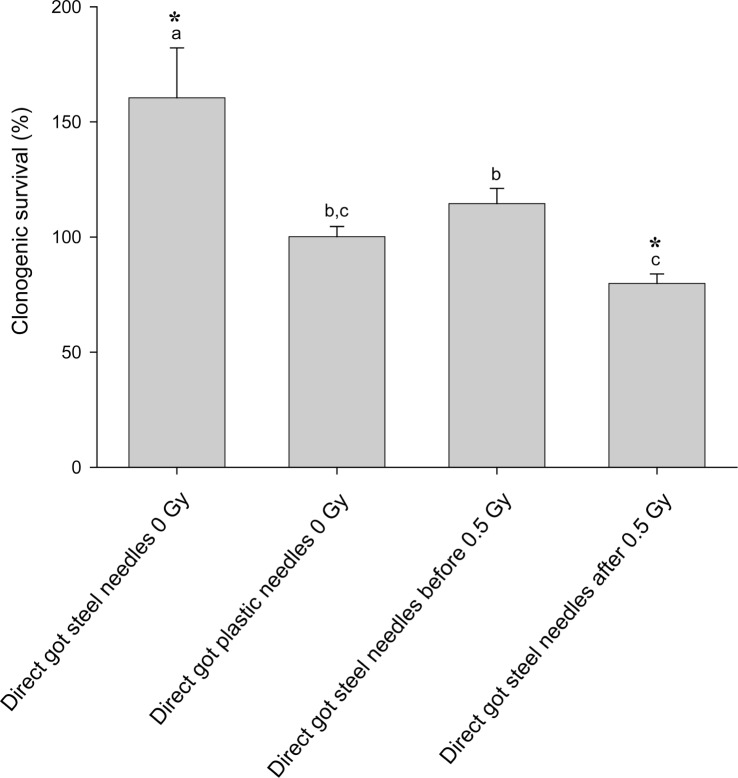
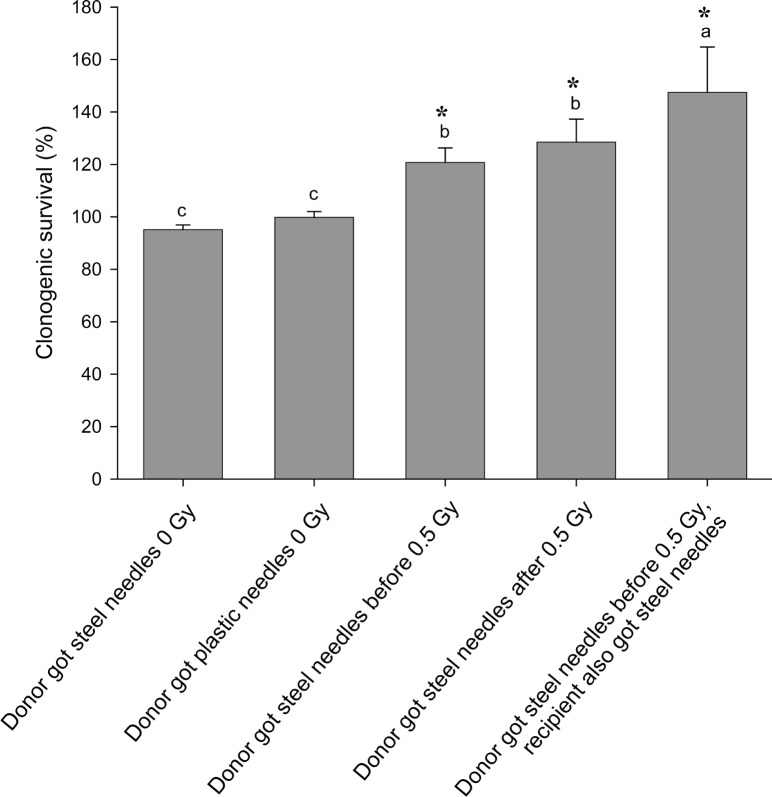
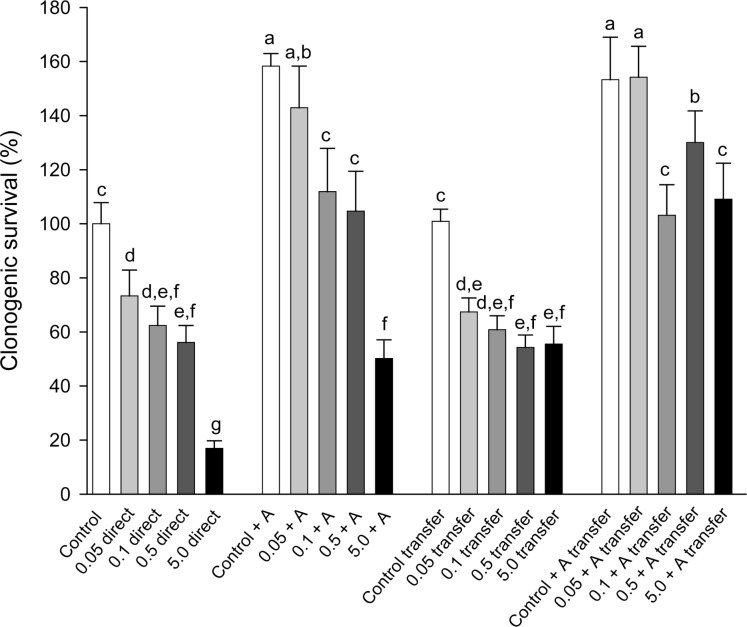
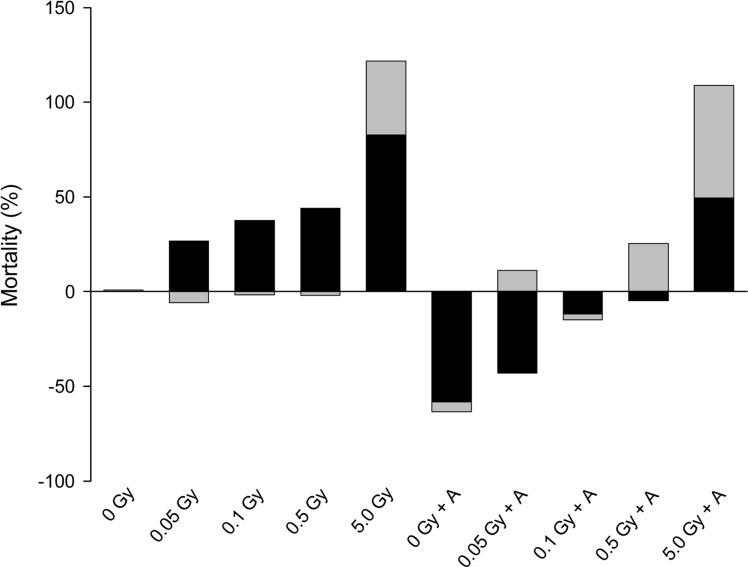
In figure 1 results of a simple experiment to determine if there was an effect of steel needles are shown. Clearly in this experiment, acupuncture treatment of cells prevented both the direct effect of 0.5Gy x-rays and also prevented the production of a bystander effect. The treatment was given to directly irradiated or donor flasks 1hr before exposure to x-rays. In figure 2(a–c) a much bigger experimental design is presented where the aim was to see if acupuncture was acting on signal production or on response to the signal. In these experiments sham controls were included for the needle treatment and treatment was given 1hr before irradiation, immediately after irradiation or given to recipient cells before medium transfer. For easy of viewing, the data are presented in 3 separate graphs although all the exposures were done in a single experiment in which the flasks were set up in triplicate and the experiment repeated 3 times. Figure 2a simply shows the irradiated and bystander medium recipient controls with no acupuncture-like treatment. The graph shows that 0.5Gy x-rays reduced the clonogenic survival of directly irradiated cells by about 40%. Medium harvested from directly irradiated cells caused a similar reduction in clonogenic survival. These values are the controls against which the effects of steel and plastic needle acupuncture-like effects should be viewed. In figure 2b the data for directly irradiated cells with or without acupuncture-like treatment are presented. All data on this graph were normalized to the absolute control (100%) in Fig 2a. The results show that treatment with steel needles increased the clonogenic survival of the unirradiated cells above the level of the absolute control i.e. the plating efficiency of the cells increased. Treatment with plastic needles had no effect. Treatment with steel needles before exposure to 0.5Gy reduced the plating efficiency to the level of the absolute control with the same proportional decrease in survival had the data been normalized to the steel needle control. This suggests that the steel needle treatment acts by increasing the survival of cells not by reducing percentage of cell death caused by exposure to ionizing radiation. Exposure to steel needle treatment after radiation exposure was less effective than treatment before exposure. Fig. 2c shows the clonogenic survival of cells which received no direct irradiation but received media from irradiated cells. These data were normalized to medium transfer recipient control in Fig.2a. The results clearly show that acupuncture treatment with steel needles to the donor cells is required to prevent the bystander–induced reduction in clonogenic survival response. Interestingly it does not matter whether the needle treatment is given before irradiation or immediately afterwards for medium transfer experiments but the steel needles have the greatest effect on cell survival if treatment is given before exposure to the donors AND after exposure to the recipients. In figure 3 a dose response is shown. The quite sensitive direct response and the saturated bystander response following medium transfer are normal for this cell line. The acupuncture data for the directly irradiated cells reveals a protective effect at all doses including the zero Gy control. The magnitude of this effect does not increase with increasing dose but is a factor of approximately 2 over the range 0.05Gy–5Gy. The bystander medium transfer data are more variable but maintain protective factors in the 1.5–2.0 range over the whole dose range including the zero Gy recipient. In fig. 4 the dose response data have been replotted to show the proportion of the cell killing which can be attributed to the direct effect of radiation alone and the percent due to the bystander component of the total effect. This plot was done by assuming full expression of the bystander effect in the directly irradiated flasks. The % death for bystander cells was then subtracted from the % death for the “direct” group which actually contain cells arrested by the actual energy deposition from the dose and the cells arrested by the bystander factors. The graphs show clearly where the net effect of the acupuncture-like treatment is to increase survival; (bars below the 0 cell death on the y axis). This plot also allows the relative contributions of bystander and direct effects to the overall cell death to be seen.
FIGURE 1:
Effect of acupuncture on the production of a bystander response following exposure to 0.5Gy x-rays. Letters above the chart columns indicate the degree of difference from the control. Errors are standard error of the mean.
FIGURE 2A:
Direct and medium transfer induced reduction in clonogenic survival of cells exposed to 0.5Gy or medium harvested from donor cells exposed to 0.5Gy ionizing radiation. Letters above the chart columns indicate the degree of difference from the control. Errors are standard error of the mean.
FIGURE 2B:
Effect of pre or post exposure acupuncture-like treatment with steel or plastic needles on the clonogenic survival of cells directly exposed to 0.5Gy ionizing radiation. Letters above the chart columns indicate the degree of difference from the control. Errors are standard error of the mean.
FIGURE 2C:
Effect of pre or post exposure acupuncture-like treatment with steel or plastic needles on the clonogenic survival of cells exposed to medium harvested from cells exposed to 0.5Gy ionizing radiation. Letters above the chart columns indicate the degree of difference from the control. Errors are standard error of the mean.
FIGURE 3:
Dose response for directly irradiated and bystander medium transfer cells treated with acupuncture before exposure to radiation. Letters above the chart columns indicate the degree of difference from the control. Errors are the standard error of the mean.
FIGURE 4:
The data in Figure 3 replotted to show the proportion of cell death attributable to the net direct effect of ionizing radiation energy deposition after subtraction of the bystander component (grey portion) and the proportion attributable to the bystander component i.e. the result of medium transfer (black portion). The control was normalized to zero% mortality. Bars below 0 % mortality indicate enhanced survival of cells due to the treatment.
Reiki experiments
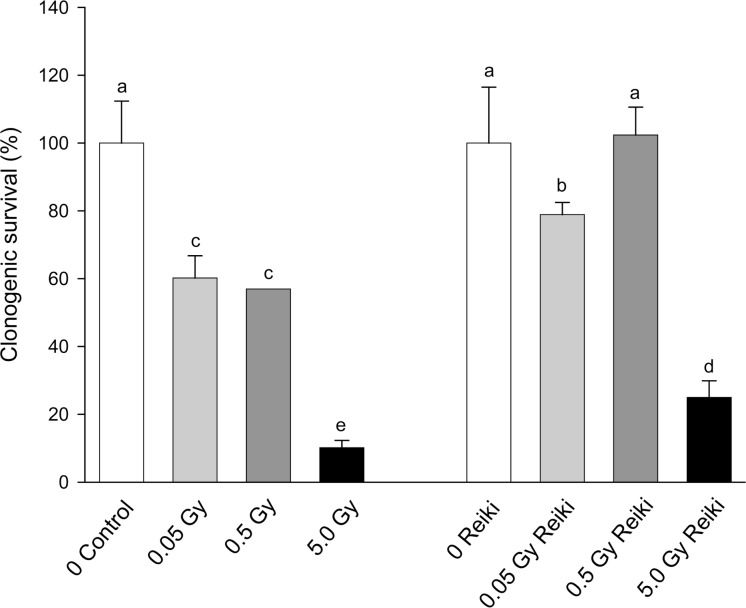
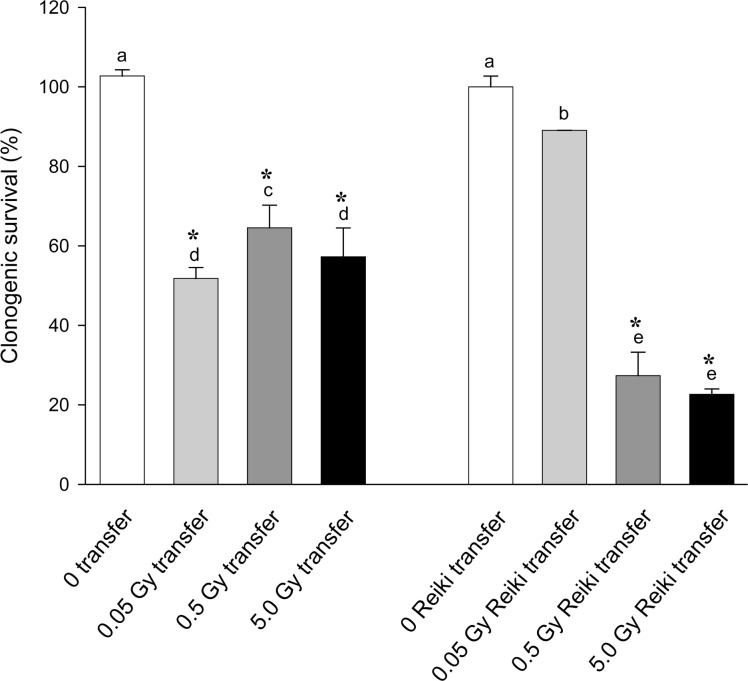
The results of the experiments where bioenergy using Reiki was administered to cells for 20 mins 1hr before irradiation (0.05, 0.5 or 5Gy acute dose) are shown in Figure 5 (a and b). In each case the data were normalized to 100% for the relevant untreated control. In case the 20 min time span outside the incubator was causing any effect, sham controls were included where cells were placed on a laboratory bench remote from the site of the Reiki practitioner. Results for the sham and untreated controls were identical. The figure shows that Reiki treatment modifies the direct and bystander response to radiation. The groups not treated with Reiki show the usual response to direct irradiation (Fig. 5a) and to transfer medium from irradiated donors (Fig.5b). However Reiki treatment significantly increases survival of directly irradiated cells at all doses tested and unlike the acupuncture experiments this is not because of any effect on the control plating efficiency. The bystander result is less clear, the low radiation dose donor medium is less toxic but the 0.5Gy and 5Gy donor media are more toxic.
FIGURE 5A:
Effect of bioenergising cells using Reiki-like techniques on the clonogenic survival of cells following direct exposure of cells to ionizing radiation. Letters above the chart columns indicate the degree of difference from the control. Errors are standard error of the mean..
FIGURE 5B:
Effect of bioenergising cells using Reiki-like techniques on the clonogenic survival of cells following direct exposure of cells to medium harvested from cells exposed to ionizing radiation. Letters above the chart columns indicate the degree of difference from the control. Errors are standard error of the mean.
DISCUSSION
The data in this paper support our hypothesis that non targeted effects of low dose irradiation would be influenced by alternative medicine therapies which balance bioenergy. At first sight it might seem a strange idea that these two popular alternative medicine techniques might and do modify response of cells in vitro to ionizing radiation.
Examination of the literature reveals a field of research dealing with bioenergy and specifically with the importance of very low energy photons in maintaining function in living organisms (Kiang et al. 2002, 2005, Van Wijk et al. 2008; Wiegant and Van Wijk, 2010). These references attribute the success of acupuncture, Reiki and meditation to the ability of trained individuals to balance or tune the energy balance (meridians) in themselves or their subjects/patients. Another series of papers attempt to identify mechanisms at the cellular level. Key among these are suggestions that membrane polarization is important and that ion-gated channels are important (Ye and Zhang 1992, Jasti et al, 2001). Others suggest ionic perturbations due to oxidative stress (Hernández-Enríquez, et al 2011, Gorman et al. 2010). All these cellular mechanisms have been seen in radiation-induced bystander experiments. Calcium fluxes were identified by Lyng et al. in 2002 and subsequent research also confirmed that ion-gated channels - particularly the 5-HT3A receptor, were implicated (Maguire et al. 2007; Poon et al. 2007; Saroya et al. 2009; Mothersill et al. 2010). There are many papers in the literature on the role of ROS and NOS in bystander mechanisms (reviewed in Averbeck, 2010). Most of these papers suggest that ROS damages DNA through short-lived free radical attack (Hei et al. 2008, Sedelnikova et al. 2010) and that NOS is important in signaling. Inhibitor studies prove that both ROS and NOS are involved as inhibition various aspects of these pathways prevents the bystander effect, however an issue with this has always been that the medium transfer experiments suggest that the signal is long-lived. Its production appears to be controlled by an on/off switch with a low dose threshold and does not show a dose dependent relationship between effect and dose (Schettino et al. 2005; Liu et al. 2006).
A neat explanation that could fit the existing data and explain the data presented here is that ionizing radiation perturbs delicately balanced bioenergy fields, resulting in homeostatic signaling aimed at rebalancing the pre-irradiation ionic gradients. Reiki and acupuncture could be seen then as activities which stop the imbalance from occurring or rebalance very quickly so that signaling pathways are not turned on. Support for this idea is that the generation of bystander signal in medium transfer experiments at least has been shown to require at least 30–60 mins after irradiation to be generated (Mothersill and Seymour, 1997) so the fact that Reiki and acupuncture applied immediately after irradiation were effective means that intervention before the signal expression is complete may stop it happening or modify the process. In the case of acupuncture but not Reiki, it appears that the treatment is able to stabilize cells irrespective of exposure to ionizing radiation because the protective effect is seen in the controls. Possibly the treatment acts in a similar way to anti-oxidants by preventing the occurrence of the perturbation or by proactively aiding the survival of cells whether or not these are subsequently stressed.
If it is accepted that the mechanisms by which these therapies modulate bystander effects is because of the bioenergy field then it is interesting to consider other known interactions between ionizing radiation and electromagnetic fields. The literature is sparse and very contradictory. With some laboratories reporting that EMF can enhance ionizing radiation-induced DNA damage. Increases in micronuclei, chromosome aberrations and apoptosis have all been reported when the ionizing radiation exposure is administered in an EMF field even in the microT range. Epidemiological studies also produce contradictory results (Johansen, 2004; Schüz et al. 2009,). However several studies find no effects (reviewed in Habash et al. 2003). This situation of apparently contradictory findings is also characteristic of the low dose radiation field (Nguyen and Wu, 2011; Kondo, 2011; Nomura, 2011; Zyuzikov, et al 2011). In the latter field however it is now clear that different cell lines, different radiation exposures and even media composition give different results (Bourguignon et al. 2005; Mothersill et al. 2006; Ponnaiya et al. 1997, Morgan and Sowa, 2009; Little, 2010; Dainiak, 2011). This could suggest that these very subtle and sensitive responses are of little relevance in risk assessment. However it could also be argued that this variability underlies the range of variation in organismal response to low dose exposures to radiation and other environmental stressors. The resulting uncertainty about the low dose risk for a given individual necessitates conservatism and precautionary regulatory decisions. Determining the important mechanisms after low dose exposure to any environmental stressor must be an essential step in assessing true risk.
Many of the critics of alternative medicine techniques claim that the treatments work for symptoms such as pain, backache, headache etc. because the patients believe in the therapy or in the therapist. This might be described as a placebo effect (Kaptchuk 2002, Benedetti et al 2003, Wager et al, 2004). Controlled studies such as (Rubin et al. 2010) show no benefit if the patients do not know they are being treated. Other studies dismiss this but do suggest that the subject and the practitioner have some sort of mental association (Yu et al. 2003). Most interestingly, people have measured weak light photons coming from practitioners when they meditate during therapy (Hou et al. 1994; Ulett et al. 1998; Lee et al. 2010; Van Wijk et al. 2008) and attribute the biological effects to this energy. Whatever may underlie human responses to these treatments, we can be sure that in vitro cellular responses cannot be due to psychosomatic effects and must have some real basis in biology and biophysics however incredible this may seem.
In conclusion, we have shown that both reiki and acupuncture can significantly reduce direct effects of ionizing radiation in human epithelial cells. Acupuncture is effective in preventing or reducing medium transfer mediated bystander effects if applied to irradiated donor cells before or immediately after treatment. Reiki treatment given to medium transfer donor cells has a more complex effect which is dose dependent. The data strongly suggest a physical component involving bioenergy in the generation of bystander signals. If the mechanisms could be harnessed they might provide new modalities for use in radiotherapy or in risk mitigation following medical or accidental low dose radiation exposures.
Acknowledgments
We gratefully acknowledge the continued support of The Natural Science and Engineering Research Council (NSERC) Discovery Grant Programme, and the Canada Research Chairs Programme.
REFERENCES
- Aaron RK, Boyan BD, Ciombor DM, Schwartz Z, Simon BJ. Stimulation of growth factor synthesis by electric and electromagnetic fields. Clin Orthop Relat Res. 2004;419:30–37. doi: 10.1097/00003086-200402000-00006. [DOI] [PubMed] [Google Scholar]
- Aaron RK, Wang S, Ciombor DM. Upregulation of basal TGFbeta1 levels by EMF coincident with chondrogenesis—implications for skeletal repair and tissue engineering. J Orthop Res. 2002;20(2):233–240. doi: 10.1016/S0736-0266(01)00084-5. [DOI] [PubMed] [Google Scholar]
- Alper T, Mothersill C, Seymour CB. Lethal mutations attributable to misrepair of Q-lesions. Int J Radiat Biol. 1988;54(4):525–530. doi: 10.1080/09553008814551961. [DOI] [PubMed] [Google Scholar]
- Averbeck D. Non-targeted effects as a paradigm breaking evidence. Mutat Res. 2010;687(1–2):7–12. doi: 10.1016/j.mrfmmm.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. J Neurosci. 2003;23(10):4315–23. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodamyali T, Bhatt B, Hughes FJ, Winrow VR, Kanczler JM, Simon B, Abbott J, Blake DR, Stevens CR. Pulsed electromagnetic fields simultaneously induce osteogenesis and upregulate transcription of bone morphogenetic proteins 2 and 4 in rat osteoblasts in vitro. Biochem and Biophys Res Commun. 1998;250(2):458–461. doi: 10.1006/bbrc.1998.9243. [DOI] [PubMed] [Google Scholar]
- Boone K, Barber D, Brown B. Imaging with electricity: Report of the European concerted action on impedance tomography. J Med Eng Technol. 1997;21:201–232. doi: 10.3109/03091909709070013. [DOI] [PubMed] [Google Scholar]
- Bourguignon MH, Gisone PA, Perez MR, Michelin S, Dubner D, Giorgio MD, Carosella ED. Genetic and epigenetic features in radiation sensitivity Part I: cell signalling in radiation response. Eur J Nuc Med Mol Imaging. 2005;32(2):229–246. doi: 10.1007/s00259-004-1730-7. [DOI] [PubMed] [Google Scholar]
- Brodsky LM, Habash RW, Leiss W, Krewski D, Repacholi M. Health risks of electromagnetic fields. Part III: Risk analysis. Crit Rev Biomed Eng. 2003;31(4):333–354. doi: 10.1615/critrevbiomedeng.v31.i4.20. [DOI] [PubMed] [Google Scholar]
- Brown DC, Trott KR. Clonal heterogeneity in the progeny of HeLa cells which survive X-irradiation. Int J Radiat Biol. 1994;66(2):151–155. doi: 10.1080/09553009414551051. [DOI] [PubMed] [Google Scholar]
- Chang WP, Little JB. Delayed reproductive death as a dominant phenotype in cell clones surviving X-irradiation. Carcinogenesis. 1992;13(6):923–928. doi: 10.1093/carcin/13.6.923. [DOI] [PubMed] [Google Scholar]
- Chang WP, Little JB. Delayed reproductive death in X-irradiated Chinese hamster ovary cells. Int J Radiat Biol. 1991;60(3):483–496. doi: 10.1080/09553009114552331. [DOI] [PubMed] [Google Scholar]
- Clutton SM, Townsend KM, Walker C, Ansell JD, Wright EG. Radiation-induced genomic instability and persisting oxidative stress in primary bone marrow cultures. Carcinogenesis. 1996;17(8):1633–1639. doi: 10.1093/carcin/17.8.1633. [DOI] [PubMed] [Google Scholar]
- Dainiak N. Recommendations for assessment of consequences and health risks of low-level exposure to ionizing radiation. Health Phys. 2011;100(3):311–312. doi: 10.1097/hp.0b013e31820a1b20. [DOI] [PubMed] [Google Scholar]
- Dini L, Abbro L. Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron. 2005;36(3):195–217. doi: 10.1016/j.micron.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Eyübog_lu BM. 2006. Magnetic Resonance - Electrical Impedance Tomography Encyclopedia of Biomedical Engineering (Metin Akay, ed.), (4): 2154-216.
- Eyuboglu M, Degirmenci E. Image reconstruction in Magnetic Resonance Conductivity Tensor Imaging (MRCTI) IEEE Trans Med Imaging. 2011 doi: 10.1109/TMI.2011.2171192. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gandhi OP. Electromagnetic fields: human safety issues. Annu Rev Biomed Eng. 2002;4:211–234. doi: 10.1146/annurev.bioeng.4.020702.153447. [DOI] [PubMed] [Google Scholar]
- Gorman S, Fox E, O’Donoghue D, Sheahan K, Hyland J, Mulcahy H, Loeb LA, O’Sullivan J. Mitochondrial mutagenesis induced by tumor-specific radiation bystander effects. J Mol Med (Berl) 2010;88(7):701–708. doi: 10.1007/s00109-010-0616-3. [DOI] [PubMed] [Google Scholar]
- Habash RW, Brodsky LM, Leiss W, Krewski D, Repacholi M. Health risks of electromagnetic fields. Part I: Evaluation and assessment of electric and magnetic fields. Crit Rev in Biomed Eng. 2003;31(3):41–195. doi: 10.1615/critrevbiomedeng.v31.i3.10. [DOI] [PubMed] [Google Scholar]
- Hei TK, Zhou H, Ivanov VN, Hong M, Lieberman HB, Brenner DJ, Amundson SA, Geard CR. Mechanism of radiation-induced bystander effects: a unifying model. The J Pharm and Pharmacol. 2008;60(8):943–950. doi: 10.1211/jpp.60.8.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Enríquez B, Guemez-Gamboa A, Morán J. Reactive oxygen species are related to ionic fluxes and volume decrease in apoptotic cerebellar granule neurons: role of NOX enzymes. J Neurochem. 2011;117(4):654–664. doi: 10.1111/j.1471-4159.2011.07231.x. [DOI] [PubMed] [Google Scholar]
- Hou TZ, Dawitof M, Wang JY, Li MD. Experimental evidence of a plant meridian system: I. Bioelectricity and acupuncture effects on electrical resistance of the soybean (Glycine max) Am J Chin Med. 1994;22(1):1–10. doi: 10.1142/S0192415X94000024. [DOI] [PubMed] [Google Scholar]
- Jasti AC, Wetzel BJ, Aviles H, Vesper DN, Nindl G, Johnson MT. Effect of a wound healing electromagnetic field on inflammatory cytokine gene expression in rats. Biomed Sci Instrum. 2001;37:209–214. [PubMed] [Google Scholar]
- Johansen C. Electromagnetic fields and health effects—epidemiologic studies of cancer, diseases of the central nervous system and arrhythmia-related heart disease. Scand J of Work Env Hea. 2004;30(Suppl 1):1–30. [PubMed] [Google Scholar]
- Kadhim MA, Lorimore SA, Hepburn MD, Goodhead DT, Buckle VJ, Wright EG. Alpha-particle-induced chromosomal instability in human bone marrow cells. Lancet. 1994;344(8928):987–988. doi: 10.1016/s0140-6736(94)91643-8. [DOI] [PubMed] [Google Scholar]
- Kadhim MA, Macdonald DA, Goodhead DT, Lorimore SA, Marsden SJ, Wright EG. Transmission of chromosomal instability after plutonium alpha-particle irradiation. Nature. 1992;355(6362):738–740. doi: 10.1038/355738a0. [DOI] [PubMed] [Google Scholar]
- Kaptchuk TJ. The placebo effect in alternative medicine: Can the performance of a healing ritual have clinical significance? Ann Intern Med. 2002;136(11):817–825. doi: 10.7326/0003-4819-136-11-200206040-00011. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Ives JA, Jonas WB. External bioenergy-induced increases in intracellular free calcium concentrations are mediated by Na+/Ca2+ exchanger and L-type calcium channel. Mol Cell Biochem. 2005;271(1–2):51–59. doi: 10.1007/s11010-005-3615-x. [DOI] [PubMed] [Google Scholar]
- Kiang JG, Marotta D, Wirkus M, Wirkus M, Jonas WB. External bioenergy increases intracellular free calcium concentration and reduces cellular response to heat stress. J Investig Med. 2002;50(1):38–45. doi: 10.2310/6650.2002.33516. [DOI] [PubMed] [Google Scholar]
- Kondo S. Hormetic effects on human cancer mortality are inducible only after long-term irradiation at low dose rates. Health Phys. 2011;100(3):340–341. doi: 10.1097/hp.0b013e3181e9b120. [DOI] [PubMed] [Google Scholar]
- Lee Y, Kim S, Son T, Kang D, Jung B. Meridian electrical potential response to acupuncture stimulation between operator and subject. JAcupunct Meridian Stud. 2010;3(4):249–254. doi: 10.1016/S2005-2901(10)60044-1. [DOI] [PubMed] [Google Scholar]
- Little MP. Do non-targeted effects increase or decrease low dose risk in relation to the linear non-threshold (LNT) model? Mutat Res. 2010;687(1–2):17–27. doi: 10.1016/j.mrfmmm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Mothersill CE, McNeill FE, Lyng FM, Byun SH, Seymour CB, Prestwich WV. A dose threshold for a medium transfer bystander effect for a human skin cell line. Radiat Res. 2006;166(1Pt 1):19–23. doi: 10.1667/RR3580.1. [DOI] [PubMed] [Google Scholar]
- Lohmann CH, Schwartz Z, Liu Y, Li Z, Simon BJ, Sylvia VL, Dean DD, Bonewald LF, Donahue HJ, Boyan BD. Pulsed electromagnetic fields affect phenotype and connexin 43 protein expression in MLO-Y4 osteocyte-like cells and ROS 17/2.8 osteoblast-like cells. J Orthop Res. 2003;21(2):326–334. doi: 10.1016/S0736-0266(02)00137-7. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Oxidative stress in cells exposed to low levels of ionizing radiation. Biochem Soc Trans. 2001;29(Pt 2):350–353. doi: 10.1042/0300-5127:0290350. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Seymour CB, Mothersill C. Early events in the apoptotic cascade initiated in cells treated with medium from the progeny of irradiated cells. Radiat Prot Dosimetry. 2002;99(14):169–172. doi: 10.1093/oxfordjournals.rpd.a006753. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Maguire P, McClean B, Seymour CB, Mothersill C. The involvement of calcium and MAP kinase signaling pathways in the production of radiation-induced bystander effects. Radiat Res. 2006;165(4):400–409. doi: 10.1667/rr3527.1. [DOI] [PubMed] [Google Scholar]
- Lyng FM, Howe OL, McClean B. Reactive oxygen species-induced release of signalling factors in irradiated cells triggers membrane signalling and calcium influx in bystander cells. Int J Radiat Biol. 2011;87(7):683–95. doi: 10.3109/09553002.2010.549533. [DOI] [PubMed] [Google Scholar]
- Maguire P, Mothersill C, McClean B, Seymour CB, Lyng FM. Modulation of radiation responses by pre-exposure to irradiated cell conditioned medium. Radiat Res. 2007;167(4):485–492. doi: 10.1667/RR0159.1. [DOI] [PubMed] [Google Scholar]
- Marder BA, Morgan WF. Delayed chromosomal instability induced by DNA damage. Mol Cell Biol. 1993;13(11):6667–6677. doi: 10.1128/mcb.13.11.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan WF, Sowa MB. Non-targeted effects of ionizing radiation: implications for risk assessment and the radiation dose response profile. Health Phys. 2009;97(5):426–432. doi: 10.1097/HP.0b013e3181ab98c7. [DOI] [PubMed] [Google Scholar]
- Mosse I, Marozik P, Seymour CB, Mothersill C. The effect of melanin on the bystander effect in human keratinocytes. Mutat Res. 2006;597(1–2):133–137. doi: 10.1016/j.mrfmmm.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Medium from irradiated human epithelial cells but not human fibroblasts reduces the clonogenic survival of unirradiated cells. International J Radiat Biol. 1997;71(4):421–427. doi: 10.1080/095530097144030. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Radiation-induced bystander effects: past history and future directions. Radiat Res. 2001;155(6):759–767. doi: 10.1667/0033-7587(2001)155[0759:ribeph]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Seymour CB. Actions of radiation on living cells in the “post bystander” era. EXS. 2006;(96):159–177. doi: 10.1007/3-7643-7378-4_7. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Lyng F, Seymour CB, Maguire P, Lorimore S, Wright E. Genetic factors influencing bystander signaling in murine bladder epithelium after low-dose irradiation in vivo. Radiat Res. 2005;163(4):391–399. doi: 10.1667/rr3320. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Bucking C, Smith RW, Agnihotri N, Oneill A, Kilemade M, Seymour CB. Communication of radiation-induced stress or bystander signals between fish in vivo. Environ Sci Technol. 2006;40(21):6859–6864. doi: 10.1021/es061099y. [DOI] [PubMed] [Google Scholar]
- Mothersill C, Moran G, McNeill F, Gow MD, Denbeigh J, Prestwich W, Seymour CB. A role for bioelectric effects in the induction of bystander signals by ionizing radiation? Dose Response. 2007;5(3):214–229. doi: 10.2203/dose-response.06-011.Mothersill. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothersill C, Saroya R, Smith RW, Singh H, Seymour CB. Serum serotonin levels determine the magnitude and type of bystander effects in medium transfer experiments. Radiat Res. 2010;174(1):119–123. doi: 10.1667/RR2036.1. [DOI] [PubMed] [Google Scholar]
- Murphy JB, Norton JJ. The effect of x-ray on the resistance to cancer in mice. Science. 1915;42(1093):842–843. doi: 10.1126/science.42.1093.842. [DOI] [PubMed] [Google Scholar]
- Nagazawa H, Little JB. Induction of sister chromatid exchanges by extremely low doses of alpha-particles. Cancer Res. 1992;52(22):6394–6396. [PubMed] [Google Scholar]
- Nguyen PK, Wu JC. Radiation exposure from imaging tests: is there an increased cancer risk? Expert Rev Cardiovasc Ther. 2011;9(2):177–183. doi: 10.1586/erc.10.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T. Biological consequences and health concerns from low-dose and low-dose-rate radiation in mice and humans. Health Phys. 2011;100(3):266–268. doi: 10.1097/hp.0b013e3181e9b10d. [DOI] [PubMed] [Google Scholar]
- Pirisi L, Creek KE, Doniger J, DiPaolo JA. Continuous cell lines with altered growth and differentiation properties originate after transfection of human keratinocytes with human papillomavirus type 16 DNA. Carcinogenesis. 1988;9(9):1573–1579. doi: 10.1093/carcin/9.9.1573. [DOI] [PubMed] [Google Scholar]
- Ponnaiya B, Cornforth MN, Ullrich RL. Radiation-induced chromosomal instability in BALB/c and C57BL/6 mice: the difference is as clear as black and white. Radiat Res. 1997;147(2):121–125. [PubMed] [Google Scholar]
- Poon RC, Agnihotri N, Seymour CB, Mothersill C. Bystander effects of ionizing radiation can be modulated by signaling amines. Environ Res. 2007;105(2):200–211. doi: 10.1016/j.envres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Rubin GJ, Nieto-Hernandez R, Wessely S. Idiopathic environmental intolerance attributed to electromagnetic fields (formerly “electromagnetic hypersensitivity”): An updated systematic review of provocation studies. Bioelectromagnetics. 2010;31(1):1–11. doi: 10.1002/bem.20536. [DOI] [PubMed] [Google Scholar]
- Rubik B. The biofield hypothesis: its biophysical basis and role in medicine. J Altern Complement Med. 2002;8(6):703–717. doi: 10.1089/10755530260511711. [DOI] [PubMed] [Google Scholar]
- Saroya R, Smith R, Seymour CB, Mothersill C. Injection of resperpine into zebrafish, prevents fish to fish communication of radiation-induced bystander signals: confirmation in vivo of a role for serotonin in the mechanism. Dose-Response. 2009;8(3):317–330. doi: 10.2203/dose-response.09-043.Saroya. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schettino G, Folkard M, Michael BD, Prise KM. Low-dose binary behavior of bystander cell killing after microbeam irradiation of a single cell with focused c(k) x rays. Radiat Res. 2005;163(3):332–336. doi: 10.1667/rr3319. [DOI] [PubMed] [Google Scholar]
- Schüz J, Lagorio S, Bersani F. Electromagnetic fields and epidemiology: an overview inspired by the fourth course at the International School of Bioelectromagnetics. Bioelectromagnetics. 2009;30(7):511–524. doi: 10.1002/bem.20510. [DOI] [PubMed] [Google Scholar]
- Sedelnikova OA, Redon CE, Dickey JS, Nakamura AJ, Georgakilas AG, Bonner WM. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat Res. 2010;704(1–3):152–159. doi: 10.1016/j.mrrev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. Lethal mutations, the survival curve shoulder and split-dose recovery. Int J Radiat Biol. 1989;56(6):999–1010. doi: 10.1080/09553008914552451. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C. All colonies of CHO-K1 cells surviving gamma-irradiation contain non-viable cells. Mutat Res. 1992;267(1):19–30. doi: 10.1016/0027-5107(92)90107-d. [DOI] [PubMed] [Google Scholar]
- Seymour CB, Mothersill C, Alper T. High yields of lethal mutations in somatic mammalian cells that survive ionizing radiation. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;50(1):167–179. doi: 10.1080/09553008614550541. [DOI] [PubMed] [Google Scholar]
- Shao C, Lyng FM, Folkard M, Prise KM. Calcium fluxes modulate the radiation-induced bystander responses in targeted glioma and fibroblast cells. Radiat Res. 2006;166(3):479–487. doi: 10.1667/RR3600.1. [DOI] [PubMed] [Google Scholar]
- Shao C, Prise KM, Folkard M. Signaling factors for irradiated glioma cells induced bystander responses in fibroblasts. Mut Res. 2008;638(1–2):139–145. doi: 10.1016/j.mrfmmm.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Swanson J, Kheifets L. Biophysical mechanisms: a component in the weight of evidence for health effects of power-frequency electric and magnetic fields. Radiat Res. 2006;165(4):470–478. doi: 10.1667/rr3522.1. [DOI] [PubMed] [Google Scholar]
- Ulett GA, Han J, Han S. Traditional and evidence-based acupuncture: history, mechanisms, and present status. Southern Med J. 1998;91(12):1115–1120. doi: 10.1097/00007611-199812000-00004. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, Davidson RJ, Kosslyn SM, Rose RM, Cohen JD. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Werneck MM, Barrientos EM. Fiberoptic transmission of biological signals. Med Prog Technol. 1994;20(1–2):59–62. [PubMed] [Google Scholar]
- Wiegant F, Van Wijk R. The similia principle: results obtained in a cellular model system. Homeopathy. 2010;99(1):3–14. doi: 10.1016/j.homp.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Van Wijk EP, Van Wijk R, Bajpai RP. Quantum squeezed state analysis of spontaneous ultra-weak light photon emission of practitioners of meditation and control subjects. Indian J Exp Biol. 2008;46(5):345–352. [PubMed] [Google Scholar]
- Yamaguchi S, Ogiue-Ikeda M, Sekino M, Ueno S. Effects of pulsed magnetic stimulation on tumor development and immune functions in mice. Bioelectromagnetics. 2006;27(1):64–72. doi: 10.1002/bem.20177. [DOI] [PubMed] [Google Scholar]
- Yu T, Tsai HL, Hwang ML. Suppressing tumor progression of in vitro prostate cancer cells by emitted psychosomatic power through Zen meditation. Am J Chin Med. 2003;31(3):499–507. doi: 10.1142/S0192415X03001132. [DOI] [PubMed] [Google Scholar]
- Ye X, Zhang S. The effect of electroacupuncture “zusanli” and “neiguan” points on membrane fluidity of red cells in rabbits. Zhen Ci Yan Jiu. 1992;17(1):42–44. [PubMed] [Google Scholar]
- Zyuzikov NA, Coates PJ, Parry JM, Lorimore SA, Wright EG. Lack of non-targeted effects in murine bone marrow after low-dose in vivo X irradiation. Radiat Res. 2011;175(3):322–327. doi: 10.1667/RR2386.1. [DOI] [PubMed] [Google Scholar]